Ergänzende Vitamin‐D‐Gabe zur Vorbeugung von Infektionen bei Kindern unter fünf Jahren
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Individual randomized trial. Location: Northern Spain. Setting: primary health care centres in a community. Duration: 12 months (enrolment from February 2007 through February 2008). | |
| Participants | Number: 102 enrolled (14 excluded before start of prophylaxis, 7 in each group). Inclusion criteria: healthy term infants presenting for a routine health visit within the first 15 days of life. Exclusion criteria: infants with chronic disease, use of medications affecting vitamin D metabolism, refusal of parents, prematurity, dark skin, sunlight exclusion for cultural, religious or other reasons, and breastfeeding by vegetarian mothers. In summary, the trial excluded infants at risk of vitamin D deficiency. | |
| Interventions | Intervention: vitamin D supplementation 402 IU/d containing 67 IU of cholecalciferol per drop (N = 41). Control: no vitamin D supplementation. No placebo was used (N = 47). | |
| Outcomes |
| |
| Sources of funding | The trial was funded partly by grant FIS ECO8/00238 from the Instituto de Salud Carlos III and by the Fundacion Nutricion y Crecimiento. | |
| Conflicts of interest | The trial authors did not report this information. | |
| Notes | We acquired data on infections (outcome 3), which were not reported in the published article, from the trial author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The principal investigator made the assignment by phone using a computer software". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The principal investigator made the assignment by phone using a computer software". Comment: adequately done. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study was not blinded to parents and investigators". Comment: unblinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear. |
| Incomplete outcome data (attrition bias) | High risk | Intervention group: 11/41 = 26.8% lost to follow‐up. Control group: 4/47 = 8.5% lost to follow‐up. The attrition rate was higher in intervention group. The trial authors mentioned the reasons for loss to follow‐up but did not give details of the distribution between groups. |
| Selective reporting (reporting bias) | High risk | The trial protocol was unavailable. However, the methods section mentions outcomes such as child's body weight, length and head circumference that the trial authors did not discuss in the results. The trial authors did not report infection outcomes and we obtained them from the unpublished data. |
| Other bias | Low risk | There was no other evidence of confounding or selection bias. |
| Methods | Double‐blind randomized prospective trial. Location: Cincinnati, Ohio. Setting: single private paediatric practice. Duration: 12 weeks. | |
| Participants | Number: 18 enrolled. Inclusion criteria: healthy, term, exclusively breast‐fed infants between the second and third weeks of life. Exclusion criteria: Infants with major congenital anomalies, bone disorders, and gastrointestinal disease were excluded. | |
| Interventions | Intervention: 400 IU of vitamin D2 per day diluted with propylene glycol to a concentration of 400 IU/ mL (N = 9). Control: a daily placebo of propylene glycol (N = 9). | |
| Outcomes |
| |
| Sources of funding | Supported in part by a grant from Ross Laboratories and the National Institute of Child Health and Human Development, HD 11725‐02. | |
| Conflicts of interest | The trial authors did not report this information. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups. The randomization was done with a random numbers table by the pharmacist after we called in" (obtained from communication with the authors). Comment: adequately done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. The trial authors did not provide enough information. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups and studied prospectively in a double‐blind fashion". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Eighteen healthy, term, exclusively breast‐fed infants were divided randomly into two groups and studied prospectively in a double‐blind fashion". Comment: adequately done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there was no attrition or lost to follow‐up in this trial. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems the trial authors reported all expected prespecified trial outcomes. |
| Other bias | High risk | There is a possibility of confounding and selection bias because the trial authors did not adequately describe the randomisation methods. |
| Methods | Double‐blind randomized prospective trial. Location: Madison, Wisconsin. Setting: private paediatric practice. Duration: 6 months (24 weeks). | |
