تاثیر مصرف مکمل ویتامین A در دوران بارداری بر پیامدهای مادر و نوزاد
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT. | |
| Participants | Inclusion criteria: 44 parturient women in good health from the lower and middle socioeconomic groups (in a population in which vitamin A deficiency occurs). | |
| Interventions | Intervention group 1: 15 women. Single intramuscular injection of 600,000 lU of vitamin A palmitate in oil at parturition. 4 samples of 2 to 3 mL of colostrum were collected. 1 antepartum sample and 3 postpartum samples, 1 on each consecutive day of hospitalisation. Intervention group 2: 11 women. Given 600,000 lU of water‐dispersible vitamin A palmitate orally shortly before delivery. 4 samples of 2 to 3 mL of colostrum were collected. 1 antepartum sample and 3 postpartum samples, 1 on each consecutive day of hospitalisation. Followed by public health nurses at their homes where bi‐weekly samples of milk were collected during the first week after discharge and then weekly samples for a total period ranging between 38 and 59 days postpartum. Control group: 18 women not given any form of vitamin A therapy prepartum. 4 samples of 2 to 3 mL of colostrum were collected. 1 antepartum sample and 3 postpartum samples, 1 on each consecutive day of hospitalisation. | |
| Outcomes | Primary outcome: levels of vitamin A and carotenoids in the maternal blood. Other outcomes: levels of vitamin A and carotenoids in the colostrum prenatal and postnatal. | |
| Notes | Vitamin A levels measured before starting supplementation in group 1 and 2. Study was done in a population in which vitamin A deficiency occurs. Study setting: American university hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description except allotted at random. |
| Allocation concealment (selection bias) | Unclear risk | No description except allotted at random. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding methods were reported. The routes of medication administration methods varied across groups. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was provided. |
| Incomplete outcome data (attrition bias) | Low risk | No exclusion or loss of follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | High risk | The 3 groups were not studied during the same period. The authors reported "subjects in groups 1 and 2 were studied in the summer and those of group 3 in the following winter". |
| Methods | Double‐blind RCT. | |
| Participants | Inclusion criteria:
(HIV‐seropositive women identified through antenatal screening programmes. All the women enrolled were black Africans.) | |
| Interventions | Intervention group: 368 women received daily dose of 5000 IU retinyl palmitate and 30 mg beta‐carotene during the third trimester of pregnancy (together corresponding to 43,400 IU vitamin A daily for 12 weeks) and 200,000 IU retinyl palmitate at delivery. Control group: 360 women received placebo on the same schedule. | |
| Outcomes | Primary outcome: effects of vitamin A on HIV viral load and HIV transmission. Other outcomes: neonatal mortality (the number of deaths during the first 28 completed days of life per 1000 live births in a given year or period) and anaemia, maternal anaemia, clinical infection (fever > 1 week at 1 week postnatally), preterm birth (delivery less than 37 completed weeks' gestational age estimated using LMP), low birthweight and morbidity. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: South Africa. Study setting: King Edward VIII Hospital and McCords Hospital, in Durban, South Africa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "double‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 57 (7.8%) women did not deliver in the hospitals and cannot be traced. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | A randomised double‐blind controlled trial. | |
| Participants | Inclusion criteria:
Exclusion criteria: HIV infection or tuberculosis. | |
| Interventions | Intervention group: 48 women received weekly capsules of 10,000 IU of vitamin A as retinyl palmitate in groundnut oil, plus tocopherol as a preservative from enrolment until 6 weeks postpartum. Suplimintation was for a minimum of 18 weeks. Control group: 50 women received groundnut oil and tocopherol only in the placebo capsules from enrolment until 6 weeks postpartum. | |
| Outcomes | Primary outcome: maternal infections (presence of placental malaria and peripheral parasitaemia). Other outcomes: Hb and birthweight. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Ghana. Study setting: Nkoranza District Hospital and 3 rural health clinics in Brong Ahafo region, Central Ghana. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "balanced block randomisation." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "double‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 12 (12%) women were excluded from the analysis: 1 false pregnancy, 1 early miscarriage, 10 missed late pregnancy visit. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Unclear risk | The most marked difference was in educational level and gestational age at enrolment. Levels of anti‐VSACSA IgG to the FCR3CSA parasite line differed between the treatment groups at baseline. There were considerably fewer data available for the placebo than the vitamin A group at the late pregnancy follow‐up. |
| Methods | Double‐blind RCT, factorial design. | |
| Participants | Inclusion criteria: all women were recruited before 20 weeks' gestational age. Exclusion criteria: twin pregnancy and congenital abnormalities that interfered with growth, development, or metabolism. | |
| Interventions | Intervention group 1: 37 women received iron and folic acid supplements together with ß ‐carotene (4.5 mg as water‐soluble granulate/d (representing 5750 IU of vitamin A)). Each woman was supplemented daily during pregnancy until delivery for a minimum of 16 weeks. Intervention group 2: 37 women received iron and folic acid supplements together with zinc (30 mg zinc as sulphate/d). Each woman was supplemented daily during pregnancy until delivery. Intervention group 3: 37 women received iron and folic acid supplements together with zinc and carotene. Each woman was supplemented daily during pregnancy until delivery. Control group: 37 women received iron and folic acid. | |
| Outcomes | Primary outcome: maternal and fetal Hb and zinc levels. Other outcomes: maternal and fetal ferritin, retinol and carotene levels. Other outcomes from this same trial were recorded by Dijkhuizen 2001‐ see notes below. | |
| Notes | Vitamin A levels were not measured before starting supplementation. Country: Indonesia. Study setting: 13 adjacent villages in a rural area in Bogor District, West Java, Indonesia. Dijkhuizen 2001 assessed maternal and neonatal complications in this same trial described above. Outcomes described included maternal puerperal fever, preterm delivery, stillbirth, neonatal mortality and birthweight. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided except factorial randomisation. |
| Allocation concealment (selection bias) | Low risk | "Capsules were indistinguishable and given a letter code." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel double‐blind". |
| Blinding of outcome assessment (detection bias) | Low risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 35 (20%) women were not followed up or included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | 2‐by‐2 factorial design. | |
| Participants | Inclusion criteria:
| |
| Interventions | Intervention group 1: 270 women received a daily (for at least 10 weeks) oral dose of multivitamins including vitamin A (30 mg b‐carotene (representing 38,000 IU vitamin A) and 5000 IU of preformed vitamin A, 20 mg of B1, 20 mg of B2, 25 mg of B6, 100 mg of niacin, 50 mg of B12, 500 mg of C, 30 mg of vitamin E, and 0.8 mg of folic acid); an additional oral dose of vitamin A (200,000 IU) at delivery. Intervention group 2: 269 women received a daily oral dose of vitamin A alone (30 mg b‐carotene and 5000 IU of preformed vitamin A), plus an additional oral dose of vitamin A (200,000 IU) at delivery. Intervention group 3: 269 women received a daily oral dose of multivitamins excluding vitamin A, plus an additional oral placebo at delivery. Intervention group 4: 267 women received a daily oral dose of placebo. An additional oral placebo at delivery. | |
