Kombinirana naspram sekvencijske kemoterapije jednim lijekom za metastatski rak dojke
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008792.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de mama
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Rachel Dear (RD), Martin Tattersall (MT) and Nicholas Wilcken (NW) conceived the study objective. RD wrote the review, which was reviewed and revised by MT, Alexandra Barratt (AB), NW, Kevin McGeechan (KMcG), and Marisa Jenkins (MJ).
Sources of support
Internal sources
-
Nil, Other
External sources
-
Nil, Other
Declarations of interest
None
Acknowledgements
We would like to thank Fergus Tai for his work identifying studies through the Cochrane Breast Cancer Group Specialised Register. We would also like to thank Melina Willson for her advice during the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 18 | Combination versus sequential single agent chemotherapy for metastatic breast cancer | Review | Rachel F Dear, Kevin McGeechan, Marisa C Jenkins, Alexandra Barratt, Martin HN Tattersall, Nicholas Wilcken | |
| 2010 Oct 06 | Combination versus sequential single agent chemotherapy for metastatic breast cancer | Protocol | Rachel F Dear, Martin HN Tattersall, Kevin McGeechan, Alexandra Barratt, Nicholas Wilcken | |
Differences between protocol and review
In the protocol we proposed only including first and second‐line chemotherapy trials. Our review includes a trial of third‐line chemotherapy (Park 2010, prior treatment with anthracyclines or taxanes) because the paper met all our other eligibility criteria.
A pre‐specified secondary outcome in the protocol was stable disease. We have not reported this outcome because overall tumour response rate (which includes complete response and partial response) is a more commonly reported outcome that can then be compared to other similar reviews on this topic.
Another pre‐specified outcome in the protocol was "QTWIST" (quality‐adjusted time without symptoms of disease and toxicity). We have not reported this in our review because this outcome was not reported in any of the trials included in this review, and toxicity data were not consistently reported so that time with side effects from chemotherapy could not accurately be extracted.
In the review, the 'Human' limit in the MEDLINE search strategy and the 'Randomised controlled trial' limit in the EMBASE search strategy were revised.
Notes
Two studies (Campone 2013; Zhang 2013) were categorised under the section 'Characteristics of studies awaiting classification' because the trials were completed but with insufficient data for inclusion at present. The results from these two small trials will be included in an updated version of this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antineoplastic Agents [*administration & dosage, adverse effects];
- Antineoplastic Combined Chemotherapy Protocols [*administration & dosage, adverse effects];
- Breast Neoplasms [*drug therapy, mortality, pathology];
- Disease Progression;
- Disease-Free Survival;
- Nausea [chemically induced];
- Neutropenia [chemically induced];
- Randomized Controlled Trials as Topic;
- Vomiting [chemically induced];
Medical Subject Headings Check Words
Female; Humans;
PICO
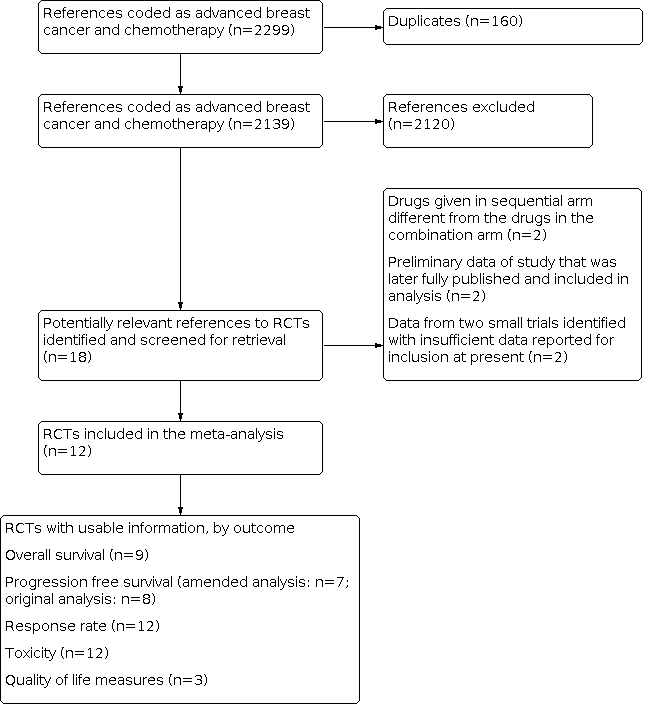
Results of search strategy applied 31 October 2013 for combination versus sequential single agent chemotherapy for metastatic breast cancer.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Overall survival, outcome: 1.1 Overall survival (all trials).

