Statines pour la stéatose hépatique non alcoolique et la stéatohépatite non alcoolique
Appendices
Appendix 1. Search strategies
| Database | Time Span | Search Strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | March 2013 | (statin* OR Lovastatin OR Atorvastatin OR Simvastatin OR Parvovastatin OR Rosuvastatin OR Fluvastatin OR Pravastatin OR 'reductase inhibitor*') AND (liver* OR hepat* OR steatohepat* OR NAFL* OR NASH*) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | Issue 1, 2013 | #1 Statins /explode all trees (MeSH) |
| MEDLINE (OvidSP) | 1946 to March 2013 | 1. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ or exp Lovastatin/ or exp Pravastatin/ 2. (statin* or Lovastatin or Atorvastatin or Simvastatin or Parvovastatin or Rosuvastatin or Fluvastatin or Pravastatin or 'reductase inhibitor*').mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp Fatty Liver/ 5. ((fatty and (liver* or hepat*)) or steatohepat* or NAFL* or NASH*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 7 and 8 |
| EMBASE (OvidSP) | 1974 to March 2013 | 1. exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ 2. (statin* or Lovastatin or Atorvastatin or Simvastatin or Parvovastatin or Rosuvastatin or Fluvastatin or Pravastatin or 'reductase inhibitor*').mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 3. 1 or 2 4. exp fatty liver/ 5. ((fatty and (liver* or hepat*)) or steatohepat* or NAFL* or NASH*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 7 and 8 |
| Science Citation Index Expanded (http://apps.isiknowledge.com) | 1900 to March 2013 | #1 TS=(statins OR HMG‐CoA reductase inhibitors OR Hydroxymethylglutaryl‐CoA reductase inhibitors OR Lovastatin OR Pravastatin OR Rosuvastatin OR Atorvastatin OR Simvastatin) #5 #4 AND #3 |
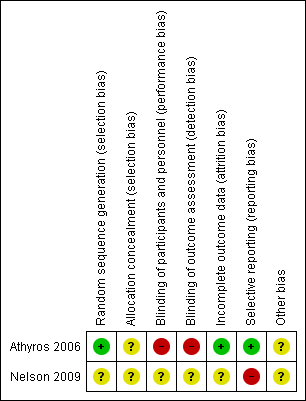
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Source of the search | Number of records |
| CHBG Controlled Trial Register | 51 |
| Cochrane Central Register of Controlled Trials | 78 |
| MEDLINE (Ovid) | 116 |
| EMBASE (Ovid) | 518 |
| Science Citation Index EXPANDED | 91 |
| Total number of references identified | 851 |
| Number of duplicates excluded | 198 |
| Number of references in final list | 653 |
|
| Statin (n = 10) | Placebo (n = 6) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 70.4 | 49.5 | 20.9 | 66.8 | 75.3 | ‐8.5 |
| AST (U/L) | 43.3 | 36.5 | 6.8 | 42.8 | 49.3 | ‐6.5 |
| ALP (U/L) | 86.1 | 89.7 | ‐3.6 | 74.3 | 73 | 1.3 |
| TG (mg/dL) | 388.7 | 490 | ‐101.3 | 335.3 | 361.7 | ‐26.4 |
| ALT = alanine aminotransferase | ||||||
|
| Statin (n = 63) | Fenofibrate (n = 62) | ||||
| Before treatment | After treatment | Mean decrease | Before treatment | After treatment | Mean decrease | |
| ALT (U/L) | 54 | 32 | 22 | 52 | 36 | 16 |
| AST (U/L) | 38 | 25 | 13 | 39 | 27 | 12 |
| ALP (U/L) | 110 | 75 | 35 | 108 | 78 | 30 |
| TG (mg/dL) | 203.8 | 142.2 | 61.6 | 194.9 | 115.6 | 79.3 |
| ALT = alanine aminotransferase | ||||||
| Outcome | Biochemical improvement
| Radiological improvement | No improvement in histology | Serious adverse effects |
| Evidence | Low‐quality systematic review | Low‐quality randomised clinical trial | Small sample size in low‐quality randomised clinical trial | Low‐quality systematic review
|
| Level of evidence | 2 | 2 | 2 | 2 |
| Grade of recommendation | B | B | B | B |