| Outcomes | Primary outcome: CD levels in both mother and fetus and HIV transmission. Other outcomes: birthweight, preterm birth (delivery less than 37 completed weeks estimated using LMP) and Hb in both mother and fetus (Hb < 10.0 g/dL). | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Tanzania. Duration: 1995‐1997. Study setting: 4 ANCs with several smaller peripheral clinics. Other secondary outcomes of interest in this review (maternal infection) from this trial are described by Arsenault 2010 and Olofin 2014. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was done in blocks of 20." |
| Allocation concealment (selection bias) | Low risk | "At enrolment, we assigned each eligible women the next numbered bottle of regimen." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "double blind". |
| Blinding of outcome assessment (detection bias) | Low risk | No information. |
| Incomplete outcome data (attrition bias) | Low risk | 117 (10.8%) women were excluded from the analysis:
|
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | Quasi‐RCT, multi‐centred. | |
| Participants | Inclusion criteria: pregnant women. Exclusion criteria: cases not delivered in hospital. | |
| Interventions | Intervention group: 275 women received 1 oz of the vitamin preparation radiostoleum, an amount equivalent in vitamins A and D roughly to 30 oz of a good cod‐liver oil (equivalent to 444,000 IU vitamin A), should have been taken daily commencing 1 month previous to the calculated day of labour. The first 76 cases prior to June 1929 were given the preparation for only 14 days before delivery (daily). It was, however, continued for the first 7 days of the puerperium. It was then decided that a more logical procedure would probably be to begin the administration earlier and thus build up a larger reserve at the time of labour. Control group: 275 women received an untreated version. | |
| Outcomes | Maternal infection (puerperal fever > 38o C) and maternal and baby mortality and morbidity. | |
| Notes | Vitamin A levels were not measured before starting supplementation. Country: UK. Study setting: the Jessop Hospital and the Nether Edge municipal hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "the first patient was given the preparation and the next due for delivery about the same time was indexed as a control." |
| Allocation concealment (selection bias) | High risk | "the first patient was given the preparation and the next due for delivery about the same time was indexed as a control." |
| Blinding of participants and personnel (performance bias) | High risk | The control group received no intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | 50 (8.3%) women delivered somewhere else and were excluded. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | Randomised, placebo‐controlled double masked community‐based trial. | |
| Participants | Inclusion: women with positive pregnancy test in the first 120 days of pregnancy. Exclusion:
| |
| Interventions | Group 1: 248 received vitamin A 2400 retinol equivalent, second group 254 received zinc 20 mg/day, third group 243 received both vitamin A and zinc, while the fourth group 263 received placebo. | |
| Outcomes | Maternal sepsis (temp ≽ 38oC between day 2‐14 postpartum), haemorrhage (bleeding during labour or within 2 days of delivery). | |
| Notes | Trial run between 1995 and 1997 in Indonesia termed the ZIBUVITA trial. Setting: Central Java, Indonesia. Of note this study information is from a draft of a publication which was not published in any peer review journal. However, 2 follow‐up studies using this original trial have been published, Prawirohartono 2011 and Prawirohartono 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1:1:1 ratio in blocks of 12 based on a list of treatment numbers derived from a pseudo‐random number generator in SAS." |
| Allocation concealment (selection bias) | Low risk | "treatment allocation sequence was prepared and held at ..a site remote from the trial." |
| Blinding of participants and personnel (performance bias) | Low risk | "supplements were packaged in ..identical opaque pink capsules." "all investigators, field and laboratory staff and participants were blinded to the treatment code." |
| Blinding of outcome assessment (detection bias) | Low risk | "a survey of sample of field workers and their supervisors revealed they were unable to identify which treatments the study participants were receiving." |
| Incomplete outcome data (attrition bias) | High risk | > 20% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of this study is not available. |
| Other bias | High risk | Of note this information is from a draft of a publication which was not published in any peer review journal. However, 2 follow‐up studies using this original trial have been published, Prawirohartono 2011 and Prawirohartono 2013. |
| Methods | Cluster‐randomised trial. | |
| Participants | Inclusion criteria: women aged 15 to 45 years giving informed consent and who planned to live in the trial area for at least 3 months were eligible for enrolment. | |
| Interventions | Intervention group: 104,484 women in 544 clusters received weekly vitamin A capsule consisted of 25,000 IU (7500 ug) retinol equivalents (equivalent to 25,000 IU vitamin A) in soybean oil in a dark red opaque soft gel for 12 weeks. Control group: 103,297 women in 542 clusters received placebo capsule consisted of soybean oil only. | |
| Outcomes | Primary outcome: maternal mortality and all‐cause female mortality. Other outcomes: maternal morbidity, perinatal and neonatal mortality (the number of deaths during the first 28 completed days of life per 1000 live births in a given year or period). | |
| Notes | Setting: 7 districts in Brong Ahafo region in Ghana. Sample size: more that 207,000 pregnant women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | "The capsules were packaged in labelled jars." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 44% of enrolled women initially reported as loss to follow‐up: 1% withdrew consent, 43% moved. However, supplementary information provided by authors in February 2011 at the time of more detailed analysis reported overall loss to follow‐up for analysis for pregnancy‐related mortality analysis as 8%: 4657 pregnancies excluded because outcome not known (with 2340 in vitamin A arm and 2317 in placebo arm). 4192 pregnancies excluded because status of woman at 42 days not known (2174 vitamin A; 2018 placebo). Before these exclusions, the total number of pregnancies captured was 111,801; after exclusions, the total number of pregnancies with a known outcome was 102,952. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | No other bias awarded. |
| Methods | RCT. | |
| Participants | Inclusion criteria:
| |
| Interventions | Intervention group: 340 women received daily doses of orally administered vitamin A (3 mg retinol equivalent (10,000 IU of vitamin A) + iron and folate for minimum of 12 weeks. Oral vitamin A (30 mg retinol equivalent) at 6 weeks' postpartum. Control group: 357 women received daily doses of iron (30 mg of elemental iron) and folate (400 mg) from the time of study enrolment until delivery. Oral vitamin A (30 mg retinol equivalent) at 6 weeks postpartum. | |
| Outcomes | Primary outcome: maternal vitamin A levels in blood and breast milk and HIV transmission in mother and baby. Other outcomes: Hb and birthweight. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Malawi. Study setting: Queen Elizabeth Central Hospital (Blantyre, Malawi). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a computer random‐number generator." |
