治疗儿童和青少年破坏性品行障碍的非典型抗精神病药物
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, randomised controlled trial of risperidone solution and placebo | |
| Participants | Setting: outpatients, multicentre Sample size: 118; 55 active treatment (reported for 52 participants); 63 placebo Sex: 97 males, 21 females Age range: 5 to 12 years Mean age: active treatment 8.7 (SD = 2.1) years; placebo 8.1 (SD = 2.3) years IQ range: 36 to 84 Inclusion criteria: ≥ 24 (70th percentile) on the Conduct Problem subscale of the Nisonger Child Behaviour Rating Form Diagnosis: DSM‐IV diagnosis of conduct disorder, oppositional defiant disorder, or disruptive behaviour disorder not otherwise specified Comorbidity: 70 ADHD (33 active treatment; 37 placebo) Withdrawn/dropouts: 31 withdrawn (12 active treatment; 19 placebo) Other interventions
| |
| Interventions | Intended dose: risperidone solution 0.01 mg/kg increasing to 0.02 mg/kg on day 3 Mean dose at endpoint: 1.16 mg/day (mean 0.037 mg/kg per day) | |
| Outcomes | Primary outcomes: Conduct Problem subscale of the Nisonger Child Behaviour Rating Form Secondary outcomes: Aberrant Behaviour Checklist, Behaviour Problem Inventory, Clinical Global Impression ‒ Severity Rating Scale, Clinical Global Impression ‒ Change Scores, Visual Analogue Scale of the target symptoms Follow‐up interval: 6 weeks | |
| Notes | Imputation method for incomplete data: last observation carried forward (LOCF) (p 1339) Funding/support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned" (p 1338) ‒ insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Appearance and taste of solutions were identical. All trial medications were labelled with the protocol number, medication number, lot number and strata. A tear‐off label was provided on each box of study medication which contained the medication code. The label was placed in the Case Report Form on the appropriate page. The code should only be broken in case of an emergency (Loy 2011b [pers comm]). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | 12 participants (22%) in the risperidone and 19 (30%) in the placebo group withdrew. 4 in the risperidone and 15 in the placebo group due to insufficient response, 2 in risperidone because of adverse events, 3 in risperidone due to non‐compliance, 1 in risperidone and 3 in placebo lost to follow‐up, 1 in risperidone and 1 in placebo withdrew consent and 1 in risperidone lost medication. No efficacy data were recorded for 3 patients in the risperidone group and hence they were not included in any efficacy analyses (but the authors stated to have used an intention‐to‐treat analysis). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | Unclear risk | 1‐week placebo run‐in "to rule out placebo responders" (p 1338). |
| Methods | Double‐blind, randomised controlled trial of risperidone and placebo, added to pre‐existing stimulants | |
| Participants | Setting: outpatients, 1 centre Sample size: 25; 12 active treatment, 13 placebo Sex: 22 males and 3 females Age range: 7 to 12 years Mean age: active treatment 7.3 (SD = 3.7) years; placebo 8.8 (SD = 3.1) years IQ range: IQ ≥ 75 Inclusion criteria: DSM‐IV criteria for ADHD; aggression criteria documented by the presence of 3 acts of aggression in the past week, 2 of which had to be acts of physical aggression against other people, objects or self. Patients had an Aggression Questionnaire Predatory ‒ Affective index score of 0 or below indicating primarily an affective or impulsive type of aggression, Clinical Global Impressions ‒ Severity score ≥ 4 (moderately ill) Comorbidity: All ADHD Withdrawn/dropouts: 2 withdrawn (1 active treatment, 1 placebo) Other interventions
| |
| Interventions | Intended dose: 0.5 mg/day at bedtime individually regulated until optimum efficacy. Max 2 mg a day Mean end dose: 1.08 mg/day | |
| Outcomes | Primary outcomes: Children's Aggression Scale ‒ Parents, Children's Aggression Scale ‒ Teachers Secondary outcomes: Conners' Parent Rating Scale, Conners' Teacher Rating Scale, Clinical Global Impression ‒ Improvement, Clinical Global Impression ‒ Severity Follow‐up interval: 28 days | |
| Notes | Imputation method for incomplete data: "data from last known prior session were used for subsequent missing time points" (LOCF) Funding/support: "This study was supported by Janssen Pharmaceuticals" (p 558). Declaration: Dr Armenteros has received research support and is on the speakers' panel of Janssen Pharmaceuticals. The other authors have no financial relationships to disclose (p 564). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assignment of subjects to treatment groups was carried out by following a table of random permutations, which balanced the number of subjects in each group. The research staff was not informed of the length of the permutations." (p 560). |
| Allocation concealment (selection bias) | Unclear risk | No stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "All of the subjects and clinical and research staff were blind to treatment condition" (p 560). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 1 participant (8%) in the risperidone group dropped out of the study after 1 week of treatment and 1 participant (8%) in the placebo group also dropped out of the study after 2 weeks of treatment. In both cases, participants failed to comply with treatment regulations. The rest of the sample completed the 28 days of treatment as per protocol (p 561). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. Possible reporting bias as dichotomous results were presented while no differences in mean scores were detected. |
| Other bias | High risk | Small number of participants therefore a limited power to detect differences. |
| Methods | Double‐blind, randomised controlled trial of risperidone and placebo | |
| Participants | Setting: inpatients (2 centres) Sample size: 38; 19 active treatment, 19 placebo Sex: 33 males, 5 females Age range: 12 to 18 years Mean age: active treatment 14 (SD = 1.5) years; placebo 13.7 (SD = 2.0) years IQ range: 60 to 90 Inclusion criteria: persistent overt aggressive behaviour as evidenced by ≥ 1 on Overt Agression Scale ‒ Modified; failure of behavioural treatment. Participants were included if "their aggressive behavior failed to respond to behavioral treatment approaches (typically, these behavioral treatments involve contingency management and social skills training delivered on an individual basis for at least 2 months)." (p 240) Diagnosis: DSM‐IV criteria conduct disorder, oppositional defiant disorder Comorbidity: ADHD (14 active treatment, 12 placebo) Withdrawn/dropouts: 2 withdrawals (2 placebo) | |
| Interventions | Intended dose: from 0.5 mg twice daily increased by 1 mg up to 5 mg. As fixed as possible, could be adjusted down if adverse event present. Mean end dose: 63% on 3 mg a day, mean 2.9 mg (1.5 to 4 mg) | |
| Outcomes | Primary outcomes: Clinical Global Impression ‒ Severity scale Secondary outcomes: Overt Aggression Scale ‒ Modified, Aberrant Behaviour Checklist (all scales nurse and teacher‐rated) Follow‐up interval: 6 weeks | |
| Notes | Imputation method for incomplete data: last observation carry forward (LOCF) (p 242) Funding/support: "Supported by Janssen‐Cilag, BV, Tilburg, the Netherlands" (p 239) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code had been generated by computer in block of four numbers" (p 241). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Dosage was adjusted by the responsible psychiatrist who was blind to the treatment" (p 241). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | "Two participants (11%) in the placebo group stopped treatment during the double‐blind period because of lack of therapeutic effects and uncontrollable aggressive behavior" (p 242). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | No reason given why 145 approached and 49 found to be eligible. "Greater severity of psychosocial stressors in the risperidone group" (p 242). |
