نقش پروبیوتیکها در پیشگیری از عفونتهای مجاری ادراری در بزرگسالان و کودکان
چکیده
پیشینه
عفونت مجاری ادراری (UTI) یک عفونت باکتریایی شایع است که میتواند منجر به بروز موربیدیتی قابل توجهی همچون تنگی (stricture)، تشکیل آبسه (abscess)، فیستول (fistula)، باکتریمی (bacteraemia)، سپسیس (sepsis)، پیلونفریت (pyelonephritis) و اختلال عملکرد کلیه شود. نرخ مورتالیتی ناشی از پیشرفت پیلونفریت در مردان تا سقف 1% و در زنان تا سقف 3% گزارش شده است. به دلیل آنکه درمان با پروبیوتیک به راحتی بدون نیاز به نسخه در دسترس است، مرور اثربخشی آنها در پیشگیری از UTI ممکن است به مصرف کنندگان در اتخاذ تصمیمهای آگاهانه درباره درمان بالقوه پروفیلاکتیک کمک کند. مؤسسات و ارائه دهندگان خدمات نیز برای اتخاذ تصمیمهای آگاهانه در رابطه با درمان بیماران به خلاصهای مبتنی بر شواهد موجود نیاز دارند.
اهداف
آیا پروبیوتیکها (با هر نوع فرمولاسیون) به هنگام استفاده برای پیشگیری از UTI در جمعیت بیماران مستعد، در مقایسه با دارونما (placebo) یا عدم درمان، از مزیت درمانی از نظر مورتالیتی و موربیدیتی برخوردار هستند؟
آیا پروبیوتیکها (با هر نوع فرمولاسیون) به هنگام استفاده برای پیشگیری از UTIها در جمعیت بیماران مستعد، در مقایسه با سایر مداخلات پروفیلاکتیک، مشتمل بر معیارهای دارویی و غیر‐دارویی (برای مثال پروفیلاکسی دارویی/پروفیلاکسی با آنتیبیوتیک به صورت مداوم، استروژن موضعی، آب آلبالو)، از مزیت درمانی از نظر مورتالیتی و موربیدیتی برخوردار هستند؟
روشهای جستوجو
از طریق برقراری ارتباط با هماهنگ کننده جستوجوی کارآزماییها (Trials' Search Co‐ordinator) و با استفاده از واژگان مرتبط با این مرور، تا تاریخ 21 سپتامبر 2015 پایگاه ثبت تخصصی گروه کلیه و پیوند در کاکرین (Cochrane Kidney and Transplant Specialised Register) را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) شامل بیماران مستعد (برای مثال دارای سابقه ابتلا به UTI در گذشته) یا افراد سالم که در آنها پروبیوتیک از هر نوع، با هر فرمولاسیون، دوز یا فراوانی، با دارونما یا سایر گروههای مقایسه فعال (active comparators) مقایسه شده بود، وارد مرور شدند.
گردآوری و تجزیهوتحلیل دادهها
تمامی RCTها و شبه‐RCTها (RCTهایی که تخصیص جهت درمان در آنها، بر اساس تناوب، استفاده از رکوردهای پزشکی جایگزین، تاریخ تولد یا سایر روشهای قابل پیشبینی است) که ناظر بر مقایسه پروبیوتیکها با عدم اقدام به درمان، دارونما، یا سایر مداخلات پروفیلاکتیک بودند، وارد مطالعه شدند. تخمینهای خلاصه از تاثیر با استفاده از مدل اثرات‐تصادفی به دست آمدند و نتایج به صورت خطرات نسبی (RR) و 95% فواصل اطمینان (CI) برای پیامدهای دو‐حالتی بیان شدند.
نتایج اصلی
ما نه مطالعه مشتمل بر 735 نفر را در این مرور وارد کردیم. چهار مطالعه به مقایسه پروبیوتیک با دارونما، دو مطالعه به مقایسه پروبیوتیک با عدم درمان، دو مطالعه به مقایسه پروبیوتیکها با آنتیبیوتیکها در بیماران مبتلا به UTI، و یک مطالعه به مقایسه پروبیوتیک با دارونما در زنان سالم پرداخته بودند. تمامی مطالعات با هدف اندازهگیری تفاوتهای موجود میان نرخ عود UTI انجام شده بودند.
در ارزیابی خطر سوگیری (bias) متوجه شدیم که حجم نمونه در بیشتر مطالعات کوچک بوده و به دلیل گزارش ناکافی جزئیات مربوط به روششناسی، امکان ارزیابی دقیق را فراهم نمیکردند. در مجموع، سوگیری در مطالعات وارد شده پُر‐خطر بود که امکان نتیجهگیری قطعی و دقیق را فراهم نکرده و اینگونه نشان میداد که هر یک از تاثیرات درمان گزارش شده ممکن است گمراه کننده بوده یا بیش از حد تخمین زده شود.
ما هیچ کاهش معناداری در خطر ابتلا به عفونت مجاری ادراری باکتریایی علامتدار راجعه (عودکننده) میان بیماران درمان شده با پروبیوتیکها و دارونما نیافتیم (6 مطالعه، 352 شرکتکننده: RR: 0.82؛ 95% CI؛ 0.60 تا 1.12؛ I2 = 23%) با فواصل اطمینان گسترده، و ناهمگونی آماری پائین. هیچ کاهش معناداری در خطر ابتلا به عفونت مجاری ادراری باکتریایی علامتدار راجعه (عودکننده) میان بیماران درمان شده با پروبیوتیک و آنتیبیوتیک یافت نشد (1 مطالعه، 223 شرکتکننده: RR: 1.12؛ 95% CI؛ 0.95 تا 1.33).