| Allocation concealment (selection bias) | Low risk | "pre packing study supplements in sequentially numbered series assigned to study identification numbers." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 63 (9%) women were excluded from the analysis: 57 moved out, 6 could not be located. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | Not aware of any other bias. |
| Methods | A randomised double‐blind community‐based trial. | |
| Participants | Inclusion criteria: 16 to 20 weeks pregnant, aged 17‐35 years and parity < 6. | |
| Interventions | Intervention group: 122 women received each week from enrolment until delivery 2 tablets each of which contained 3000 RE vitamin A in addition to the ferrous sulphate and folic acid. So intervention was 6000 RE vitamin A (20,000 IU) weekly for a minimum of 16 weeks. Control group: 121 women received each week from enrolment until delivery 2 tablets each containing 60 mg elemental iron as ferrous sulphate and 250 mg folic acid. | |
| Outcomes | Primary outcome: infant growth in 1 year of life. Other outcomes: maternal Hb and fetal morbidity. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Indonesia. Study setting: 9 villages in the rural subdistrict of Leuwiliang, West Java, Indonesia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned randomly." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | High risk | Out of 243 pregnant women initially enrolled, 18 dropped out during pregnancy, 5 gave birth to a stillborn child, 1 had twins (only 1 survived), 7 had infants who died before reaching 3 months of age and 11 moved from the research area. Among the remaining 201 eligible participants, 182 participants attended the postpartum examination. Overall, the loss to follow‐up of the intervention group was 32.8 % and the control group was 27.3%. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | Not aware of any other bias. |
| Methods | "double‐blinded, randomized, controlled study." | |
| Participants | Inclusion criteria:
Exclusion criteria: women with recurrent pregnancy loss or earlier preterm delivery and those with diabetes, hypertension, or any other metabolic disorder. | |
| Interventions | Intervention group: 85 women received red palm oil providing 2173 to 2307 µg of β‐carotene per day with a dosage schedule of 1 sachet per day (8 mL), which provided 91% to 96% of the daily requirement of vitamin A in pregnancy, (i.e. 2400 µg of β‐carotene which is equivalent to 3000 IU of vitamin A) daily for a period of 8 weeks. Control group: 85 women received 1 sachet of groundnut oil (8 mL) for a period of 8 weeks. | |
| Outcomes | Primary outcome: maternal and neonatal vitamin A status. Other outcomes: Hb levels in mother and baby, preterm birth (delivery less than 37 completed weeks as confirmed by ultrasound examination), birthweight and gestational age. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: India. Study setting: the outpatient department of Niloufer Hospital, Hyderabad, India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 41 (24.1%) women were excluded from the analysis: 23 were not available for supplementation, while 18 dropped out after initiating supplementation. Overall, the loss to follow‐up of the intervention group was 9.5 % and the control group was 15.1%. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | A randomised, double‐blind, controlled clinical trial. | |
| Participants | Inclusion criteria:
| |
| Interventions | Intervention group: 109 women received daily supplement containing iron (30 mg elemental iron), folate (400 mg), and vitamin A (3000 µg retinol equivalent, which is 10,000 IU of vitamin A) until delivery for a minimum of 8 weeks. Control group: 94 women received daily supplement containing iron (30 mg) and folate (400 mg) until delivery. | |
| Outcomes | Primary outcome: Hb concentrations and plasma erythropoietin concentrations. Other outcomes: levels of ferritin, ? 1‐acid glycoprotein, CRP and plasma vitamin A. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Malawi. Study setting: the Queen Elizabeth Central Hospital in Blantyre, Malawi. Semba 2001 is linked to the Semba 2000 trial; the difference being that Semba 2000 investigated slightly different outcomes in HIV‐positive women and Semba 2001 assessed HIV negative women. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a computer random‐number generator." |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered opaque bottle." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | High risk | 66 (32.5%) women were excluded from the analysis: 42 missed the study visit, 9 did not have their Hb analysed, 15 moved out. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | Double‐blinded RCT. | |
| Participants | Inclusion criteria:
| |
| Interventions | Intervention group 1: 63 women received vitamin A (2.4 mg retinol as retinyl palmitate) (equivalent to 8000 IU of vitamin A) and placebo iron tablets daily for 8 weeks. Intervention group 2: 63 women received iron tablets (60 mg ferrous sulphate) and placebo vitamin A daily for 8 weeks. Intervention group 3: 63 women received vitamin A and iron daily for 8 weeks. Control group: 62 women received both placebo daily for 8 weeks. | |
| Outcomes | Maternal anaemia indices. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Indonesia. Study setting: rural villages in 3 subdistricts of Bogo, West Java. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly." |
| Allocation concealment (selection bias) | Low risk | 'An independent researcher randomly labelled' the preparations. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "Double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) | Low risk | 54 (17%) women were excluded from the analysis: 11 moved, 23 taken supplement less than 8 weeks, 10 refused blood sample, 10 not available for 2nd blood sample. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | Double‐blind RCT. | |
| Participants | Inclusion criteria:
| |
| Interventions | 4 groups:
| |
| Outcomes | Primary outcomes:
| |
| Notes | Short intervention time of 2 months duration. Patient were recruited between March 2004 ‐ September 2005. Setting: Shen county in a central rural area of China. Total number of patients ‐ 186. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomised in order of enrolment." |
| Allocation concealment (selection bias) | High risk | "Patients were randomised in order of enrolment." |
| Blinding of participants and personnel (performance bias) | Low risk | Adequate blinding of participants and personnel reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) | Low risk | Loss of participants < 5% ‐ 6 women did not complete the trial due to moving to other villages (3), and stopped taking supplements during the trial (3). |
| Selective reporting (reporting bias) | Unclear risk | No details given. |
| Other bias | Unclear risk | No details given. |
| Methods | Quasi‐RCT. A double‐blind, placebo, controlled trial. | |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention group 1: 22 women; group IFR received iron‐folate tablets + 5 mg riboflavin 7 days a week for 60 days. Intervention group 2: 29 women; group IFA received iron‐folate tablets + 2.75 mg retinyl palmitate (equal to 5000 IU vitamin A) 7 days a week for 60 days. Intervention group 3: 23 women; group IFRA received iron‐folate tablets + 5 mg riboflavin + 2.75 mg retinyl palmitate 7 days a week for 60 days. Control group: 29 women; group IF received iron‐folate tablets + 5 mg glucose 7 days a week for 60 days. | |
| Outcomes | Maternal levels of vitamin A and riboflavin. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Indonesia. Study setting: health centre ANC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "allocated alternately." |
| Allocation concealment (selection bias) | High risk | "allocated alternately." |
