Techniques d'anesthésie pour les risques de récidive de tumeurs malignes
Referencias
References to studies included in this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Secondary data analysis of a single‐centre double‐blinded RCT | |
| Participants | 89 adult patients scheduled for major abdominal surgery for cancer | |
| Interventions | Intraoperative and postoperative epidural analgesia vs general anaesthesia alone (IV opioids) | |
| Outcomes | Overall survival; recurrence‐free survival | |
| Notes | 163 participants were randomly assigned in the prospective trial; 153 completed the study, 132 of those had malignancy and 89 of those underwent primary tumour resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The original trial was described as randomized, but no information on the randomization process was given. Analysed subgroup might not be perfectly balanced |
| Allocation concealment (selection bias) | Unclear risk | No information was given |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants was attempted with sham SC catheter, but placement of epidural took place preoperatively in awake participants, while SC catheter was placed postoperatively while participant was anaesthetised. Blinding of caregivers was not reported. Incomplete blinding likely did not influence the outcome |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that outcome assessment was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was properly described Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. Outcomes are reported in accordance with the methods section of the published study |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Methods | Secondary data analysis of multi‐centre RCT (subgroup) | |
| Participants | 112 male adult ASA III patients undergoing surgery for colon cancer | |
| Interventions | Intraoperative and postoperative epidural analgesia with bupivacaine, epinephrine and morphine vs general anaesthesia alone (IV or IM opioids) | |
| Outcomes | Overall survival | |
| Notes | 1021 participants randomly assigned in prospective multi‐centre trial; 982 completed the study; of those 177 with colon cancer and available pathology staging data; of those, 112 without metastasis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Original study: adaptive randomization scheme within each site (balanced variables: type of surgery, age, Goldman index). However, even distribution of subgroup analysed is not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were not blinded. However, it was judged unlikely that the outcome was influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that the outcome assessment was influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Pathological staging data could not be obtained for 70 participants and were excluded from the analysis. The survival experience for these 70 participants was similar to that for the 177 participants for whom staging data were available Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. Outcomes are reported in accordance with the methods section of the published study |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Methods | Secondary data analysis of a multi‐centre RCT (subgroup) | |
| Participants | 446 adult patients scheduled for major abdominal surgery for primary cancer without metastasis | |
| Interventions | Intraoperative and postoperative epidural analgesia vs general anaesthesia alone (IV opioids) | |
| Outcomes | Primary endpoint: progression‐free survival; secondary endpoint: overall survival, time to tumour progression | |
| Notes | 915 participants were included in the prospective multi‐centre trial; 506 of those had undergone surgery for cancer, and 3 were unclassified/excluded. 31 participants were excluded because of metastasis at the time of surgery. 26 additional participants were excluded because they were lost to follow‐up or refused to provide consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization for the original study was based on use of random permuted blocks within each institution, and was maintained and allocated to each institution by a central trial secretariat at the Department of Public Health. Baseline characteristics of the analysed subgroup are reported and comparable |
| Allocation concealment (selection bias) | Low risk | Random permuted blocks used, assigned by central allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and caregivers were not blinded. Likely no influence on outcome |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that outcome assessment was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | In all, 26 cases (14 participants in the study group and 12 in the control group) were lost to follow‐up and were not included in the data analysis. Characteristics of this group have not been reported Only 4 (< 1%) of the participants had incomplete (censored) data within the first 5 years after surgery Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. One secondary outcome (TTR) was not specified or defined in the methods section of the published article but was reported in the results section. Primary outcome was reported in accordance with the methods section of the published study |