Forest plot of comparison: 2 Progression‐free survival, outcome: 2.1 Progression‐free survival (amended analysis).

Forest plot of comparison: 3 Overall response, outcome: 3.1 Overall response (all trials).

Forest plot of comparison: 24 Overall response ‐ schema 1 versus schema 2, outcome: 24.1 Overall response ‐ schema 1 versus schema 2.

Comparison 1: Overall survival, Outcome 1: Overall survival (all trials)

Comparison 2: Progression‐free survival, Outcome 1: Progression‐free survival (amended analysis)

Comparison 2: Progression‐free survival, Outcome 2: Progression‐free survival (all trials)

Comparison 3: Overall response, Outcome 1: Overall response (all trials)

Comparison 4: Treatment‐related deaths, Outcome 1: Treatment‐related deaths (all trials)

Comparison 5: Neutropenia, Outcome 1: Neutropaenia

Comparison 6: Febrile neutropenia, Outcome 1: Febrile neutropenia

Comparison 7: Nausea and vomiting, Outcome 1: Nausea and vomiting

Comparison 8: Overall survival ‐ risk of bias, Outcome 1: Overall survival ‐ risk of bias

Comparison 9: Progression‐free survival ‐ risk of bias, Outcome 1: Progression‐free survival ‐ risk of bias (amended analysis)

Comparison 9: Progression‐free survival ‐ risk of bias, Outcome 2: Progression‐free survival ‐ risk of bias

Comparison 10: Overall response ‐ risk of bias, Outcome 1: Overall response ‐ risk of bias

Comparison 11: Treatment‐related deaths ‐ risk of bias, Outcome 1: Treatment‐related deaths ‐ risk of bias

Comparison 12: Neutropenia ‐ risk of bias, Outcome 1: Neutropaenia ‐ risk of bias

Comparison 13: Febrile neutropenia ‐ risk of bias, Outcome 1: Febrile neutropenia ‐ risk of bias

Comparison 14: Nausea and vomiting ‐ risk of bias, Outcome 1: Nausea and vomiting ‐ risk of bias

Comparison 15: Overall survival ‐ line of chemotherapy, Outcome 1: Overall survival ‐ line of chemotherapy

Comparison 16: Progression‐free survival ‐ line of chemotherapy, Outcome 1: Progression free survival ‐ line of chemotherapy (amended analysis)

Comparison 16: Progression‐free survival ‐ line of chemotherapy, Outcome 2: Progression free survival ‐ line of chemotherapy

Comparison 17: Overall response ‐ line of chemotherapy, Outcome 1: Overall response ‐ subgroup analysis, line of chemotherapy

Comparison 18: Treatment‐related deaths ‐ line of chemotherapy, Outcome 1: Treatment‐related deaths ‐ line of chemotherapy

Comparison 19: Neutropenia ‐ line of chemotherapy, Outcome 1: Neutropaenia ‐ line of chemotherapy

Comparison 20: Febrile neutropenia ‐ line of chemotherapy, Outcome 1: Febrile neutropenia ‐ line of chemotherapy

Comparison 21: Nausea and vomiting ‐ line of chemotherapy, Outcome 1: Nausea and vomiting ‐ line of chemotherapy

Comparison 22: Overall survival ‐ schema 1 versus schema 2, Outcome 1: Overall survival ‐ Schema 1 versus Schema 2

Comparison 23: Progression‐free survival ‐ schema 1 versus schema 2, Outcome 1: Progression‐free survival ‐ Schema 1 versus Schema 2 (amended analysis)

Comparison 23: Progression‐free survival ‐ schema 1 versus schema 2, Outcome 2: Progression‐free survival ‐ Schema 1 versus Schema 2

Comparison 24: Overall response ‐ schema 1 versus schema 2, Outcome 1: Overall response ‐ Schema 1 versus Schema 2

Comparison 25: Treatment‐related deaths ‐ schema 1 versus schema 2, Outcome 1: Treatment‐related deaths ‐ Schema 1 versus Schema 2

Comparison 26: Neutropenia ‐ schema 1 versus schema 2, Outcome 1: Neutropaenia ‐ subgroup analysis, Schema 1 versus Schema 2

Comparison 27: Febrile neutropenia ‐ schema 1 versus schema 2, Outcome 1: Febrile neutropenia ‐ Schema 1 versus Schema 2