شایعترین عوارض جانبی گزارش شده عبارت بود از اسهال، تهوع، استفراغ، یبوست و نشانههای واژینال. هیچ یک از مطالعات وارد شده، تعداد شرکتکنندگان را با حداقل یک مورد ابتلا به UTI باکتریایی بدون نشانه، مورتالیتی به هر علتی یا شرکتکنندگان با حداقل یک مورد ابتلا به باکتریمی یا عفونت خون با قارچ (fungaemia) را گزارش نکرده بودند. در دو مطالعه انصراف از ادامه همکاری در مطالعه به دلیل بروز عوارض جانبی و تعداد شرکتکنندگان با حداقل یک مورد عارضه جانبی گزارش شده بود. یک مطالعه انصراف شش نفر از شرکتکنندگان تحت درمان با پروبیوتیک (5.2%) و 15 نفر از شرکتکنندگانِ تحت درمان با آنتیبیوتیک (12.2%) را گزارش کرده بود، در حالی که مطالعه دوم، درمان نیمه رها کرده یک شرکتکننده از گروه دارونما را به دلیل بروز عارضه جانبی گزارش کرده بود.
نتیجهگیریهای نویسندگان
هیچ مزیت معناداری برای پروبیوتیکها در مقایسه با دارونما یا عدم درمان ثابت نشد، اما این مزیت را نمیتوان منتفی دانست زیرا دادهها محدود و از مطالعات کوچک با گزارشدهی ضعیف روششناسی حاصل شده بودند.
دادههای محدودی درباره خطرات و مورتالیتی مرتبط با پروبیوتیکها وجود داشت و هیچ شواهدی در رابطه با اثر پروبیوتیکها بر عوارض جانبی جدی وجود نداشت. شواهد موجود نمیتوانند کاهش یا افزایش UTI راجعه در زنان مبتلا به UTI راجعه که از پروبیوتیکهای پروفیلاکتیک استفاده میکنند، منتفی بدانند. شواهد ارائه شده از یک RCT برای اظهار نظر درباره تاثیر پروبیوتیکها در برابر آنتیبیوتیکها ناکافی بودند.
PICO
خلاصه به زبان ساده
نقش پروبیوتیکها در پیشگیری از عفونتهای مجاری ادراری در بزرگسالان و کودکان
پیشینه
عفونتهای مجاری ادراری (urinary tract infections; UTIs) در کلیهها، حالبها، مجاری ادراری یا مثانه اتفاق میافتد. UTIها یکی از شایعترین عفونتهای باکتریایی بوده و میتوانند منجر به بروز سایر مشکلات مرتبط با سلامت شوند.
تصور میشود که پروبیوتیکها (میکروارگانیسمهای زندهای که وقتی به مقادیر کافی استفاده میشوند، برای میزبان خود مزیتی به همراه دارند) به واسطه پیشگیری از ابتلای مجاری ادراری به سایر عفونتهای باکتریایی، در بروز عفونت تاثیرگذار هستند. ما علاقهمند بودیم هر یک از انواع پروبیوتیکها (استفاده از باکتریها برای تغییر تعادل باکتریها) را در مقایسه با عدم درمان، آنتیبیوتیکها، هورموندرمانی، آب آلبالو یا سایر مداخلات در افراد در معرض خطر ابتلا به UTI، مطالعه کنیم. در صورت مثبت بودن نتیجه ارزیابی اثربخشی پروبیوتیکها، ما برنامهریزی کرده بودیم تعداد افراد مبتلا به UTIهای راجعه را اندازهگیری کنیم.
ویژگیهای مطالعه
تا سپتامبر 2015 به جستوجو در منابع علمی پرداختیم و نه مطالعه به لحاظ انطباق با معیار انتخاب ما، واجد شرایط بودند. این نه مطالعه به گزارش دادههای مربوط به 735 شرکتکننده پرداخته و اثرات پروبیوتیکها را در پیشگیری از UTI تحقیق و بررسی کرده بودند:هفت مطالعه دربرگیرنده زنان یا دختران مبتلا به UTIها بوده، یک مطالعه به بررسی موضوع در کودکان مبتلا به ناهنجاریهای مجاری ادراری پرداخته بود و یک مطالعه به تحقیق و بررسی UTI در زنان سالم اقدام کرده بود.
نتایج کلیدی
به طور کلی، کیفیت مطالعات در سطح پائین و خطر سوگیری (bias) در آنها بالا بود. فارغ از جمعیتهای متفاوت، انواع بسیار مختلفی از پروبیوتیکها و انواع مختلفی از دوزها به صورت واژینال و خوراکی داده شده بود و پروبیوتیکها برای دورههای زمانی متنوعی تجویز شده بودند. تمامی این عوامل ممکن است روی نتایج ما تاثیر گذاشته باشد.
بیشتر مطالعات دادههای مربوط به عوارض جانبی را گردآوری نکرده بودند، بنابراین ما نمیتوانستیم خطرات مرتبط با درمانهای مختلف را بر پایه پروبیوتیک تخمین بزنیم. هیچ کاهش معناداری در رابطه با خطر ابتلا به عفونت مجاری ادراری باکتریایی علامتدار راجعه (عودکننده) میان بیماران درمان شده با پروبیوتیک و دارونما (placebo) و هیچ کاهش معناداری در رابطه با خطر ابتلا به این عفونت میان پروبیوتیک و بیماران درمان شده با آنتیبیوتیکها نیافتیم.
کیفیت شواهد
شواهد موجود در دسترس هیچ کاهشی در UTI به دنبال استفاده از پروبیوتیکها نشان ندادند.
