Técnicas anestésicas para el riesgo de recidiva de tumores malignos
Appendices
Appendix 1. Search strategies
Search strategy for PubMed (1950 to present)
| Search #9 AND #11 | |
| Search randomized controlled trial OR randomized controlled trials OR controlled clinical trial OR controlled clinical trials OR random* OR trial OR trials OR groups OR double blind method OR double blind methods OR single blind method OR single blind methods OR clinical trial OR clinical trials OR research design OR controlled study OR controlled studies OR "clinical study" OR "clinical studies" OR control OR controlled OR controls | |
| Search #8 AND (animals[mh] NOT humans[mh]) | |
| Search #8 NOT (animals[mh] NOT humans[mh]) | |
| Search #7 NOT (editorial[pt] OR letter[pt] OR case reports[pt] OR news[pt] OR newspaper article[pt]) | |
| Search #3 OR #5 OR #6 | |
| Search #4 AND (neoplasm*[ti] OR tumor*[ti] OR tumour*[ti] OR cancer*[ti]) AND (recur*[ti] OR risk*[ti] OR metasta*[ti]) | |
| Search #2 AND #4 AND neoplasm[mh] AND adverse effects[sh] | |
| Search opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl alcohol" OR bumecain OR bupivacaine OR butamben OR carbizocaine OR carticaine OR chloralose OR chloroprocaine OR cyclopropane OR desflurane OR diazepam OR dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine OR "magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane OR anesthe*[ti] OR anaesthe*[ti] OR analges*[ti] | |
| Search #1 AND #2 | |
| Search neoplasm recurrence, local[mh] OR neoplasm invasiveness[mh] OR neoplasm metastasis[mh] OR cocarcinogenesis[mh] | |
| Search "anesthesia and analgesia"[mh:noexp] OR anesthesia[mh] OR analgesia[mh:noexp] OR analgesia, epidural[mh] OR analgesia, patient‐controlled[mh] OR anesthetics[majr] OR anesthetics/adverse effects OR anesthetics/immunology OR anesthetics/pharmacology OR analgesics[majr] OR analgesics/adverse effects OR analgesics/immunology OR analgesics/pharmacology OR adjuvants, anesthesia[mh] |
The PubMed search will use a combination of Medical Subject Headings and Keyword terms.
Search strategy for EMBASE (1974 to present)
| #14 | #11 AND #13 |
| #13 | 'randomized controlled trial' OR 'randomized controlled trials' OR 'controlled clinical trial' OR 'controlled clinical trials' OR random*:ab,ti OR 'double blind procedure' OR 'double blind procedures' OR 'single blind procedure' OR 'single blind procedures' OR 'clinical trial' OR 'clinical trials' OR 'controlled study' OR 'controlled studies' OR 'clinical study'/de OR 'major clinical study'/exp |
| #12 | #10 AND [animals]/lim NOT [humans]/lim |
| #11 | #10 NOT ([animals]/lim NOT [humans]/lim) |
| #10 | #9 NOT ('editorial'/de OR 'letter'/de OR 'case report'/de) |
| #9 | #3 OR #6 OR #7 OR #8 |
| #8 | anesthe*:ti OR anaesthe*:ti OR analges*:ti AND metasta*:ti |
| #7 | anesthe*:ti OR anaesthe*:ti OR analges*:ti AND (neoplasm*:ti OR tumor*:ti OR tumour*:ti OR cancer*:ti) AND (recur*:ti OR risk*:ti OR metasta*:ti) |
| #6 | #4 AND #5 |
| #5 | 'cancer recurrence'/exp OR 'recurrent cancer'/exp OR 'tumor recurrence'/exp OR 'metastasis'/de OR 'cocarcinogenesis'/de OR 'cancer invasion'/exp |
| #4 | 'anesthesiological techniques'/exp/mj OR 'anesthetic agent'/exp/mj OR 'analgesic agent'/exp/mj OR 'local anesthetic agent'/exp/mj OR 'anesthesia complication'/exp/mj |
| #3 | #1 AND #2 |
| #2 | 'cancer recurrence'/exp/mj OR 'recurrent cancer'/exp/mj OR 'tumor recurrence'/exp/mj OR 'metastasis'/mj OR 'cocarcinogenesis'/mj OR 'cancer invasion'/exp/mj |
| #1 | 'anesthesiological techniques'/exp OR 'anesthetic agent'/exp OR 'analgesic agent'/exp OR 'local anesthetic agent'/exp OR 'anesthesia complication'/exp |
The EMBASE search will use EMTREE subject headings and select Title Word terms.