| Participants | Number: 46 enrolled from October 1985 to January 1987. Inclusion criteria: healthy, term, breast‐fed white infants during the first week of life. Exclusion criteria:Infants with major congenital anomalies, bone disorders, and gastrointestinal disease were excluded. | |
| Interventions | Intervention: 400 IU of vitamin D2 per day diluted with propylene glycol to a concentration of 400 IU/ mL (N = 22). Control: a daily placebo of propylene glycol (N = 24). All participating families were given a small supply of vitamin D‐free formula to be used only for emergency situations. | |
| Outcomes |
| |
| Sources of funding | Supported by U.S. Department of Agriculture grant No. 85‐CRCR‐1‐1712. | |
| Conflicts of interest | This trial authors did not report this information. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups. The randomization was done with a random numbers table by the pharmacist after we called in" (obtained from communication with the authors). Comment: adequately done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear risk. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups and studied in a double‐blind fashion". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Forty‐six term, breast‐fed infants were divided randomly into two groups and studied in a double‐blind fashion". Comment: adequately done. |
| Incomplete outcome data (attrition bias) | Low risk | Intervention group: 3/22 = 13.6% lost to follow‐up at 6 months. Control group: 5/24 = 20.8% lost to follow‐up at 6 months. The attrition rate was high but almost similar across the two groups. The trial authors mentioned the reasons for loss to follow‐up and these were distributed equally between the two groups. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems that the trial authors reported all expected prespecified outcomes from the trial. |
| Other bias | High risk | There is a possibility of confounding and selection bias because the trial authors did not adequately describe the randomization methods. |
| Methods | Individual randomized placebo‐controlled superiority trial. Location: catchment area of the Maiwand Teaching Hospital, serving an inner‐city population in Kabul, Afghanistan. Setting: community‐based trial. Duration: 18 months, enrolment between 4 November and 4 December 2008 with follow‐up until May 2009. | |
| Participants | Number: 3046 enrolled. Inclusion criteria: infants aged 1 to 11 months and living in the trial region. Exclusion criteria: children expected to migrate within 18 months, with diagnosis of rickets or past history of vitamin D treatment, or having kwashiorkor or marasmus. | |
| Interventions | Intervention: quarterly supplementation of 100,000 IU (2.5 mg) of vitamin D (cholecalciferol) in olive oil (2 mL) (N = 1524). Control: 2 mL placebo (olive oil) (N = 1522). | |
| Outcomes |
| |
| Sources of funding | The Wellcome Trust and British Council Delphi programme funded this trial. USAID, Afghanistan and Washington State University also supported the trial author(s). | |
| Conflicts of interest | Trial authors have no known conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician (Shabbar Jaffar) randomised unique identification numbers individually in fixed blocks of 20 to the vitamin D. or placebo group by use of a random number generator with the SAS routine". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistician (Shabbar Jaffar) randomised unique identification numbers individually in fixed blocks of 20 to the vitamin D. or placebo group by use of a random number generator with the SAS routine." Comment: adequately done. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The vitamin D3 and the placebo were the same colour (pale yellow), taste, and quantity (0·5 mL) and therefore the study staff and the families did not know to which group the children were assigned". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The masked radiographs were read by two independent paediatric radiologists". Comment: adequately done. |
| Incomplete outcome data (attrition bias) | High risk | Intervention group: 436/1524 = 28.6% lost to follow‐up and 10/1524 died. Control group: 445/1522 = 29.2% lost to follow‐up and 7/1522 died. The attrition rate was high but similar across the two groups. The trial authors did not mention the reasons for loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was unavailable; but based on 'methods' section, it seems that the trial authors reported all expected prespecified trial outcomes. |
| Other bias | Low risk | There was no other evidence of confounding or information bias (misclassification). |