Authors' conclusions
Summary of findings
| Probiotics compared with placebo or antibiotics for urinary tract infections (UTI) | ||||||
| Patient or population: adults and children at risk of UTI Settings: outpatient Intervention: probiotics Comparison: placebo or antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
| Symptomatic bacterial UTI in adults and children in patients with and without recurrent UTI Probiotics versus placebo (follow‐up) | 395 per 1000 | 296 per 1000 | RR 0.75 (0.50, 1.13) | 352 (6) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
| Symptomatic bacterial UTI in adults and children with recurrent UTI Probiotics versus placebo (follow‐up) | 421 per 1000 | 315 per 1000 | RR 0.74 (0.54, 1.01) | 275 (4) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
| Symptomatic bacterial UTI in women with recent UTI Probiotics versus antibiotics (follow‐up) | 666 per 1000 | 745 per 1000 | RR 1.12 (0.95, 1.33) | 223 (1) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus antibiotics. Imprecision also due to small sample from only one RCT |
| Symptomatic bacterial UTI in children with VUR Probiotics versus placebo (follow‐up) | 270 per 1000 | 145 per 1000 | RR 0.54 (0.24, 1.23) | 96 (1) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains of and suggest that results are imprecise or overestimate probiotic effects versus placebo. Imprecision also due to small sample from only one RCT |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| UTi ‐ urinary tract infection | ||||||
Background
Description of the condition
Urinary tract infections (UTIs) are defined as infections of kidneys, ureters, urethra, or bladder due to bacterial colonisation. UTIs are one of the most common bacterial infections and can lead to significant morbidity including strictures, abscess formation, fistulas, bacteraemia, sepsis, pyelonephritis, and kidney dysfunction. Mortality rates are reported to be as high as 1% in men and 3% in women due to development of pyelonephritis. One in two women experience UTI at some point in their lifetime. UTI incidence in men is related to age (1.1% to 1.6% in the first 10 years of life, 5 to 8 infections/year/10,000 men up to age 50 years, and higher after age 50 due to prostate enlargement and subsequent complications) (Foxman 2003, Howes 2009; Howes 2010). Elderly people are more susceptible to asymptomatic UTI; prevalence is 30% in women and 10% in men per year in women and men (Richards 2004).
Several interventions have been studied for preventing UTI. Mixed results have been seen for intravaginal hormonal therapy for women and management of incontinence (Perrotta 2008; Ouslander 1995; Schnelle 1995). Improved urinary catheter technology and catheter management strategies have demonstrated efficacy in reducing UTI incidence (CDC 2000; Christensen 2001; Maki 2001; Richards 2001; Saint 2000). A systematic review of randomised controlled trials (RCTs) concluded that there is some evidence that cranberry juice reduces the incidence of UTIs in women (Jepson 2012). Prophylactic antibiotics have been shown to reduce the incidence of UTIs in non‐pregnant women with recurrent UTIs (Albert 2004) and may reduce asymptomatic UTIs in children (Williams 2011).
Description of the intervention
Probiotics are defined as "a preparation of, or a product containing viable, defined micro‐organisms in sufficient numbers, which alter the microflora (by implantation or colonisation) in a compartment of the host and by that exert beneficial health effects in this host" (Schrezenmeir 2001). There are a number of species and strains of probiotics available that are used in many formulations administered via several different routes.
How the intervention might work
Probiotic organisms (e.g. lactobacillus) are thought to establish a barrier against infectious pathogens ascending the urinary tract, colonising, and subsequently causing infection. The protective effects thought to be exerted by probiotics are thought to include reducing pathogen adherence, growth and colonisation, and modulating host defences (Bruce 1988; Hawthorn 1990; Heineman 2000; Osset 2001; Velraeds 1998).
Why it is important to do this review
A 2006 systematic review concluded that carefully selected strains of probiotics when tested in case‐control studies and RCTs had mixed effects in terms of UTI prophylaxis (Falagas 2006). The authors concluded that there was some in vitro and in vivo evidence that probiotics restore normal vaginal flora and prevent recurrent UTI in women (Falagas 2006).
Probiotic therapy is readily available without prescription. A review of their efficacy in preventing UTIs may aid consumers and healthcare providers to make informed decisions about potential prophylactic therapy.
Objectives
Our review aimed to assess:
-
Compared to placebo or no therapy, do probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality, when used to prevent UTIs in susceptible patient populations?
-
Compared to other prophylactic interventions, including drug and non‐drug measures (e.g. continuous antibiotic prophylaxis, topical oestrogen, cranberry juice), do probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality when used to prevent UTIs in susceptible patient populations?
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at comparing probiotics to no therapy, placebo, or other prophylactic interventions were included.
Types of participants
-
Men, women, and children with histories of recurrent bacterial UTI (two episodes within the last two months)
-
Men and women over the age of 60 years
-
Pregnant women
-
Men, women and children with an indwelling catheter or requiring intermittent catheterisation
-
Men, women and children with an abnormal urinary tract (for example vesicoureteric reflux, urinary obstruction, dysfunctional voiding)
-
Men and women resident in residential and long‐term care facilities
-
Men and women with asymptomatic bacteriuria.
Studies exclusively involving critically ill or immunosuppressed patients were excluded. Applicable patient data were extracted from studies with mixed populations.
Types of interventions
-
All available probiotics in any formulation including tablets, capsules, food products (i.e. shakes, yogurt) for preventing UTIs in adults and children.
-
Any study in which probiotics were used for the treatment (versus prevention) of suspected or proven bacterial UTI was excluded.
-
Studies investigating prophylaxis with probiotics in combination with antibiotics were not included. These topics were beyond the scope of this review.