| Methods | Double‐blind randomised controlled trial of oral quetiapine and placebo | |
| Participants | Setting: single site, outpatients Sample size: 19; 9 active treatment, 10 placebo Sex: 74% male Age range: 12 to 17 years Mean age: 14.1 (1.6) years IQ range: excluded significantly sub‐average IQ, assessed by the clinician, based on school history Inclusion criteria: primary psychiatric diagnosis of conduct disorder, moderate to severe aggressive behaviour as evidenced by Overt Aggression Scale score ≥ 25, Clinical Global Impressions ‒ Severity score ≥ 4 Comorbidity: ADHD in active 8 and control 7 Withdrawn/dropouts: 8 withdrawals (1 active treatment, 7 placebo) Other interventions: current psychosocial therapies were allowed in the protocol as long as therapy was not changed during the study | |
| Interventions | Intended dose: 25 mg twice daily, by day 14 at least 200 mg, after day 14 up to 800 mg at the discretion of a clinician Mean dose at endpoint: 294 (± 78) mg/day, range 200 to 600 mg/day, average weight adjusted 4.5 (± 2.5) mg/kg per day | |
| Outcomes | Primary outcomes: Clinical Global Impression ‒ Severity, Clinical Global Impression ‐ Improvement scales Secondary outcomes: Overt Aggression Scale ‒ parent‐rated, Conners' Parent Rating Scale ‒ conduct problem subscale, Quality of Life Enjoyment and Satisfaction Questionnaire Follow‐up interval: 6 weeks | |
| Notes | Imputation method for incomplete data: used mixed‐effect longitudinal analysis (p 146) Funding/support: this study was supported by an Investigator Initiated Grant from Astra Zeneca Pharmaceuticals (p 153). Dr Connor and McLaughlin analysed all the data and completed all the writing of this submission (p 153). First author is a consultant for Shire Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "Study medication was blinded and encapsulated by placing whole tablets into identical‐looking tablets by institutional research pharmacist" (p 144). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | 1 participant (11%) withdrew from the medication (quetiapine) group due to side effects. 5 participants withdrew from the placebo group due to lack of efficacy and 2 withdrew due to protocol violation (70%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | 1‐week single‐blind placebo to start with. Small sample size and therefore limited power to detect differences. |
| Methods | Randomised double‐blind control trial of risperidone and placebo | |
| Participants | Setting: outpatients, 1 centre, inner city academic outpatient medical centre Sample size: 20; 10 active treatment, 10 placebo Sex: 19 male, 1 female Age range: 5 to 15 years Mean age: 9.2 (2.9) years (range 6 to 14 years) IQ range: IQ more than 70 Diagnosis: DSM‐IV of conduct disorder Inclusion criteria: Clinical Global Impression ‒ Severity score moderate severity; Child Behaviour Checklist aggression subscale T‐score 2 SD or more above mean for age and gender‐matched peers Comorbidity: moderate to severe ADHD excluded Withdrawn/dropouts: 11 withdrew (4 active treatment, 7 placebo) Other Interventions: psychosocial interventions not mentioned | |
| Interventions | Intended dose: for under 50 kg, 0.25 mg increasing to 1.5 mg; and for over 50 kg, 0.5 mg increasing to 3 mg Mean dose at endpoint: 0.028 (± 0.004) mg/kg per day (range 0.75 to 1.50 mg/day) | |
| Outcomes | Primary outcomes: Rating of Aggression Against People and Property Scale Secondary outcomes: Conners' Parent Rating Scale ‒ conduct problem subscale, Child Behaviour Checklist, Clinical Global Impression ‐ Severity, Clinical Global Impression ‐ Improvement Follow‐up interval: 10 weeks | |
| Notes | Imputation method for incomplete data: unclear from the published study Funding/support
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number list. The list was kept in the Center for Drug Research and not was not accessible to either PI or other study raters. |
| Allocation concealment (selection bias) | Low risk | A random number list. The list was kept in the Center for Drug Research and not was not accessible to either PI or other study raters. |
| Blinding of participants and personnel (performance bias) | Low risk | Risperidone and placebo matched in appearance. The blind was not broken during the course of the trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Of the 10 youths assigned to risperidone, 6 completed the entire study (40% attrition): "3 youths who were assigned to receive risperidone were withdrawn by their guardian because of lack of effect, and 1 youth who received risperidone was withdrawn from the study during week 4 because of the development of a rash" (p 511). Only 3 youths who received placebo finished the trial (70% attrition): "4 patients assigned to placebo were withdrawn from the protocol by their guardians because of lack of benefit, 2 more were withdrawn from the study by the PI because of non‐compliance with study procedures, and 1 youth randomly assigned to placebo was lost to follow‐up" (p 511). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | Small sample size and therefore limited power to detect differences. |
| Methods | Randomised double‐blind control trial of ziprasidone and placebo | |
| Participants | Setting: outpatients; single site Sample size: 50; 25 active treatment and 25 placebo Sex: not reported Age range: 7 to 17 years Mean age: active treatment 11.5 (± 2.4) years, placebo 12.2 (±2.5) years IQ range: no average IQ reported; IQ greater than 55 Inclusion criteria: primary diagnosis of conduct disorder or oppositional defiant disorder or disruptive disorder not otherwise specified; score of > 21 on Nisonger Child Behavior Rating Form Typical IQ Version scales for conduct and oppositional behaviour disorders at screening Comorbidity: no specific report on the assessment of comorbid ADHD. Withdrawn/dropouts: 22 (44%) participants completed the study; 12 in the active arm and 10 in the placebo arm Other interventions: psychosocial interventions not mentioned | |
| Interventions | Intended dose: oral suspension of ziprasidone (Zeldox) vs placebo. Patients with a body weight ≤ 50 kg received an initial oral course starting with 5 mg/d Ziprasidone or placebo titrated over a course of 3 weeks to a maximum daily dose of 20 mg. Patients with a body weight > 50 kg received 10 mg/d Ziprasidone or placebo, which was titrated over 3 weeks to a maximum of 40 mg/day. The total dose was split into 2 half doses for administration twice a day (morning and evening) Mean dose at endpoint: none reported | |
| Outcomes | Primary outcome: Nisonger Child Behaviour Rating Form ‒ Typical IQ Version D‐Total (conduct disorder and oppositional defiant disorder subscales) rated by the participant’s primary caregiver. Secondary outcomes: included Clinical Global Impressions ‐ Severity of Illness and the Clinical Global Impressions‐Improvement scales. Follow up interval: the study consisted of a 3‐week baseline period for finding the best individual dose followed by a 6‐week treatment period and 2‐week washout period. | |