Search strategy for ISI Web of Science (1965 to present)
| #9 | #8 AND #7 |
| #8 | Topic=(random* OR "controlled clinical trial" OR "controlled clinical trials" OR "double blind method" OR "double blind methods" OR "single blind method" OR "single blind methods" OR "clinical trial" OR "clinical trials" OR "research design" OR "controlled study" OR "controlled studies" OR "clinical study" OR "clinical studies") |
| #7 | #5 OR #6 |
| #6 | Title=(anesthe* or anaesthe* or analges*) AND Title=(metasta*) |
| #5 | #3 and #4 |
| #4 | Topic=(neoplasm* or tumor* or tumour* or cancer*) AND Topic=(recur* or risk* or metasta*) |
| #3 | #1 OR #2 |
| #2 | Title=("magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane) |
| #1 | Title=(anesthe* OR anaesthe* OR analges* OR opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine) |
Search strategy for BIOSIS (1926 to present)
| # 11 | #10 AND Document Type=(Article OR Meeting OR Meeting Paper) AND Taxa Notes=(Humans) Databases=PREVIEWS Timespan=All Years |
| # 10 | #9 AND Document Type=(Article OR Meeting OR Meeting Paper) Databases=PREVIEWS Timespan=All Years |
| # 9 | #8 AND #7 Databases=PREVIEWS Timespan=All Years |
| # 8 | Topic=(random* OR "controlled clinical trial" OR "controlled clinical trials" OR "double blind method" OR "double blind methods" OR "single blind method" OR "single blind methods" OR "clinical trial" OR "clinical trials" OR "research design" OR "controlled study" OR "controlled studies" OR "clinical study" OR "clinical studies") Databases=PREVIEWS Timespan=All Years |
| # 7 | #6 OR #5 Databases=PREVIEWS Timespan=All Years |
| # 6 | Title=(anesthe* or anaesthe* or analges*) AND Title=(metasta*) Databases=PREVIEWS Timespan=All Years |
| # 5 | #3 and #4 Databases=PREVIEWS Timespan=All Years |
| # 4 | Topic=(neoplasm* or tumor* or tumour* or cancer*) AND Topic=(recur* or risk* or metasta*) Databases=PREVIEWS Timespan=All Years |
| # 3 | #1 OR #2 Databases=PREVIEWS Timespan=All Years |
| # 2 | Title=("magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane) Databases=PREVIEWS Timespan=All Years |
| # 1 | Title=(anesthe* OR anaesthe* OR analges* OR opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl alcohol" OR bumecain OR bupivacaine OR butamben OR carbizocaine OR carticaine OR chloralose OR chloroprocaine OR cyclopropane OR desflurane OR diazepam OR dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine) Databases=PREVIEWS Timespan=All Years |
The Biosis search will use a simplified RCT strategy.
Search strategy for The Cochrane Library
#1 MeSH descriptor Anesthesia and Analgesia explode all trees
#2 MeSH descriptor Analgesia, this term only
#3 MeSH descriptor Analgesia, Patient‐Controlled explode all trees
#4 MeSH descriptor Analgesia, Epidural explode all trees
#5 MeSH descriptor Anesthetics, this term only
#6 MeSH descriptor Analgesics explode all trees
#7 MeSH descriptor Adjuvants, Anesthesia explode all trees
#8 MeSH descriptor Anesthesia, Epidural explode all trees
#9 MeSH descriptor Anesthesia, Spinal explode all trees
#10 MeSH descriptor Anesthesia, Local explode all trees
#11 MeSH descriptor Anesthesia, Conduction explode all trees
#12 MeSH descriptor Nerve Block explode all trees
#13 (opioid* or opiate* or morphin* or alfentanil or alphadolone or alphaxalone or benoxinate or benzocaine or "benzyl alcohol" or bumecain or bupivacaine or butamben or carbizocaine or carticaine or chloralose or chloroprocaine or cyclopropane or desflurane or diazepam or dibucaine or diphenhydramine or dyclonine or emla or enflurane or entonox or etidocaine or etomidate or ether or fentanyl or halothane or heptacaine or innovar or isoflurane or ketamine or levobupivacaine or lidocaine or lignocaine or "magnesium sulfate" or mepivacaine or methohexital or methoxyflurane or methyleugenol or midazolam or minaxolone or "nitrous oxide" or norflurane or pentacaine or phenoxyethanol or pregnanolone or prilocaine or procaine or propanidid or propisomide or propofol or propoxycaine or proxymetacaine or remifentanil or romifidine or ropivacaine or sevoflurane or "sodium oxybate" or sufentanil or "tec solution" or tetracaine or tetrahydrodeoxycorticosterone or tetrodotoxin or thiamylal or thiopental or tiletamine or tribromoethanol or tricaine or trichloroethylene or trimecaine or urethane):ti,ab
#14 (ane?sthe* or analges*):ti
#15 ((an?esth* or analg* or neuraxial or nerve block*) near (technique* or method*))
#16 ((intercostal or paravertebral) near nerve block*)
#17 ((an?esth* or analg*) near complicat*)
#18 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17)
#19 MeSH descriptor Neoplasm Recurrence, Local explode all trees
#20 MeSH descriptor Neoplasm Invasiveness explode all trees
#21 MeSH descriptor Neoplasm Metastasis explode all trees
#22 MeSH descriptor Cocarcinogenesis explode all trees
#23 ((neoplasm* or tumo?r* or cancer* or malignant*) near (recur* or risk* or metasta* or growth* or intensif* or escalat* or develop* or invasion))
#24 carcinogenesis or metastas*:ti,ab
#25 (#19 OR #20 OR #21 OR #22 OR #23 OR #24)
#26 (#18 AND #25)
Appendix 2. Study eligibility screening form
Study eligibility screening form
| Study title | Screener | |
|
| OSC | KK |
| First study author | Source (e.g. journal, abstract) | Publication year |
|
|
|
|
Study eligibility
|
| Inclusion criteria | Yes | No | Unknown |
| Study design | RCT, CCT? |
|
|
|
