Auscultación intermitente (AI) de la frecuencia cardíaca fetal durante el trabajo de parto para el bienestar fetal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008680.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ruth Martis wrote the protocol with Ova Emilia and Detty Nurdiati providing feedback. Julie Brown joined the review team during the editorial process for this review to assist with data extractions of the newly added studies and finalising the review for publication. All review authors contributed to data extraction, presentation and interpretation of the results, and approved the final version of the review.
Sources of support
Internal sources
-
Christchurch Polytechnic Institute of Technology, CPIT, New Zealand.
The Department of Health Science and Allied Health, ARC committee, provided financial support for Ruth Martis to travel to Australia to finish the review.
-
The Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
provided financial support for Ova Emilia and Detty Nurdiati to travel to Australia to finish the review.
-
Liggins Institute, Univeristy of Auckland, New Zealand.
External sources
-
Australasian Cochrane Centre, Monash University, Melbourne, Australia.
Technical support from the Australasian Cochrane Centre, which included an accommodation grant for OE and DN to stay in Melbourne for a week to complete the review.
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Ruth Martis: received support from Cochrane Health Promotion and Public Health Field in the form of a bursary supporting travel to meet with review authors for review progress and data analysis. The bursary was administrated by the SeaOrchid project.
Ova Emilia: received support (for travel and accommodation) from the SeaOrchid Project to be trained in preparing Cochrane Systematic Review in Australia.
Detty S Nurdiati: received support (for travel and accommodation) from the SeaOrchid Project to be trained in preparing Cochrane Systematic Review in Australia. She also received support from the Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia to attend a workshop on completing systematic reviews.
Julie Brown: none known.
Acknowledgements
The review authors would like to thank the staff from the Australasian Cochrane Centre for technical support. We also sincerely acknowledge Steve McDonald, Philippa Middleton and Miranda Cumpston for their editorial assistance and support. The Department of Health Science and Allied Health, ARC committee, Christchurch Polytechnic Institute of Technology provided support for Ruth Martis to travel to Australia to finish the review. The Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia, provided support for Ova Emilia and Detty Nurdiati to travel to Australia to finish the review.
We also would like to acknowledge and thank Associate Professor Malinee Laopaiboon, Department of Biostatistics and Demography, Faculty of Public Health, Khon Kaen University, Khon Kaen, Thailand, for her invaluable statistical guidance.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 13 | Intermittent auscultation (IA) of fetal heart rate in labour for fetal well‐being | Review | Ruth Martis, Ova Emilia, Detty S Nurdiati, Julie Brown | |
| 2010 Sep 08 | Intermittent auscultation (IA) of fetal heart rate in labour for fetal well‐being | Protocol | Ruth Martis, Ova Emilia, Detty S Nurdiati | |
Differences between protocol and review
The methods section has been updated to reflect the latest guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Pregnancy and Childbirth Group's methodological guidelines. The electronic searches section was updated to include Embase and email alerts.
We did not anticipate that one trial (Mahomed 1994) would include electronic fetal monitoring (EFM) with a cardiotocograph (CTG) as an intermittent auscultation (IA) modality. After discussion, we decided to include this arm of the intervention as well, as it appears that only the fetal heart rate (FHR) was used for clinical management decisions, rather than interpreting the paper tracing. This trial reported Apgar score < six at five minutes as a measure of newborn condition; although our prespecified outcome was Apgar score < seven, we decided to use the data from this trial, as overall only two studies are included in the review and we wanted to use all available data.
Two secondary outcomes for the mother have been removed, as recommended by the peer reviewers. These were: (5) Types of induction of labour, e.g. prostaglandin gel, oxytocin infusion, amniotomy and (6) Spontaneous onset of labour. The reasons provided for this request were that participants are in labour already therefore neither intervention could affect the outcome.
The maternal secondary outcome, 'length of ruptured membranes' was edited to 'duration of ruptured membranes'.
Spontaneous onset of labour versus induction of labour (3) was replaced with first stage versus second stage of labour for subgroup analysis and investigation of heterogeneity.
In this version of the review, we have included a new infant outcome: mortality or serious morbidity, and this outcome is included in our 'Summary of findings' tables.