Types of outcome measures
Primary outcomes
Numbers of patients with at least one symptomatic bacterial UTI in each group (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen and defined as > 105 CFU/mL, or as defined by authors).
Secondary outcomes
-
Numbers with at least one asymptomatic bacterial UTI (confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen)
-
Withdrawal due to adverse events
-
Total adverse events
-
All‐cause mortality
-
Numbers with at least one non‐fatal serious adverse events
-
Numbers with at least one confirmed case of bacteraemia or fungaemia.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 21 September 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that potentially included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and where necessary, the full text of these studies to determine which satisfied inclusion criteria. There were no language restrictions.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies among published versions was planned to be highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes results were expressed as risk ratio (RR) with 95% confidence intervals (CI). All prespecified outcomes were dichotomous; therefore no analysis of continuous outcome data was necessary.
Unit of analysis issues
Data from all patients individually randomised to each intervention were included in the analyses. Care was taken to identify situations in which data had been censored or excluded or if data presented were the total number of events or the total number of patients with a first event. Authors were contacted for clarification if necessary. The rates of each outcome in the probiotic groups group were compared to the rate of that outcome in control groups to calculate risk differences. If the rates for an outcome were not provided, a narrative summary of data was presented. UTI rates were extracted for numbers of patients experiencing at least one UTI, not the number of UTIs in a treatment group.
Dealing with missing data
In general if there were missing data, the authors of the study were contacted for clarification to determine if details were available. If not, or if authors did not respond to requests, the worst outcome was imputed for all missing data points in the experimental treatment group (i.e. worst case scenario). A sensitivity analysis was performed to see if the effect size for any particular outcome was sensitive to conducting the worst case scenario with imputed data versus ignoring the missing data (i.e. using only the available data).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
A funnel plot was not created because of the few included studies; the resulting analysis would likely be underpowered to detect possible publication bias (Higgins 2011).
Data synthesis
Data were pooled using relative risks with the random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted for studies comparing probiotics with placebo or active comparators. In addition, a post‐hoc subgroup analysis was conducted for different patient characteristics: adult women; children, and children with vesicoureteral reflux.
Sensitivity analysis
Sensitivity analysis was performed to test for robustness of the results. Analysis of the following categories was undertaken separately.
-
Studies without proper randomisation or concealment of allocation compared to those without these characteristics.
-
Studies performed without intention‐to‐treat (ITT) analysis compared to those with an ITT analysis.
-
Unblinded studies versus blinded studies.
-
Studies using different probiotic formulations.
-
The effects of probiotics when there is missing data for patients receiving probiotics, these patients are assumed to have had the worst possible outcome.
Results
Description of studies
Results of the search
We identified 389 records. Following assessment of titles and abstracts, 28 full‐text records were screened. Of these, nine studies (14 records) were included and eight studies (10 records) were excluded. Two ongoing studies were identified (NCT00781625; ProSCIUTTU Study 2014), one study is awaiting translation (Skerk 2010), and one study was identified prior to publication ( Reid 1995). These four studies and will be assessed in a future update of this review (Figure 1).
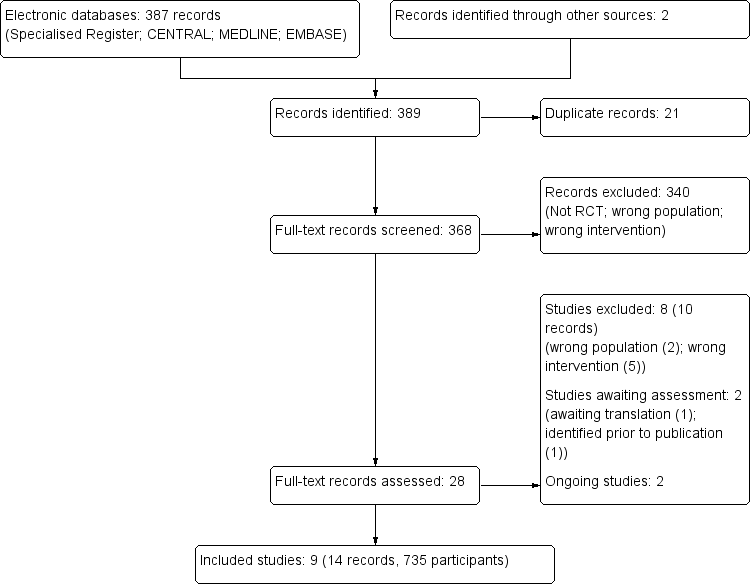
Study flow diagram
Included studies
We included nine studies in this review (Baerheim 1994; Czaja 2007; Ferrara 2009; Kontiokari 2001; Lee 2007a; NAPRUTI Study II 2006; Reid 1992; Reid 2003; Stapleton 2011).
Six studies compared probiotics with placebo (Baerheim 1994; Czaja 2007; Reid 1992; Stapleton 2011) or no comparator (Ferrara 2009; Kontiokari 2001); two studies compared probiotics with antibiotic prophylaxis in patients with UTI (one in adults (NAPRUTI Study II 2006) and one in children with VUR (Lee 2007a)); and one study compared probiotics with placebo in healthy women (Reid 2003).
Design
The included studies were parallel RCTs with a mix of active comparators, placebo or no comparators. Efficacy of the probiotics in placebo‐controlled studies (Baerheim 1994; Czaja 2007; Ferrara 2009; Kontiokari 2001; Reid 1992; Stapleton 2011) could not be compared to studies that used effective prophylactic measures such as antibiotics (NAPRUTI Study II 2006). Placebo‐controlled studies were therefore analysed separately from active comparator studies. The 'no comparator' arms of the Kontiokari 2001 and Ferrara 2009 studies were used to include them with the four studies comparing probiotics with placebo.