| Participants | Tumour surgery in adults and/or children? |
|
|
|
| Intervention | General anaesthesia (GA) vs regional anaesthesia (RA) or vs combination (GA + RA)? or opioid anaesthesia vs opioid‐free anaesthesia? |
|
|
|
| Outcome | Mortality and/or tumour recurrence? |
|
|
|
If you answer any of the questions above ‘NO,’
-
Exclude the study
-
Provide a reason for exclusion
| Reason for exclusion |
|
|
If you answer all questions above with ‘YES’ or ‘UNKNOWN,’ proceed with the data abstraction form.
Appendix 3. Data extraction form
Data extraction form
| First study author | Year | |
| Study title: | Initials of review author: | |
| Source (Journal, Abstract…): | ||
| Study design: | RCT | CCT |
| Participants | Group 1 (control) | Group 2 (intervention) |
| N per group |
|
|
| Age (mean) |
|
|
| Paediatric patients (n) |
|
|
| Male/female (n) |
|
|
| Type of cancer, site |
| |
| Type of cancer, histology |
| |
| TNM clinical |
|
|
| TNM pathological |
|
|
| Stage (0–IV) |
|
|
| Type(s) of surgery |
| |
| Resection of the primary tumour (yes/no) | ||
| Preceding chemotherapy (n) |
|
|
| Preceding radiation (n) |
|
|
| Following chemotherapy (n) |
|
|
| Following radiation (n) |
|
|
| Additional information/notes: | ||
| Intervention | |
| Type of intervention (RA or combination GA + RA) |
|
| Type of RA (block technique) (name all that apply) |
|
| Single shot or catheter technique? |
|
| Local anaesthetic (LA) used for RA |
|
| Dose of LA (% mL) (mean) used for RA |
|
| Time RA administered (preop/postop) |
|
| Duration of RA (for catheter techniques) (mean per group) |
|
| Control group GA? |
|
| Type of GA (TIVA, BAL) | |
| If BAL: type of volatile anaesthetic used | |
| Amount of opioid used perioperatively per group (mean) | |
| Continuous IV lidocaine infusion used perioperatively? If yes: give duration and dosage | |
| Any opioids administered intrathecally, epidurally or peripherally? If yes: give route of administration, dose and time | |
| Outcome: overall survival | Group 1 (control) | Group 2 (intervention) | total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox | |||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haenszel, Cox) | |||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up: Minimum Maximum Median Time period of recruitment | |||
| Interval censoring method | |||
| Outcome: progression‐free survival | Group 1 (control) | Group 2 (intervention) | total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox | |||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haensel, Cox) | |||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up: Minimum Maximum Median Time period of recruitment | |||
| Interval censoring method | |||
| Outcome: time to tumour progression | Group 1 (control) | Group 2 (intervention) | total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox | |||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haensel, Cox) | |||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up (months): Minimum Maximum Median Time period of recruitment | |||
| Interval censoring method | |||
| Adverse events reported (in‐hospital) | Group 1 (control) | Group 2 (intervention) |
| PONV | ||
| Postoperative respiratory complications (i.e. pneumonia, respiratory insufficiency, aspiration, pulmonary embolism) | ||
| Postoperative cardiovascular events (i.e. myocardial ischaemia, myocardial infarction, heart failure, cardiac arrest) |
| Trial characteristics | ||
| Single‐centre/multi‐centre |
| |
| Country/Countries |
| |
| How was participant eligibility defined? |
| |
| Was the outcome of interest (tumour recurrence) defined as a primary or secondary outcome in the original protocol? | ||
| If 'NO': | When was the decision made to assess tumour recurrence? | |
| Was there a formal study protocol amendment? If 'YES': When? What was the amendment? | ||
| How was tumour recurrence assessed? ‐ Follow‐up visits were part of the original study design and were used to assess tumour recurrence ‐ Assessment of tumour recurrence was extracted from cancer registry ‐ Assessment of tumour recurrence was extracted from hospital records | ||
| Additional information/notes: | ||
| Methodological quality: | Adequate (random)
| Inadequate (e.g. alternate) | Unclear |
| Allocation of intervention |
|
|
|
| Describe method: | |||
| Concealment of allocation |
|
|
|
| Describe method: | |||
| Blinding | Yes | No | Unclear |
| Caregiver |
|
| |
| Participant |
|
| |
| Outcome assessor |
|
| |
| Intention‐to‐treat | |||
| All participants entering trial |
|
| |
| 15% or fewer excluded |
|
| |
| More than 15% excluded |
|
| |
| Not analysed as intention‐to‐treat |
|
| |
| Unclear |
|
| |
| Withdrawals described? |
|
| |
| Additional notes: | |||
Appendix 4. The Cochrane Collaboration's tool for assessing risk of bias
Description
| Domain | Description | Review authors’ judgement |
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Was the allocation sequence adequately generated? |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessorsAssessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information regarding whether the intended blinding was effective | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomly assigned participants), reasons for attrition/exclusions when reported and any re‐inclusions in analyses performed by the review authors | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting | State how the possibility of selective outcome reporting was examined by the review authors, and describe what was found | Are reports of the study free of suggestion of selective outcome reporting? |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool If particular questions/entries were prespecified in the review protocol, responses should be provided for each question/entry | Was the study apparently free of other problems that could put it at high risk of bias? |