The GRADE approach to examine the quality of the body of evidence was added to this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Infant, Newborn; Pregnancy;
PICO
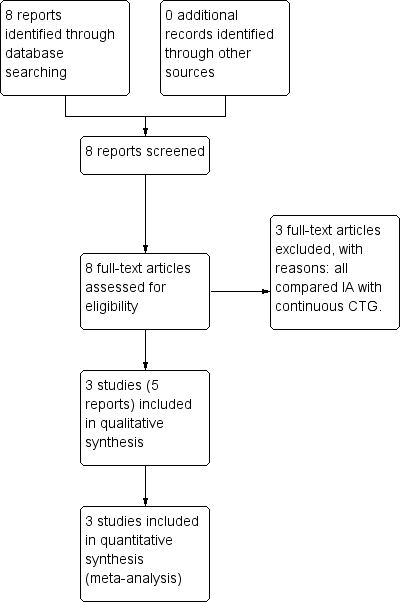
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included trial.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
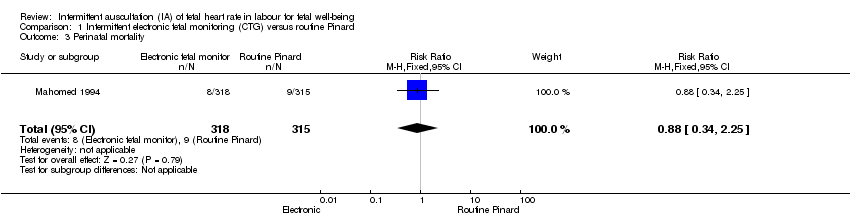
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 6 Admission to NICU/NNU.
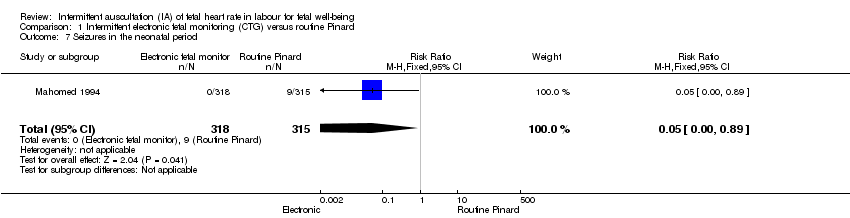
Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 7 Seizures in the neonatal period.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 9 Caesarean section.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 1 Intermittent electronic fetal monitoring (CTG) versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 2 Doppler versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 2 Doppler versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
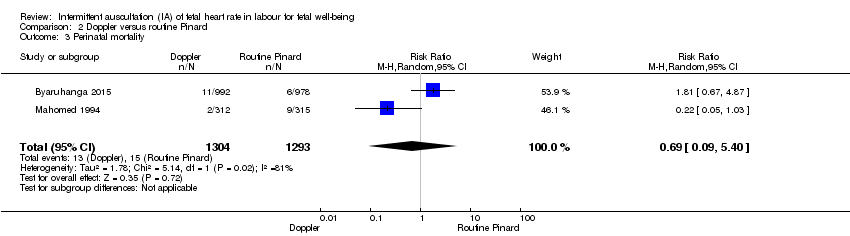
Comparison 2 Doppler versus routine Pinard, Outcome 3 Perinatal mortality.

Comparison 2 Doppler versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
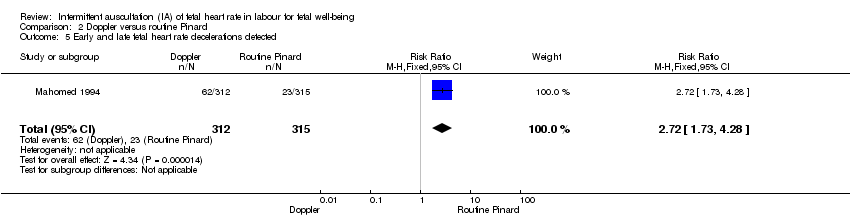
Comparison 2 Doppler versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.

Comparison 2 Doppler versus routine Pinard, Outcome 6 Admission to NICU/NNU.