| Notes | The authors of this paper queried whether the chosen dosage level was too low. Funding/support: the study medication and the trial were financially supported by a pharmaceutical company (it was an investigator‐initiated trial). Christian Fleischhaker received grants from government (Federal Ministry of Education and Research [BMBF]) and by Bristol‐Myers‐Squibb, Novartis, Shire, Otsuka and Pfizer. Eberhard Schulz received grants from government (Federal Ministry of Education and Research [BMBF]) and by Janssen‐Cilag, Eli Lilly, Novartis, Shire und Pfizer (as described in the Conflict of interest section) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment assignments were made in accordance with the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | Authors reported that 22/50 participants (44%) completed the study but the reasons for withdrawal or dropout were not stated for 16 of those who had not completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. A number of factual errors noted in the AACAP abstract and conference poster provided to the reviewers via correspondence (Stasiak 2015 [pers comm]). |
| Other bias | High risk | Unpublished data and thus not peer reviewed. Small sample size and no power calculation described, thus the study may have been underpowered. Quality of reporting consistent with a poster presentation but not to the level of a peer‐review journal article. |
| Methods | Randomised double‐blind controlled trial of risperidone and placebo | |
| Participants | Setting: international, multicentre, 3‐stage; outpatients Sample size: 527 (acute, 6 weeks, open label); 436 (continuation, 6 weeks, single blind, risperidone), 335 (maintenance, 6 months, double‐blind, RCT). N = 335 (for the 6‐month, double blind, maintenance treatment); 172 active treatment, 163 placebo Sex: 290 male, 45 female Age range: 5 to 17 years Mean age: risperdone 10.9 (SD 2.93) years; placebo 10.8 (SD 2.94) years IQ range: 216 with IQ > 84; 119 with IQ < 84 Inclusion criteria: Nisonger Child Behaviour Rating Form score > 24 Diagnosis: DSM‐IV for conduct disorder, oppositional defiant disorder or disruptive behaviour disorder not otherwise specified Comorbidity: 227 with comorbid ADHD (overall, 24% treated with concomitant stimulant, p 409) Withdrawn/dropouts: 162 completed treatment; 124 experienced symptom recurrence; 49 discontinued (out of these, 8 experienced an adverse event) Other interventions: pre‐existing or comorbid psychosocial interventions not mentioned | |
| Interventions | Oral risperidone solution Intended dose: 1 mg/ml oral solution once or twice daily, same dose as in continuation phase, max < 50 kg = 0.75 mg or > 50 kg = 1.5 mg Mean dose at endpoint: < 50 kg = 0.81 mg (0.34 mg), > 50 kg = 1.22 mg (0.36 mg) | |
| Outcomes | Primary outcomes: time to symptom recurrence, deterioration of ≥ 2 points on Clinical Global Impression ‒ Improvement score or 7 points on conduct problem subscale Secondary outcomes: rate of discontinuation, Nisonger Children's Behaviour Rating Form, Clinical Global Impression ‒ Severity, visual analogue scale of the most troublesome symptoms, Children's Global Assessment Scale Follow‐up interval: 6 months | |
| Notes | Imputation method for incomplete data: LOCF (page 405) Funding/support: this study was supported by Johnson & Johnson R&D (p 410). The first author's correspondence address is at J & J Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was generated by the study sponsor" (p 403‐4). |
| Allocation concealment (selection bias) | Low risk | "...treatment numbers allocated at each investigative centre in chronological order" (p 404). |
| Blinding of participants and personnel (performance bias) | Low risk | "Placebo and risperidone oral solutions were identical in appearance and flavour" (p 404). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | In the risperidone group, 24 discontinued treatment and that included 4 participants who experienced an adverse event. In the placebo group, 25 discontinued and that included 4 participants who had experienced an adverse event. The reasons for discontinuations (besides those stopping due to adverse events) were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | Unclear risk | "...only patients who responded to initial treatment were randomized, potentially introducing a selection bias. This was, in part, addressed by including a single‐blind period prior to double‐blind randomization..." (p 409). |
| Methods | Randomised double‐blind controlled trial of risperidone and placebo | |
| Participants | Setting: outpatients; multicentre (10 sites in Canada, 4 in USA and 2 in South Africa) Sample size: 110; 53 active treatment, 57 placebo Sex: 83 male, 27 female Age range: 5 to 12 years IQ range: 36 to 84 (n = 53 borderline, n = 42 mild, n = 15 moderate ID) Inclusion criteria: Nisonger Childrens Behaviour Rating Form ‒ parent version > 24 Diagnosis: DSM‐IV for conduct disorder, oppositional defiant disorder, or disruptive behaviour disorder not otherwise specified Comorbidity: ADHD 80% (n = 84), 45 were treated Withdrawn/dropouts: 25 withdrawals (6 risperidone, 19 placebo) Other interventions: psychosocial intervention was not mentioned but its design was stated to be identical to Aman 2002; behavioural therapy was permitted if it was initiated at least 30 days before the start of the study; no changes to behavioural therapy were allowed during the trial. | |
| Interventions | Intended dose: max 0.06 mg/kg in the morning Mean dose at endpoint: 0.98 mg/day (SE = 0.06), which equalled 0.033 mg/kg (SE = 0.001) range 0.40 to 3.80 mg/day | |
| Outcomes | Primary outcome: Nisonger Childrens Behaviour Rating Form ‒ Conduct Disorder subscale Secondary outcomes: Aberrant Behaviour Checklist, Behaviour Problem Inventory, Clinician Global Impression ‒ Improvement, visual analogue scale symptom (parent‐rated) Follow‐up interval: 6 weeks | |
| Notes | Imputation method for incomplete data: "last post randomisation assessments were used in endpoint analysis" (LOCF) Funding/support: Janssen Research Foundation provided randomisation and training, company central co‐ordination | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Janssen Research Foundation prepared the randomization list". (p 1029) |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Appearance and taste of solutions were identical. All trial medications were labelled with the protocol number, medication number, lot number and strata. A tear‐off label was provided on each box of study medication, which contained the medication code. The label was placed in the Case Report Form on the appropriate page. The code should only be broken in case of an emergency (Loy 2011b [pers comm]). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | 6 (11.3%) participants dropped out of the risperidone group and 19 (33%) dropped out from the placebo group. Reasons for discontinuance included: (1) insufficient response (2 from risperidone group and 19 from placebo group); (2) loss to follow‐up (1 from risperidone group); and (3) loss of parental consent (3 from risperidone group). Discrepancy in dropouts (n = 6) between the table data and the narrative and graph. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | 1‐week placebo run‐in. 2 sites in South Africa ‒ unclear if followed the same protocol. |
| Methods | 2‐stage 9 week parallel group, double‐blind, randomised controlled trial of risperidone ('augmented' = active treatment) and placebo ('basic' = placebo) added to parent training and stimulant. Stage 1: 3 weeks, open‐label stimulant and parent training; Stage 2: 6 weeks of a double‐blinded, placebo‐controlled comparison of added risperidone versus placebo. | |