| Blinding of participants and personnel (performance bias) | Low risk | "participants and personnel double‐blind." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | Low risk | 19 (18.4%) were excluded from the analyses: 9 premature labour, 1 stillbirth, 1 migration, 1 refusal to give blood, 2 nausea and vomiting and 5 incorrect dates given for last menstruation but with normal deliveries. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | High risk | Women in group IFRA were shorter and lighter than those in other groups. |
| Methods | RCT. | |
| Participants | Inclusion criteria:
| |
| Interventions | Intervention group 1: 5 women received 1.07 mmol (60 mg) ferrous sulphate with a vitamin A placebo daily for 8 weeks. Intervention group 2: 8 women received vitamin A plus iron. Intervention group 3: 7 women received 8.4 µmol (8000 IU) vitamin A as retinyl palmitate with an iron placebo. Control group: 7 women received placebo. | |
| Outcomes | Maternal Hb and retinol levels. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Indonesia. Study setting: local health posts the suburban areas of Bogor in West Java. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | "Subjects and village volunteers were unaware of group assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) | Low risk | No loss of follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Unclear risk | Not enough information provided. |
| Methods | RCT. | |
| Participants | Inclusion criteria:
Exclusion criteria: twin pregnancy. | |
| Interventions | Intervention group 1: 234 women; 5000 IU vitamin A and iron tablets daily (60 mg elemental iron as ferrous sulphate with 0.25 mg folic acid) and antimalarial prophylaxis as 2 doses of Fansidar (500 mg sulphadoxine with 25 mg pyrimethamine. Tablets given daily from enrolment till delivery minimum of 8 weeks. Intervention group 2: 234 women; 10,000 IU vitamin A and iron tablets daily (60 mg elemental iron as ferrous sulphate with 0.25 mg folic acid) and antimalarial prophylaxis as 2 doses of Fansidar (500 mg sulphadoxine with 25 mg pyrimethamine). Control group: 232 women; placebo and iron tablets daily (60 mg elemental iron as ferrous sulphate with 0.25 mg folic acid) and antimalarial prophylaxis as 2 doses of Fansidar (500 mg sulphadoxine with 25 mg pyrimethamine. | |
| Outcomes | Primary outcome: Hb concentrations and anaemia. Other outcomes: iron status, preterm birth (delivery less than 37 completed weeks as confirmed by ultrasound examination), markers of infections included CRP, malaria parasitaemia and HIV status. | |
| Notes | Vitamin A levels were measured before starting supplementation. Country: Malawi. Study setting: rural southern Malawi attending ANC at Health Centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a random‐generation procedure." |
| Allocation concealment (selection bias) | Low risk | "consecutive numbers" "in sealed envelopes." |
| Blinding of participants and personnel (performance bias) | Low risk | "The supplements in vitamin A and placebo treatments allocated were prepared in identical capsules." |
| Blinding of outcome assessment (detection bias) | Low risk | "The supplements in vitamin A and placebo treatments allocated were prepared in identical capsules." |
| Incomplete outcome data (attrition bias) | Low risk | 96 (13.7%) women were excluded from the analyses: 18 women moved out from the area, 68 declined to continue, 10 missed appointment. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | Not aware of other bias. |
| Methods | Double‐blind cluster RCT. | |
| Participants | Inclusion criteria:
Exclusion criteria: women who were already married who had moved into study wards. | |
| Interventions | Intervention group 1: 15,305 women in 90 wards received opaque, gelatinous capsules containing peanut oil and 23,300 IU of preformed vitamin A (7000 µg retinol equivalents) as retinyl palmitate weekly for a minimum of 12 weeks. Intervention group 2: 14,536 women in 90 wards received 42 mg of all trans‐b carotene (7000 µg retinol equivalents, assuming a conversion ratio to retinol of 6 to 1 after uptake) weekly. Control group: 14,805 women in 90 wards received no vitamin A or b carotene (placebo) weekly. | |
| Outcomes | Primary outcome: mortality of mother and baby (the number of deaths during the first 28 completed days of life per 1000 live births in a given year or period). Other outcomes: maternal vitamin A and retinol levels, and maternal morbidity. | |
| Notes | Vitamin A levels were not measured before starting supplementation. Country: Nepal. Study setting: 270 wards in 30 subdistricts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All wards were assigned in Kathmandu by a random draw of numbered chits, blocked on subdistrict, for eligible women to receive one of three identical coded supplements." |
| Allocation concealment (selection bias) | Low risk | "'three identical coded supplements." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel "double‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Details not included. |
| Incomplete outcome data (attrition bias) | Low risk | 1136 (2.5%) women were excluded because they emigrated before becoming pregnant or dying or because they declined to be recruited. 157 women were lost to follow‐up during the postpartum period (their median follow‐up time postpartum was around 2 weeks in each group). |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study is not available at the moment. |
| Other bias | Low risk | No other bias awarded. |
| Methods | Double‐blind cluster‐randomised placebo‐controlled trial. | |
| Participants | Inclusion criteria: all married women of reproductive age (13‐45 years of age) were under surveillance living in the study settings and all who became pregnant were included in this study. Exclusion criteria: > first trimester of pregnancy; women who during surveillance:
Sample size: 60,294. | |
| Interventions | 7000 ug of retinol equivalent as retinyl palmitate, 42 mg of all‐trans beta‐carotene or placebo. | |
| Outcomes | Primary outcome: all‐cause mortality of women related to pregnancy, stillbirth, and infant mortality up to 12 weeks (84 days) following pregnancy outcome. | |
| Notes | Vitamin A levels were not measured before starting supplementation. Country: Bangladesh. Duration: 2001‐2007 termed the JiVitA‐1 trial. Study setting: 596 sectors in the rural northwestern district of Gaibandha and Rangpur between 2001 and 2007. Christian 2013 is a follow‐up study of this trials that describes secondary outcomes of interest (low birthweight and preterm birth) in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "cluster randomization" "sectors were randomized in blocks of nine, to one of three codes ‐ 1,2,3." "field supervisor [were engaged] to in the process of randomization to increase the transparency of sector allocation." |
| Allocation concealment (selection bias) | Low risk | "three sets of 3 identical coins on which the numbers 1, 2 or 3 were written were placed into a container, mixed and removed randomly, without replacement and the 3 digit code of each sector was read aloud sequentially." |
| Blinding of participants and personnel (performance bias) | Low risk | "study participants, interviewers, field supervisors, and investigators remained masked to treatment assignments until the end of the trial." |
| Blinding of outcome assessment (detection bias) | Low risk | "study participants, interviewers, field supervisors, and investigators remained masked to treatment assignments until the end of the trial." |
| Incomplete outcome data (attrition bias) | Low risk | Loss of participants < 5%, except for outcome of low birthweight, which was only measured for less than 36%. |
| Selective reporting (reporting bias) | Low risk | Nil reported in the protocol. |
| Other bias | Low risk | No other bias awarded. |
ANC: antenatal clinic
CRP: C‐reactive protein
Hb: haemoglobin
IU: international unit
LMP: last menstrual period
RCT: randomised controlled trial
RE: retinol equivalents
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The intervention did not include vitamin A. | |
