Terapias osmóticas agregadas a los antibióticos para la meningitis bacteriana aguda
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008806.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 febrero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2018 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
- This is an open access article under the terms of the Creative Commons Attribution Licence, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Emma Wall (EW) was responsible for writing the main text, extracting data from studies and reviewing the analyses; Paul Garner (PG) was responsible for methodological input, help with interpretation and writing the review; Katherine Ajdukiewicz (KA) was responsible for updating the main text, extracting data from studies and updating the analyses with new data, and commented on the text; Robert Heyderman (RH) provided comments on the text; Hannah Bergman carried out data extraction and updated this review.
Sources of support
Internal sources
-
Clare Dooley, Australia.
Editorial and secretarial
-
Liz Dooley, Australia.
Editorial
-
Sarah Thorning, Australia.
Library and searching support
-
Dr David Sinclair, UK.
Data support
External sources
-
Wellcome Trust, UK.
Award: 089671/B/09/Z
-
UKAID Grant 5242, UK.
UKAID does not participate in the selection of topics, in the conduct of the review or in the interpretation of findings
Declarations of interest
Emma Wall: none known.
Katherine Ajdukiewicz: none known.
Hanna Bergman: works for Cochrane Response, a healthcare evidence consultancy that take commissions from healthcare guideline developers and policy makers. Hanna received payment for updating this review from UKAID through the grant held by PG.
Robert Heyderman: none known
Paul Garner: This review and the salary of PG is supported by UKAID aimed at ensuring systematic reviews, particularly Cochrane Reviews, are completed on topics relevant to the poor in low‐ and middle‐income countries (grant number 5242). UKAID does not participate in the selection of topics, in the conduct of the review or in the interpretation of findings.
Acknowledgements
We wish to thank Sarah Thorning for assistance with the search strategy and support and Dr David Sinclair for his help synthesising the 'Summary of findings' table. We thank the following people for commenting on the draft protocol: Anne Lyddiatt, Teenah Handiside, Amit Kumar, Max Bulsara and Diederik van de Beek. We also thank the following people for commenting on the original review: Sylvia Beamon, Kameshwar Prasad, Matthijs Brouwer, Teresa Neeman and Diederik van de Beek.