| Participants | Setting: outpatients, multicentre (University clinics) Sample size: 168; 84 active treatment, 84 placebo Sex: 129 boys (77%) and 39 girls; 65 boys and 19 girls in the active arm and 64 boys and 20 girls in the placebo arm Age range: 6 to 12 years Mean age: active treatment 9.03 (SD = 2.05) years; placebo 8.75 (SD = 1.98) years IQ range: not reported. Mean IQ 97.1 (SD = 14.1) Inclusion criteria: DSM‐IV disruptive behaviour disorder diagnosis (conduct disorder or oppositional defiant disorder); DSM‐IV diagnosis of ADHD (any subtype); evidence of serious physical aggression as rated on the Overt Aggression Scale–M (score greater or equal to 3 on assaults against other people, objects, or self); and evidence of seriously disruptive behaviour as determined by a parent or guardian rating of at least 27 (90th percentile) on the Nisonger Child Behaviour Rating Form ‒ Typical IQ Version D‐Total (Conduct Disorder and Oppositional Defiant Disorder subscales combined). In addition, a Clinical Global Impressions ‒ Severity scale score of at least 4 (“moderately ill” or higher) for aggression was required by blinded clinicians. Participants needed to be free of psychotropic medicines for 2 weeks for most drugs (such as most antidepressants, a‐agonists, b‐blockers, anxiolytics, mood stabilizers, oral antipsychotics, and antihistamines) and 4 weeks for depot antipsychotics or fluoxetine. This rule was occasionally relaxed (to as few as 3 to 7 days) for extreme cases who could not tolerate being unmedicated the full time, as approved by the cross‐site steering committee. Diagnosis: 124 (74%) oppositional defiant disorder; 44 (265) conduct disorder Comorbidity: All have ADHD Sample characteristics: 53% White European ancestry; living with working parents (52% mothers, 53% fathers); some college education (66% mothers; 35% fathers); family incomes of USD 40,000 or less a year (57%) Withdrawn/dropouts: 22 participants dropped out before active treatment was introduced or because they were deemed not to need it (i.e. they were clinical responders to methylphenidate alone). In the active arm, 11 participants dropped out in the first 3 weeks (5 were clinical responders to methylphenidate alone) and in the placebo arm 3 dropped out in the first 3 weeks (3 were clinical responders to methylphenidate alone). Other Interventions:
| |
| Interventions | Intended dose: if residual symptoms remained, then randomised placebo or risperidone was added to treatment at weeks 4 through 6. For children weighing less than 25 kg, risperidone was dosed at 0.5 to 2.5 mg/day; for children heavier than 25 kg, dosing ranged from 0.5 to 3.5 mg/day. The risperidone titration schemes allowed for dose increases every 3 to 7 days, following a schedule that specified maximum dose increases over 29 days of titration; doses could always be held constant or decreased if a satisfactory clinical response occurred or if indicated by adverse event. Mean dose at endpoint: at week 9 (endpoint), in the active group the mean risperidone dose was 1.7 (± 0.75) mg/day and in the placebo group it was 1.9 (± 0.72) mg/day. | |
| Outcomes | Primary outcomes: Nisonger Child Behavior Rating Form ‐ Typical IQ version D‐Total Secondary outcomes: Positive Social, Overly sensitive ADHD, Withdrawn‐dysphoric subscales of the Nisonger Child Behavior Rating Form, Antisocial Behavior Scale consisting of Proactive and Reactive subscales; Clinical Global Impression ‒ Improvement and Clinical Global Impression ‒ Severity scales Follow‐up interval: 9 weeks (risperidone and placebo were introduced in week 4) | |
| Notes | Funding/support: the trial was funded by National Institute of Mental Health (NIMH). From correspondence (Loy 2016a [pers comm]), the authors originally requested a pharmaceutical company to sponsor the study medication. Due to lack of agreement over certain issues, this fell through except for 1 trial site. Subsequently, the authors obtained funding from the NIMH to purchase the study medications (both stimulant and risperidone and placebo) from an independent pharmacy. Only 1 study site, from which approximately 50 participants were recruited, received medication sponsored by the pharmaceutical company. It is possible that the centre used pharmaceutical‐supplied medication for 1 or 2 participants only. There were 168 participants in the trial. The lead author's view was that the pharmaceutical involvement was virtually nonexistent. Disclosures: the lead author has "received research contracts, consulted with, or served on advisory boards" of various pharmaceutical companies as listed on p 59. Full disclosure for other co‐authors is available on p 59. Long‐term outcomes to be published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation was used: blocks of size 2 were allocated to each stratum formed by the cross‐classification of the levels of the stratifying factors: site and DSM‐IV diagnosis (conduct disorder versus no conduct disorder) (Loy 2016b [pers comm]). |
| Allocation concealment (selection bias) | Low risk | Randomisation assignment was completed through a secured website. The unblinded medication dispenser entered the appropriate information into the website and an email with the participant’s treatment assignment was sent to the dispenser and to the statistician at the time. Randomisation assignment for each participant was printed and sealed in an envelope for study emergency only (Loy 2016b [pers comm]). |
| Blinding of participants and personnel (performance bias) | Low risk | Each child was followed by a primary clinician and a blinded clinician. Risperidone and placebo were absolutely identical. Different colour capsules were used to signify different doses (Loy 2016b [pers comm]). No details published about the details of the blinding procedure of the participants (children and parents) (Loy 2016b [pers comm]). |
| Blinding of outcome assessment (detection bias) | Low risk | Each child was followed by a primary clinician and a blinded clinician. The primary clinician assessed the participants for adverse events and titrated dosage, whereas the blinded clinician assessed the children for clinical improvement (i.e. was responsible for monitoring therapeutic effects on Nisonger Child Behaviour Rating Form ‒ Typical IQ Version, Clinical Global Impression‐Improvement (CGI‐Improvement), Clinical Global Impression‐Severity (CGI‐Severity). Parents and participating children were told not to discuss side effects or (when the blind was broken) medication assignment with the blinded clinicians. Blinded clinicians were barred from asking about side effects, appetite, sleep patterns, or seeing any of the completed Adverse Effect forms. There was a Medication Knowledge Form to determine both the parents' and blinded clinicians' knowledge regarding the identity of risperidone recipients. Blinded clinicians were never unblinded until the entire study was completed and all study data were locked (Loy 2016b [pers comm]). |
| Incomplete outcome data (attrition bias) | Low risk | Detailed reasons for participant attrition are displayed in table S1 and S2 (p 60 e1). |
| Selective reporting (reporting bias) | Unclear risk | Published results are based on completers‐only sample (i.e. no imputation of missing data was carried out in the published manuscript). Sensitivity analysis was said to be conducted (Loy 2016b [pers comm]) but not published or available. The reported outcome measures are consistent with those listed on the Clinical Trials registry (NCT00796302). |
| Other bias | Low risk | Completers analysis published only. |
| Methods | Double‐blind randomised controlled trial of risperidone and placebo | |