| Other bias | Low risk | The number of included participants varies between 445 (forest plot), 446 (text) and 447 (flow chart), most likely as the result of calculation error. However, as the difference is only 1 among more than 450 participants, we find that this most likely does not introduce bias |
| Methods | Secondary data analysis of a single‐centre RCT | |
| Participants | 99 adult male patients undergoing radical prostatectomy and bilateral lymphadenectomy for adenocarcinoma of the prostate | |
| Interventions | Intraoperative general anaesthesia + epidural analgesia vs general anaesthesia alone (IV morphine) | |
| Outcomes | Clinical evidence or biochemical recurrence of prostate cancer (defined as PSA > 0.2 ng/mL) | |
| Notes | 102 participants randomly assigned in prospective single‐centre trial; 99 completed the protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated table of random numbers, participants were block randomly assigned (block size = 10) |
| Allocation concealment (selection bias) | Low risk | Blinded study envelopes, which were opened immediately before surgery. Then, treatment allocation is predictable towards the end of a block of 10 if block size is known |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and caregivers were not blinded. Likely no influence on outcome |
| Blinding of outcome assessment (detection bias) | Low risk | Treatment allocation was temporarily removed from the data set during the censoring process in an attempt to make it as non‐informative as possible |
| Incomplete outcome data (attrition bias) | Low risk | 102 participants were randomly assigned, 51 to each group. It was not possible to site the epidural catheter for two participants in the epidural group, and one participant with renal failure was recruited to the control group in violation of protocol. These 3 participants were excluded from the study (no data available) In 22 cases (14 participants in the study group and eight in the control group), outcome data (PSA) were not available Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Unclear risk | Published article uses 'survival,' 'disease‐free survival' and 'recurrence' to describe outcomes. It remains unclear whether these terms are used interchangeably or if 'survival' as used in the methods section was a predefined outcome that then was not reported |
| Other bias | Unclear risk | Progress of participants through the trial flow chart (Figure 1) is inconsistent in terms of numbers of included patients. The breakdown of the general anaesthesia group (n = 50) calculates to 54 participants, and it is unclear how this mismatch might influence data analysis Outcome definition does not match any of the standardized outcomes used for this review. The wording appears to come closest to our outcome of 'time to tumour progression (TTP)' = time elapsed between surgery and tumour progression, with the difference that prostate cancer–related deaths were considered to show tumour progression. However, only one participant in each group died of prostate cancer and was included in the calculation of TTP |
RCT = randomized controlled trial.
ASA = American Society of Anesthesiologists physical status classification.
IV = intravenous.
IM = intramuscular.
PSA = prostate‐specific antigen.
TTP = time to progression.
TTR = time to recurrence
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Trial name or title | Perioperative Epidural Analgesia for Short‐term and Long‐term Outcomes of Pancreatic Cancer Surgery—Randomized Trial |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 20‐85 years of age with pancreatic cancer, scheduled for curative Whipple procedure Estimated enrolment: 150 participants |
| Interventions | Epidural patient‐controlled analgesia (PCEA) with bupivacaine + fentanyl vs intravenous patient‐controlled analgesia (PCA) with morphine for postoperative pain |
| Outcomes | 1‐year survival rate (secondary outcome) |
| Starting date | 2012 |
| Contact information | National Taiwan University Hospital, Department of Anesthesiology. PI: Kuang Cheng Chan, MD |
| Notes |
| Trial name or title | Comparing Local Anesthesia With General Anesthesia for Breast Cancer Surgery |
| Methods | Randomized single‐blinded trial |
| Participants | Female patients 21‐75 years of age, ASA I‐II, diagnosed with biopsy‐proven breast cancer, scheduled for mastectomy and axillary node dissection in a single procedure Estimated enrolment: 40 participants |
| Interventions | Local anaesthesia + sedation vs general anaesthesia |
| Outcomes | Disease‐free survival up until 5 years after surgery |
| Starting date | 2008 |
| Contact information | Mackay Memorial Hospital, Taipei/Taiwan. PI: Yuan‐Ching Chang, MD |
| Notes |