Based on Jepson 2012, it appears that cranberry juice cannot be recommended for the prevention of UTI due to small effect sizes and studies with significant biases that limit the reliability of the data. There is also no identified evidence that lingonberry juice alone or in combination with cranberry juice has proven efficacy or safety versus placebo for the prevention of UTI. It is for this reason that probiotics were not compared versus cranberry juice (Ferrara 2009) or cranberry‐lingonberry juice (Kontiokari 2001).
Sample sizes
The smallest study included 30 participants (Czaja 2007) and the largest 252 participants (NAPRUTI Study II 2006). Most studies (60%) included 100 participants or fewer (Baerheim 1994; Czaja 2007; Ferrara 2009; Reid 1992; Reid 2003; Stapleton 2011).
Setting
All nine studies took place in outpatient settings.
Participants
Patient populations differed in terms of time since the last acute UTI and previous use of prophylactic antibiotics. Only Reid 2003 included exclusively healthy women. Three studies required participants with acute UTI at inclusion to be treated before commencing the study (Ferrara 2009; Kontiokari 2001; Stapleton 2011), Reid 2003 randomised women to acute antibiotic therapy before randomising them to prophylaxis; and three studies listed acute UTI or recent antibiotic use as exclusion criteria (Baerheim 1994; Czaja 2007; NAPRUTI Study II 2006). The study in children with VUR included children with persistent primary VUR following 12 months of antibiotic prophylaxis (Lee 2007a).
Interventions
The species and mode of administration of the probiotic intervention varied widely among studies, as did duration of therapy (eight weeks to 12 months). Details of formulations and species of probiotics in the studies are presented in Characteristics of included studies.
Outcomes
Only Reid 2003 did not report our primary outcome of UTI, although definitions varied by study (Characteristics of included studies). Few studies reported on our prespecified secondary outcomes. Four studies reported on adverse events (Czaja 2007; Reid 1992; Reid 2003; Stapleton 2011), and only two considered serious adverse events (Czaja 2007; NAPRUTI Study II 2006).
Excluded studies
Seven studies were excluded (Characteristics of excluded studies). Dani 2002 was conducted in a neonatal population; Colodner 2003 did not include an arm where participants did not receive a probiotic; Molander 1990 did not include investigation of a probiotic; and NCT00900653 studied a combination of probiotic and hormonal therapies. Manley 2007 and Pushkarev 2005 studied treatment rather than prophylaxis; Ranganathan 2009 enrolled people with chronic kidney disease (CKD) to determine if probiotics exerted renoprotective effects and reduced uraemia symptoms.
Risk of bias in included studies
NAPRUTI Study II 2006 was large and methods and events were adequately reported; however, most studies had small sample sizes and reported insufficient methodological detail to enable robust assessment (Figure 2; Figure 3).
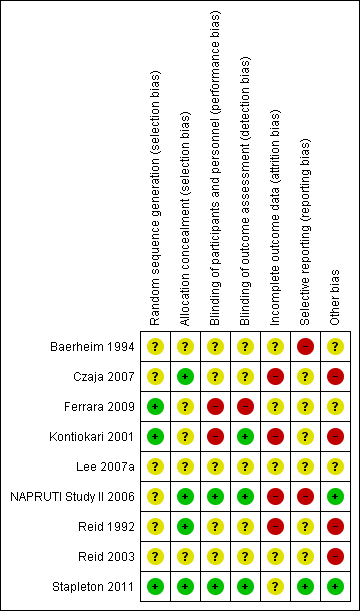
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
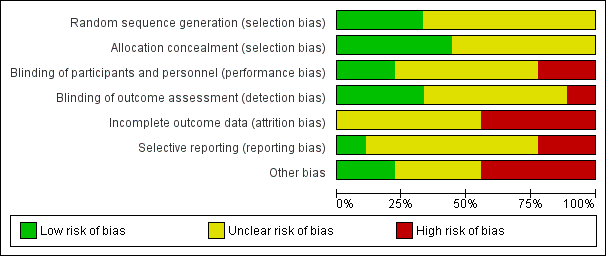
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Overall, there was a high risk of bias among the included studies. This meant that we were unable to draw firm conclusions or determine if any reported treatment effects may be misleading or represent overestimates.
Allocation
Three studies (Ferrara 2009; Kontiokari 2001; Stapleton 2011) reported on adequate methods of sequence generation and the other six studies (Baerheim 1994; Czaja 2007; Lee 2007a; NAPRUTI Study II 2006; Reid 1992; Reid 2003) did not describe sequence generation methods.
Four studies reported adequate allocation concealment (Czaja 2007; NAPRUTI Study II 2006; Reid 1992; Stapleton 2011). Allocation concealment was unclear for the remaining five studies.
Blinding
Two included studies were adequately described as double blind (NAPRUTI Study II 2006; Stapleton 2011), two were open label (Ferrara 2009; Kontiokari 2001) and there were insufficient data to assess blinding for the remaining studies; blinding was either not reported or lacked sufficient detail to determine who was blinded.
Incomplete outcome data
Satisfactory explanation was provided for changes in the number of participants for only one of the studies assessed (Czaja 2007). Reid 2003 had a significant proportion of their small study population excluded from analysis; attrition was significant and not explained satisfactorily in three studies (Kontiokari 2001; NAPRUTI Study II 2006, Stapleton 2011). Remaining studies lacked sufficient information to determine if attrition was likely to result in significant bias. Due to this incomplete follow‐up, worst case scenarios were undertaken for both the probiotics in comparison to placebo analysis and the probiotics in comparison to antibiotics analysis.