| Participants | Setting: residential care Sample size: 13; 6 active treatment, 7 placebo Sex: 5 males and 8 females Age range: 6 to 18 years Mean age: active treatment 10.5 (range 6 to 14) years; placebo 11 (range 7 to 14) years IQ range: 45 to 85 Inclusion criteria: "Persistent behavioural disturbance" (hostility, aggressive behaviour, irritability, agitation, hyperactivity) symptoms. Primary psychiatric diagnoses were not specified. Comorbidity: ADHD comorbidity not reported other that 1 participant in placebo group was on ritalin but this was discontinued during the trial; and 1 (in the active group) received concurrent antiepileptic (valproate) Withdrawn/dropouts: no withdrawals Other interventions: no description of pre‐existing or comorbid psychosocial interventions | |
| Interventions | Intended dose: once daily, evenings, week 1 0.01 to 0.04 mg/kg/day, week 2 to 4 flexible dosing Mean dose at endpoint: 0.05 mg/kg (range 0.03 to 0.06 mg/kg or 1.2 mg/day) | |
| Outcomes | Primary outcome: no pre‐specified primary endpoint (from email correspondence) Secondary outcomes: Aberrant Behaviour Checklist, Personal Assessment Checklist, Clinical Global Impression, visual analogue scale for the most disturbing symptom Follow‐up interval: 4 weeks | |
| Notes | Imputation method for incomplete data: LOCF (page 7) Funding/support: "Support for this work was received from Janssen Pharmaceutica, Berchem, Belgium" (p 5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | "All patients completed the study" (p 7). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | Pilot study, limited by a small sample size and thus limited power to detect differences. No SDs or SEs reported. |
AACAP: American Academy of Child and Adolescent Psychiatry; ADHD: attention deficit hyperactivity disorder; DSM IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; IQ: intelligence quotient; LOCF: last observation carried forward; PI: principal investigator; RCT: randomised controlled trial; SD: standard deviation; SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Divalproex is considered to be a mood stabiliser. Ineligible intervention. | |
| Open titration and optimisation of stimulant monotherapy in 160 children. Ineligible study design (not randomised) and ineligible intervention. | |
| Open‐label study of risperidone treatment in 26 hospitalised children and youths with borderline or sub‐average IQs with mixed diagnoses and aggressive behaviour. Ineligible study design (not randomised). | |
| 1‐year, multi‐site, open‐label study looking at safety and effectiveness of risperidone in 504 children and youths aged 5 to 14 with disruptive behaviour disorders and below average IQs. Ineligible study design (not randomised). | |
| 48‐week open‐label study of risperidone in 107 children aged 5 to 12 with severe disruptive behaviour disorders and below average IQs. Ineligible study design (not randomised). | |
| 8‐week pilot, open‐label, outpatient trial of quetiapine in 17 aggressive children with conduct disorder aged 6 to 12 years old. Ineligible study design (not randomised). | |
| 1‐year, open‐label, safety extension study in 232 children with disruptive behaviour disorders treated with risperidone. Ineligible study design (not randomised). | |
| Open‐label trial of olanzapine in 16 youths with sub‐average IQ and disruptive behaviour disorders. Ineligible study design (not randomised). | |
| Open‐label atomoxetine and olanzapine in ADHD with comorbid disruptive behaviour disorder in children and adolescents. Ineligible study design (not randomised). | |
| Study on hold since 2013. Relapse prevention in children and adolescents with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Conduct Disorder treated with risperidone: a randomised double‐blind, placebo‐controlled, discontinuation study (www.isrctn.com/ISRCTN95609637). Discontinued study/no data available. | |
| Open‐label trial of aripiprazole in the treatment of conduct disorders in adolescents. Ineligible study design (not randomised). | |
| A retrospective chart review of olanzapine treatment in adolescents with conduct disorder. Ineligible study design (not randomised). | |
| Treatment of Children With ADHD Who do Not Fully Respond to Stimulants (TREAT). Active comparator (also called the "combination arm") consists of parent training plus continued treatment on a stimulant, plus augmentation with aripiprazole. Placebo comparator (also called the "simple treatment" arm) will consist of parent training plus continued treatment on a stimulant plus a placebo matching aripiprazole. Study was terminated due to slow rate of recruitment. Discontinued study/no data available. ClinicalTrials.gov Identifier: NCT00279409. | |
| An open‐label study of quetiapine added to OROS methylphenidate in the treatment of ADHD and aggressive behaviour. Ineligible study design (not randomised). | |
| Open‐label study over a cumulative period of 3 years, looking at safety and tolerability of risperidone in 35 children with disruptive behaviour disorders and borderline and sub‐average IQs. Ineligible study design (not randomised). | |
| Ineligible study design (single‐blind design). | |
| A clinical case series of 6 extremely aggressive youths treated with olanzapine. Ineligible study design (not randomised). | |
| Open, naturalistic study of clozapine in 7 boys with severe conduct disorder over 26 weeks. Ineligible study design (not randomised). | |
| Response to treatment with aripiprazole in children and adolescents with bipolar disorder and comorbid ADHD. Ineligible study design (not randomised). | |
| 48‐week open‐label study of risperidone for the treatment of disruptive behaviour disorders in 77 children with sub‐average IQs. Ineligible study design (not randomised). | |
| RCT in adults of risperidone, haloperidol and placebo in the treatment of aggressive challenging behaviour in adult patients with intellectual disability. Ineligible population (adults). |
ADHD: attention deficit hyperactivity disorder.
IQ: intelligence quotient.
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double blinded, randomised controlled trial, interventional study |
| Participants | Children aged 6 to 12 years with ADHD, male and female |
| Interventions | Active treatment: methylphenidate with maximum dose of 30 mg per day and risperidone with maximum dose of 1 mg per day for 8 weeks Comparator: methylphenidate with maximum dose of 30 mg per day and placebo for 8 weeks |
| Outcomes | Improvement in ADHD symptoms as assessed using the Clinical Global Impression (CGI) scale and Conners' Parents Rating Scale (CDRS) for ADHD. |
| Notes | IRCT identifier: IRCT201211051743N10: A double‐blind, placebo‐controlled study on the efficacy of risperidone adjunctive to methylphenidate in patients with ADHD. Limitation is that this is an Iranian study and whether it will be published in English or Persian. It may not measure the outcomes relevant to the review, which are aggression and conduct problems. |
| Methods | Randomised controlled trial, open label |
| Participants | Children and adolescents with attention deficit/hyperactivity disorder, oppositional defiant disorder or conduct disorder |
| Interventions | Drug 1: methylphenidate Drug 2: risperidone |
| Outcomes | Primary outcome measure: change from baseline of aggressive behaviours. The Retrospective Modified Overt Aggression Scale (R‐MOAS) will be used for the assessment of aggressive behaviours and their response to treatment. Time frame: baseline, 2 weeks, 4 weeks, 8 weeks |
| Notes | ClinicalTrials.gov identifier: NCT02063945: Methylphenidate vs. Risperidone for the Treatment of Children and Adolescents With ADHD and Disruptive Disorders. Open label rather than double blinded according to the ClinicalTrials.gov web site. Likely to end up as an excluded trial. (clinicaltrials.gov/ct2/show/NCT02063945) |
ADHD: attention deficit hyperactivity disorder.
IQ: intelligence quotient.
IRCT: Iranian Registry of Clinical Trials.