Abbreviations: N: number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This trial compared 2 different doses of vitamin D in adolescents. | |
| This trial included children above 5 years of age. | |
| This trial included children with acute diarrhoea. | |
| This trial included HIV‐infected children aged 6 to 16 years. | |
| This trial provided v itamin D supplementation to mothers and not to children. | |
| This trial targeted children above 5 years of age. | |
| This trial included children with a topic dermatitis. | |
| This trial included children with hypophosphataemic rickets. | |
| This trial included children with severe pneumonia. | |
| This trial evaluated the impact of fortified juices. | |
| This trial did not have a placebo/control group and it compared different dosages of vitamin D. | |
| This trial included children above 5 years of age. | |
| This trial did not have a placebo/control group. | |
| The trial supplemented b oth mothers from 27 weeks' gestation and their infants with vitamin D. | |
| This trial included preterm infants (< 32 weeks' gestational age) during initial hospitaliz ation. | |
| This trial included HIV infected youth aged 18 to 24 years. | |
| This trial evaluated the impact of fortified cereal‐based food, not supplementation. | |
| This trial included children aged 3 to 15 years with juvenile arthritis. | |
| This trial evaluated the impact of vitamin D obtained through sunshine and not through supplements. | |
| This trial did not have a placebo/control group and compared different dosages of vitamin D. | |
| This trial included HIV‐infected children. | |
| This study evaluated the levels of Vitamin D in children diagnosed with tuberculosis . | |
| This trial included children aged 9 to 18 years with developmental disabilities. | |
| This trial included low birthweight term infants. | |
| This trial included children with nutritional rickets. | |
| This trial included epileptic children aged 5 to 14 years of age. | |
| This trial included children diagnosed with tuberculosis . | |
| This trial did not have a placebo/control group and targeted preterm infants. | |
| This trial targeted children aged 10 to 17 years. | |
| Vitamin D supplementation not the only difference between intervention and control groups (cluster‐ randomised controlled trial (cluster‐RCT)). The intervention group was infants of immigrant origin 6 weeks old who received free drops of vitamin D2 plus customiz ed information handouts compared to control group who received usual care. | |
| This trial targeted asthmatic children aged 6 to 12 years. | |
| This trial included children with clinical episode of pneumonia at baseline. | |
| This trial included otitis‐prone children and evaluated otitis media as outcome. | |
| This trial included children with tuberculosis. | |
| This trial did not have a placebo/control group and included children with rickets. | |
| This trial compared different doses of vitamin D and did not have a suitable placebo/control group. | |
| This trial did not have a suitable placebo/control group. | |
| This trial included very‐low birthweight infants and did not have a suitable placebo/control group. | |
| This RCT included children 8 to 14 years old. | |
| This trial included mother‐infant pairs, however all the infants received v itamin D and there was no control group. | |
| This trial did not have a suitable placebo/control group. | |
| Th is RCT included schoolchildren aged 8 to 15 years. | |
| This trial included asthmatic children above 5 years of age. | |
| This trial evaluated the impact of foodLETS (fortification in infant food), not supplementation. | |
| This trial included children with atopic dermatitis. | |
| This trial did not have suitable placebo/control group. | |
| This trial included human immunodeficiency virus (HIV)‐ infected children above 5 years of age. | |
| This trial included HIV‐ infected and HIV‐ exposed infants and did not have a suitable placebo/control group. | |
| The study i ncluded Aboriginal children under 16 years of age. This was a RCT of vitamin D given as oral daily or single‐dose stoss therapy but had no placebo or control group. | |
| This trial included children with rickets. | |
| This trial compared 2 doses of vitamin D among children with rickets. | |
| This trial included children above 5 years of age. | |
| This trial supplemented mothers with different doses of v itamin D and did not have a suitable placebo/control group. | |
| This trial compared different doses of vitamin D and did not have a suitable placebo/control group. |
Abbreviations: HIV : human immun odeficiency virus; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | PREVARID ‐ PREVention of Acute Respiratory Infections with Vitamin D. Does vitamin D supplementation prevent acute respiratory infection health care visits among children under 2 years old? A randomized controlled trial |