Paul Garner and David Sinclair received support from the Effective Health Care Research Consortium, which is funded by UK aid from the UK Government Department for International Development (grant number 5242).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Feb 06 | Osmotic therapies added to antibiotics for acute bacterial meningitis | Review | Emma CB Wall, Katherine MB Ajdukiewicz, Hanna Bergman, Robert S Heyderman, Paul Garner | |
| 2013 Mar 28 | Osmotic therapies added to antibiotics for acute bacterial meningitis | Review | Emma CB Wall, Katherine MB Ajdukiewicz, Robert S Heyderman, Paul Garner | |
| 2010 Nov 10 | Osmotic therapies as adjuncts to antibiotics for acute bacterial meningitis | Protocol | Emma CB Wall, Katherine MB Ajdukiewicz, Robert S Heyderman, Paul Garner | |
Differences between protocol and review
In the 2017 update, we presented death and disability separately. In the earlier version of this review, this was a composite outcome. We believe this provides greater clarity for patients and clinicians.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adrenal Cortex Hormones [therapeutic use];
- Anti‐Bacterial Agents [therapeutic use];
- Combined Modality Therapy [methods];
- Community‐Acquired Infections [complications, metabolism, mortality, therapy];
- Deafness [epidemiology, prevention & control];
- Dexamethasone [therapeutic use];
- Diuretics, Osmotic [adverse effects, *therapeutic use];
- Epilepsy [prevention & control];
- Gastrointestinal Hemorrhage [prevention & control];
- Glucose [therapeutic use];
- Glycerol [adverse effects, *therapeutic use];
- Intracranial Pressure [physiology];
- Meningitis, Bacterial [complications, metabolism, mortality, *therapy];
- Nervous System Diseases [epidemiology, prevention & control];
- Osmosis [physiology];
- Osmotic Pressure [physiology];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans;
PICO
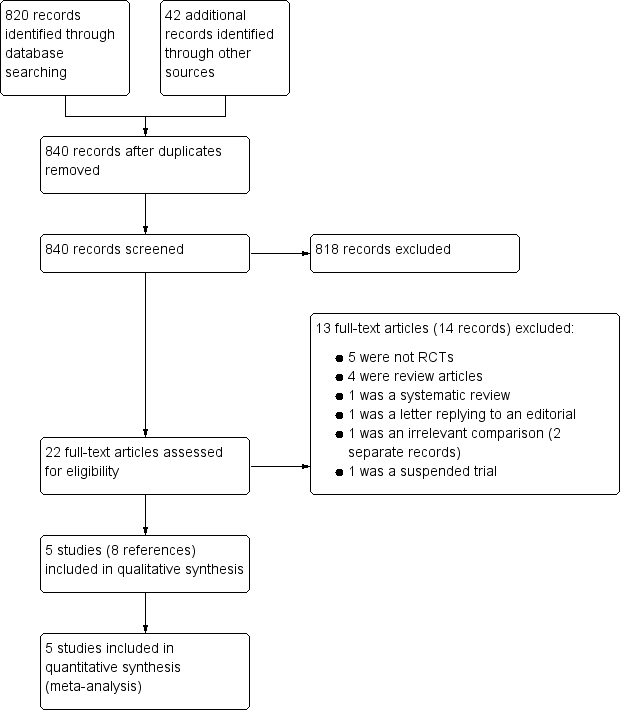
Study screening flow diagram
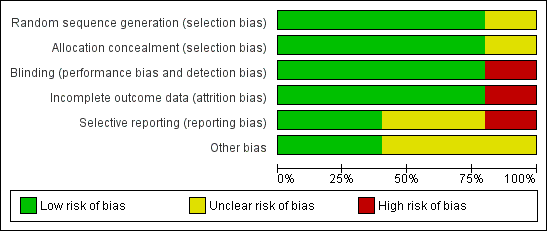
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
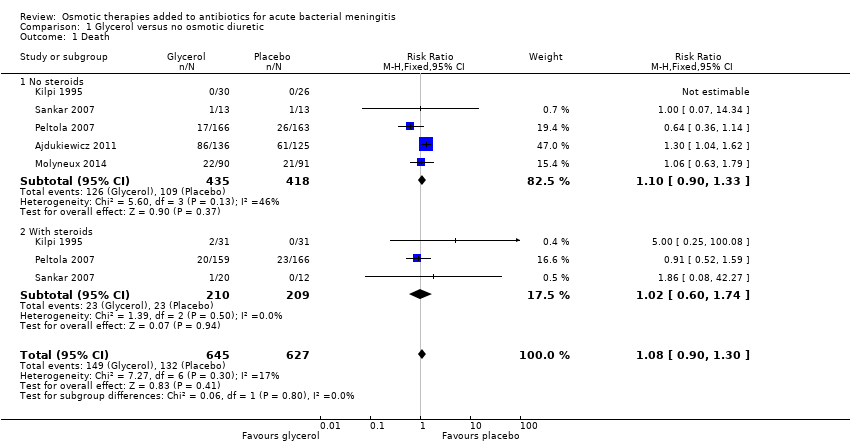
Comparison 1 Glycerol versus no osmotic diuretic, Outcome 1 Death.