Comparison 28: Nausea and vomiting ‐ schema 1 versus schema 2, Outcome 1: Nausea and vomiting ‐ Schema 1 versus Schema 2

Comparison 29: Overall survival ‐ relative dose intensity, Outcome 1: Overall survival ‐ relative dose intensity

Comparison 30: Progression‐free survival ‐ relative dose intensity, Outcome 1: Progression‐free survival ‐ relative dose intensity (amended analysis)

Comparison 30: Progression‐free survival ‐ relative dose intensity, Outcome 2: Progression‐free survival ‐ relative dose intensity

Comparison 31: Overall response ‐ relative dose intensity, Outcome 1: Overall response ‐ relative dose intensity

Comparison 32: Treatment‐related deaths ‐ relative dose intensity, Outcome 1: Treatment‐related deaths ‐ relative dose intensity

Comparison 33: Neutropenia ‐ relative dose intensity, Outcome 1: Neutropaenia ‐ relative dose intensity

Comparison 34: Febrile neutropenia ‐ relative dose intensity, Outcome 1: Febrile neutropenia ‐ relative dose intensity

Comparison 35: Nausea and vomiting ‐ relative dose intensity, Outcome 1: Nausea and vomiting ‐ relative dose intensity
| Combination | Sequential | Number of trials |
| doxorubicin + docetaxel | doxorubicin → docetaxel | Cresta 2004(included alternating regimen) Koroleva 2001 (included 2 combination arms with different doses) |
| 5‐fluorouracil + cyclophosphamide + vincristine | 5‐fluorouracil → cyclophosphamide → vincristine | |
| capecitabine + docetaxel or paclitaxel | capecitabine → docetaxel or paclitaxel | |
| 5‐fluorouracil + cyclophosphamide + prednisone + thyroxine or vincristine | 5‐fluorouracil → cyclophosphamide → thyroxine or vincristine → prednisone | |
| epirubicin + paclitaxel | epirubicin → paclitaxel | |
| epirubicin + paclitaxel | dose dense epirubicin→ paclitaxel | |
| gemcitabine + vinorelbine | gemcitabine → vinorelbine | |
| doxorubicin + paclitaxel | doxorubicin → paclitaxel or paclitaxel → doxorubicin | |
| docetaxel + gemcitabine | docetaxel → gemcitabine |
| Trial name | Arm I | Arm II |
| Alba 2004 | Arm I: AT= Doxorubicin 50 mg/m2 and docetaxel 75 mg/m2 both on day 1. Cycles repeated every 21 days for 6 cycles. If prior anthracyclines: given 3 cycles of AT at above doses followed by 3 cycles of docetaxel 100 mg/m2 | Arm II: A→T= Doxorubicin 75 mg/m2 intravenously day 1 for 3 cycles followed by docetaxel 100 mg/m2 intravenously day 1 for 3 cycles. If prior anthracyclines given 2 cycles of doxorubicin 75 mg/m2 followed by 4 cycles of docetaxel 100 mg/m2. Cycles repeated every 21 days |
| Baker 1974 | Arm I: FCV= 5‐fluorouracil 7.5 mg/kg intravenously days 1‐5 plus cyclophosphamide 4 mg/kg intravenously days 1 to 5 plus vincristine 0.015 mg/kg intravenously days 1 and 8. Cycles repeated every 28 days until disease progression | Arm II: F→C→V= 5‐fluorouracil 15 mg/kg intravenously days 1 to 5 every 28 days until disease progression then cyclophosphamide 8 mg/kg intravenously days 1 to 5 every 28 days until disease progression then vincristine 0.02 mg/kg intravenously weekly until disease progression |
| Beslija 2006 | Arm I: XT= Capecitabine (Xeloda, X) 1250 mg/m2 twice daily orally from days 1 to 14 and docetaxel (Taxotere, T) 75 mg/m2 intravenously day 1. Cycle repeated every 21 days until disease progression | Arm II: T→X= Docetaxel 100 mg/m2 intravenously day 1 until disease progression then capecitabine 1250 mg/m2 twice daily orally from days 1 to 14 until disease progression. Cycles repeated every 21 days |
| Chlebowski 1989 | WCSG Arm I: CMFTP= Cyclophosphamide 2 mg/kg/day orally, plus 5‐fluorouracil 15 mg/kg every 2 weeks intravenously from day 1, plus methotrexate 30 mg/m2 every 2 weeks intravenously beginning on day 8, plus prednisone 0.5 mg/kg/day orally, plus triiodothyronine 0.005 mg daily. Cycle repeated until disease progression. SECSG Arm I: Cyclophosphamide 400 mg/m2 intravenously day 1 every 28 days, plus 5‐fluorouracil 400 mg/m2 intravenously day 1 and day 8 every 28 days, plus methotrexate 30 mg/m2 intravenously day 1 and day 8 every 28 days, plus vincristine 1 mg/m2 intravenously day 1 and day 8 every 28 days, plus prednisone 80mg orally daily from days 1 to 7 every 28 days. Cycle repeated until disease progression. or Cyclophosphamide 100 mg orally daily, plus 5‐fluorouracil 400 mg/m2 intravenously weekly, plus methotrexate 20 mg/m2 orally weekly, plus vincristine 1 mg/m2 intravenously weekly, plus prednisone 45 mg orally