| Methods | Parallel randomized controlled trial. |
| Participants | Children who are residents of New Zealand, are < 2 years old a the time of their acute lower respiratory tract infection (ALRI) hospital admission and reside in the Auckland District Health Board catchment area. |
| Interventions | Weekly vitamin D supplementation (5000 IU) for 12 months after ALRI hospital admission. |
| Outcomes | Number of ARI hospital admissions Number of ARI presentations to health care Number of ARI presentations to hospital emergency departments Number of antibiotic prescriptions dispensed during 12 month follow‐up Serum 25(OH)D concentration at baseline and 6 months, plus at 12 months in a 10% subsample |
| Starting date | 1 July 2016 |
| Contact information | |
| Notes | The results are expected to be available by next year. No mention about stratification of ARI into pneumonia, bronchiolitis, upper respiratory tract infection, etc. |
| Trial name or title | Evaluation of the Effectiveness of Vitamin D Supplementation to Pregnant Women and Their Infants in Pakistan |
| Methods | Double‐blind randomized controlled trial. |
| Participants | Pregnant women from 20 to 22 weeks of gestation and their infants |
| Interventions | Vitamin D supplement versus placebo |
| Outcomes | Vitamin D deficiency |
| Starting date | February 2010 |
| Contact information | |
| Notes | No other details yet available |
| Trial name or title | Vitamin D and Its Affect on Growth Rates and Bone Mineral Density Until Age 5 |
| Methods | Double‐blind randomized controlled trial |
| Participants | Children between 9 to 12 months of age with normal 25(OH)D levels and those with 25(OH)D deficiency. Children with vitamin D deficiency were randomized. |
| Interventions | Vitamin D supplementation of 800 IU for one year versus placebo |
| Outcomes | Height at the age of 3 years. Bone densitometry by ultrasound. |
| Starting date | September 2011 |
| Contact information | |
| Notes | The outcomes may be irrelevant to this Cochrane review |
| Trial name or title | A Randomized, Double‐blind, Controlled Trial of Vitamin D for the Prevention of Acute Respiratory Infections in Children Aged 18 to 36 Months in Santiago, Coyhaique and Punta Arenas, Chile. |
| Methods | Double‐blind randomized controlled trial, efficacy study, parallel assignment. |
| Participants | 276 preschool children aged 18 to 36 months attending daycare in Santiago, Coyhaique, or Punta Arenas. |
| Interventions | Oral 5600 IU vitamin D3 versus oral 11200 IU vitamin D3 versus oral placebo in liquid weekly during 6 months. |
| Outcomes | Incidence of acute respiratory tract infections at 6 months. Adverse events during 6 months. Hospitalizations due to acute respiratory tract infections during 6 months. Serum cathelicidin levels at baseline and 6 months. Serum 25(OH)D levels at baseline and 6 months. Viral etiology of acute respiratory tract infections during 6 months. Bone metabolism parameters, that is serum measurement of parathyroid hormone, alkaline phosphatases, calcium, phosphorus, and urinary calcium/creatinine ratio at baseline and 6 months. |
| Starting date | February 2014 |
| Contact information | |
| Notes | Results of the trial not yet available. Estimated study completion date: May 2016. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence rate radiologically confirmed first or only episode of pneumonia Show forest plot | 2 | 3134 | Rate Ratio (Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| Analysis 1.1 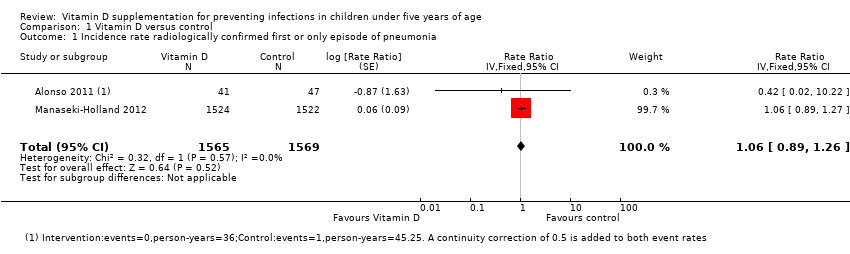 Comparison 1 Vitamin D versus control, Outcome 1 Incidence rate radiologically confirmed first or only episode of pneumonia. | ||||
| 2 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Vitamin D versus control, Outcome 2 All‐cause mortality. | ||||
| 3 Any hospital admission Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.20, 3.62] |
| Analysis 1.3  Comparison 1 Vitamin D versus control, Outcome 3 Any hospital admission. | ||||
| 4 End of supplementation mean serum vitamin D concentrations in ng/mL Show forest plot | 4 | 266 | Mean Difference (IV, Random, 95% CI) | 7.72 [0.50, 14.93] |
| Analysis 1.4  Comparison 1 Vitamin D versus control, Outcome 4 End of supplementation mean serum vitamin D concentrations in ng/mL. | ||||
| 5 Baseline mean serum vitamin D concentrations in ng/mL Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐3.30, 3.98] |
| Analysis 1.5  Comparison 1 Vitamin D versus control, Outcome 5 Baseline mean serum vitamin D concentrations in ng/mL. | ||||
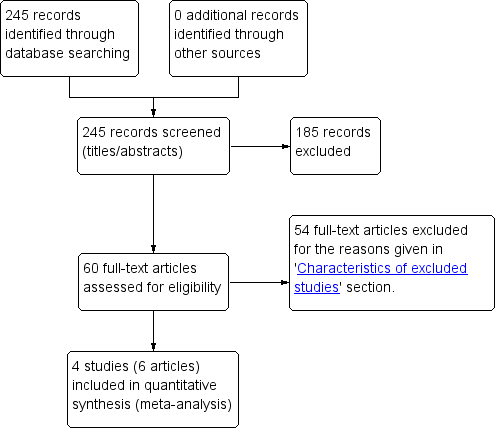
Study flow diagram.