vs: versus.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effectiveness of Combined Medication Treatment for Aggression in Children With Attention Deficit With Hyperactivity Disorder (The SPICY Study) |
| Methods | Intervention; double‐blinded randomised controlled trial |
| Participants | Children aged 6 to 12 years with attention deficit disorder with hyperactivity, male and female |
| Interventions | Drug 1: valproate Drug 2: risperidone Drug 3: placebo Drug 4: stimulant medication Behavioral: behavioural family counselling |
| Outcomes | Primary outcome: aggressive behaviour Secondary outcome: ADHD symptoms Time frame: measured weekly for 11 to 16 weeks |
| Starting date | November 2008 Estimated completion date: April 2013 |
| Contact information | Name: Joseph Blader Email: [email protected] Address: University of Texas, Northwell Health |
| Notes | Purpose: this study will determine the advantages and disadvantages of adding 1 of 2 different types of drugs to stimulant treatment for reducing aggressive behaviour in children with ADHD. During phase 1, participants will receive a stimulant medication. If they do not respond to the stimulant, valproate and behavioural family counselling will be added to their treatment during phase 2. If they do not respond to valproate, they will be switched to risperidone. The recruitment status of this study is unknown because the information has not been verified recently |
ADHD: attention deficit hyperactivity disorder.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aggression: ABC irritability (mean change scores) Show forest plot | 3 | 238 | Mean Difference (IV, Random, 95% CI) | ‐6.49 [‐8.79, ‐4.19] |
| Analysis 1.1 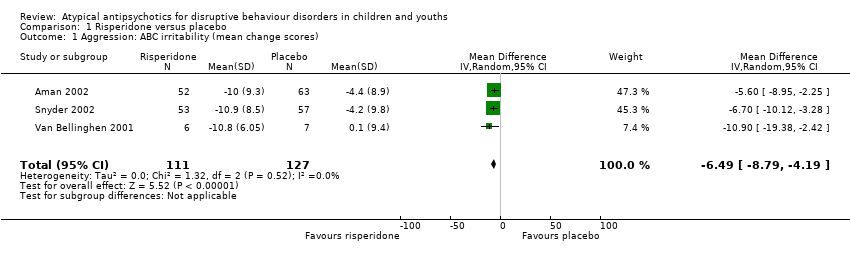 Comparison 1 Risperidone versus placebo, Outcome 1 Aggression: ABC irritability (mean change scores). | ||||
| 2 Aggression: OAS‐M, ABS Reactive subscale (final scores) Show forest plot | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.21, ‐0.40] |
| Analysis 1.2 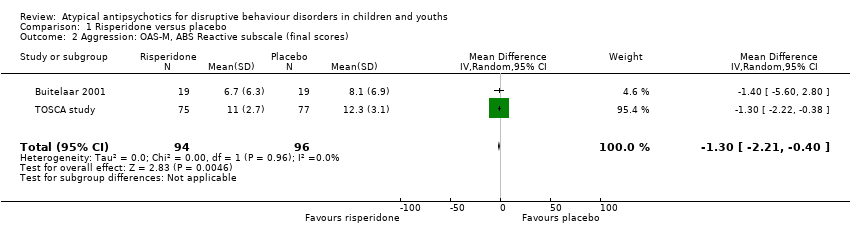 Comparison 1 Risperidone versus placebo, Outcome 2 Aggression: OAS‐M, ABS Reactive subscale (final scores). | ||||
| 3 Aggression: OAS‐M, ABS Proactive subscale (final scores) Show forest plot | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.30, 0.06] |
| Analysis 1.3  Comparison 1 Risperidone versus placebo, Outcome 3 Aggression: OAS‐M, ABS Proactive subscale (final scores). | ||||
| 4 Conduct: NCBR‐CP (mean change scores) Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐8.61 [‐11.49, ‐5.74] |
| Analysis 1.4 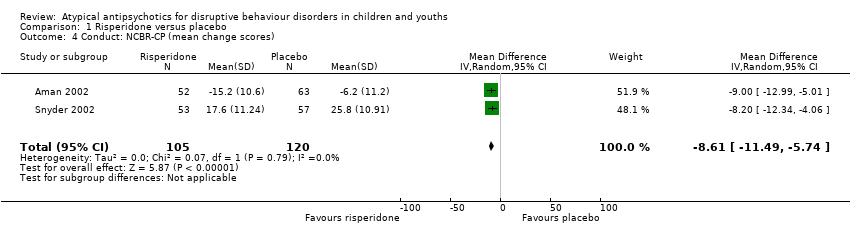 Comparison 1 Risperidone versus placebo, Outcome 4 Conduct: NCBR‐CP (mean change scores). | ||||
| 5 Weight gain (antipsychotic only): Kg (mean change scores) Show forest plot | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 2.37 [0.26, 4.49] |
| Analysis 1.5 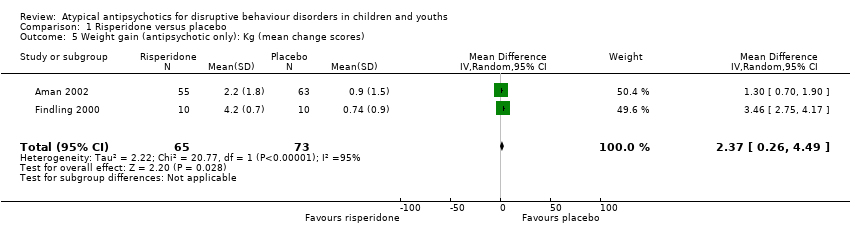 Comparison 1 Risperidone versus placebo, Outcome 5 Weight gain (antipsychotic only): Kg (mean change scores). | ||||
| 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores) Show forest plot | 3 | 305 | Mean Difference (IV, Random, 95% CI) | 2.14 [1.04, 3.23] |
| Analysis 1.6  Comparison 1 Risperidone versus placebo, Outcome 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores). | ||||
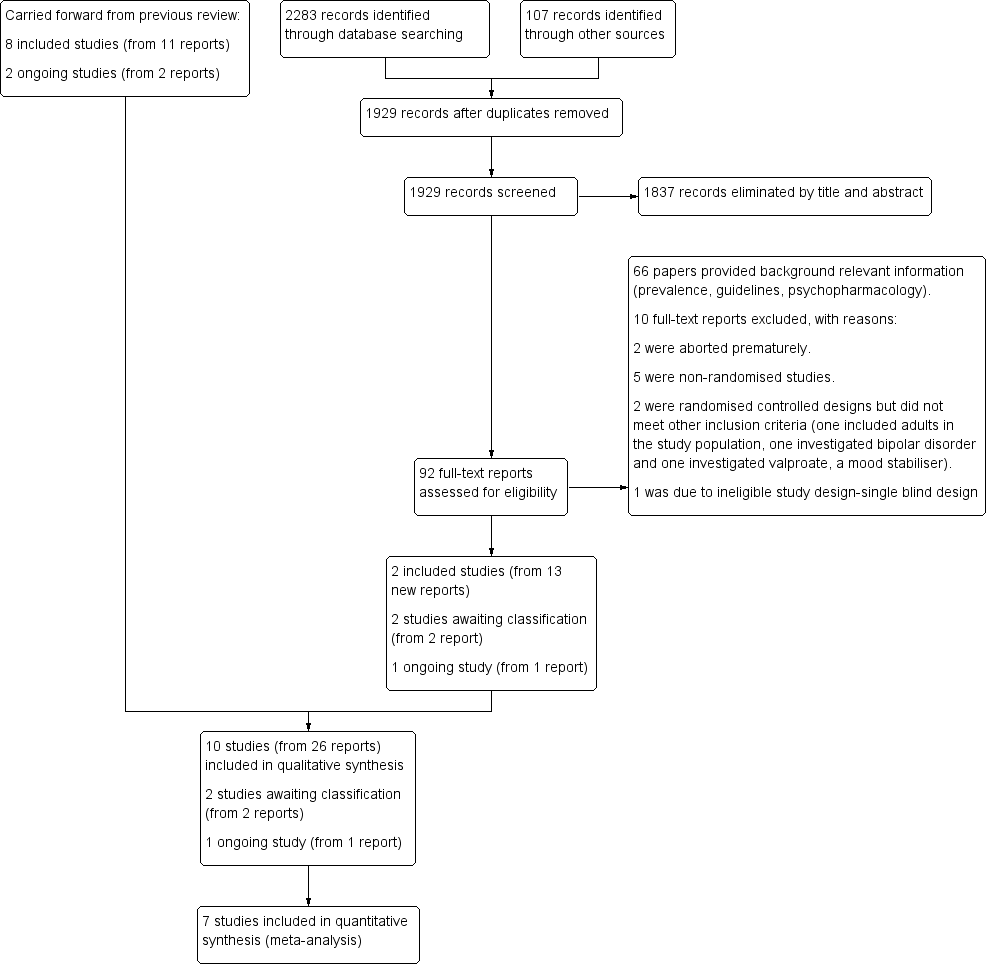
study flow diagram
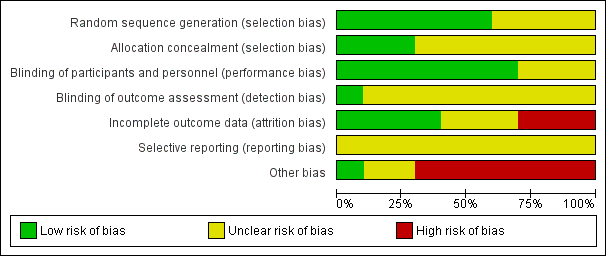
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
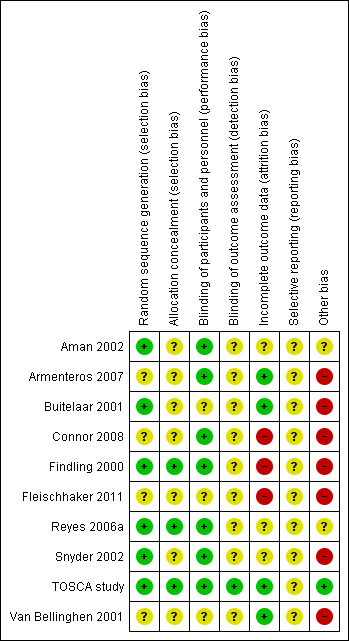
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
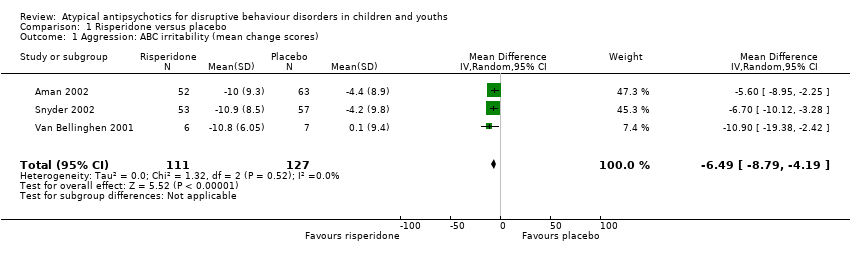
Comparison 1 Risperidone versus placebo, Outcome 1 Aggression: ABC irritability (mean change scores).

Comparison 1 Risperidone versus placebo, Outcome 2 Aggression: OAS‐M, ABS Reactive subscale (final scores).

Comparison 1 Risperidone versus placebo, Outcome 3 Aggression: OAS‐M, ABS Proactive subscale (final scores).

Comparison 1 Risperidone versus placebo, Outcome 4 Conduct: NCBR‐CP (mean change scores).

Comparison 1 Risperidone versus placebo, Outcome 5 Weight gain (antipsychotic only): Kg (mean change scores).

Comparison 1 Risperidone versus placebo, Outcome 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores).
| Risperidone compared to placebo for disruptive behaviours in children and youths | ||||||
| Patient or population: Disruptive behaviours in children and youths | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with risperidone | |||||
| Aggression | The mean aggression ABC‐I score ranged across control groups from −4.40 to 0.10 | The mean aggression ABC‐I score in the intervention groups was, on average, 6.49 lower (8.79 lower to 4.19 lower) | ‐ | 238 | ⊕⊕⊝⊝ | Included studies: Aman 2002; Snyder 2002; Van Bellinghen 2001 |