Judgement
|
SEQUENCE GENERATION Was the allocation sequence adequately generated? [Short form: Adequate sequence generation?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Investigators describe a random component in the sequence generation process such as: · Referring to a random number table; · Using a computer random number generator; · Coin tossing; · Shuffling of cards or envelopes; · Throwing of dice; · Drawing of lots; · Minimization*.
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · Sequence generated by odd or even date of birth; · Sequence generated by some rule based on date (or day) of admission; · Sequence generated by some rule based on hospital or clinic record number.
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example: · Allocation by judgement of the clinician; · Allocation by preference of the participant; · Allocation based on the results of a laboratory test or a series of tests; · Allocation by availability of the intervention. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’ |
|
ALLOCATION CONCEALMENT Was allocation adequately concealed? [Short form: Allocation concealment?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · Central allocation (including telephone, web‐based and pharmacy‐controlled, randomization); · Sequentially numbered drug containers of identical appearance; · Sequentially numbered, opaque, sealed envelopes. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · Use of an open random allocation schedule (e.g. a list of random numbers); · Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or were not sequentially numbered); · Alternation or rotation; · Date of birth; · Case record number; · Any other explicitly unconcealed procedure. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘Yes’ or ‘No.’ This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement—for example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
|
BLINDING OF PARTICIPANTS, PERSONNEL AND OUTCOME ASSESSORS Was knowledge of the allocated interventions adequately prevented during the study? [Short form: Blinding?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any one of the following: · No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; · Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; · Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; · Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; · Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Any one of the following: · Insufficient information to permit judgement of ‘Yes’ or ‘No’; · The study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Were incomplete outcome data adequately addressed? [Short form: Incomplete outcome data addressed?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any one of the following: · No missing outcome data; · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · Missing data have been imputed using appropriate methods. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; · Potentially inappropriate application of simple imputation. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Any one of the following: · Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomly assigned not stated, no reasons for missing data provided); · The study did not address this outcome. |
|
SELECTIVE OUTCOME REPORTING Are reports of the study free of suggestion of selective outcome reporting? [Short form: Free of selective reporting?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any of the following: · The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; · The study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · Not all of the study’s prespecified primary outcomes have been reported; · One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; · One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered into a meta‐analysis; · The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘Yes’ or ‘No.’ It is likely that most studies will fall into this category |
|
OTHER POTENTIAL THREATS TO VALIDITY Was the study apparently free of other problems that could put it at risk of bias? [Short form: Free of other bias?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | The study appears to be free of other sources of bias |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | There is at least one important risk of bias. For example, the study: · Had a potential source of bias related to the specific study design used; or · Stopped early because of some data‐dependent process (including a formal‐stopping rule); or · Had extreme baseline imbalance; or · Has been claimed to have been fraudulent; or · Had some other problem. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | There may be a risk of bias, but there is either: · Insufficient information to assess whether an important risk of bias exists; or · Insufficient rationale or evidence that an identified problem will introduce bias. |

Study flow diagram.