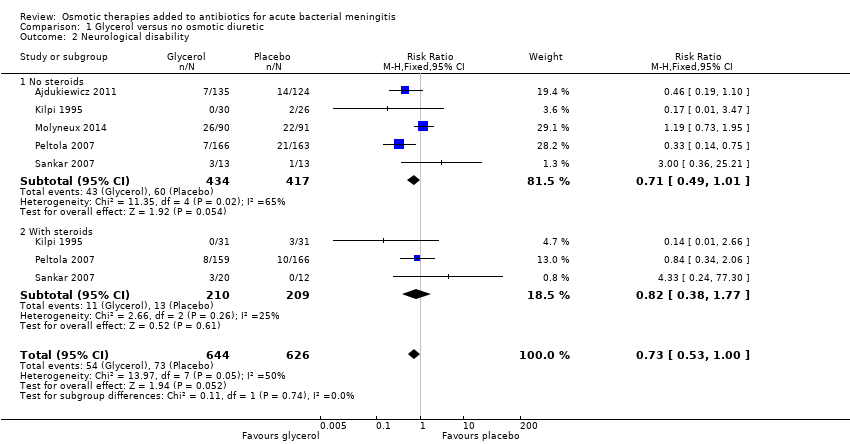
Comparison 1 Glycerol versus no osmotic diuretic, Outcome 2 Neurological disability.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 3 Seizures.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 4 Hearing loss.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 5 Adverse effects: nausea, vomiting, diarrhoea.

Comparison 1 Glycerol versus no osmotic diuretic, Outcome 6 Adverse effects: gastrointestinal bleeding.
| Glycerol for acute bacterial meningitis | ||||||
| Patient or population: children and adults with acute bacterial meningitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Glycerol | |||||
| Death | 19 per 100 | 21 per 100 | RR 1.08 | 1272 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably has little or no effect on death |
| Neurological disability | 9 per 100 | 6 per 100 (5 to 9) | RR 0.73 (0.53 to 1.00) | 1270 (5 studies) | ⊕⊕⊝⊝ Low1,3,4,5 | Downgraded for imprecision and inconsistency. Glycerol may reduce disability |
| Seizures | 32 per 100 | 35 per 100 | RR 1.08 | 1090 | ⊕⊕⊝⊝ | Downgraded for inconsistency and imprecision. Glycerol may have little or no effect on seizures |
| Hearing loss | 16 per 100 | 10 per 100 | RR 0.64 | 922 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably reduces hearing loss |
| Adverse effects: nausea, vomiting, diarrhoea | 47 per 100 | 51 per 100 (38 to 69) | RR 1.09 (0.81 to 1.47) | 851 | ⊕⊝⊝⊝ | Downgraded for serious inconsistency and imprecision. The effect of glycerol on adverse events: nausea, vomiting and diarrhoea is uncertain |
| Adverse effects: gastrointestinal bleeding | 3 per 100 | 3 per 100 (13 to 8) | RR 0.93 (0.39 to 2.19) | 607 | ⊕⊕⊕⊝ | Downgraded for imprecision. Glycerol probably has little or no effect on adverse events: gastrointestinal bleeding |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval (CI)) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: allocation concealment was adequate in four trials and unclear (not reported) in one trial. 3Not downgraded for indirectness. The five trials were conducted in Finland, Malawi, India and South America. Four were in children and one in adults. All included patients with suspected meningitis and cerebrospinal fluid (CSF) changes suggestive of bacterial infection. 5Downgraded by one level for inconsistency: in the Finnish trial the risk of neurological sequelae was reduced with glycerol (RR 0.50, 95% CI 0.32 to 0.78, N = 329), but this was not found in the other studies and the meta‐analysis did not detect a difference (I² = 59%). 6Downgraded by one level for inconsistency: in the trial with adults the risk of seizures was higher with glycerol (RR 1.62, 95% CI 1.18 to 2.23, N = 250), but this was not found in the other studies and the meta‐analysis did not detect a difference (I² = 62%). 8Another two trials reported on this outcome but the results could not be added to the meta‐analysis; one reported more cases of vomiting with glycerol and the other that the incidence of vomiting was "similar" in the treatment groups. 9Downgraded by two levels for inconsistency: in the South American and Finnish trials the risk of adverse effects was increased with glycerol, but this was not found in the Malawi and India trials, and the meta‐analysis did not detect a difference (I² = 79%). | ||||||
| Drug | Class | Mechanism of action | Dose range and route | Studied/used in |
| Glycerol | Sugar alcohol | Probably osmosis plus possible vascular and metabolic benefit | IV 5% to 10% solution or 50 g Oral 1.5 g/kg | Meningitis (Peltola 2007), stroke (Righetti 2004) |