Selective reporting
Many of the included studies either did not report secondary outcomes, or lacked sufficient detail in reporting secondary outcomes; many of our prespecified secondary outcomes were not addressed, including all‐cause mortality and number with at least one confirmed case of bacteraemia or fungaemia.
We searched for published protocols for the included studies. Protocols were found for NAPRUTI Study II 2006 and Stapleton 2011. The outcomes reported in Stapleton 2011 aligned completely with the published protocol. The published report of the NAPRUTI Study II 2006 included several outcomes that were not prespecified in the protocol: mean number of antibiotic prescriptions for UTI treatment; and a subgroup analysis of mean number of clinical recurrences in women with complicated versus uncomplicated UTI.
Other potential sources of bias
Several studies were funded by manufacturing companies (Czaja 2007; Reid 1992), and one had an issue with supply that resulted in treatment duration inequality between the study arms (Kontiokari 2001). UTI definitions were fairly consistent among studies, although microbiological criteria ranged from at least 103 CFU/mL to 105 CFU/mL and clinical criteria were more stringent in some studies compared with others.
Effects of interventions
See: Summary of findings for the main comparison
Primary outcome
The primary outcome was to assess numbers of participants with at least one symptomatic bacterial UTI in each group (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen, and defined as > 105 CFU/mL or as defined by triallists).
Analyses were conducted according to probiotics in women and probiotics in children with VUR. Placebo‐controlled studies were subdivided into studies that enrolled women and children who had recently been treated with antibiotics for UTI (Ferrara 2009; Kontiokari 2001; Reid 1992; Stapleton 2011) and studies enrolling participants who had previous UTIs (Baerheim 1994; Czaja 2007). We decided to first determine if probiotics exerted a positive effect versus placebo before analysing studies versus active comparators.
A meta‐analysis of six studies that involved 352 randomised women and children demonstrated no significant reduction in the risk of recurrent symptomatic bacterial UTI between probiotics and placebo (Analysis 1.1 (6 studies, 352 participants): RR 0.82, 95% CI 0.60 to 1.12; I2 = 23%), heterogeneity was low. The confidence interval for all studies suggests a range that includes a 15.8 % absolute decrease to a 4.7% absolute increase in the risk of recurrent bacterial UTI with probiotics versus placebo given that the placebo rate of recurrence was 39.5% over 8 to 52 weeks.
NAPRUTI Study II 2006 reported no significant difference in the rate of recurrent symptomatic bacterial UTI in women between probiotics and antibiotics (Analysis 2.1 (1 study, 223 women): RR 1.12, 95% CI 0.95 to 1.33).
Lee 2007a included children with VUR. There was no significant difference in the rate of recurrent symptomatic bacterial UTI between probiotics and antibiotics (Analysis 3.1 (1 study, 96 children): RR 0.54, 95% CI 0.24 to 1.23).
We analysed six month recurrent UTI data from Kontiokari 2001. However, the authors also reported recurrent UTI at 12 months; a sensitivity analysis demonstrated that the results of the meta‐analysis did not change meaningfully if 12 month data were used. Three studies did not report on this outcome and this may have improved the precision of the effect size if the data were available. Stapleton 2011 reported 17 and 13 recurrent symptomatic bacterial UTIs for probiotics and placebo respectively. However, correspondence with the lead author indicated that two women in each arm of the study had symptomatic UTI but these were not confirmed with positive cultures; therefore, these events were excluded. We included these unconfirmed UTI in our analysis. In a sensitivity analysis, the results of the meta‐analysis did not change meaningfully if only culture‐confirmed UTI data were used.
Secondary outcomes
None of the included studies reported numbers of participants with at least one asymptomatic bacterial UTI, all‐cause mortality or those with at least one confirmed case of bacteraemia or fungaemia. The only secondary outcomes of interest reported by the included studies were withdrawal due to adverse events, total adverse events and numbers of participants with at least one non‐fatal serious adverse event.
Withdrawal due to adverse events was reported by NAPRUTI Study II 2006 and Stapleton 2011. NAPRUTI Study II 2006 reported that withdrawals due to adverse events occurred in six probiotic participants (5.2%) versus 15 antibiotic participants (12.2%). Stapleton 2011 noted that one placebo group participant discontinued treatment due to an adverse event.
Ferrara 2009, Kontiokari 2001, and Reid 1992 did not report adverse events. NAPRUTI Study II 2006 and Stapleton 2011 reported participants who experienced at least one adverse event but data were not meta‐analysed because participants in the NAPRUTI Study II 2006 control group received an antibiotic, and Stapleton 2011 provided a placebo to control group participants. NAPRUTI Study II 2006 reported that 66 probiotic (57.4%) and 72 antibiotic participants (58.5%) respectively experienced at least one adverse event (the most commonly reported adverse effects in both groups were diarrhoea, nausea, vomiting, constipation and vaginal symptoms). Reid 2003 identified adverse events through a questionnaire that was sent to patients. They reported that no probiotic patients reported an adverse event however it is unclear how many comparator group patients experienced at least one adverse event; they only reported that 2 comparator patients reported yeast infections.
Stapleton 2011 reported that 28 probiotic (56%) and 25 placebo participants (50%) experienced at least one adverse event (the most common were vaginal discharge, itching and moderate abdominal discomfort).
Baerheim 1994 stated that treatment was well tolerated in both groups; four participants from the probiotic arm and one from placebo complained of discharge, with no other adverse effects noted.
Czaja 2007 documented a range of self‐reported adverse effects including abnormal vaginal discharge, external genital irritation, vaginal candidiasis, vaginal odour, abdominal pain and dysuria. Abnormal vaginal discharge occurred in about half of all participants, but the overall frequency of adverse effects was low.