| Trial name or title | Epidural Versus Patient‐Controlled Analgesia for Reduction in Long‐term Mortality Following Colorectal Cancer Surgery (EPICOL) |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 40‐80 years of age, ASA I‐III, undergoing elective surgery for colorectal cancer Estimated enrolment: 300 participants |
| Interventions | Epidural analgesia with ropivacaine and opioid vs PCA with morphine |
| Outcomes | All‐cause mortality and cancer recurrence up until 5 years after surgery |
| Starting date | 2011 |
| Contact information | Örebro University, Sweden. PI: Anil Gupta |
| Notes |
| Trial name or title | Prevention of Post‐Mastectomy Breast Pain Using Ambulatory Continuous Paravertebral Blocks |
| Methods | Randomized double‐blind controlled trial |
| Participants | Female patients 18 years of age and older, undergoing unilateral or bilateral mastectomy Estimated enrolment: 60 participants |
| Interventions | Postoperative paravertebral catheter analgesia with ropivacaine vs placebo (normal saline) |
| Outcomes | Cancer recurrence up until 3 years after surgery |
| Starting date | 2010 |
| Contact information | University of California, San Diego. PI: Brian Ilfeld, MD, MS |
| Notes |
| Trial name or title | Regional Anesthesia in Patients Undergoing Colon‐Rectal Surgery |
| Methods | Randomized controlled double‐blind clinical trial |
| Participants | Patients scheduled for open laparoscopic or laparoscopic assisted surgery for colon cancer Estimated enrolment: 2500 participants |
| Interventions | General anaesthesia followed by postoperative opioid analgesia vs intraoperative and postoperative regional anaesthesia and analgesia (epidural or paravertebral anaesthesia) plus intraoperative general anaesthesia |
| Outcomes | Cancer recurrence up to 5 years after surgery |
| Starting date | 2007 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Andrea Kurz, MD |
| Notes | Multi‐centre study |
| Trial name or title | The Effect of Adding Intraoperative Regional Anesthesia on Cancer Recurrence in Patients Undergoing Lung Cancer Resection |
| Methods | Randomized double‐blinded controlled clinical trial |
| Participants | Male and female patients 18‐85 years of age diagnosed with primary non‐small cell lung cancer and scheduled for potentially curative tumour resection Estimated enrolment: 1532 participants |
| Interventions | Intraoperative and postoperative general anaesthesia + epidural anaesthesia and analgesia vs general anaesthesia and postoperative intravenous analgesia |
| Outcomes | Disease‐free survival up to 5 years after surgery |
| Starting date | 2010 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Andrea Kurz, MD |
| Notes |
| Trial name or title | Thoracoscopic Lobectomy Using Thoracic Epidural Anesthesia Versus General Anesthesia for Lung Cancer Patients |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 25‐80 years of age diagnosed with non‐small cell lung cancer with clinical staging of I or II for whom thoracoscopic lobectomy (VATS) is feasible Estimated enrolment: 100 participants |
| Interventions | Intraoperative general anaesthesia vs intraoperative thoracic epidural anaesthesia |
| Outcomes | Overall survival up until 5 years after surgery |
| Starting date | 2010 |
| Contact information | National Taiwan University Hospital. PI: Yung‐Chie Lee, MD, PhD |
| Notes |
| Trial name or title | Regional Anesthesia and Breast Cancer Recurrence |
| Methods | Randomized controlled trial |
| Participants | Female participants 18‐85 years of age diagnosed with primary breast cancer without known extension beyond the breast and with axillary nodes scheduled for unilateral or bilateral mastectomy with or without implant or isolated "lumpectomy" with axillary node dissection (anticipated removal of at least 5 nodes) Estimated enrolment: 1100 participants |
| Interventions | Regional anaesthesia and analgesia (epidural or paravertebral), combined with deep sedation or general anaesthesia (sevoflurane) vsgeneral anaesthesia (sevoflurane) followed by opioid administration |
| Outcomes | Cancer recurrence rate up until 10 years after surgery |
| Starting date | 2007 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Daniel I. Sessler, MD |
| Notes | Multi‐centre study |
| Trial name or title | Anesthesia and Cancer Recurrence im Malignant Melanoma |
| Methods | Randomized single blinded (outcome assessor) clinical trial |
| Participants | Patients scheduled for inguinal lymph node dissection because of malignant melanoma of the lower limb Estimated enrolment: 230 participants |
| Interventions | Spinal anaesthesia vs general anaesthesia |
| Outcomes | Overall survival up to 5 years after surgery |
| Starting date | 2012 |
| Contact information | University Hospital Muenster, Department of Anesthesia, Intensive Care and Pain Therapy. Study Chair: Hugo K. van Aken, MD, PhD |
| Notes |
ASA = American Society of Anesthesiologists.
PCA = patient‐controlled analgesia.
VATS = video‐assisted thoracic surgery.