| Mannitol | Sugar alcohol | Osmotic diuretic | IV 20% solution 1 mL/kg to 10 mL/kg or 1 g/kg | Brain trauma (Wakai 2013), cerebral malaria (Namutangula 2007), stroke (Bereczki 2007)
|
| Sorbitol | Sugar alcohol | Osmotic diuretic (weak) | Oral, IV | Experimental brain perfusion, stroke |
| Hypertonic saline | Hypertonic solutions | Osmosis | IV | Brain trauma (Choi 2005), stroke (Schwarz 2002) |
| Sodium lactate | Hydroxy acids | Osmosis (weak) | IV | Brain trauma (Ichai 2009) |
| IV: intravenous | ||||
| Name of study | Population | Intervention and dose | Control used | Treatment duration | Study arms |
| Children in Finland | Oral glycerol 4.5 g/kg max 180 g/24 h in 3 divided doses Dexamethasone (dex) 1.5 mg/kg max 60 mg/day | No oral placebo IV saline | 3 days | 4 arms: IV dexamethasone + glycerol, oral glycerol, IV dexamethasone, neither treatment | |
| Children in India | Oral glycerol 1.5 g/kg 3 x daily Dexamethasone 0.15 mg/kg 3 x daily | Oral carboxymethylcellulose 2% IV saline | Not detailed | 4 arms: placebo oral and IV, IV dexamethasone + oral glycerol, IV placebo + oral glycerol, IV dexamethasone + oral placebo | |
| Children in South America | Oral glycerol 1.5 g/kg 3 x daily Dexamethasone 0.15 mg/kg 3 x daily | Oral carboxymethylcellulose 2% IV saline | 2 days | 4 arms: oral and IV placebo, IV dexamethasone + oral glycerol, IV placebo + oral glycerol, IV dexamethasone + oral placebo | |
| Adults in Malawi, Southern Africa | Oral glycerol 75 mg 4 x daily diluted in water or 50% dextrose solution | Oral 50% dextrose solution | 4 days | Oral glycerol versus oral 50% dextrose | |
| Children in Malawi, Southern Africa | Oral glycerol 25 mL/dose (maximum dose) = 100 mL/24 hours. Acetaminophen 35 mg/kg 6‐hourly | Oral carboxymethylcellulose 2% | 2 days | 3 arms: oral glycerol and oral acetaminophen, oral placebo and glycerol, oral acetaminophen and oral placebo | |
| IV: intravenous | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 5 | 1272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 1.1 No steroids | 5 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.90, 1.33] |
| 1.2 With steroids | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.60, 1.74] |
| 2 Neurological disability Show forest plot | 5 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.53, 1.00] |
| 2.1 No steroids | 5 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.01] |
| 2.2 With steroids | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.77] |
| 3 Seizures Show forest plot | 4 | 1090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 3.1 No steroids | 4 | 755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.44] |
| 3.2 With steroids | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.33] |
| 4 Hearing loss Show forest plot | 5 | 922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.93] |
| 4.1 No steroids | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.41, 0.99] |
| 4.2 With steroids | 3 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.35] |
| 5 Adverse effects: nausea, vomiting, diarrhoea Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 5.1 No steroids | 2 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 5.2 With steroids | 1 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.13] |
| 6 Adverse effects: gastrointestinal bleeding Show forest plot | 3 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 6.1 No steroids | 3 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.06, 2.60] |
| 6.2 With steroids | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.44, 3.04] |