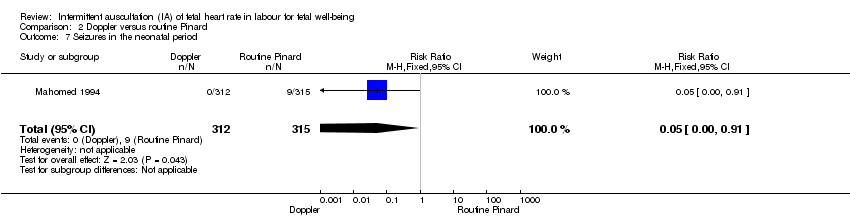
Comparison 2 Doppler versus routine Pinard, Outcome 7 Seizures in the neonatal period.
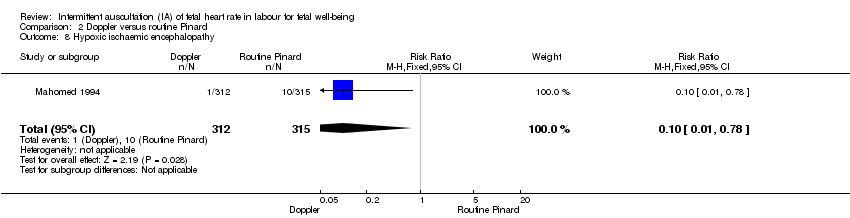
Comparison 2 Doppler versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 2 Doppler versus routine Pinard, Outcome 9 Caesarean section.
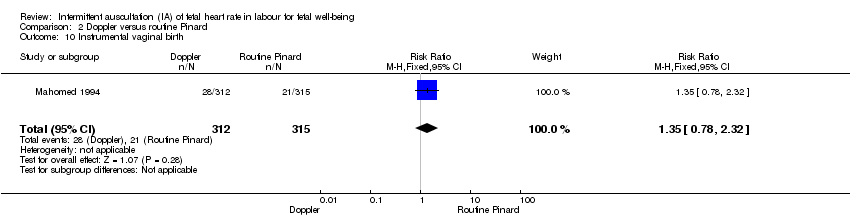
Comparison 2 Doppler versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 2 Doppler versus routine Pinard, Outcome 11 Length of labour (hours).

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 1 Apgar < 7 at 5 minutes after birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 2 Caesarean section for fetal distress.
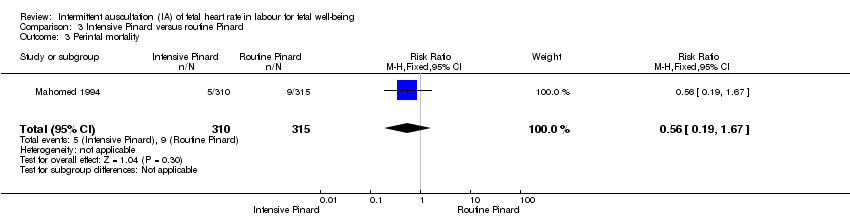
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 3 Perintal mortality.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 4 Fetal heart rate abnormality detected.
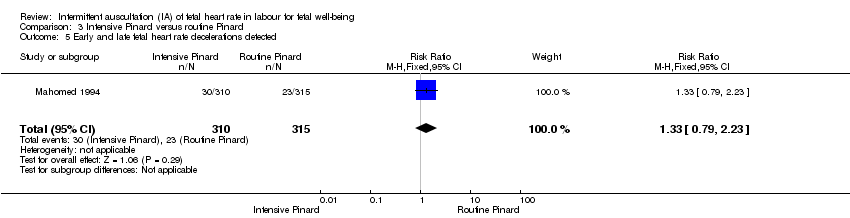
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 5 Early and late fetal heart rate decelerations detected.
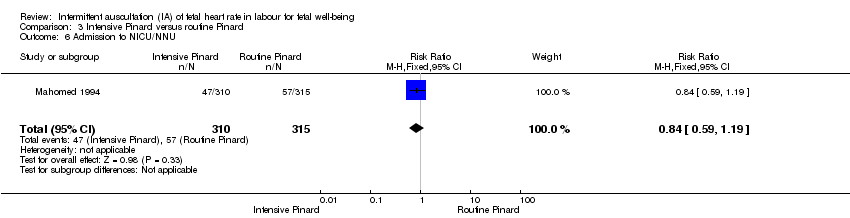
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 6 Admission to NICU/NNU.