daily for 14 days, then 30 mg daily for 14 days then 15 mg daily for 28 days. Cycle repeated until disease progression | WCSG Arm II: F→C→TP→M= 5‐fluorouracil 15 mg/kg weekly intravenously from day 1 for a minimum of 4 weeks until disease progression then cyclophosphamide 2 mg/kg/day orally for a minimum of 4 weeks until disease progression then triiodothyronine 0.005 mg daily plus prednisone 0.5 mg/kg/day for a minimum of 6 weeks until disease progression then methotrexate 30 mg/m2 intravenously weekly for a minimum of 4 weeks SECSG Arm II: F→MC→V→P= 5‐fluorouracil 600 mg/m2 intravenously weekly until disease progression then methotrexate 20 mg/m2 orally biweekly until disease progression then cyclophosphamide 100 mg/m2 orally daily until disease progression then vincristine 1 mg/m2 intravenously weekly until disease progression then prednisone 45 mg orally daily for 14 days then 30 mg daily for 14 days then 15 mg daily for 30 days |
| Conte 2004 | Arm I: EP= Epirubicin 90 mg/m2 plus paclitaxel 200 mg/m2 intravenously day 1. Cycles repeated every 21 days for 8 cycles | Arm II: E→P= Epirubicin 120 mg/m2 intravenously day 1 for 4 cycles then paclitaxel 250 mg/m2 intravenously day 1 for 4 cycles. Cycles repeated every 21 days |
| Cresta 2003 | Arm I: AT= Doxorubicin 60 mg/m2 plus docetaxel 60 mg/m2 intravenously day. Cycles repeated every 21 days for 8 cycles | Arm II: A→T (sequential regimen)= Doxorubicin 75 mg/m2 intravenously on day 1 for 4 cycles followed by docetaxel 75 mg/m2 intravenously on day 1 for 4 cycles. Cycles repeated every 21 days. Maximum 8 cycles. Arm III: T then A (alternating regimen)= Docetaxel 100 mg/m2 intravenously on day 1 for 4 cycles alternating with doxorubicin 75 mg/m2 intravenously on day 1 for 4 cycles. Cycles repeated every 21 days. Maximum 8 cycles |
| Fountzilas 2001 | Arm I: P= Epirubicin 80 mg/m2 plus paclitaxel 175 mg/m2 intravenously day 1. Cycles repeated every 21 days for 6 cycles | Arm II: E→P Epirubicin 110 mg/m2 intravenously day 1 for 4 cycles followed by paclitaxel 225 mg/m2 intravenously day 1 for 4 cycles. Cycles repeated every 14 days with G‐CSF support (dose dense regimen) |
| Koroleva 2001 | Arm I: AT= Doxorubicin 50 mg/m2 plus docetaxel 75 mg/m2 intravenously day 1. Cycles repeated every 21 days for 8 cycles | Arm II: T→A= Docetaxel 100 mg/m2 intravenously day 1 for 4 cycles followed by doxorubicin 75 mg/m2 intravenously day 1 for 4 cycles. Cycles repeated every 21 days. Arm III: AT= Doxorubicin 60 mg/m2 plus docetaxel 60 mg/m2 intravenously day 1. Cycles repeated every 21 days for 8 cycles |
| Park 2010 | Arm I: GV= Gemcitabine 1,000 mg/m2 plus vinorelbine 25 mg/m2 intravenously days 1 and 8. Cycles repeated every 21 days until disease progression | Arm II: G→V= Gemcitabine 1,200 mg/m2 intravenously on days 1 and 8 until disease progression then vinorelbine 30 mg/m2 intravenously days 1 and 8 until disease progression. Cycles repeated every 21 days |
| Sledge 2003 | Arm I: AT= Doxorubicin 50 mg/m2 plus paclitaxel 150 mg/m2 over 24 hours intravenously day 1. Cycles repeated every 21 days until disease progression | Arm II: A (→P)= Doxorubicin 60 mg/m2 intravenously day 1 for a maximum of 8 cycles. If disease progressed crossed over to paclitaxel 175 mg/m2 intravenously over 24 hours on day 1. Cycles repeated every 21 days. Arm III: P (→A)= Paclitaxel 175 mg/m2 intravenously over 24 hours on day 1. If disease progressed crossed over to doxorubicin 60 mg/m2 intravenously day 1. Cycles repeated every 21 days |
| Soto 2006 | Arm I: XT= Capecitabine 825 mg/m2 twice daily orally from days 1 to 14 plus docetaxel 75 mg/m2 intravenously on day 1 until disease progression. Cycles repeated every 21 days | Arm II: X→T (docetaxel or paclitaxel)= Capecitabine 1250 mg/m2 twice daily orally days 1 to 14 until disease progression then docetaxel 100 mg/m2 or paclitaxel 175 mg/m2 intravenously on day 1 until disease progression. Cycles repeated every 21 days. Arm III: XP= Capecitabine 825 mg/m2 twice daily orally from days 1 to 14 plus paclitaxel 175 mg/m2 intravenously on day 1 until disease progression. Cycles repeated every 21 days |