| Intervention uses lycopene which is a compound that lacks beta‐ion ring (in the β‐carotene), so lycopene cannot form vitamin A and its biological effects are due to mechanism other than forming vitamin A. | |
| Not a randomised trial. | |
| Double‐blind randomised trial with outcomes on HIV transmission and HIV complications, only abstract available. | |
| Cluster‐randomised trial with all arms of intervention containing vitamin A and no comparison for vitamin A. | |
| Intervention started after delivery. | |
| Both arms of intervention containing vitamin A and no comparison for vitamin A. | |
| Not a randomised trial. | |
| Intervention started after delivery. | |
| Not a randomised trial and vitamin A present in both arms of intervention. | |
| Both arms of intervention containing vitamin A and no comparison for vitamin A. | |
| Both arms of intervention containing iron and folic acid and no comparison for vitamin A. | |
| Intervention started after delivery. | |
| Intervention uses lycopene which is a compound that lacks beta‐ion ring (in the β‐carotene), so lycopene cannot form vitamin A and its biological effects are due to mechanism other than forming vitamin A. | |
| Participants are non‐pregnant women. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Vitamin A and maternal‐infant flu vaccine response |
| Methods | Placebo‐controlled double‐masked and randomised trial. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Weekly 10,000 IU vitamin A or placebo. |
| Outcomes | Main outcomes: IgG cord blood plasma; vitamin A cord blood; plasma influenza IgG; colostrum vitamin A; colostrum influenza sIgA. Other outcomes:
|
| Starting date | February 2009. |
| Contact information | International Centre for Diarrhoeal Disease Research, Bangladesh. |
| Notes | 66 women randomised. Setting: Dhaka, Bangladesh. 3 urban maternity clinics. 2010 ‐ no results reported. |
IgG: immunoglobulin G
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 0.88 [0.65, 1.20] | |
| Analysis 1.1  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 1 Maternal mortality. | ||||
| 2 Perinatal mortality Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.95, 1.07] | |
| Analysis 1.2  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 2 Perinatal mortality. | ||||
| 3 Neonatal mortality Show forest plot | 3 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.90, 1.05] | |
| Analysis 1.3  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 3 Neonatal mortality. | ||||
| 4 Stillbirth Show forest plot | 2 | 122850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| Analysis 1.4  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 4 Stillbirth. | ||||
| 5 Maternal anaemia Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 0.64 [0.43, 0.94] | |
| Analysis 1.5  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 5 Maternal anaemia. | ||||
| 6 Maternal clinical infection Show forest plot | 5 | Risk Ratio (Random, 95% CI) | 0.45 [0.20, 0.99] | |
| Analysis 1.6 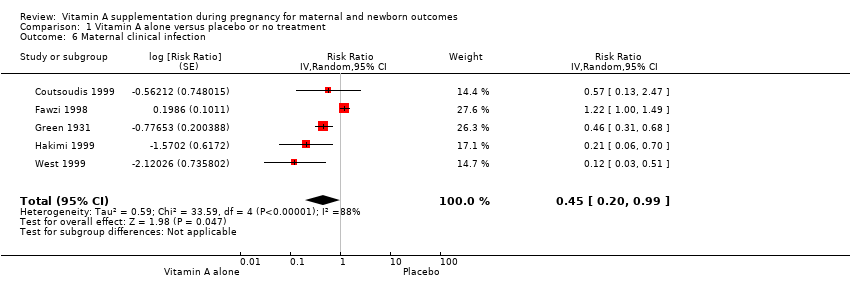 Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 6 Maternal clinical infection. | ||||
| 7 Maternal night blindness Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 0.79 [0.64, 0.98] | |
| Analysis 1.7  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 7 Maternal night blindness. | ||||
| 8 Preterm birth Show forest plot | 5 | 40137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.01] |
| Analysis 1.8  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 8 Preterm birth. | ||||
| 9 Neonatal anaemia Show forest plot | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.08] |
| Analysis 1.9 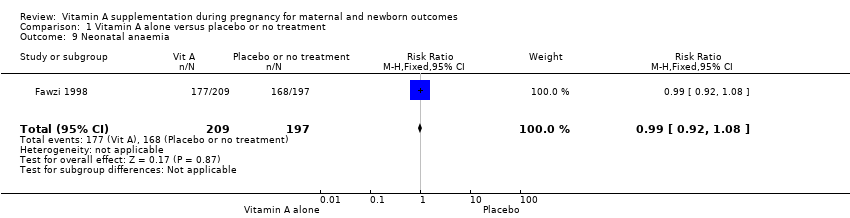 Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 9 Neonatal anaemia. | ||||
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Low birthweight Show forest plot | 4 | 14599 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] |
| Analysis 1.12  Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 12 Low birthweight. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Stillbirth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal anaemia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal clinical infection Show forest plot | 2 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| Analysis 2.6 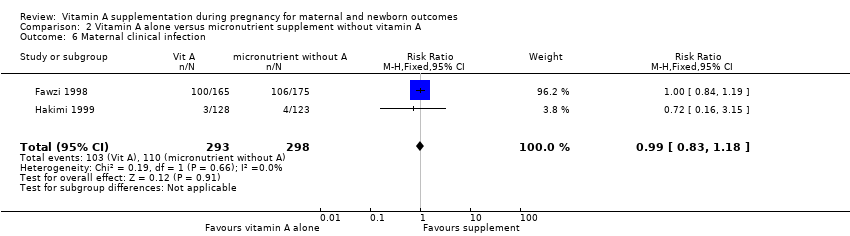 Comparison 2 Vitamin A alone versus micronutrient supplement without vitamin A, Outcome 6 Maternal clinical infection. | ||||
| 7 Maternal night blindness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Preterm birth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal anaemia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Low birthweight | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 3.2  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 2 Perinatal mortality. | ||||
| 3 Neonatal mortality Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.31] |
| Analysis 3.3  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 3 Neonatal mortality. | ||||
| 4 Stillbirth Show forest plot | 2 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.57, 3.47] |
| Analysis 3.4  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 4 Stillbirth. | ||||
| 5 Maternal anaemia Show forest plot | 3 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| Analysis 3.5  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 5 Maternal anaemia. | ||||
| 6 Maternal clinical infection Show forest plot | 2 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| Analysis 3.6  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 6 Maternal clinical infection. | ||||
| 7 Maternal night blindness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Preterm birth Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.93] |
| Analysis 3.8 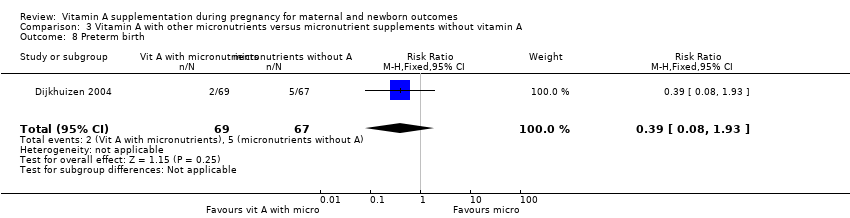 Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 8 Preterm birth. | ||||
| 9 Neonatal anaemia Show forest plot | 2 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.38, 1.51] |
| Analysis 3.9 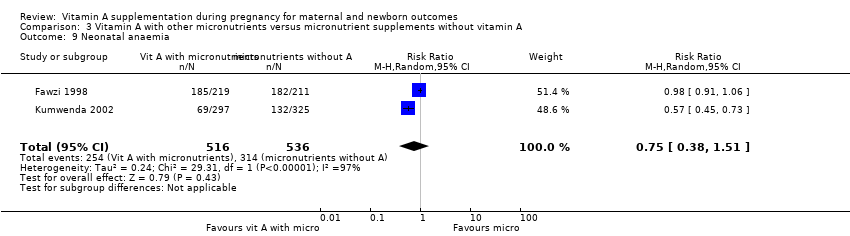 Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 9 Neonatal anaemia. | ||||
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.18] |
| Analysis 3.11 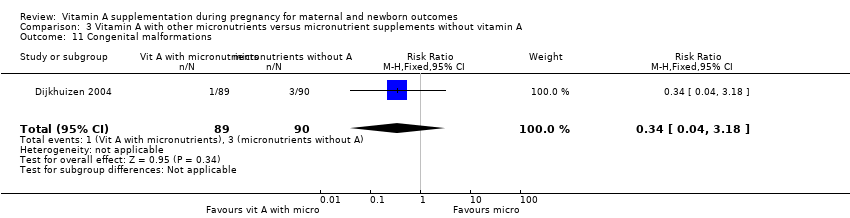 Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 11 Congenital malformations. | ||||
| 12 Low birthweight Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.96] |
| Analysis 3.12  Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 12 Low birthweight. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality (infant mortality level) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.1 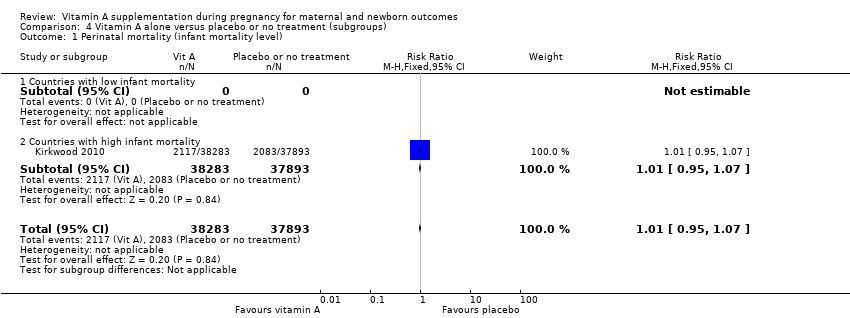 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 1 Perinatal mortality (infant mortality level). | ||||
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 2 Maternal mortality (infant mortality level) Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 0.88 [0.65, 1.20] | |
| Analysis 4.2  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 2 Maternal mortality (infant mortality level). | ||||
| 2.1 Countries with low infant mortality | 1 | Risk Ratio (Random, 95% CI) | 0.33 [0.01, 9.44] | |
| 2.2 Countries with high infant mortality | 3 | Risk Ratio (Random, 95% CI) | 0.89 [0.64, 1.23] | |
| 3 Maternal mortality (maternal mortality level) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| Analysis 4.3 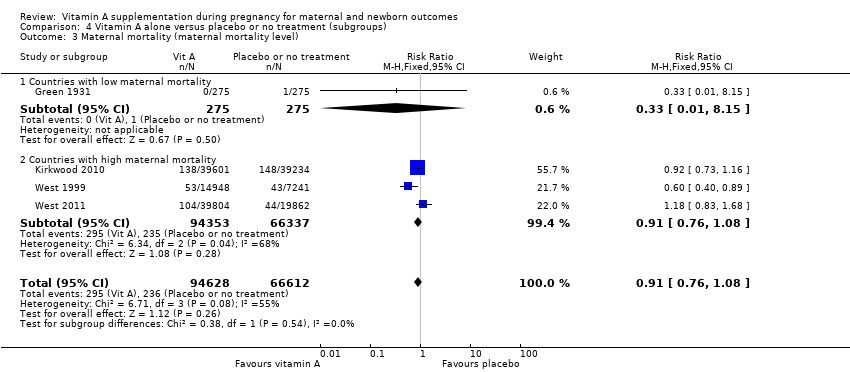 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 3 Maternal mortality (maternal mortality level). | ||||
| 3.1 Countries with low maternal mortality | 1 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 3.2 Countries with high maternal mortality | 3 | 160690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 4 Perinatal mortality (maternal mortality level) Show forest plot | 1 | 73743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| Analysis 4.4  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 4 Perinatal mortality (maternal mortality level). | ||||
