子宮内膜癌の既治療患者に対するホルモン補充療法
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. Randomisation was stratified across 3 strata (FIGO stage 1A, IB/IC and II endometrial cancer). Median follow‐up: 35.7 months. | |
| Participants | 1236 participants with surgically staged FIGO IA, IB, IC or occult stage II endometrial cancer. Inclusion criteria: indication for treatment with HRT due to symptomatic hypo‐oestrogenic state or as prophylaxis in the presence of increased cardiovascular or osteoporotic risk Exclusion criteria: liver dysfunction, history of thromboembolic disease or other cancer within 5 years, with the exception of non‐melanoma of the skin | |
| Interventions | Intervention: oestrogen replacement therapy (unspecified formulation) Control: placebo | |
| Outcomes | Tumour recurrence (2.3% of participants in the HRT arm versus 1.9% of participants in the placebo arm Time to recurrence: not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given regarding the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding the measures undertaken to ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding was reported, though with minimal detail. However, outcomes were unlikely to have been significantly affected by deficiencies in the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding was reported, though with minimal detail. However, outcomes were unlikely to have been significantly affected by deficiencies in the blinding of participants and personnel. |
| Incomplete outcome data (attrition bias) | High risk | There was significant departure from the assigned treatment with very poor compliance (41.1%) among participants in the treatment arm. |
| Selective reporting (reporting bias) | High risk | The trial could not report all relevant oncological outcomes due to premature closure. |
| Other bias | High risk | The trial was closed prior to achievement of its accrual goal. |
FIGO: International Federation of Gynecology and Obstetrics; HRT: hormone replacement therapy.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐randomised study | |
| Non‐randomised study | |
| Non‐randomised study | |
| Non‐randomised study | |
| Review | |
| Non‐randomised study |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
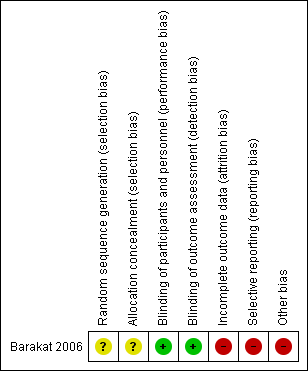
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
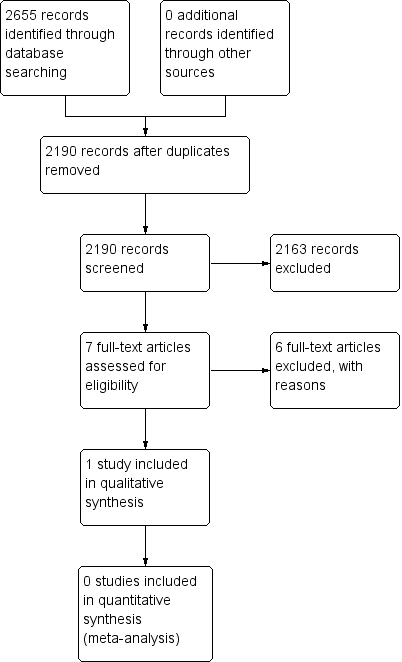
Study flow diagram.
| Oestrogen replacement therapy compared to placebo for women previously treated for endometrial cancer | ||||||
| Patient or population: women previously treated for endometrial cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty (quality) of the evidence | Comments | |
| Risk with placebo | Risk with oestrogen replacement therapy | |||||
| Rate of symptom relief | — | — | — | — | — | — |
| Rate of tumour recurrence | Study population | RR 1.17 (0.54 to 2.50) | 1236 | ⊕⊝⊝⊝ | — | |
| 19 per 1000 | 14 per 1000 | |||||
| Rate of appearance of a new malignancy | Study population | RR 0.80 (0.32 to 2.01) | 1236 | ⊕⊝⊝⊝ | — | |
| 16 per 1000 | 13 per 1000 | |||||
| Rate of survival: overall survival | — | — | — | — | — | The single study did not report overall survival of control and intervention groups individually, though it did report the percentage of participants alive at the end of follow‐up (median follow‐up: 35.7 months; 94.3% in the HRT group and 95.6% in the placebo group). |
| Rate of survival: progression‐free survival | — | — | — | — | — | The study did not report progression‐free survival of control and intervention groups individually, though it did report the percentage of participants alive, with no evidence of disease at the end of follow‐up (median follow‐up: 35.7 months; 95.8% in the HRT group and 96.9% in the placebo group). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level as the single RCT was closed prior to achieving its accrual goal. This was a serious departure from the study design and a serious risk of bias. 2Downgraded one level as there were insufficient data with respect to allocation concealment and description of the intervention, along with a significant risk of attrition bias. 3Downgraded one level for imprecision, as the single included study was underpowered to detect significant differences in the primary outcomes. | ||||||