| Tomova 2010 | Arm I: TG= Docetaxel 75 mg/m2 intravenously on day 1 plus gemcitabine 1,000 mg/m2 intravenously on days 1 and 8. Cycles repeated every 21 days for 8 cycles | Arm II: T→G= Docetaxel 100 mg/m2 intravenously on day 1 for 4 cycles followed by gemcitabine 1,250 mg/m2 intravenously on days 1 and 1 for 4 cycles. Cycles repeated every 21 days |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall survival (all trials) Show forest plot | 9 | 1786 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Progression‐free survival (amended analysis) Show forest plot | 7 | 1483 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.99, 1.25] |
| 2.2 Progression‐free survival (all trials) Show forest plot | 8 | Hazard Ratio (IV, Fixed, 95% CI) | 1.16 [1.03, 1.31] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Overall response (all trials) Show forest plot | 12 | 2140 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [1.06, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Treatment‐related deaths (all trials) Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.71, 3.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Neutropaenia Show forest plot | 12 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.87, 1.02] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Febrile neutropenia Show forest plot | 9 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.06, 1.65] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Nausea and vomiting Show forest plot | 8 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.57, 1.34] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Overall survival ‐ risk of bias Show forest plot | 9 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.93, 1.16] | |
| 8.1.1 Low risk of bias | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.21 [0.93, 1.58] | |
| 8.1.2 High/unclear risk of bias | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.01 [0.90, 1.14] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Progression‐free survival ‐ risk of bias (amended analysis) Show forest plot | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.99, 1.25] | |
| 9.1.1 Low risk of bias | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.15 [0.94, 1.41] | |
| 9.1.2 High/unclear risk of bias | 5 | Hazard Ratio (IV, Fixed, 95% CI) | 1.09 [0.94, 1.26] | |
| 9.2 Progression‐free survival ‐ risk of bias Show forest plot | 8 | Hazard Ratio (IV, Fixed, 95% CI) | 1.16 [1.03, 1.31] | |
| 9.2.1 Low risk of bias | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.15 [0.94, 1.41] | |
| 9.2.2 High/unclear risk of bias | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 1.17 [1.01, 1.35] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Overall response ‐ risk of bias Show forest plot | 12 | 2140 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [1.06, 1.28] |
| 10.1.1 Low risk of bias | 2 | 381 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 10.1.2 High/unclear risk of bias | 10 | 1759 | Risk Ratio (IV, Fixed, 95% CI) | 1.26 [1.13, 1.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Treatment‐related deaths ‐ risk of bias Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.71, 3.29] |
| 11.1.1 Low risk of bias | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.28, 24.60] |
| 11.1.2 High/unclear risk of bias | 6 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.62, 3.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Neutropaenia ‐ risk of bias Show forest plot | 12 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.87, 1.02] | |
| 12.1.1 Low risk of bias | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.62 [0.50, 0.76] | |
| 12.1.2 High/unclear risk of bias | 10 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.92, 1.09] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Febrile neutropenia ‐ risk of bias Show forest plot | 9 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.06, 1.65] | |
| 13.1.1 Low risk of bias | 2 | Risk Ratio (IV, Fixed, 95% CI) | 1.60 [0.72, 3.57] | |
| 13.1.2 High/unclear risk of bias | 7 | Risk Ratio (IV, Fixed, 95% CI) | 1.30 [1.03, 1.64] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 Nausea and vomiting ‐ risk of bias Show forest plot | 8 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.57, 1.34] | |
| 14.1.1 Low risk of bias | 1 | Risk Ratio (IV, Fixed, 95% CI) | 1.03 [0.21, 4.99] | |
| 14.1.2 High/unclear risk of bias | 7 | Risk Ratio (IV, Fixed, 95% CI) | 0.87 [0.56, 1.35] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 Overall survival ‐ line of chemotherapy Show forest plot | 9 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.93, 1.16] | |