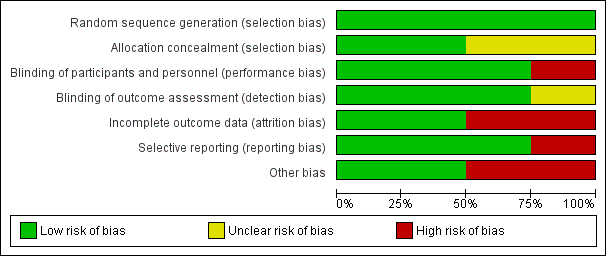
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials.
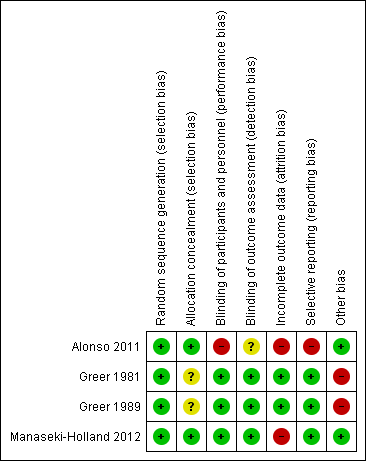
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
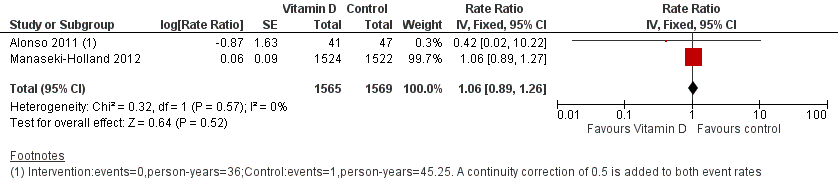
Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.1 Incidence rate radiologically confirmed first or only episode of pneumonia.

Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.2 All‐cause mortality.
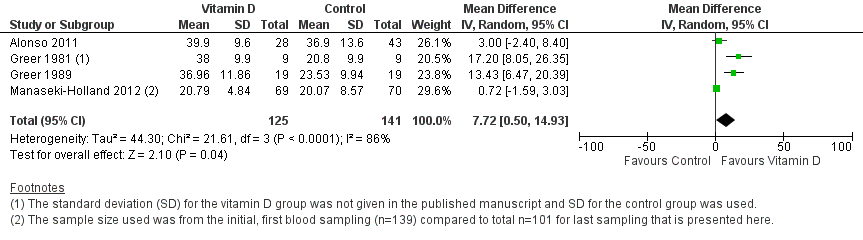
Forest plot of comparison: 1 Vitamin D versus control, outcome: 1.4 Mean serum vitamin D concentrations in ng/mL.
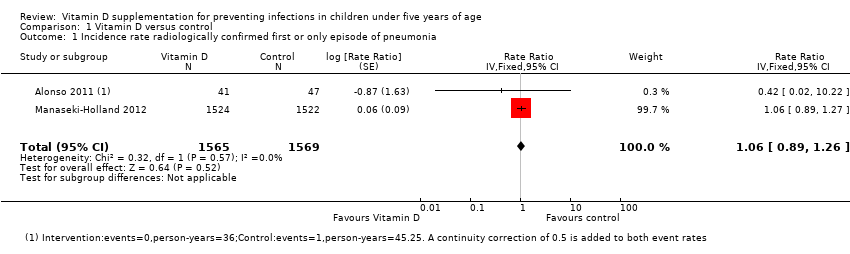
Comparison 1 Vitamin D versus control, Outcome 1 Incidence rate radiologically confirmed first or only episode of pneumonia.