NAPRUTI Study II 2006 and Czaja 2007 reported serious adverse events. In NAPRUTI Study II 2006, no significant difference in serious adverse events was noted; 17 probiotic (14.8%) and 14 antibiotic participants (11.4%) experienced at least one serious adverse event.
Subgroup analyses
In a post‐hoc subgroup analysis, there was no significant difference in recurrence of UTI between the subgroups of women without a UTI prior to enrolment compared (Analysis 1.1.1 (2 studies, 77 participants): RR 1.12, 95% CI 0.65 to 1.93; I2 = 0%) with those with UTI being treated with antimicrobials at enrolment (Analysis 1.1.2 (4 studies, 275 participants): RR 0.74, 95% CI 0.52 to 1.05; I2 = 16%). The overall pooled estimate for all studies in both subgroups was not significantly different from the pooled estimate of each subgroup (Test for subgroup differences: Chi2 = 1.57, df = 1 (P = 0.21), I2 = 36.5%).
Sensitivity analyses
Sensitivity analyses were planned to determine if treatment effects on recurrent symptomatic bacterial UTI rates differed in studies based on a number of variables. Removal of studies that were open label or that had unclear allocation concealment did not change the effect of probiotics on recurrent symptomatic bacterial UTI however only few studies had unclear allocation concealment (Baerheim 1994; Ferrara 2009; Kontiokari 2001) or were open label (Ferrara 2009; Kontiokari 2001) and it was felt that sensitivity analyses would not be informative and maybe misleading.
Sensitivity analysis was also conducted for symptomatic bacterial UTI involving Czaja 2007, NAPRUTI Study II 2006 and Stapleton 2011 as these three studies suggested that not all randomised patients were included in the UTI analysis; hence we wanted to see the impact of imputing data for a worst case scenario. In this analysis all missing patients in one group were assumed to have had UTI, and those missing from the other group were assumed to not have had UTI. For the comparison of probiotics versus placebo, the effect did not change using a worst case scenario for probiotics (Analysis 1.2: RR 0.94, 95% CI 0.63 to 1.39; I2 = 49%). For the comparison of probiotics versus antibiotics, a worst case scenario for antibiotics of the NAPRUTI Study II 2006 now demonstrated that fewer probiotic patients experienced a recurrent symptomatic bacterial UTI versus antibiotics (25 antibiotic patients, Analysis 2.3; RR 0.83, 95% CI 0.74 to 0.94). When the worst case scenario analysis for probiotics was conducted, more probiotic patients had recurrent symptomatic bacterial UTI (5 probiotic patients, Analysis 2.2; RR 1.19, 95% CI 1.01 to 1.40).
Discussion
Summary of main results
This review included nine studies involving a total of 735 participants. No significant difference in risk of recurrent UTI was seen for probiotics in comparison to placebo or antibiotic prophylaxis in either women or children. There was no significant difference found between probiotics and either placebo or antibiotic prophylaxis for harms.
The studies included in this review were generally small and of poor quality with inconsistent and limited reporting of harm, and as such the data are insufficient to exclude either a benefit or harm from probiotics versus either placebo or antibiotic prophylaxis.
Only NAPRUTI Study II 2006 compared probiotics and antibiotics; no significant difference in the rate of recurrent symptomatic bacterial UTI or harm was found between groups.
Adverse events, when reported, were poorly described with insufficient data to perform statistical evaluation. Overall the frequency of reported side effects was low and mild in nature (e.g. vaginal discomfort).
We suggest caution when interpreting the lack of a subgroup difference in Analysis 1.1, as there were too few studies to be able to confidently conclude the presence or absence of subgroup differences.
There was insufficient evidence to comment on the differences in effects of probiotics in children and women as only one study included only children (Ferrara 2009) and a subgroup analysis may be misleading.
Overall completeness and applicability of evidence
We included nine studies in this review ranging from 30 to 252 participants totalling a relatively small overall sample of 735 participants. No statistically significant difference was seen in recurrent symptomatic bacterial UTI. Given the low overall quality and quantity of data available a decrease or increase in recurrent symptomatic UTI cannot be ruled out.
There was some reporting bias in that several studies did not report on symptomatic bacterial UTI, and very limited information on harm. For example, Reid 2003 did not report symptomatic bacterial UTI recurrence; these data would have been valuable because the meta‐analysis of studies that did report this outcome did not rule out a clinically important increased or decreased risk. These studies also included different patient populations resulting in the analysis of separate small groups of studies instead of the evidence base as a whole.
There was insufficient evidence to determine if probiotics provide a therapeutic advantage over placebo for susceptible patient populations (e.g. previous history of UTI, women, school‐aged girls, men with enlarged prostates, and the elderly). Included studies randomised primarily women and young girls with no studies enrolling men with enlarged prostates or the elderly.
Quality of the evidence
Our assessments suggested an unclear or high risk of bias (Figure 2; Figure 3, summary of findings Table for the main comparison). As such, evidence has been downgraded to low for all outcomes listed in the summary of findings Table for the main comparison. This suggested that treatment effects were likely overestimated and that better methodological control is required in future research. Future studies should model methods from Stapleton 2011. Adequate allocation concealment was described in four of the eight included studies; only two studies were double blinded. Attrition bias was of concern in all but one included study.
The available evidence varied in terms of probiotic used, route of delivery and duration of therapy. These differences make drawing specific conclusions difficult and likely contribute to the heterogeneity seen in the pooled estimates. Most included studies did not systematically collect adverse event information, thus we could not draw conclusions regarding the potential harms associated with these therapies.
Potential biases in the review process
Our literature search included several international databases and search criteria were intentionally broad to identify as many potentially relevant articles as possible. We did not exclude studies published in languages other than English. We contacted study authors for missing information.