| Aggression | The mean aggression OAS‐M and ABS Proactive score ranged across control groups from 8.10 to 15.10 | The mean aggression OAS‐M and ABS Proactive score in the intervention groups was, on average, 1.12 lower (2.30 lower to 0.06 higher) | ‐ | 190 | ⊕⊕⊕⊝ | Included studies: Buitelaar 2001; TOSCA study |
| Conduct | The mean conduct score ranged across control groups from −6.20 to 25.80 | The mean conduct score in the intervention groups was, on average, 8.61 lower (11.49 lower to 5.74 lower) | ‐ | 225 | ⊕⊕⊕⊝ | Included studies: Aman 2002; Snyder 2002 |
| Weight gain (treatment with antipsychotic only) | The mean weight gain (treatment with antipsychotic only) score in the control groups ranged from 0.74 to 0.90 | The mean weight gain score in the intervention groups was, on average, 2.37 higher (0.26 higher to 4.49 higher) | ‐ | 138 | ⊕⊕⊕⊝ | Included studies: Aman 2002; Findling 2000 |
| Weight gain (treatment with antipsychotic and stimulant) | The mean weight gain (treatment with antipsychotic and stimulant) score in the control groups ranged from −1.20 to 0.90 | The mean weight gain score in the intervention groups was, on average, 2.14 higher (1.04 higher to 3.23 higher) | ‐ | 305 | ⊕⊕⊝⊝ | Included studies: Aman 2002; Findling 2000; TOSCA study |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABS: Antisocial Behavior Scale;CI: Confidence interval; MD: Mean difference;OAS: Overt Aggression Scale;OAS‐M: Overt Aggression Scale ‒ Modified; RCT: Randomised controlled trial; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 2 levels because of unclear risk of bias due to lack of information on selection bias and detection bias in 2 studies, and unclear risk of bias due to lack of information and poor reporting standards in 1 study. 2 trials assessed outpatients, 1 trial assessed patients in residential care. 2 Unclear allocation concealment and unclear blinding of outcome assessment for 1 study and potential reporting bias in both studies. 3 Downgraded 1 level because of unclear allocation concealment and unclear blinding of outcome assessment for both studies and unclear attrition and potential reporting bias. 4 Downgraded 1 level because of unclear blinding of outcome assessment and potential reporting bias. Heterogeneity: Tau² = 2.22; Chi² = 20.77, df = 1 (P < 0.00001); I² = 95%. 5 Downgraded 2 levels because of unclear blinding of outcome assessment in 2 studies, potential reporting bias in 3 studies, and potential attrition bias in 2 studies. Heterogeneity: Tau² = 0.85; Chi² = 23.32, df = 2 (P < 0.00001); I² = 91%. | ||||||
| Analysis | Method |
| Measures of treatment effect | For dichotomous data, we planned to analyse data on the intention‐to‐treat principle with dropouts included in the analysis. Out of the 10 studies, 1 used dichotomous outcomes (Armenteros 2007), therefore we were not able to perform further analyses. |
| Unit of analysis issues | For cross‐over trials, we planned to do paired analysis if data were presented. Otherwise, we planned to take all measurements from intervention periods and all measurements from control periods and analyse these as if the trial was a parallel‐group trial, acknowledging that there might be unit of analysis errors that could underestimate the precision of the estimate of the treatment effect (Deeks 2011). However, no cross‐over trials were identified. Also, there were no cluster‐randomised controlled trials, so we did not have to take this into account in our analyses. |
| Dealing with missing data ‒ missing participants | We intended to calculate the best‐ and worst‐case scenarios for the clinical response outcome, if possible. For example, the best‐case scenario assumed that dropouts in the intervention group had positive outcomes and those in the control group had negative outcomes. In the worst‐case scenario, dropouts in the intervention group had negative outcomes and those in the control group had positive outcomes. |
| Assessment of heterogeneity | Chapter 9 in the Cochrane Handbook recommends using a range for I² and a guide to interpretation (Deeks 2011). Had we found either moderate heterogeneity (I² in the range of 30% to 60%) or substantial heterogeneity (I² in the range of 50% to 90%), as specified in our protocol (Loy 2010), we planned to examine it using specified subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity and Sensitivity analysis). |
| Assessment of reporting bias | We intended to draw funnel plots (effect size versus standard error) to assess publication bias if sufficient studies were found. Asymmetry of the plots may indicate publication bias, although they may also represent a true relationship between trial size and effect size. If such a relationship were identified, we planned to examine the clinical diversity of the studies as a possible explanation (Egger 1997). There were insufficient studies in our meta‐analysis to perform a funnel plot. |
| Subgroup analysis and investigation of heterogeneity | It was our intention to conduct separate analyses on the following subgroups, where possible.