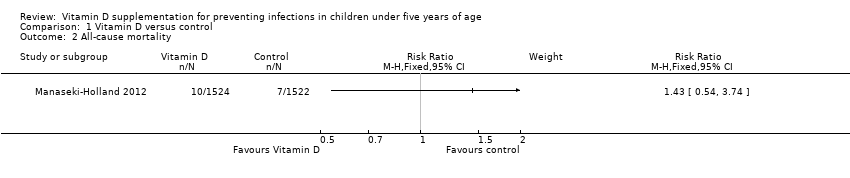
Comparison 1 Vitamin D versus control, Outcome 2 All‐cause mortality.
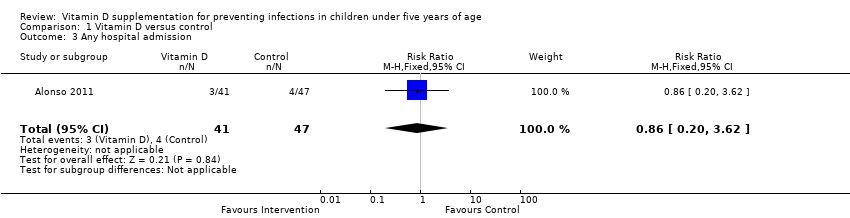
Comparison 1 Vitamin D versus control, Outcome 3 Any hospital admission.
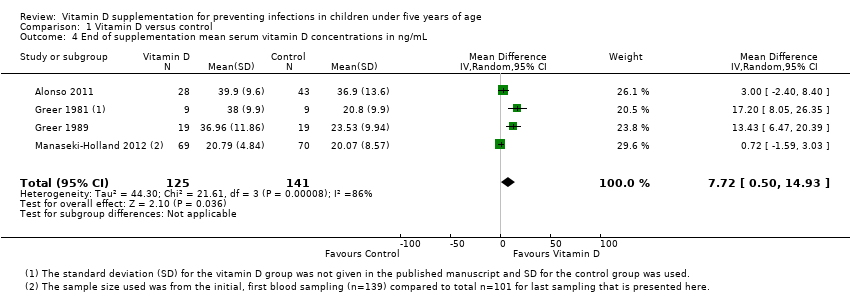
Comparison 1 Vitamin D versus control, Outcome 4 End of supplementation mean serum vitamin D concentrations in ng/mL.

Comparison 1 Vitamin D versus control, Outcome 5 Baseline mean serum vitamin D concentrations in ng/mL.
| Vitamin D versus control for preventing infections in children under five years of age | |||||
| Patient or population: children under five years of age Control: placebo or no supplementation | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative/absolute effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Vitamin D | ||||
| All‐cause mortality | 5 per 1000 | 7 per 1000 | Risk ratio 1.43 | 3046 (1) | ⊕⊕⊝⊝ |
| Cause‐specific mortality | 3 per 1000 | 5 per 1000 (1 to 16) | Risk ratio 1.50 | 3046 (1) | ⊕⊕⊝⊝ |
| Incidence rate radiologically confirmed first or only episode of pneumonia | 157 episodes per 1000 person‐years | 166 episodes per 1000 person years | Rate ratio 1.06 | 3134 | ⊕⊕⊕⊝ |
| Any hospital admission | 9 per 100 | 8 per 100 (2 to 33`) | Risk ratio 0.86 (0.20 to 3.62) | 88 (1) | ⊕⊝⊝⊝ |
| TB cases | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Diarrhoea cases | ‐ | ‐ | ‐ | 2 studies7 | ‐ |
| Malaria cases | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Febrile illness | ‐ | ‐ | ‐ | 0 studies | ‐ |
| Mean serum vitamin D concentrations | 141 | 125 | Mean difference 7.72ng/mL higher (0.50 higher to 14.93 higher) | 266 (4) | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for imprecision: the estimate varies from 46% decrease to over 3‐fold increase for all‐cause mortality; and from 58% decrease to over 5‐fold increase for cause‐specific mortality. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence rate radiologically confirmed first or only episode of pneumonia Show forest plot | 2 | 3134 | Rate Ratio (Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| 2 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Any hospital admission Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.20, 3.62] |
| 4 End of supplementation mean serum vitamin D concentrations in ng/mL Show forest plot | 4 | 266 | Mean Difference (IV, Random, 95% CI) | 7.72 [0.50, 14.93] |
| 5 Baseline mean serum vitamin D concentrations in ng/mL Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐3.30, 3.98] |