PCEA = epidural patient‐controlled analgesia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 647 | Hazard Ratio (Random, 95% CI) | 1.02 [0.78, 1.34] |
| Analysis 1.1  Comparison 1 GA + RA versus GA, Outcome 1 Overall survival. | ||||
| 2 Progression‐free survival Show forest plot | 2 | 535 | Hazard Ratio (Random, 95% CI) | 0.88 [0.56, 1.38] |
| Analysis 1.2 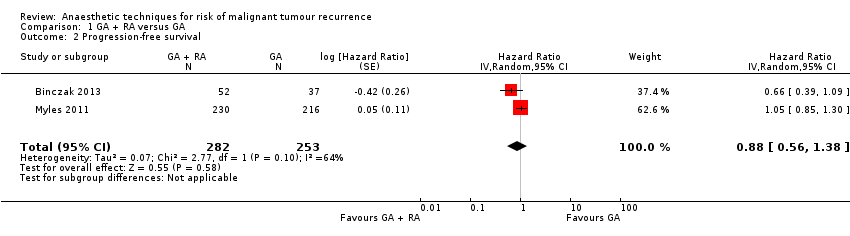 Comparison 1 GA + RA versus GA, Outcome 2 Progression‐free survival. | ||||
| 3 Time to tumour progression Show forest plot | 2 | 545 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.00, 2.25] |
| Analysis 1.3 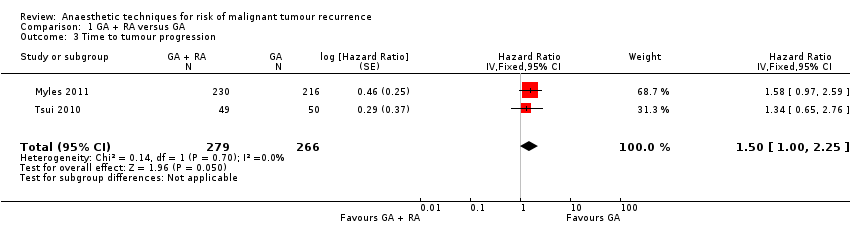 Comparison 1 GA + RA versus GA, Outcome 3 Time to tumour progression. | ||||

Study flow diagram.
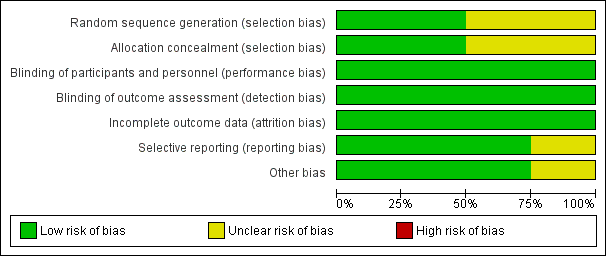
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.1 overall survival.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.2 progression‐free survival.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.3 time to tumour progression.

Comparison 1 GA + RA versus GA, Outcome 1 Overall survival.

Comparison 1 GA + RA versus GA, Outcome 2 Progression‐free survival.

Comparison 1 GA + RA versus GA, Outcome 3 Time to tumour progression.
| Epidural anaesthesia in addition to general anaesthesia compared with general anaesthesia alone for patients undergoing primary tumour surgery | |||||
| Patient or population: patients undergoing primary tumour surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect† | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| General anaesthesia alone (control) | Epidural anaesthesia in addition to general anaesthesia (intervention) | ||||
| Death from all causes 7.8‐14.8 years (Myles) 8.3‐10.75 years (Christopherson) | Study population | HR 1.03 | 647 | ⊕⊕⊝⊝ | |
| 805 per 1000a | 815 per 1000 | ||||
| Tumour progression or death from all causes 7.8‐14.8 yearsd | Study population | HR 0.88 | 535 | ⊕⊝⊝⊝ | |
| 944 per 1000d | 921 per 1000 | ||||
| Tumour progression 4.5 yearsf | Study population | HR 1.50 | 545 | ⊕⊝⊝⊝ | |
| 360 per 1000g | 488 per 1000 | ||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| HR = hazard ratio, defined as intervention/control. †HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group. aThe assumed risk and the range of follow‐up times are based on data reported by Myles and Christophersen. Data on absolute events per group were not reported by Binczak. | |||||
| Number of participants | Recruitment site(s) | Age (years) | Male sex | ASA | Type of surgery | Outcome data derived from | |
| 112 | USA; multi‐centre | Control group: 69.1 ± 7.8 Epidural group: 68.6 ± 7.7 | Male only | IIIa | Elective surgery for colon cancer | Veterans Affairs Beneficiary Information and Records Locator System (VA BIRLS) | |
| 446 | Australia, East Asia, Middle East; multi‐centre (MASTERS trial) | Control group: 70 ± 11 Epidural group: 71 ± 9.5 | Control group: 53% Epidural group: 60% | 'High risk patients'b | Major abdominal surgery for cancer | 1. Medical hospital record 2. Contact with participant's general practitioner 3. State‐based cancer registry or National Health Index 4. Participant contact 5. Contact with next of kin | |
| 99 | Canada; single‐centre | Control group: 63.9 ± 6.1 Epidural group: 63.0 ± 5.5 | Male only | ASA I‐III | Radical prostatectomy and bilateral pelvic lymphadenectomy | Participant's hospital charts and medical records | |