There were too few studies in any of the analyses for us to carry out any subgroup analyses. |
| Sensitivity analysis | We intended to perform sensitivity analyses to explore whether the results of the review were robust in relation to certain study characteristics. We intended to exclude trials with 'no' or 'unclear' ratings for allocation concealment and use the fixed‐effect model for our primary outcome. We identified a limited number of trials and we did not exclude any of them based on the ratings of allocation concealment. We were not able to carry out a sensitivity analysis due to the small number of trials. |
| ADHD: attention deficit hyperactivity disorder | |
| Name of rating scale | Description | Construction | Study | Source of Information used in the study |
| Aberrant Behaviour Checklist (ABC) (Aman 1985a; Aman 1985b) | Symptom checklist for assessing problem behaviours of children and adults with mental retardation. It is also used for classifying problem behaviours of children and adolescents with mental retardation. | 58 items, 5 scales.
| Parent/caregiver | |
| Child Behaviour Checklist (CBCL) (Achenbach 1991)
| Checklist for evaluating maladaptive behavioural and emotional problems. | 113 items, 8 subscales.
| Parent | |
| Overt Aggression Scale (OAS) (Yudofsky 1986) | Assesses the severity and frequency of overt aggression. | 25 items, 4 subscales.
Within each category, aggressive behaviour is rated according to its severity. | Parent | |
| Overt Aggression Scale ‒ Modified (OAS‐M) (Kay 1988) | Assesses the severity and frequency of overt aggression. | 20 items, 4 subscales.
5‐point interval scale that represents increasing level of aggression. The total aggression score is obtained by multiplying the 4 individual scales by weights of 1, 2, 3 or 4 and then summing the 4 weighted scores. | Nurse or teacher | |
| Rating of aggression against people and/or property scale (RAAP) (Kemph 1993) | ‐ | Global rating scale, 1 item. Scored from 1 (no aggression reported) to 5 (intolerable behaviour). | Clinician | |
| Children's Aggression Scale ‒ Parent (CAS‐P; Halperin 2002) and Teacher (CAS‐T; Halperin 2003) | Retrospectively measures the frequency and severity of 4 categories of aggression: verbal aggression; aggression against objects and animals; provoked physical aggression; and initiated physical aggression | Respondents (parents/guardians and teachers) complete a Likert scale to evaluate the frequency of an act. The frequency of aggressive events is multiplied by its designated severity weight factor and then summed to yield a total score. | Parent and teacher | |
| Antisocial Behavior Scale (ABS) Proactive and Reactive Subscales (Brown 1996) | Instrument used to differentiate reactive/affective from proactive subtypes of aggression | 28 items. Proactive Aggression subscale: 5 proactive items and 5 covert antisocial items. Reactive Aggression subscale: 6 items. | Parent |
| Name of rating scale | Description | Construction | Study | Source of information used in the study |
| Conners' Parent Rating Scale (CPRS) (Conners 1989) | Checklist for assessing behavioural and emotional difficulties. | 48 items, 6 subscales.
| Parent | |
| Nisonger Child Behaviour Rating Form (NCBRF) (Aman 1996; Tassé 1996) | Assesses behaviour of children and adolescents with intellectual disability or autism spectrum disorders, or both. | 76 items, 8 subscales.
| Parent | |
| Nisonger Child Behavior Rating Form ‒ Typical IQ D‐Total (includes conduct problems and oppositional subscales) | Typical IQ version: assesses behaviour of children and adolescents with normal IQ. | 10 items, 1 prosocial subscale.
54 items, 6 problem behaviour subscales.
| Parent | |
| IQ: intelligence quotient. | ||||
| Study ID | General | Neurological | Gastrointestinal | Respiratory | Cardiovascular/Metabolic | Serious adverse event (unspecified) | Other |
| (risperidone = 12, placebo = 13) |
|
|
| ‐ | Not reported | ‐ | ‐ |
| (risperidone = 19, placebo = 19) |
|
|
|
| Not reported | ‐ | ‐ |
| (quetiapine = 9, placebo = 10) |
|
| ‐ | ‐ | No differences across groups found on ECG QRS or QTc intervals. | ‐ | ‐ |
| (risperidone = 10, placebo = 10) |
| ‐ |
| ‐ | No clinically significant changes in ECG. | ‐ |
|
| (risperidone = 6, placebo = 7) | No side effects reported in any category. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| (risperidone = 55, placebo = 63) |
|
|
|
|
| ‐ | ‐ |
| (risperidone = 172, placebo = 163) |
|
|
|
|
|
| ‐ |
| (risperidone = 53, placebo = 57) |
|
|
|
|
|
|
|
| (risperidone = 73, placebo = 80) |
|
|
|
|
| ‐ |
|
| (ziprasidone = 25, placebo = 25) |
|
|
|
|
|
|
|
| Bpm: beats per minute; ECG: electrocardiogram; URTI: upper respiratory tract infection; EPSE: Extrapyramidal side effects; QRS: the name for the 3 waves (Q wave, R wave and S wave) on an electrocardiogram; QTc: correct QT (start of Q wave to end of T wave) interval | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aggression: ABC irritability (mean change scores) Show forest plot | 3 | 238 | Mean Difference (IV, Random, 95% CI) | ‐6.49 [‐8.79, ‐4.19] |
| 2 Aggression: OAS‐M, ABS Reactive subscale (final scores) Show forest plot | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.21, ‐0.40] |
| 3 Aggression: OAS‐M, ABS Proactive subscale (final scores) Show forest plot | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.30, 0.06] |
| 4 Conduct: NCBR‐CP (mean change scores) Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐8.61 [‐11.49, ‐5.74] |
| 5 Weight gain (antipsychotic only): Kg (mean change scores) Show forest plot | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 2.37 [0.26, 4.49] |
| 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores) Show forest plot | 3 | 305 | Mean Difference (IV, Random, 95% CI) | 2.14 [1.04, 3.23] |