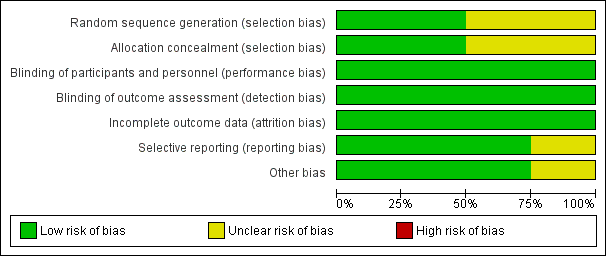
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.1 overall survival.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.2 progression‐free survival.

Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.3 time to tumour progression.

Comparison 1 GA + RA versus GA, Outcome 1 Overall survival.

Comparison 1 GA + RA versus GA, Outcome 2 Progression‐free survival.

Comparison 1 GA + RA versus GA, Outcome 3 Time to tumour progression.
| Epidural anaesthesia in addition to general anaesthesia compared with general anaesthesia alone for patients undergoing primary tumour surgery | |||||
| Patient or population: patients undergoing primary tumour surgery | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect† | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| General anaesthesia alone (control) | Epidural anaesthesia in addition to general anaesthesia (intervention) | ||||
| Death from all causes 7.8‐14.8 years (Myles) 8.3‐10.75 years (Christopherson) | Study population | HR 1.03 | 647 | ⊕⊕⊝⊝ | |
| 805 per 1000a | 815 per 1000 | ||||
| Tumour progression or death from all causes 7.8‐14.8 yearsd | Study population | HR 0.88 | 535 | ⊕⊝⊝⊝ | |
| 944 per 1000d | 921 per 1000 | ||||
| Tumour progression 4.5 yearsf | Study population | HR 1.50 | 545 | ⊕⊝⊝⊝ | |
| 360 per 1000g | 488 per 1000 | ||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| HR = hazard ratio, defined as intervention/control. †HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group. aThe assumed risk and the range of follow‐up times are based on data reported by Myles and Christophersen. Data on absolute events per group were not reported by Binczak. | |||||
| Number of participants | Recruitment site(s) | Age (years) | Male sex | ASA | Type of surgery | Outcome data derived from | |
| 112 | USA; multi‐centre | Control group: 69.1 ± 7.8 Epidural group: 68.6 ± 7.7 | Male only | IIIa | Elective surgery for colon cancer | Veterans Affairs Beneficiary Information and Records Locator System (VA BIRLS) | |
| 446 | Australia, East Asia, Middle East; multi‐centre (MASTERS trial) | Control group: 70 ± 11 Epidural group: 71 ± 9.5 | Control group: 53% Epidural group: 60% | 'High risk patients'b | Major abdominal surgery for cancer | 1. Medical hospital record 2. Contact with participant's general practitioner 3. State‐based cancer registry or National Health Index 4. Participant contact 5. Contact with next of kin | |
| 99 | Canada; single‐centre | Control group: 63.9 ± 6.1 Epidural group: 63.0 ± 5.5 | Male only | ASA I‐III | Radical prostatectomy and bilateral pelvic lymphadenectomy | Participant's hospital charts and medical records | |
| 89 | France; single‐centre | Not reported for subcohort (mean for full cohort 58 years) | Not reported for subcohort (full cohort includes > 62% male) | Not reported | Major abdominal surgery for cancer | 1. Hospital intern cancer registry 2. Participant contact 3. French National Registry | |
| ASA = American Society of Anesthesiologists physical status classification. USA = United States of America. aThe study by Christopherson 2008 reports that ASA I‐III patients were included. However, the original trial included only ASA III patients (Park 2001). bAccording to the inclusion criteria noted in the original study (Rigg 2002), 'high risk' translates to ASA II‐III. | |||||||
| GA maintenance | Epidural catheter level | Time placed | Duration | LA used | Epidural medications intraoperatively | Epidural medications postoperatively | Intraoperative IV opioids | Postoperative IV opioids | |
| Isoflurane 0.9% (mean) + N2O | Thoracic or lumbar epidural catheter | Preoperatively | "as long as needed" | Bupivacaine 0.5% | 3‐6 mg morphine; 25‐50 mg boluses bupivacaine/3‐5 hours as needed; epinephrine | 25‐50 mg boluses bupivacaine/3‐5 hours as needed; morphine 3‐6 mg/12‐24hours as needed | Fentanyl for both groups | Morphine, meperidine as needed (IV in epidural group, IV or IM in control group) | |