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 1 | 73743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| Analysis 4.5 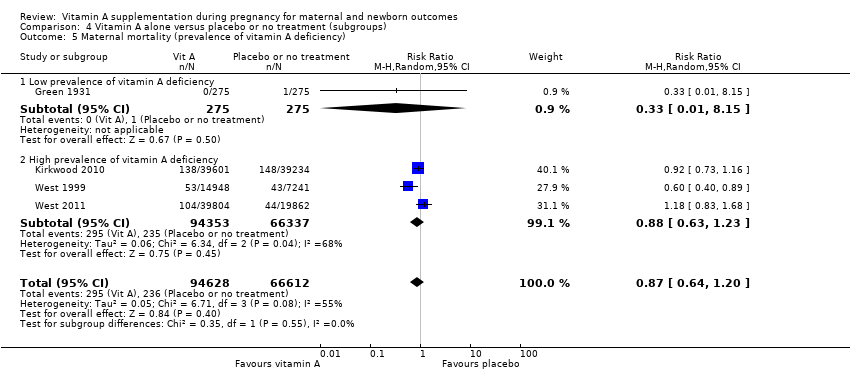 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 5 Maternal mortality (prevalence of vitamin A deficiency). | ||||
| 5.1 Low prevalence of vitamin A deficiency | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 5.2 High prevalence of vitamin A deficiency | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.6  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 6 Perinatal mortality (prevalence of vitamin A deficiency). | ||||
| 6.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High prevalence of vitamin A deficiency | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 7 Maternal mortality (prevalence of HIV in the general population) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| Analysis 4.7  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 7 Maternal mortality (prevalence of HIV in the general population). | ||||
| 7.1 Countries with low HIV prevalence | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.8 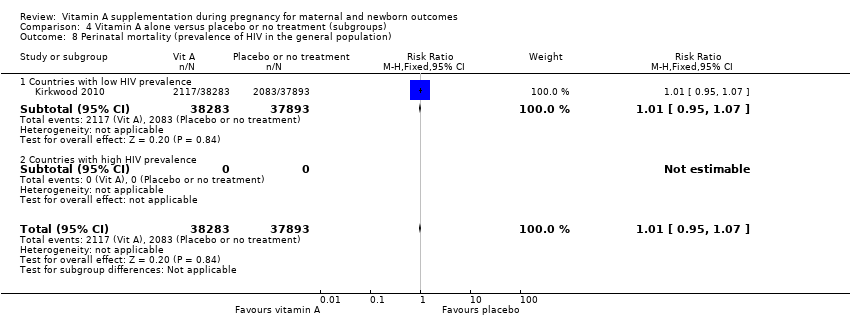 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 8 Perinatal mortality (prevalence of HIV in the general population). | ||||
| 8.1 Countries with low HIV prevalence | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) Show forest plot | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| Analysis 4.9  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 9 Maternal mortality (dose). | ||||
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
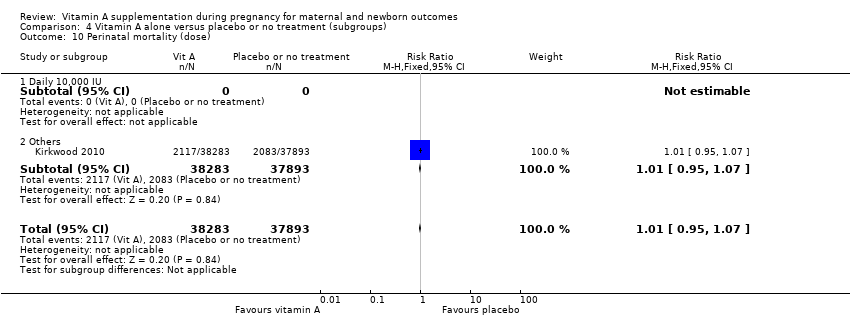
| 10 Perinatal mortality (dose) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.10  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 10 Perinatal mortality (dose). | ||||
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 11 Maternal mortality (regimen) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| Analysis 4.11 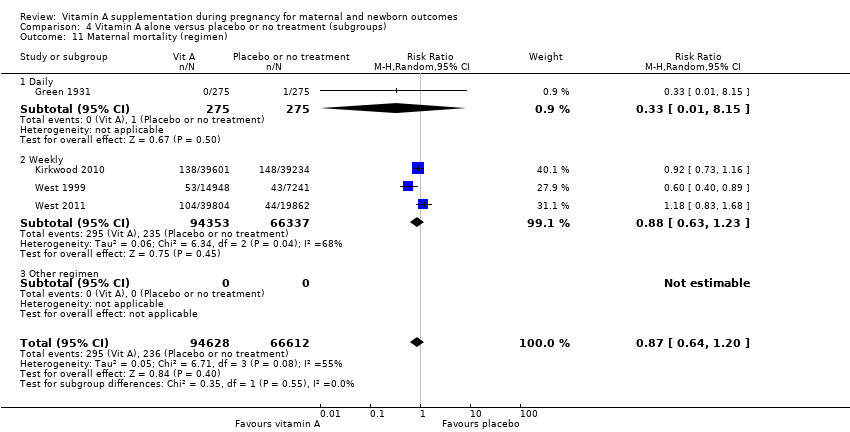 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 11 Maternal mortality (regimen). | ||||
| 11.1 Daily | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 11.2 Weekly | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
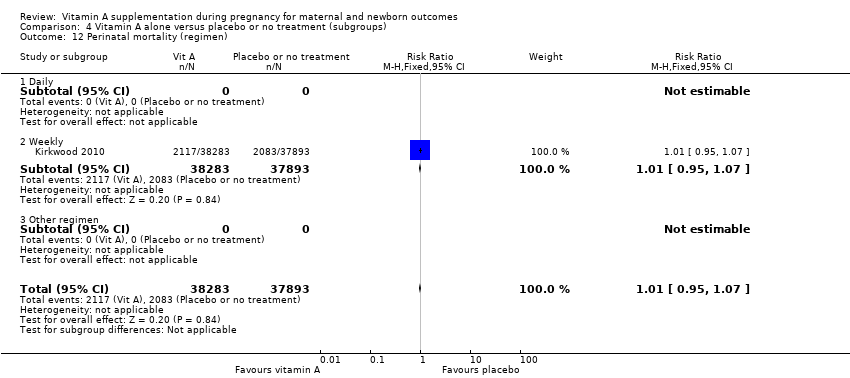
| 12 Perinatal mortality (regimen) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.12  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 12 Perinatal mortality (regimen). | ||||
| 12.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Weekly | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
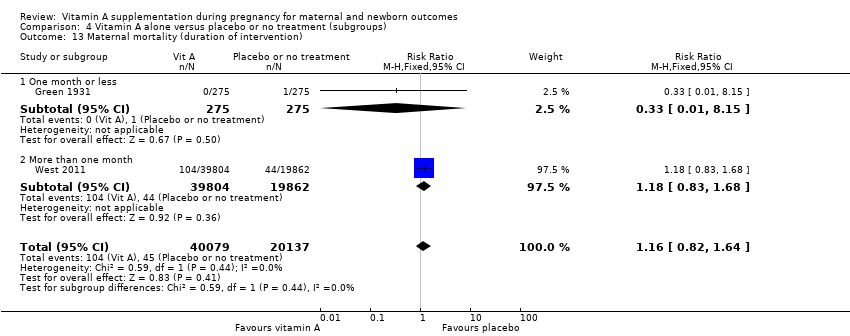
| 13 Maternal mortality (duration of intervention) Show forest plot | 2 | 60216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.64] |
| Analysis 4.13  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 13 Maternal mortality (duration of intervention). | ||||
| 13.1 One month or less | 1 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 13.2 More than one month | 1 | 59666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.68] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| Analysis 4.15 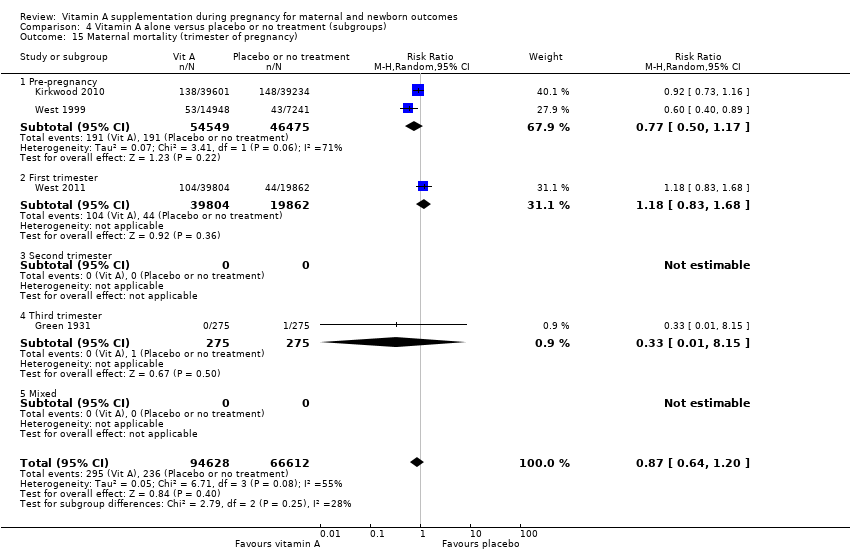 Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 15 Maternal mortality (trimester of pregnancy). | ||||
| 15.1 Pre‐pregnancy | 2 | 101024 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.17] |
| 15.2 First trimester | 1 | 59666 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.68] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal mortality (trimester of pregnancy) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.16  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 16 Perinatal mortality (trimester of pregnancy). | ||||
| 16.1 Pre‐pregnancy | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal mortality (randomisation) Show forest plot | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| Analysis 4.17  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 17 Maternal mortality (randomisation). | ||||
| 17.1 Cluster‐randomised | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
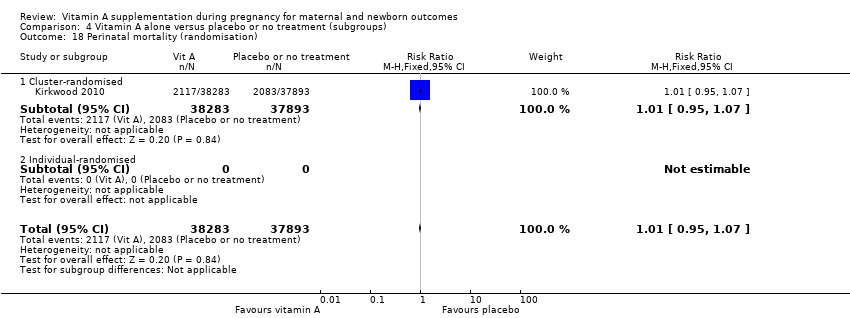
| 18 Perinatal mortality (randomisation) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| Analysis 4.18  Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 18 Perinatal mortality (randomisation). | ||||
| 18.1 Cluster‐randomised | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 18.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perinatal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perinatal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Perinatal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Perinatal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality (infant mortality level) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.2  Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 2 Perinatal mortality (infant mortality level). | ||||
| 2.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Countries with high infant mortality | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 3 Maternal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
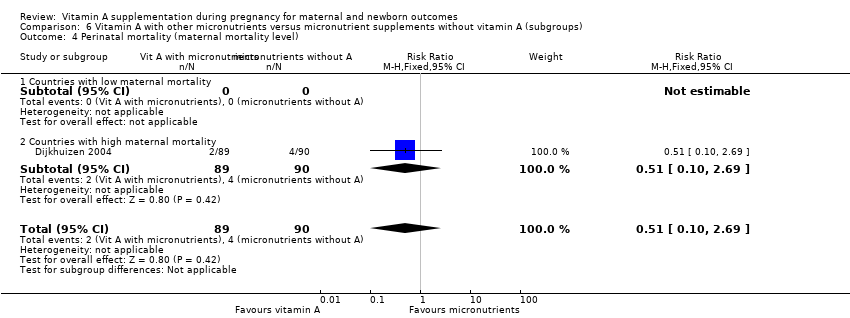
| 4 Perinatal mortality (maternal mortality level) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.4 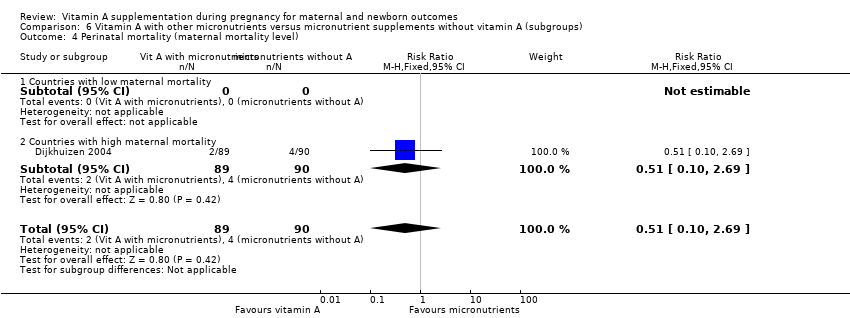 Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 4 Perinatal mortality (maternal mortality level). | ||||
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.5  Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 5 Maternal mortality (prevalence of vitamin A deficiency). | ||||
| 5.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 High prevalence of vitamin A deficiency | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.6 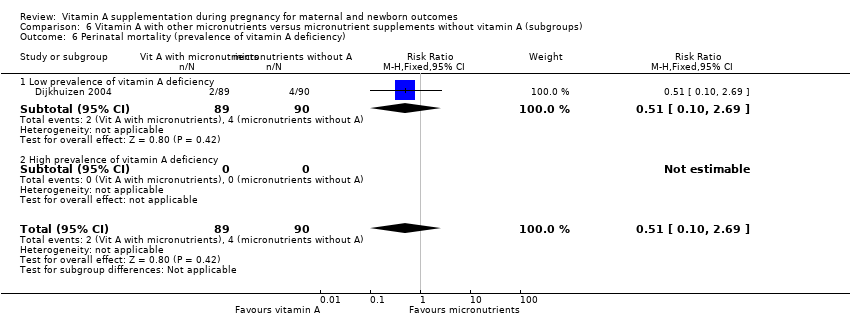 Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 6 Perinatal mortality (prevalence of vitamin A deficiency). | ||||
| 6.1 Low prevalence of vitamin A deficiency | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 6.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.8 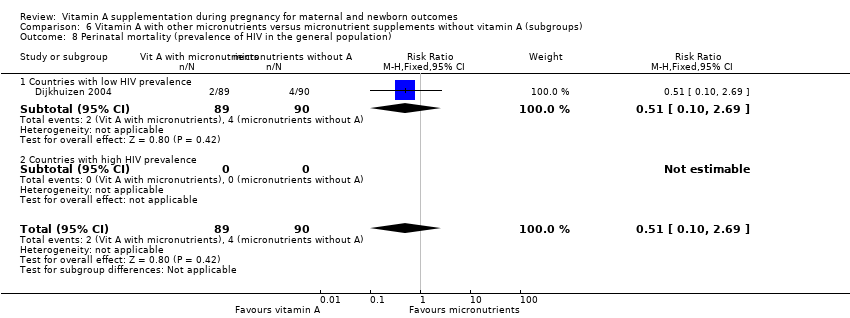 Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 8 Perinatal mortality (prevalence of HIV in the general population). | ||||
| 8.1 Countries with low HIV prevalence | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
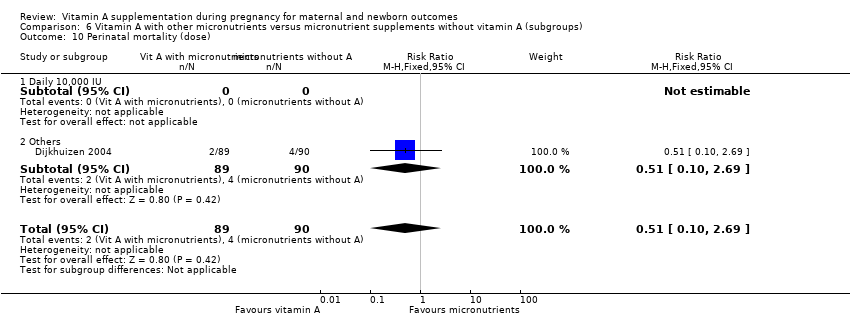
| 10 Perinatal mortality (dose) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.10  Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 10 Perinatal mortality (dose). | ||||
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 11 Maternal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
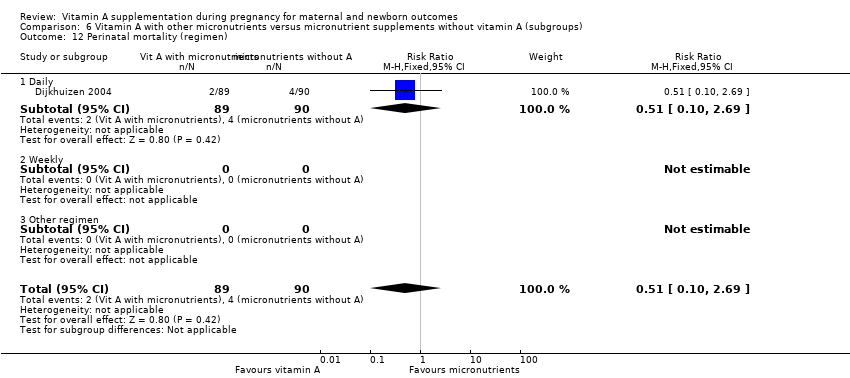
| 12 Perinatal mortality (regimen) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.12  Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 12 Perinatal mortality (regimen). | ||||
| 12.1 Daily | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 12.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
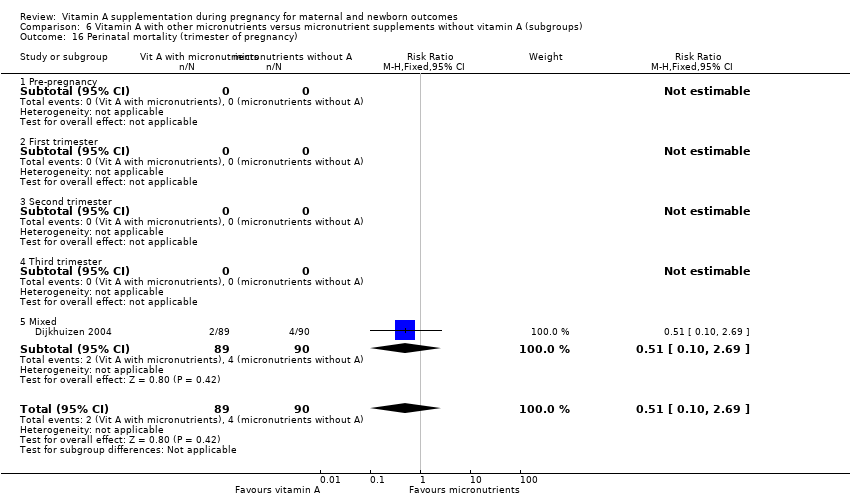
| 16 Perinatal mortality (trimester of pregnancy) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.16  Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 16 Perinatal mortality (trimester of pregnancy). | ||||
| 16.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 17 Maternal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Perinatal mortality (randomisation) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| Analysis 6.18 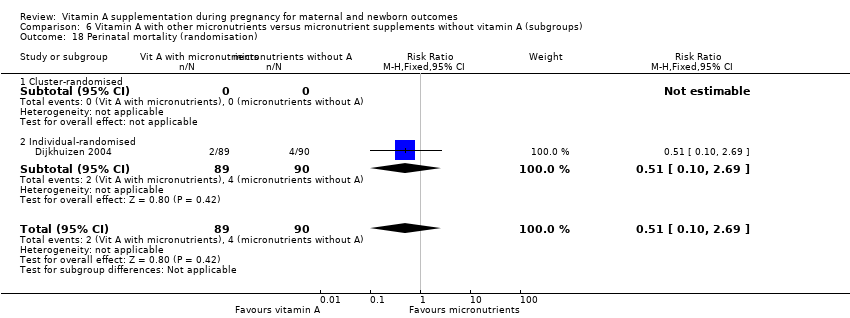 Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 18 Perinatal mortality (randomisation). | ||||
| 18.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Individual‐randomised | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 1 Maternal mortality.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 2 Perinatal mortality.