| 89 | France; single‐centre | Not reported for subcohort (mean for full cohort 58 years) | Not reported for subcohort (full cohort includes > 62% male) | Not reported | Major abdominal surgery for cancer | 1. Hospital intern cancer registry 2. Participant contact 3. French National Registry | |
| ASA = American Society of Anesthesiologists physical status classification. USA = United States of America. aThe study by Christopherson 2008 reports that ASA I‐III patients were included. However, the original trial included only ASA III patients (Park 2001). bAccording to the inclusion criteria noted in the original study (Rigg 2002), 'high risk' translates to ASA II‐III. | |||||||
| GA maintenance | Epidural catheter level | Time placed | Duration | LA used | Epidural medications intraoperatively | Epidural medications postoperatively | Intraoperative IV opioids | Postoperative IV opioids | |
| Isoflurane 0.9% (mean) + N2O | Thoracic or lumbar epidural catheter | Preoperatively | "as long as needed" | Bupivacaine 0.5% | 3‐6 mg morphine; 25‐50 mg boluses bupivacaine/3‐5 hours as needed; epinephrine | 25‐50 mg boluses bupivacaine/3‐5 hours as needed; morphine 3‐6 mg/12‐24hours as needed | Fentanyl for both groups | Morphine, meperidine as needed (IV in epidural group, IV or IM in control group) | |
| Balanced anaesthesia (volatile anaesthetic not specified), N2O use not specified or recorded, but usual practice was to include it | At discretion of the anaesthesiologist "With the exception of some pelvic operations, all epidural catheters were inserted in the thoracic region" | Preoperatively | 3 days after surgery | Bupivacaine or ropivacaine | Bupivacaine or ropivacaine | Continuous infusion of ropivacaine or bupivacaine, supplemented with fentanyl or pethidine | Fentanyl pethidine | Postoperative opioids, mostly PCA in control group (fentanyl, pethidine) | |
| Isoflurane 1‐2% + N2O 60% | Low thoracic or high lumbar epidural catheter | Preoperatively | Not reported | Ropivacaine | Ropivacaine bolus + continuous infusion; fentanyl | Not reported | Morphine for control group | Not reported | |
| Isoflurane 1‐2% + N2O 70% | Thoracic 7‐11 | Preoperatively | Until 5th postoperative day | Bupivacaine | 50 mg bupivacaine as needed; epinephrine | 12.5 mg/h bupivacaine; 0.25 mg/h morphine | Fentanyl for both groups | Epidural group: morphine boluses SC as needed; control group: 2.5 mg/h morphine SC via catheter | |
| GA = general anaesthesia. LA = local anaesthetic. IV = intravenous. IM = intramuscular. SC = subcutaneous. | |||||||||
| Tumour stage (TNM) | Clinical vs pathologic staging | Median overall survival (95% CI) | Median progression‐free survival | Median time to tumour progression | 5‐Year survival | Follow‐up time | Statistical test used (uni‐ vs. multivariable) | |
| All T, N0, M0 | Pathological | 6.14 (5.22 to 7.99) | Not reported | Not reported | Not reported | Up to 9 years | Data extracted from Kaplan‐Meier curve; HR and SEHR calculated according to Tierney (Tierney 2007) | |
| All T, all N, no distant metastasis (M0) 'complete surgical excision' | Not reported | Epidural group: 3.3 (95% CI 2.1 to 4.5) Control group: 3.7 (95% 2.0 to 5.4) | Epidural group: 2.6 (IQR 0.7 to 8.7) Control group: 2.8 (IQR 0.7 to 8.7) | Epidural group: 1.1 (95% CI 0.7 to 1.6) Control group: 1.4 (95% CI 0.6 to 2.3) | Epidural group: 42% Control group: 44% | Up to 12 years | Univariable testing, log‐rank statistics, intention‐to‐treat analysis | |