Agreements and disagreements with other studies or reviews
Grin 2013 is the most recent systematic review on this topic. Grin 2013 included five RCTs, against nine included in this review. Our assessment is that Grin 2013 limited their search to include only studies that included premenopausal women with history of UTI. Our inclusion criteria did not limit to a particular population nor did it exclude studies in healthy people (e.g. Reid 2003 enrolled healthy women and met our inclusion criteria). Grin 2013 concluded that it was possible that certain strains lactobacillus‐containing suppositories could prevent recurrent UTI in premenopausal women. Grin 2013 suggested that more RCTs were required to be certain of the effect on recurrent UTI. In addition, Grin 2013 suggested that current RCTs did not enable definitive conclusions to be made about the safety of probiotics.
In general, our conclusions are similar to Grin 2013 in that more RCTs are required to determine the net health impact of probiotics, although our conclusions apply to a broader patient population.

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Probiotics versus placebo in adults and children, Outcome 1 Symptomatic bacterial UTI.

Comparison 1 Probiotics versus placebo in adults and children, Outcome 2 Worst case scenario ‐ symptomatic bacterial UTI.

Comparison 2 Probiotics versus antibiotics in women, Outcome 1 Symptomatic bacterial UTI.
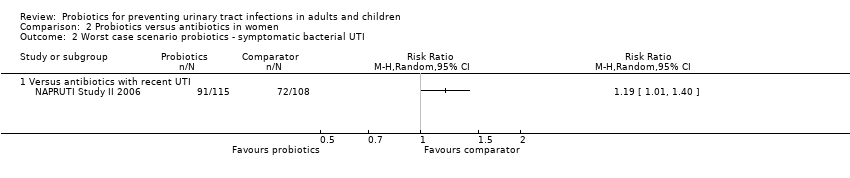
Comparison 2 Probiotics versus antibiotics in women, Outcome 2 Worst case scenario probiotics ‐ symptomatic bacterial UTI.

Comparison 2 Probiotics versus antibiotics in women, Outcome 3 Worst case scenario antibiotics ‐ symptomatic bacterial UTI.

Comparison 3 Probiotics versus control in children with vesicoureteric reflux, Outcome 1 Symptomatic bacterial UTI.
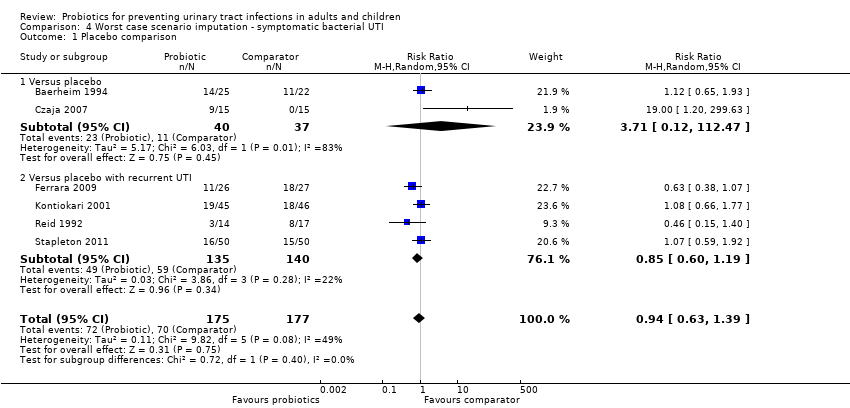
Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 1 Placebo comparison.

Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 2 Antibiotic comparison ‐ worst case probiotics.

Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 3 Antibiotic comparison ‐ worst case antibiotics.
| Probiotics compared with placebo or antibiotics for urinary tract infections (UTI) | ||||||
| Patient or population: adults and children at risk of UTI Settings: outpatient Intervention: probiotics Comparison: placebo or antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
| Symptomatic bacterial UTI in adults and children in patients with and without recurrent UTI Probiotics versus placebo (follow‐up) | 395 per 1000 | 296 per 1000 | RR 0.75 (0.50, 1.13) | 352 (6) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
| Symptomatic bacterial UTI in adults and children with recurrent UTI Probiotics versus placebo (follow‐up) | 421 per 1000 | 315 per 1000 | RR 0.74 (0.54, 1.01) | 275 (4) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
| Symptomatic bacterial UTI in women with recent UTI Probiotics versus antibiotics (follow‐up) | 666 per 1000 | 745 per 1000 | RR 1.12 (0.95, 1.33) | 223 (1) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus antibiotics. Imprecision also due to small sample from only one RCT |
| Symptomatic bacterial UTI in children with VUR Probiotics versus placebo (follow‐up) | 270 per 1000 | 145 per 1000 | RR 0.54 (0.24, 1.23) | 96 (1) | ⊕⊕⊝⊝ | Risk of bias was assessed at unclear or high in most domains of and suggest that results are imprecise or overestimate probiotic effects versus placebo. Imprecision also due to small sample from only one RCT |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| UTi ‐ urinary tract infection | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic bacterial UTI Show forest plot | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.12] |
| 1.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.65, 1.93] |
| 1.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 Worst case scenario ‐ symptomatic bacterial UTI Show forest plot | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 2.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.12, 112.47] |
| 2.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic bacterial UTI Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Worst case scenario probiotics ‐ symptomatic bacterial UTI Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Worst case scenario antibiotics ‐ symptomatic bacterial UTI Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic bacterial UTI Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placebo comparison Show forest plot | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 1.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.12, 112.47] |
| 1.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.19] |
| 2 Antibiotic comparison ‐ worst case probiotics Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Antibiotic comparison ‐ worst case antibiotics Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