| Balanced anaesthesia (volatile anaesthetic not specified), N2O use not specified or recorded, but usual practice was to include it | At discretion of the anaesthesiologist "With the exception of some pelvic operations, all epidural catheters were inserted in the thoracic region" | Preoperatively | 3 days after surgery | Bupivacaine or ropivacaine | Bupivacaine or ropivacaine | Continuous infusion of ropivacaine or bupivacaine, supplemented with fentanyl or pethidine | Fentanyl pethidine | Postoperative opioids, mostly PCA in control group (fentanyl, pethidine) | |
| Isoflurane 1‐2% + N2O 60% | Low thoracic or high lumbar epidural catheter | Preoperatively | Not reported | Ropivacaine | Ropivacaine bolus + continuous infusion; fentanyl | Not reported | Morphine for control group | Not reported | |
| Isoflurane 1‐2% + N2O 70% | Thoracic 7‐11 | Preoperatively | Until 5th postoperative day | Bupivacaine | 50 mg bupivacaine as needed; epinephrine | 12.5 mg/h bupivacaine; 0.25 mg/h morphine | Fentanyl for both groups | Epidural group: morphine boluses SC as needed; control group: 2.5 mg/h morphine SC via catheter | |
| GA = general anaesthesia. LA = local anaesthetic. IV = intravenous. IM = intramuscular. SC = subcutaneous. | |||||||||
| Tumour stage (TNM) | Clinical vs pathologic staging | Median overall survival (95% CI) | Median progression‐free survival | Median time to tumour progression | 5‐Year survival | Follow‐up time | Statistical test used (uni‐ vs. multivariable) | |
| All T, N0, M0 | Pathological | 6.14 (5.22 to 7.99) | Not reported | Not reported | Not reported | Up to 9 years | Data extracted from Kaplan‐Meier curve; HR and SEHR calculated according to Tierney (Tierney 2007) | |
| All T, all N, no distant metastasis (M0) 'complete surgical excision' | Not reported | Epidural group: 3.3 (95% CI 2.1 to 4.5) Control group: 3.7 (95% 2.0 to 5.4) | Epidural group: 2.6 (IQR 0.7 to 8.7) Control group: 2.8 (IQR 0.7 to 8.7) | Epidural group: 1.1 (95% CI 0.7 to 1.6) Control group: 1.4 (95% CI 0.6 to 2.3) | Epidural group: 42% Control group: 44% | Up to 12 years | Univariable testing, log‐rank statistics, intention‐to‐treat analysis | |
| All T, all N, M not reported | Pathological | Not reported | Not reported | 1644 days | Not reported | Up to 3403 days (˜9.3 years) | Unadjusted Cox model, no intention‐to‐treat analysis | |
| Primary tumour resection (all stages) with or without residual disease postoperatively | Not reported | Not reported | Not reported | Not reported | Not reported | Up to 17 years | Unadjusted HR (reported by the contact author through personal communication) | |
| IQR = interquartile range. TNM classification of malignant tumours: T = tumour size, N = lymph node involvement, M = distant metastasis. HR = hazard ratio. SEHR = standard error of hazard ratio. CI = confidence interval. | ||||||||
| Study PI | Start date (clinical trials.gov) | Population | Sample size | Intervention | Control group |
| 2007 | Female patients 18‐85 years of age, diagnosed with primary breast cancer without known extension beyond the breast and axillary nodes, scheduled for unilateral or bilateral mastectomy with or without implant or isolated "lumpectomy" with axillary node dissection (anticipated removal of at least 5 nodes) | 1100 | Regional anaesthesia and analgesia (epidural or paravertebral), combined with deep sedation or general anaesthesia (sevoflurane) | General anaesthesia (sevoflurane) followed by opioid administration | |
| 2008 | Patients scheduled for open, laparoscopic or laparoscopic‐assisted surgery for colon cancer without known extension beyond colon | 2500 | Intraoperative and postoperative regional anaesthesia and analgesia (epidural or paravertebral anaesthesia) plus intraoperative general anaesthesia | General anaesthesia followed by postoperative opioid analgesia | |
| 2009 | Female patients 21‐75 years of age, ASA I‐II, diagnosed with biopsy‐proven breast cancer, scheduled for mastectomy and axillary node dissection in a single procedure | 40 | Local anaesthesia + sedation | General anaesthesia | |
| 2010 | Male and female patients 18‐85 years of age, diagnosed with primary non‐small cell lung cancer and scheduled for potentially curative tumour resection | 1532 | Intraoperative and postoperative general anaesthesia + epidural anaesthesia and analgesia | General anaesthesia and postoperative intravenous analgesia | |