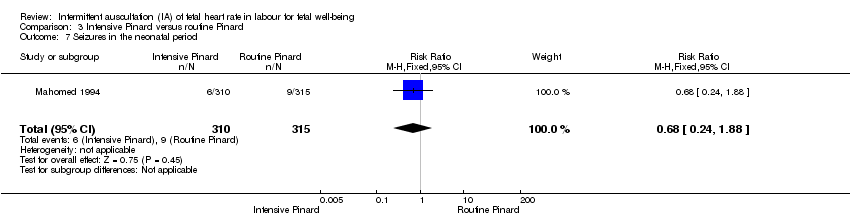
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 7 Seizures in the neonatal period.
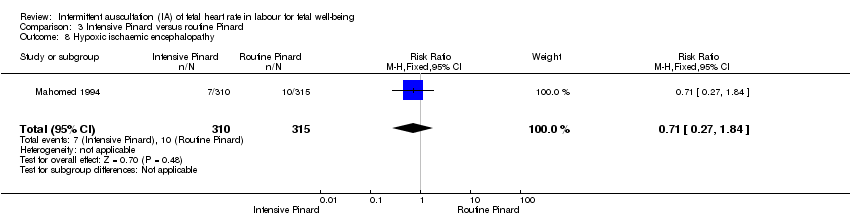
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 8 Hypoxic ischaemic encephalopathy.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 9 Caesarean section.
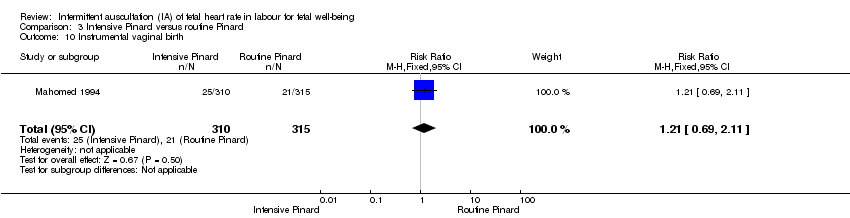
Comparison 3 Intensive Pinard versus routine Pinard, Outcome 10 Instrumental vaginal birth.

Comparison 3 Intensive Pinard versus routine Pinard, Outcome 11 Length of labour (hours).
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus routine Pinard (outcomes for the baby). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 19 per 1000 | RR 0.66 | 633 | ⊕⊕⊝⊝ | Low event rate. Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 1 per 1000 | RR 0.05 | 633 | ⊕⊕⊝⊝ LOW 1,3 | Low event rates. Routine Pinard group (9/315) compared to the intermittent EFM (CTG) group (0/318). |
| Perinatal mortality | 29 per 1000 | 25 per 1000 | RR 0.88 | 633 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. Low event rates 8/318 for intermittent EFM (CTG) group and 9/315 for routine Pinard group. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies at either 6 months or 1 year. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision; single trial with low event rate and wide 95% CI crossing the line of no effect. Downgraded two levels. 3 Evidence of imprecision, evidence based on a single trial with low event rates. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intermittent electronic fetal monitoring (CTG) (inconsistent/ opportunistic paper tracing) versus Routine Pinard (outcomes for the mother). | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intermittent electronic fetal monitoring intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 176 per 1000 | RR 2.92 | 633 | ⊕⊕⊕⊝ MODERATE 1, | |
| Instrumental vaginal birth | 67 per 1000 | 97 per 1000 | RR 1.46 | 633 | ⊕⊕⊝⊝ LOW 1,2, | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐ pharmacological analgesia use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide confidence intervals. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Routine Pinard | Risk with Doppler | |||||
| Apgar < 7 at 5 minutes | 20 per 1000 | 15 per 1000 | RR 0.76 | 2598 | ⊕⊝⊝⊝ | One of the studies contributing data reported Apgar score < 6. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Seizures in the neonatal period | 29 per 1000 | 1 per 1000 | RR 0.05 | 627 | ⊕⊝⊝⊝ | Event rates are low 0/312 for Doppler and 9/315 for routine Pinard. |
| Perinatal mortality | 12 per 1000 | 8 per 1000 | RR 0.69 | 2597 | ⊕⊕⊝⊝ | Event rates 13/1304 for Doppler and 15/1293 for routine Pinard. Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Evidence of imprecision with wide 95% CI crossing the line of no effect. Downgraded one level. 3 There was high heterogeneity for this outcome. 4 Evidence of imprecision, with wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. 5 There was high heterogeneity for this outcome. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Doppler versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Doppler | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 163 per 1000 | RR 2.71 | 627 | ⊕⊕⊕⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 90 per 1000 | RR 1.35 | 627 | ⊕⊕⊝⊝ | |
| Maternal mortality | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal mortality in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for any pharmacological or non‐pharmacological use excluding epidural in the included studies. | |
| Epidural anaesthesia for pain relief excluding for caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding for caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for postnatal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Wide confidence interval. Downgraded one level. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the baby) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Apgar < 7 at 5 minutes | 29 per 1000 | 26 per 1000 | RR 0.90 | 625 | ⊕⊝⊝⊝ | Study reported Apgar score < 6 at 5 minutes. |
| Cord blood acidosis | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cord blood acidosis in the included studies. | |
| Neonatal seizures | 29 per 1000 | 19 per 1000 | RR 0.68 | 625 | ⊕⊝⊝⊝ | |
| Perinatal mortality | 29 per 1000 | 16 per 1000 | RR 0.56 | 625 | ⊕⊝⊝⊝ | Neonatal deaths included, unable to separate out from reported data. |
| Composite of mortality and serious morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for a composite of mortality and serious morbidity in the included studies. | |
| Cerebral palsy | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for cerebral palsy in the included studies. | |