| All T, all N, M not reported | Pathological | Not reported | Not reported | 1644 days | Not reported | Up to 3403 days (˜9.3 years) | Unadjusted Cox model, no intention‐to‐treat analysis | |
| Primary tumour resection (all stages) with or without residual disease postoperatively | Not reported | Not reported | Not reported | Not reported | Not reported | Up to 17 years | Unadjusted HR (reported by the contact author through personal communication) | |
| IQR = interquartile range. TNM classification of malignant tumours: T = tumour size, N = lymph node involvement, M = distant metastasis. HR = hazard ratio. SEHR = standard error of hazard ratio. CI = confidence interval. | ||||||||
| Study PI | Start date (clinical trials.gov) | Population | Sample size | Intervention | Control group |
| 2007 | Female patients 18‐85 years of age, diagnosed with primary breast cancer without known extension beyond the breast and axillary nodes, scheduled for unilateral or bilateral mastectomy with or without implant or isolated "lumpectomy" with axillary node dissection (anticipated removal of at least 5 nodes) | 1100 | Regional anaesthesia and analgesia (epidural or paravertebral), combined with deep sedation or general anaesthesia (sevoflurane) | General anaesthesia (sevoflurane) followed by opioid administration | |
| 2008 | Patients scheduled for open, laparoscopic or laparoscopic‐assisted surgery for colon cancer without known extension beyond colon | 2500 | Intraoperative and postoperative regional anaesthesia and analgesia (epidural or paravertebral anaesthesia) plus intraoperative general anaesthesia | General anaesthesia followed by postoperative opioid analgesia | |
| 2009 | Female patients 21‐75 years of age, ASA I‐II, diagnosed with biopsy‐proven breast cancer, scheduled for mastectomy and axillary node dissection in a single procedure | 40 | Local anaesthesia + sedation | General anaesthesia | |
| 2010 | Male and female patients 18‐85 years of age, diagnosed with primary non‐small cell lung cancer and scheduled for potentially curative tumour resection | 1532 | Intraoperative and postoperative general anaesthesia + epidural anaesthesia and analgesia | General anaesthesia and postoperative intravenous analgesia | |
| 2010 | Female patients 18 years of age and older, undergoing unilateral or bilateral mastectomy | 60 | Postoperative paravertebral catheter analgesia with ropivacaine | Placebo (normal saline) | |
| 2011 | Male and female patients 40‐80 years of age, ASA I‐III, undergoing elective surgery for colorectal cancer | 300 | Epidural analgesia with ropivacaine and opioid | PCA with morphine | |
| 2011 | Male and female patients 25‐80 years of age, diagnosed with non‐small cell lung cancer with clinical staging of I or II for whom thoracoscopic lobectomy (VATS) is feasible | 100 | Intraoperative thoracic epidural anaesthesia | Intraoperative general anaesthesia | |
| 2012 | Patients scheduled for inguinal lymph node dissection because of malignant melanoma of the lower limb | 230 | Spinal anaesthesia | General anaesthesia | |
| 2013 | Male and female patients 20‐85 years of age with pancreatic cancer, expected to receive curative Whipple operation | 150 | Epidural analgesia with ropivacaine and opioid | PCA with opioid | |
| VATS = video‐assisted thoracic surgery. ASA = American Society of Anesthesiologists physical status classification. PCA = patient‐controlled analgesia. | |||||
| Author year | Type of cancer | Type of surgery | Intervention 1 (n) | Intervention 2 (n) | Control (n) | Endpoint | Statistical method | Result* | Date of surgery | Follow‐up until |