| 2010 | Female patients 18 years of age and older, undergoing unilateral or bilateral mastectomy | 60 | Postoperative paravertebral catheter analgesia with ropivacaine | Placebo (normal saline) | |
| 2011 | Male and female patients 40‐80 years of age, ASA I‐III, undergoing elective surgery for colorectal cancer | 300 | Epidural analgesia with ropivacaine and opioid | PCA with morphine | |
| 2011 | Male and female patients 25‐80 years of age, diagnosed with non‐small cell lung cancer with clinical staging of I or II for whom thoracoscopic lobectomy (VATS) is feasible | 100 | Intraoperative thoracic epidural anaesthesia | Intraoperative general anaesthesia | |
| 2012 | Patients scheduled for inguinal lymph node dissection because of malignant melanoma of the lower limb | 230 | Spinal anaesthesia | General anaesthesia | |
| 2013 | Male and female patients 20‐85 years of age with pancreatic cancer, expected to receive curative Whipple operation | 150 | Epidural analgesia with ropivacaine and opioid | PCA with opioid | |
| VATS = video‐assisted thoracic surgery. ASA = American Society of Anesthesiologists physical status classification. PCA = patient‐controlled analgesia. | |||||
| Author year | Type of cancer | Type of surgery | Intervention 1 (n) | Intervention 2 (n) | Control (n) | Endpoint | Statistical method | Result* | Date of surgery | Follow‐up until |
| Breast CA | Mastectomy + LND | GA + paravertebral catheter (50) | ‐ | GA (79) | Time to tumour recurrence (local or metastasis) | Adjusted Cox regression | HR 0.21 (0.06‐0.71) | 2001‐2002 | 2005 | |
| Cervical CA | First brachytherapy (of several) | SPA or EC (63) | ‐ | GA (69) | 1. Time to tumour recurrence 2. Overall survival | Adjusted Cox regression | 1. HR 0.95 (0.54‐1.67) 2. HR 1.46 (0.81‐2.61) | 1996‐2003 | nr | |
| Colon CA | Colorectal cancer surgery (open) | GA + EC preop (302) | ‐ | GA (58) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.82 (0.30‐2.19) | 2004‐2009 | 2009 | |
| Rectal CA | Colorectal cancer surgery (open) | GA + EC preop (260) | ‐ | GA (35) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.45 (0.22‐0.90) | 2004‐2009 | 2009 | |
| Vogelaar 2012 (abstract) | Colon CA | Surgery for colon CA | EC 'perioperative' (407) | ‐ | GA (198) | Overall survival | Adjusted Cox regression | HR 0.93 (0.93‐0.98) | 1995‐2003 | 2011 |
| (abstract) | Colon CA | Primary colon surgery | GA + EC (182) | ‐ | GA (931) | Tumour recurrence | Univariable | HR 1.33 (0.94‐1.87) | 2001‐2006 | 2009 |
| Colorectal CA | Colorectal cancer surgery | GA + EC preop (256) | ‐ | GA (253) | Time to tumour recurrence | Adjusted Cox model with stratification on propensity score quintiles | HR 0.74 (0.45‐1.22) | 2000‐2007 | 2008 | |
| Colorectal CA w/no metastases | Open colectomy | EC (Medicare code) (9670) | ‐ | No EC (Medicare code) (32481) | 1. Overall survival 2. 4‐Year tumour recurrence | 1. Adjusted marginal Cox model with propensity score as co‐variate 2. Adjusted logistic regression | 1. HR 0.92 (0.88‐0.96) 2. OR 1.05 (0.95‐1.15) | 1996‐2005 | 2009 | |
| Colorectal CA | Laparoscopic resection | EC preop (107) | SPA (144) | GA + PCA (173) | 1. Overall survival 2. Disease‐free survival | KM estimate, log‐rank test | 1. P value 0.622 2. P value 0.490 | 2003‐2010 | ||
| Hepatocellular CA | Percutaneous radiofrequency ablation | GA + EC preop (62) | ‐ | GA (117) | 1. Recurrence‐free survival 2. Overall survival | Adjusted Cox model with propensity score as co‐variate | 1. 3.66 (2.59‐5.15) 2. 0.77 (0.50‐1.18) | 1999‐2008 | 2011 | |
| Malignant melanoma | Lymph node dissection | SPA (52) | ‐ | GA (221) | Long‐term survival | Mean survival (months) of matched pairs (52 pairs) | 95.9 (81.2‐110.5) SPA 70.4 (53.6‐87.1) GA P value 0.087 | 1998‐2005 | 2009 | |
| Malignant melanoma | Melanoma resection | Local anaesthesia (376) | ‐ | GA (190) | Survival | KM estimate, log‐rank test | P value 0.51 (stage pT1/2, n = 237) P value 0.006 (stage pT3a, n = 195) in favour of local anaesthesia P value 0.47 (stage pT3b/4, n = 134) | Control: 1972‐1980 Intervention: 1981‐88 | 1988 | |