| 15.1.1 First line chemotherapy | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.93, 1.18] | |
| 15.1.2 Second/third line chemotherapy | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.03 [0.76, 1.40] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Progression free survival ‐ line of chemotherapy (amended analysis) Show forest plot | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.99, 1.25] | |
| 16.1.1 First line chemotherapy | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.99, 1.26] | |
| 16.1.2 Second/third line chemotherapy | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.85 [0.24, 2.99] | |
| 16.2 Progression free survival ‐ line of chemotherapy Show forest plot | 8 | Hazard Ratio (IV, Fixed, 95% CI) | 1.16 [1.03, 1.31] | |
| 16.2.1 First line chemotherapy | 6 | Hazard Ratio (IV, Fixed, 95% CI) | 1.16 [1.02, 1.31] | |
| 16.2.2 Second/third line chemotherapy | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.25 [0.80, 1.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 Overall response ‐ subgroup analysis, line of chemotherapy Show forest plot | 12 | 2140 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [1.06, 1.28] |
| 17.1.1 First line chemotherapy | 9 | 1778 | Risk Ratio (IV, Fixed, 95% CI) | 1.10 [1.00, 1.22] |
| 17.1.2 Second/third line chemotherapy | 3 | 362 | Risk Ratio (IV, Fixed, 95% CI) | 1.54 [1.22, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 Treatment‐related deaths ‐ line of chemotherapy Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.71, 3.29] |
| 18.1.1 First line chemotherapy | 5 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.70, 4.18] |
| 18.1.2 Second/third line chemotherapy | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.23, 4.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 19.1 Neutropaenia ‐ line of chemotherapy Show forest plot | 12 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.84, 1.01] | |
| 19.1.1 First‐line chemotherapy | 9 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.87, 1.05] | |
| 19.1.2 Second/third‐line chemotherapy | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.48, 0.85] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 20.1 Febrile neutropenia ‐ line of chemotherapy Show forest plot | 9 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.06, 1.65] | |
| 20.1.1 First‐line chemotherapy | 7 | Risk Ratio (IV, Fixed, 95% CI) | 1.34 [1.07, 1.68] | |
| 20.1.2 Second/third‐line chemotherapy | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.24, 3.15] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 21.1 Nausea and vomiting ‐ line of chemotherapy Show forest plot | 8 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.57, 1.34] | |
| 21.1.1 First‐line chemotherapy | 5 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.55, 1.33] | |
| 21.1.2 Second/third‐line chemotherapy | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.27, 5.74] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 22.1 Overall survival ‐ Schema 1 versus Schema 2 Show forest plot | 9 | Hazard Ratio (IV, Fixed, 95% CI) | 1.04 [0.93, 1.16] | |
| 22.1.1 Schema 1 | 5 | Hazard Ratio (IV, Fixed, 95% CI) | 0.98 [0.86, 1.12] | |
| 22.1.2 Schema 2 | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.22 [0.99, 1.49] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 23.1 Progression‐free survival ‐ Schema 1 versus Schema 2 (amended analysis) Show forest plot | 7 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.99, 1.25] | |
| 23.1.1 Schema 1 | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 1.11 [0.94, 1.31] | |
| 23.1.2 Schema 2 | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.12 [0.94, 1.33] | |
| 23.2 Progression‐free survival ‐ Schema 1 versus Schema 2 Show forest plot | 8 | Hazard Ratio (IV, Fixed, 95% CI) | 1.16 [1.03, 1.31] | |
| 23.2.1 Schema 1 | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.20 [1.03, 1.40] | |
| 23.2.2 Schema 2 | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.12 [0.94, 1.33] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 24.1 Overall response ‐ Schema 1 versus Schema 2 Show forest plot | 12 | 2140 | Risk Ratio (IV, Fixed, 95% CI) | 1.16 [1.06, 1.28] |
| 24.1.1 Schema 1 | 6 | 1344 | Risk Ratio (IV, Fixed, 95% CI) | 1.46 [1.28, 1.65] |
| 24.1.2 Schema 2 | 6 | 796 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.80, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 25.1 Treatment‐related deaths ‐ Schema 1 versus Schema 2 Show forest plot | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.71, 3.29] |