| Neurosensory disability | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for neurosensory disability in the included trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded 1 level. 2 Evidence was imprecise; wide 95% CI crossing the line of no effect and low event rate. Downgraded 2 levels. | ||||||
| Intermittent ausculation of fetal heart rate in labour for fetal well‐being ‐ Intensive Pinard versus Routine Pinard (outcomes for the mother) | ||||||
| Patient or population: women in established labour and their babies. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with routine Pinard | Risk with Intensive Pinard | |||||
| Caesarean section for fetal distress and/or fetal acidosis | 60 per 1000 | 42 per 1000 | RR 0.70 | 625 | ⊕⊕⊝⊝ | |
| Instrumental vaginal birth | 67 per 1000 | 81 per 1000 | RR 1.21 | 625 | ⊕⊕⊝⊝ | |
| Maternal morbidity | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for maternal morbidity in the included studies. | |
| Any pharmacological or non‐pharmacological analgesia use excluding epidural | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No reported data for any pharmacological or non‐pharmacological analgesia use excluding epidural. | |
| Epidural anaesthesia for pain relief excluding caesarean section | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for epidural anaesthesia for pain relief excluding caesarean section in the included studies. However, 1 trial reported that no epidural analgesia was available in the labour ward. | |
| Mobility or restriction during labour | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for mobility or restriction during labour in the included studies. | |
| Postnatal depression | 0 per 1000 | 0 per 1000 | not estimable | (0 studies) | No data reported for post natal depression in the included studies. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Blinding of participants and health professionals not possible; high risk of performance bias and it is unclear if outcome assessors were blinded. Downgraded one level. 2 Some imprecision with wide CI crossing the line of no effect. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.24, 1.83] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.78, 4.80] |
| 3 Perinatal mortality Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.25] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [4.21, 8.79] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.82, 4.45] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.63, 1.25] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 9 Caesarean section Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.39, 2.64] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.86, 2.49] |
| 11 Length of labour (hours) Show forest plot | 1 | 633 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.05, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.20, 2.87] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| 3 Perinatal mortality Show forest plot | 2 | 2597 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.40] |
| 4 Fetal heart rate abnormality detected Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.09, 5.29] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.73, 4.28] |
| 6 Admission to NICU/NNU Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.91] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.91] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.78] |
| 9 Caesarean section Show forest plot | 2 | 2598 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.81, 2.05] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.32] |
| 11 Length of labour (hours) Show forest plot | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Apgar < 7 at 5 minutes after birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.31] |
| 2 Caesarean section for fetal distress Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.38] |
| 3 Perintal mortality Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.67] |
| 4 Fetal heart rate abnormality detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.10, 2.65] |
| 5 Early and late fetal heart rate decelerations detected Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.23] |
| 6 Admission to NICU/NNU Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.19] |
| 7 Seizures in the neonatal period Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.88] |
| 8 Hypoxic ischaemic encephalopathy Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
| 9 Caesarean section Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.08] |
| 10 Instrumental vaginal birth Show forest plot | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 11 Length of labour (hours) Show forest plot | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.52, 1.52] |

