Intravenous immunoglobulins for epilepsy
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008557.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 04 julio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Epilepsia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Geng JS ‐ all correspondence; drafting protocol and review versions; searching for trials; selection of trials for inclusion and exclusion; extraction of data; interpretation of data analyses; updating review.
Dong JC ‐ arbiter of selection of trials for inclusion and exclusion; drafting review versions; extracting data; updating review.
Li YP ‐ methodology expert.
Ni HJ ‐ obtaining copies of trial reports.
Jiang K ‐ search for trials; updating review.
Shi LL ‐ selection of trials for inclusion and exclusion.
Wang GH ‐ conduct of analyses; data entry.
Sources of support
Internal sources
-
Project of Humanities and Social Sciences (Project No. 14YJCZH035), Ministry of Education in China, China.
-
National Natural Science Foundation of China (Project No. 71603138), China.
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
JG: none known
JD: none known
YL: none known
HN: none known
LS: none known
GW: none known
Acknowledgements
We are grateful for technical assistance from the Cochrane Epilepsy Group. We sincerely thank Professor Sridharan Ramaratnam (editorial mentor) for important advice on how to write and revise the protocol. We acknowledge the contribution of Rachael Kelly (Managing Editor), Markus Reuber (subject expert), Ann Johnston (subject expert), Karla Hemming (statistician) and Keven Hearn (consumer). We thank Professor Marcel Delire for providing us with additional information about the included trial. We thank Professor Taixiang Wu and Professor GuanJian Liu at the Chinese Cochrane Center and Chinese Evidence‐based Medicine Center for guidance on how to teach and carry out research in evidence‐based medicine. We acknowledge the help of the teachers at the Chinese Cochrane Center and Chinese Evidence‐based Medicine Center, West China Hospital and Department of Medical informatics, Medical School of Nantong University.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Dec 02 | Intravenous immunoglobulins for epilepsy | Review | JinSong Geng, JianCheng Dong, Youping Li, HengJian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | |
| 2017 Jul 04 | Intravenous immunoglobulins for epilepsy | Review | JinSong Geng, JianCheng Dong, Youping Li, Hengjian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | |
| 2011 Jan 19 | Intravenous immunoglobulins for epilepsy | Review | JinSong Geng, JianCheng Dong, Youping Li, HengJian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | |
| 2010 Jun 16 | Intravenous immunoglobulins for epilepsy | Protocol | JinSong Geng, JianCheng Dong, Youping Li, HengJian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | |
Differences between protocol and review
Although 'global assessment' was not stated in the review protocol, it was reported as an important outcome in the included study. The global assessment was considered to integrate several clinical aspects including reduction in the number and severity of seizures, evolution of EEG, interictal status, and perception of the participants and caregivers.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Comparison 1 IVIg versus placebo, Outcome 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case.

Comparison 1 IVIg versus placebo, Outcome 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol.

Comparison 1 IVIg versus placebo, Outcome 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case.
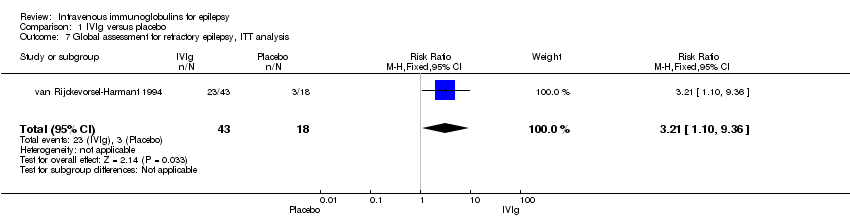
Comparison 1 IVIg versus placebo, Outcome 7 Global assessment for refractory epilepsy, ITT analysis.

Comparison 1 IVIg versus placebo, Outcome 8 Global assessment for refractory epilepsy, per‐protocol.
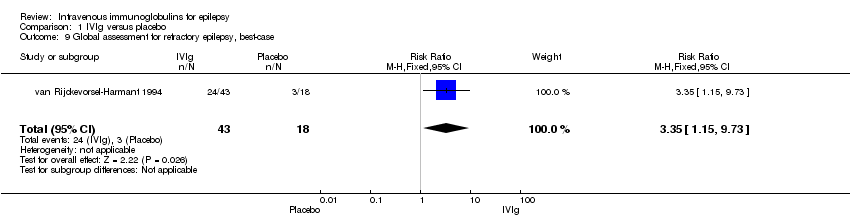
Comparison 1 IVIg versus placebo, Outcome 9 Global assessment for refractory epilepsy, best‐case.
| IVIg compared to placebo for refractory epilepsy | ||||||
| Patient or population: patients with refractory epilepsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IVIg | |||||
| Seizure freedom | Not reported | |||||
| Satisfactory seizure control | 278 per 1000 | 525 per 1000 | RR 1.89 | 58 | ⊕⊕⊝⊝ | |
| Incidence of adverse or harmful effects | Not reported | |||||
| Global assessment | 167 per 1000 | 548 per 1000 | RR 3.29 | 60 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias: no information was obtained on generation of the random sequence or concealment of allocation of the randomisation. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% or greater reduction in seizure frequency for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.79, 3.93] |
| 2 50% or greater reduction in seizure frequency for refractory epilepsy, per‐protocol Show forest plot | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.85, 4.21] |
| 3 50% or greater reduction in seizure frequency for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.91, 4.43] |
| 4 50% or greater reduction in seizure frequency for refractory partial epilepsy, ITT analysis Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.84, 11.34] |
| 5 50% or greater reduction in seizure frequency for refractory partial epilepsy, per‐protocol Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.91, 12.30] |
| 6 50% or greater reduction in seizure frequency for refractory partial epilepsy, best‐case Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.98, 13.00] |
| 7 Global assessment for refractory epilepsy, ITT analysis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [1.10, 9.36] |
| 8 Global assessment for refractory epilepsy, per‐protocol Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.13, 9.57] |
| 9 Global assessment for refractory epilepsy, best‐case Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.15, 9.73] |