| Breast CA | Mastectomy + LND | GA + paravertebral catheter (50) | ‐ | GA (79) | Time to tumour recurrence (local or metastasis) | Adjusted Cox regression | HR 0.21 (0.06‐0.71) | 2001‐2002 | 2005 | |
| Cervical CA | First brachytherapy (of several) | SPA or EC (63) | ‐ | GA (69) | 1. Time to tumour recurrence 2. Overall survival | Adjusted Cox regression | 1. HR 0.95 (0.54‐1.67) 2. HR 1.46 (0.81‐2.61) | 1996‐2003 | nr | |
| Colon CA | Colorectal cancer surgery (open) | GA + EC preop (302) | ‐ | GA (58) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.82 (0.30‐2.19) | 2004‐2009 | 2009 | |
| Rectal CA | Colorectal cancer surgery (open) | GA + EC preop (260) | ‐ | GA (35) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.45 (0.22‐0.90) | 2004‐2009 | 2009 | |
| Vogelaar 2012 (abstract) | Colon CA | Surgery for colon CA | EC 'perioperative' (407) | ‐ | GA (198) | Overall survival | Adjusted Cox regression | HR 0.93 (0.93‐0.98) | 1995‐2003 | 2011 |
| (abstract) | Colon CA | Primary colon surgery | GA + EC (182) | ‐ | GA (931) | Tumour recurrence | Univariable | HR 1.33 (0.94‐1.87) | 2001‐2006 | 2009 |
| Colorectal CA | Colorectal cancer surgery | GA + EC preop (256) | ‐ | GA (253) | Time to tumour recurrence | Adjusted Cox model with stratification on propensity score quintiles | HR 0.74 (0.45‐1.22) | 2000‐2007 | 2008 | |
| Colorectal CA w/no metastases | Open colectomy | EC (Medicare code) (9670) | ‐ | No EC (Medicare code) (32481) | 1. Overall survival 2. 4‐Year tumour recurrence | 1. Adjusted marginal Cox model with propensity score as co‐variate 2. Adjusted logistic regression | 1. HR 0.92 (0.88‐0.96) 2. OR 1.05 (0.95‐1.15) | 1996‐2005 | 2009 | |
| Colorectal CA | Laparoscopic resection | EC preop (107) | SPA (144) | GA + PCA (173) | 1. Overall survival 2. Disease‐free survival | KM estimate, log‐rank test | 1. P value 0.622 2. P value 0.490 | 2003‐2010 | ||
| Hepatocellular CA | Percutaneous radiofrequency ablation | GA + EC preop (62) | ‐ | GA (117) | 1. Recurrence‐free survival 2. Overall survival | Adjusted Cox model with propensity score as co‐variate | 1. 3.66 (2.59‐5.15) 2. 0.77 (0.50‐1.18) | 1999‐2008 | 2011 | |
| Malignant melanoma | Lymph node dissection | SPA (52) | ‐ | GA (221) | Long‐term survival | Mean survival (months) of matched pairs (52 pairs) | 95.9 (81.2‐110.5) SPA 70.4 (53.6‐87.1) GA P value 0.087 | 1998‐2005 | 2009 | |
| Malignant melanoma | Melanoma resection | Local anaesthesia (376) | ‐ | GA (190) | Survival | KM estimate, log‐rank test | P value 0.51 (stage pT1/2, n = 237) P value 0.006 (stage pT3a, n = 195) in favour of local anaesthesia P value 0.47 (stage pT3b/4, n = 134) | Control: 1972‐1980 Intervention: 1981‐88 | 1988 | |
| Malignant melanoma w/no metastases | Primary melanoma excision | Local anaesthesia (2185) | ‐ | GA (2136) | Survival | Log‐rank test on matched pairs (1501 pairs) | P value < 0.01 in favour of local anaesthesia | 1976‐1986 | nr | |
| Ovarian CA | Surgery for ovarian cancer | GA + EC preop (26) | GA intraop/EC postop (29) | GA (127) | 1. Overall survival 2. Time to recurrence | 1. Median survival time (months), log‐rank test 2. Adjusted Cox model | 1. 71 m (62‐80) for GA 96 m (84‐109) for EC intraop 70 m (58‐83) for EC postop P value 0.01 for GA vs EC intraop (favours EC intraop) 2. HR 0.37 (0.19‐0.73) for intraop EC HR 0.86 (0.52‐1.41) for postop EC | 2000‐2006 | 2009 | |