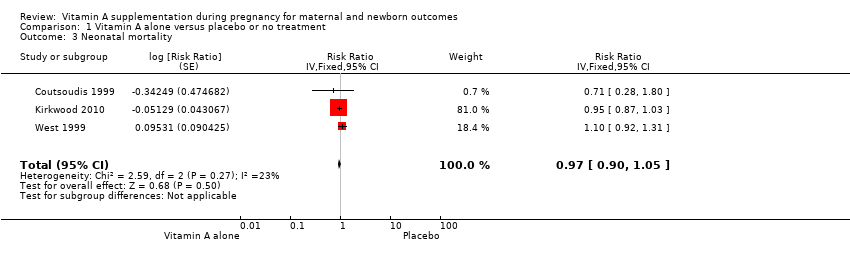
Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 3 Neonatal mortality.
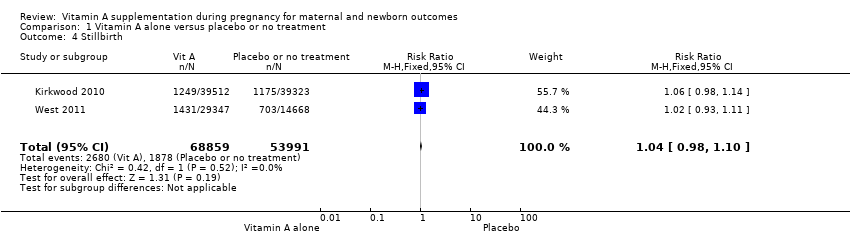
Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 4 Stillbirth.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 5 Maternal anaemia.
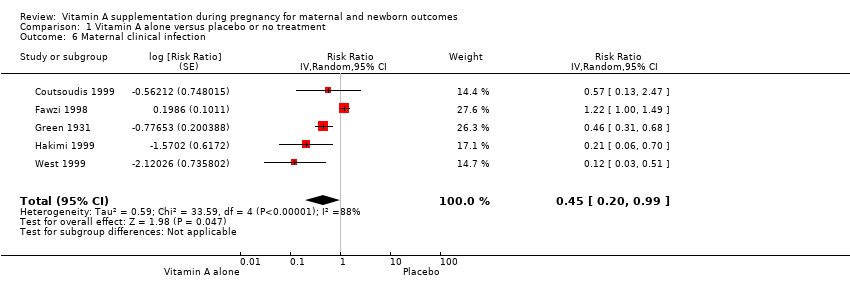
Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 6 Maternal clinical infection.
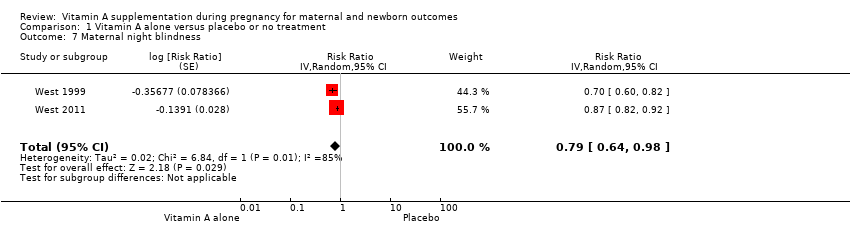
Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 7 Maternal night blindness.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 8 Preterm birth.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 9 Neonatal anaemia.

Comparison 1 Vitamin A alone versus placebo or no treatment, Outcome 12 Low birthweight.

Comparison 2 Vitamin A alone versus micronutrient supplement without vitamin A, Outcome 6 Maternal clinical infection.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 2 Perinatal mortality.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 3 Neonatal mortality.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 4 Stillbirth.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 5 Maternal anaemia.
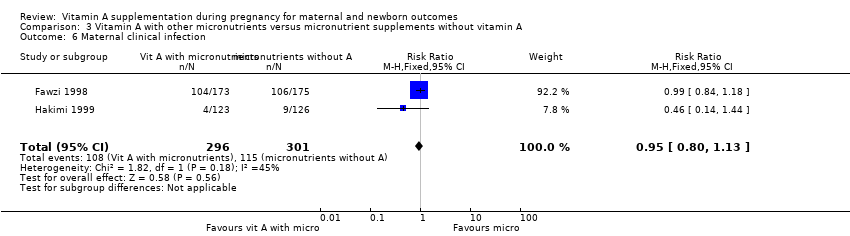
Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 6 Maternal clinical infection.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 8 Preterm birth.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 9 Neonatal anaemia.
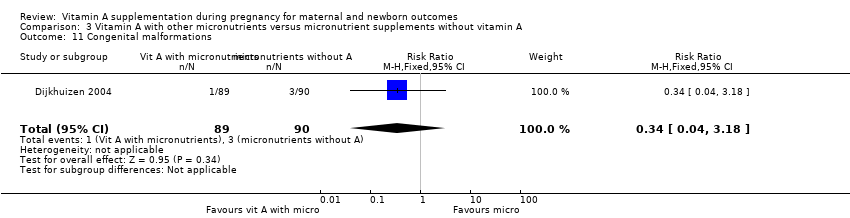
Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 11 Congenital malformations.

Comparison 3 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A, Outcome 12 Low birthweight.
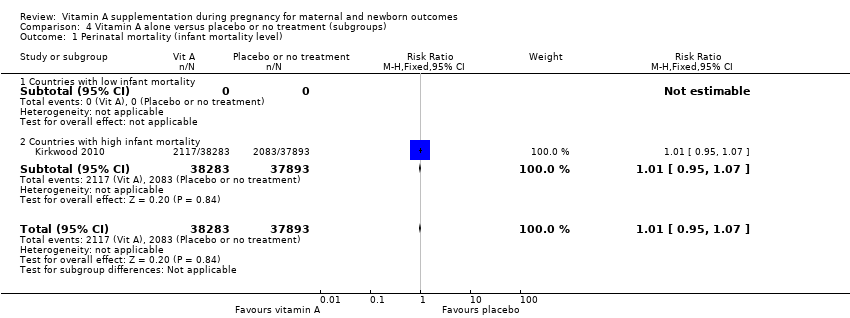
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 1 Perinatal mortality (infant mortality level).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 2 Maternal mortality (infant mortality level).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 3 Maternal mortality (maternal mortality level).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 4 Perinatal mortality (maternal mortality level).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 5 Maternal mortality (prevalence of vitamin A deficiency).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 6 Perinatal mortality (prevalence of vitamin A deficiency).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 7 Maternal mortality (prevalence of HIV in the general population).
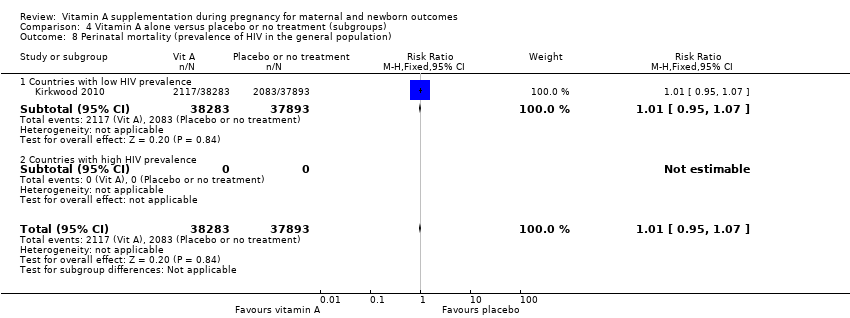
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 8 Perinatal mortality (prevalence of HIV in the general population).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 9 Maternal mortality (dose).