| Malignant melanoma w/no metastases | Primary melanoma excision | Local anaesthesia (2185) | ‐ | GA (2136) | Survival | Log‐rank test on matched pairs (1501 pairs) | P value < 0.01 in favour of local anaesthesia | 1976‐1986 | nr | |
| Ovarian CA | Surgery for ovarian cancer | GA + EC preop (26) | GA intraop/EC postop (29) | GA (127) | 1. Overall survival 2. Time to recurrence | 1. Median survival time (months), log‐rank test 2. Adjusted Cox model | 1. 71 m (62‐80) for GA 96 m (84‐109) for EC intraop 70 m (58‐83) for EC postop P value 0.01 for GA vs EC intraop (favours EC intraop) 2. HR 0.37 (0.19‐0.73) for intraop EC HR 0.86 (0.52‐1.41) for postop EC | 2000‐2006 | 2009 | |
| Ovarian CA | Surgery for ovarian cancer | EC only preop (106) | ‐ | GA (37) | Survival time | Adjusted Cox regression on propensity matched pairs (29 pairs) | HR 0.83 (0.67‐0.99) | 1994‐2006 | 2008 | |
| (abstract) | Ovarian CA | Primary radical tumour debulking | EC preop + GA (72) | GA (33) | 1. Recurrence‐free survival 2. Overall survival | KM estimate, log‐rank test | 1. HR 1.52 (1.4‐1.56), P value 0.008 2. nr | 2003‐2010 | nr | |
| Ovarian cancer (Figo IIIc‐IV) | Exploratory laparotomy | EC preop or postop + GA (37) | GA (43) | 1. Time to recurrence 2. Cancer‐specific survival | Adjusted Cox regression with propensity score weighting | 1. HR 0.65 (0.40‐1.08) 2. HR 0.59 (0.32‐1.08) | 2000‐2011 | nr | ||
| Kienbaum 2010/Alexander 2009 (abstracts) | Pancreatic CA | Radical pancreatic tumour resection | GA + EC (71) | ‐ | GA (29) | Overall survival | Log‐rank | P value 0.05 (P value 0.025 in favour of control for participants receiving high‐dose epidural opioids) | 2005‐2008 | nr |
| Prostate CA | Open radical prostatectomy | GA + EC preop (102) | ‐ | GA (123) | BCR‐free survival | Univariable Cox regression on propensity matched pairs (71 pairs) | HR 0.48 (0.23‐1.00) | 1994‐2003 | 2006 | |
| Prostate CA w/no metastasis | Radical prostatectomy | GA + EC preop (578) | ‐ | GA (533) | BCR‐free survival | Adjusted Cox model | HR 0.84 (0.52‐1.17) | 1993‐2006 | 2006 | |
| Prostate CA (all stages) | Open radical retropubic prostatectomy w/LND | GA + EC preop (103) | ‐ | GA (158) | 1. BCR‐free survival 2. Clinical progression‐free survival 3. Cancer‐specific survival 4. Overall survival | Adjusted Cox model with propensity score as co‐variate | 1. HR 0.82 (0.50‐1.34) 2. HR 0.40 (0.20‐0.79) 3. HR 0.95 (0.36‐2.47) 4. HR 1.01 (0.44‐2.32) | Intervention: 1994‐1997 Control: 1997‐2000 | nr | |
| Prostate CA (pT3/4) | Retropubic radical prostatectomy w/LND | GA + EC preop (67) | ‐ | GA (81) | 1. BCR‐free survival 2. Local recurrence‐free survival 3. Distant recurrence‐free survival 4. Cancer‐specific survival 5. Overall survival | Univariable Cox regression on matched pairs (67 pairs) | 1. HR 1.00 (0.69‐1.47) 2. HR 1.16 (0.41‐3.29) 3. HR 0.56 (0.26‐1.25) 4. HR 0.96 (0.45‐2.05) 5. HR 1.17 (0.63‐2.17) | 1994‐2000 | nr | |
| Several statistical methods were used in most studies. We weighted reported results in the following descending order: adjusted regression with propensity score or matched pairs, adjusted regression, univariable analysis. Only the highest weighted analysis is reported in the table. HR = hazard ratio, defined as intervention/control. *HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group. We adjusted the HR derived from individual trials accordingly, as needed. bold font denotes significant results in favour of the intervention group (EC). italic font denotes significant results in favour of the control group (GA). CA = cancer. pT = pathological tumour staging. EC = epidural catheter. SPA = spinal anaesthesia. GA = general anaesthesia. LND = lymph node dissection. preop = preoperatively. postop = postoperatively. n = number of participants. OR = odds ratio. n.s. = non‐significant. BCR = biochemical recurrence. nr = not reported. m = months. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 647 | Hazard Ratio (Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Progression‐free survival Show forest plot | 2 | 535 | Hazard Ratio (Random, 95% CI) | 0.88 [0.56, 1.38] |
| 3 Time to tumour progression Show forest plot | 2 | 545 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.00, 2.25] |