| Ovarian CA | Surgery for ovarian cancer | EC only preop (106) | ‐ | GA (37) | Survival time | Adjusted Cox regression on propensity matched pairs (29 pairs) | HR 0.83 (0.67‐0.99) | 1994‐2006 | 2008 | |
| (abstract) | Ovarian CA | Primary radical tumour debulking | EC preop + GA (72) | GA (33) | 1. Recurrence‐free survival 2. Overall survival | KM estimate, log‐rank test | 1. HR 1.52 (1.4‐1.56), P value 0.008 2. nr | 2003‐2010 | nr | |
| Ovarian cancer (Figo IIIc‐IV) | Exploratory laparotomy | EC preop or postop + GA (37) | GA (43) | 1. Time to recurrence 2. Cancer‐specific survival | Adjusted Cox regression with propensity score weighting | 1. HR 0.65 (0.40‐1.08) 2. HR 0.59 (0.32‐1.08) | 2000‐2011 | nr | ||
| Kienbaum 2010/Alexander 2009 (abstracts) | Pancreatic CA | Radical pancreatic tumour resection | GA + EC (71) | ‐ | GA (29) | Overall survival | Log‐rank | P value 0.05 (P value 0.025 in favour of control for participants receiving high‐dose epidural opioids) | 2005‐2008 | nr |
| Prostate CA | Open radical prostatectomy | GA + EC preop (102) | ‐ | GA (123) | BCR‐free survival | Univariable Cox regression on propensity matched pairs (71 pairs) | HR 0.48 (0.23‐1.00) | 1994‐2003 | 2006 | |
| Prostate CA w/no metastasis | Radical prostatectomy | GA + EC preop (578) | ‐ | GA (533) | BCR‐free survival | Adjusted Cox model | HR 0.84 (0.52‐1.17) | 1993‐2006 | 2006 | |
| Prostate CA (all stages) | Open radical retropubic prostatectomy w/LND | GA + EC preop (103) | ‐ | GA (158) | 1. BCR‐free survival 2. Clinical progression‐free survival 3. Cancer‐specific survival 4. Overall survival | Adjusted Cox model with propensity score as co‐variate | 1. HR 0.82 (0.50‐1.34) 2. HR 0.40 (0.20‐0.79) 3. HR 0.95 (0.36‐2.47) 4. HR 1.01 (0.44‐2.32) | Intervention: 1994‐1997 Control: 1997‐2000 | nr | |
| Prostate CA (pT3/4) | Retropubic radical prostatectomy w/LND | GA + EC preop (67) | ‐ | GA (81) | 1. BCR‐free survival 2. Local recurrence‐free survival 3. Distant recurrence‐free survival 4. Cancer‐specific survival 5. Overall survival | Univariable Cox regression on matched pairs (67 pairs) | 1. HR 1.00 (0.69‐1.47) 2. HR 1.16 (0.41‐3.29) 3. HR 0.56 (0.26‐1.25) 4. HR 0.96 (0.45‐2.05) 5. HR 1.17 (0.63‐2.17) | 1994‐2000 | nr | |
| Several statistical methods were used in most studies. We weighted reported results in the following descending order: adjusted regression with propensity score or matched pairs, adjusted regression, univariable analysis. Only the highest weighted analysis is reported in the table. HR = hazard ratio, defined as intervention/control. *HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group. We adjusted the HR derived from individual trials accordingly, as needed. bold font denotes significant results in favour of the intervention group (EC). italic font denotes significant results in favour of the control group (GA). CA = cancer. pT = pathological tumour staging. EC = epidural catheter. SPA = spinal anaesthesia. GA = general anaesthesia. LND = lymph node dissection. preop = preoperatively. postop = postoperatively. n = number of participants. OR = odds ratio. n.s. = non‐significant. BCR = biochemical recurrence. nr = not reported. m = months. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 647 | Hazard Ratio (Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Progression‐free survival Show forest plot | 2 | 535 | Hazard Ratio (Random, 95% CI) | 0.88 [0.56, 1.38] |
| 3 Time to tumour progression Show forest plot | 2 | 545 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.00, 2.25] |