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 10 Perinatal mortality (dose).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 11 Maternal mortality (regimen).
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 12 Perinatal mortality (regimen).
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 13 Maternal mortality (duration of intervention).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 15 Maternal mortality (trimester of pregnancy).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 16 Perinatal mortality (trimester of pregnancy).

Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 17 Maternal mortality (randomisation).
Comparison 4 Vitamin A alone versus placebo or no treatment (subgroups), Outcome 18 Perinatal mortality (randomisation).

Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 2 Perinatal mortality (infant mortality level).
Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 4 Perinatal mortality (maternal mortality level).

Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 5 Maternal mortality (prevalence of vitamin A deficiency).

Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 6 Perinatal mortality (prevalence of vitamin A deficiency).

Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 8 Perinatal mortality (prevalence of HIV in the general population).
Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 10 Perinatal mortality (dose).
Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 12 Perinatal mortality (regimen).
Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 16 Perinatal mortality (trimester of pregnancy).

Comparison 6 Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (subgroups), Outcome 18 Perinatal mortality (randomisation).
| Vitamin A alone versus placebo or no treatment | ||||||
| Patient or population: Pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment | Risk with vitamin A alone | |||||
| Maternal mortality | Study population | RR 0.88 | 154,039 | ⊕⊕⊕⊕ | Inverse variance. | |
| 5 per 1000 | 5 per 1000 (4 to 7) | |||||
| Perinatal mortality | Study population | RR 1.01 | 76,176 | ⊕⊕⊕⊕ | Inverse variance. | |
| 54 per 1000 | 54 per 1000 (51 to 57) | |||||
| Maternal anaemia | Study population | RR 0.64 | 15,649 | ⊕⊕⊕⊝ | Inverse variance. | |
| 191 per 1000 | 122 per 1000 (82 to 180) | |||||
| Maternal clinical infection | Study population | RR 0.45 | 17,313 | ⊕⊕⊝⊝ | Inverse variance. | |
| 18 per 1000 | 8 per 1000 (4 to 18) | |||||
| Preterm birth | Study population | RR 0.98 | 48,007 | ⊕⊕⊕⊕ | ||
| 249 per 1000 | 244 per 1000 | |||||
| Moderate | ||||||
| 190 per 1000 | 186 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The authors considered that the pooled effect estimate was not biased by the design of the studies or their analysis of data. Following correspondence received from the trialists for Kirkwood 2010, the loss to follow‐up for this study was 8%: the data from this study are not at risk of attrition bias. 2 Statistical Heterogeneity (I² > 60%). 3 Most studies contributing data had design limitations. | ||||||
| Combination vitamin A and micronutrients for maternal and newborn mortality and morbidity | ||||||
| Patient or population: Pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with micronutrient supplements without vitamin A | Risk with vitamin A with other micronutrients | |||||
| Maternal mortality | Study population | not estimable | (0 studies) | See comment | No study reported results for this outcome. | |
| not pooled | not pooled | |||||
| Perinatal mortality | Study population | RR 0.51 | 179 | ⊕⊕⊝⊝ | ||
| 44 per 1000 | 23 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 23 per 1000 | |||||
| Maternal anaemia | Study population | RR 0.86 | 706 | ⊕⊕⊝⊝ | ||
| 269 per 1000 | 231 per 1000 | |||||
| Moderate | ||||||
| 346 per 1000 | 298 per 1000 | |||||
| Maternal clinical infection | Study population | RR 0.95 | 597 | ⊕⊕⊝⊝ | ||
| 382 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 339 per 1000 | 322 per 1000 | |||||
| Preterm birth | Study population | RR 0.39 | 136 | ⊕⊕⊝⊝ | ||
| 75 per 1000 | 29 per 1000 | |||||
| Moderate | ||||||
| 75 per 1000 | 29 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, few events & small sample size. 2 Wide confidence interval crossing the line of no effect & small sample size. | ||||||
| Retinol supplementation in mcg | Vitamin A in IU |
| 1 | 3.33 |
| 2 | 6.66 |
| 3 | 9.99 |
| IU: international units | |
| Serum retinol mcg/dL | Serum retinol mc mol/L |
| 10 | 0.35 |
| 20 | 0.7 |
| 30 | 1.05 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 0.88 [0.65, 1.20] | |
| 2 Perinatal mortality Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.95, 1.07] | |
| 3 Neonatal mortality Show forest plot | 3 | Risk Ratio (Fixed, 95% CI) | 0.97 [0.90, 1.05] | |
| 4 Stillbirth Show forest plot | 2 | 122850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 5 Maternal anaemia Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 0.64 [0.43, 0.94] | |
| 6 Maternal clinical infection Show forest plot | 5 | Risk Ratio (Random, 95% CI) | 0.45 [0.20, 0.99] | |
| 7 Maternal night blindness Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 0.79 [0.64, 0.98] | |
| 8 Preterm birth Show forest plot | 5 | 40137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.01] |
| 9 Neonatal anaemia Show forest plot | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.08] |
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Low birthweight Show forest plot | 4 | 14599 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Neonatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Stillbirth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal anaemia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal clinical infection Show forest plot | 2 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 7 Maternal night blindness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Preterm birth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal anaemia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Low birthweight | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 3 Neonatal mortality Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.31] |
| 4 Stillbirth Show forest plot | 2 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.57, 3.47] |
| 5 Maternal anaemia Show forest plot | 3 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| 6 Maternal clinical infection Show forest plot | 2 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 Maternal night blindness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Preterm birth Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.93] |
| 9 Neonatal anaemia Show forest plot | 2 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.38, 1.51] |
| 10 Neonatal clinical infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Congenital malformations Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.18] |
| 12 Low birthweight Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality (infant mortality level) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 2 Maternal mortality (infant mortality level) Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 0.88 [0.65, 1.20] | |
| 2.1 Countries with low infant mortality | 1 | Risk Ratio (Random, 95% CI) | 0.33 [0.01, 9.44] | |
| 2.2 Countries with high infant mortality | 3 | Risk Ratio (Random, 95% CI) | 0.89 [0.64, 1.23] | |
| 3 Maternal mortality (maternal mortality level) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 3.1 Countries with low maternal mortality | 1 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 3.2 Countries with high maternal mortality | 3 | 160690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.08] |
| 4 Perinatal mortality (maternal mortality level) Show forest plot | 1 | 73743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 1 | 73743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 5.1 Low prevalence of vitamin A deficiency | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 5.2 High prevalence of vitamin A deficiency | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 6.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High prevalence of vitamin A deficiency | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 7 Maternal mortality (prevalence of HIV in the general population) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 7.1 Countries with low HIV prevalence | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 8.1 Countries with low HIV prevalence | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) Show forest plot | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 10 Perinatal mortality (dose) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 11 Maternal mortality (regimen) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 11.1 Daily | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 11.2 Weekly | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Perinatal mortality (regimen) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 12.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Weekly | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal mortality (duration of intervention) Show forest plot | 2 | 60216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.64] |
| 13.1 One month or less | 1 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 13.2 More than one month | 1 | 59666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.68] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) Show forest plot | 4 | 161240 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.20] |
| 15.1 Pre‐pregnancy | 2 | 101024 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.17] |
| 15.2 First trimester | 1 | 59666 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.68] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal mortality (trimester of pregnancy) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 16.1 Pre‐pregnancy | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal mortality (randomisation) Show forest plot | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 17.1 Cluster‐randomised | 3 | 160690 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.23] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Perinatal mortality (randomisation) Show forest plot | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 18.1 Cluster‐randomised | 1 | 76176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 18.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perinatal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perinatal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Perinatal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Perinatal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality (infant mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Countries with high infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality (infant mortality level) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 2.1 Countries with low infant mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Countries with high infant mortality | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 3 Maternal mortality (maternal mortality level) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Countries with high maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Perinatal mortality (maternal mortality level) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 4.1 Countries with low maternal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Countries with high maternal mortality | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 5 Maternal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 5.1 Low prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 High prevalence of vitamin A deficiency | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 6 Perinatal mortality (prevalence of vitamin A deficiency) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 6.1 Low prevalence of vitamin A deficiency | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 6.2 High prevalence of vitamin A deficiency | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal mortality (prevalence of HIV in the general population) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Countries with low HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal mortality (prevalence of HIV in the general population) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 8.1 Countries with low HIV prevalence | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 8.2 Countries with high HIV prevalence | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal mortality (dose) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Others | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perinatal mortality (dose) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 10.1 Daily 10,000 IU | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Others | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 11 Maternal mortality (regimen) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Perinatal mortality (regimen) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 12.1 Daily | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 12.2 Weekly | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Perinatal mortality (duration of intervention) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal mortality (trimester of pregnancy) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Mixed | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal mortality (trimester of pregnancy) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 16.1 Pre‐pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 First trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Second trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Third trimester | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Mixed | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 17 Maternal mortality (randomisation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Individual‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Perinatal mortality (randomisation) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |
| 18.1 Cluster‐randomised | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Individual‐randomised | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.69] |