| 25.1.1 Schema 1 | 3 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.36, 2.44] |
| 25.1.2 Schema 2 | 4 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.87, 18.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 26.1 Neutropaenia ‐ subgroup analysis, Schema 1 versus Schema 2 Show forest plot | 12 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.87, 1.02] | |
| 26.1.1 Schema 1 | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.87, 1.14] | |
| 26.1.2 Schema 2 | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.84, 1.01] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 27.1 Febrile neutropenia ‐ Schema 1 versus Schema 2 Show forest plot | 9 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.06, 1.65] | |
| 27.1.1 Schema 1 | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.76 [1.14, 2.73] | |
| 27.1.2 Schema 2 | 6 | Risk Ratio (IV, Fixed, 95% CI) | 1.19 [0.92, 1.55] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 28.1 Nausea and vomiting ‐ Schema 1 versus Schema 2 Show forest plot | 8 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.57, 1.34] | |
| 28.1.1 Schema 1 | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.39, 1.46] | |
| 28.1.2 Schema 2 | 5 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.56, 1.71] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 29.1 Overall survival ‐ relative dose intensity Show forest plot | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.14 [0.92, 1.39] | |
| 29.1.1 Similar dose intensity | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.23 [0.93, 1.62] | |
| 29.1.2 Different dose intensity | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.03 [0.76, 1.40] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 30.1 Progression‐free survival ‐ relative dose intensity (amended analysis) Show forest plot | 3 | Hazard Ratio (IV, Fixed, 95% CI) | 1.13 [0.90, 1.43] | |
| 30.1.1 Similar dose intensity | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.15 [0.91, 1.45] | |
| 30.1.2 Different dose intensity | 1 | Hazard Ratio (IV, Fixed, 95% CI) | 0.85 [0.24, 2.99] | |
| 30.2 Progression‐free survival ‐ relative dose intensity Show forest plot | 4 | Hazard Ratio (IV, Fixed, 95% CI) | 1.17 [0.95, 1.44] | |
| 30.2.1 Similar dose intensity | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.15 [0.91, 1.45] | |
| 30.2.2 Different dose intensity | 2 | Hazard Ratio (IV, Fixed, 95% CI) | 1.25 [0.80, 1.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 31.1 Overall response ‐ relative dose intensity Show forest plot | 6 | 679 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 31.1.1 Similar dose intensity | 3 | 355 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 31.1.2 Different dose intensity | 3 | 324 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.72, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 32.1 Treatment‐related deaths ‐ relative dose intensity Show forest plot | 4 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.45, 6.10] |
| 32.1.1 Similar dose intensity | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 32.1.2 Different dose intensity | 3 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.45, 6.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 33.1 Neutropaenia ‐ relative dose intensity Show forest plot | 6 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.92, 1.12] | |
| 33.1.1 Similar dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.08 [0.97, 1.20] | |
| 33.1.2 Different dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.51, 0.87] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 34.1 Febrile neutropenia ‐ relative dose intensity Show forest plot | 6 | Risk Ratio (IV, Fixed, 95% CI) | 1.21 [0.93, 1.57] | |
| 34.1.1 Similar dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 2.36 [1.21, 4.62] | |
| 34.1.2 Different dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.07 [0.80, 1.42] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 35.1 Nausea and vomiting ‐ relative dose intensity Show forest plot | 6 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.56, 1.71] | |
| 35.1.1 Similar dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [0.50, 2.60] | |
| 35.1.2 Different dose intensity | 3 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.40, 1.83] | |

