進行體外人工受孕療程的婦女,口服藥物clomiphene citrate或aromatase抑制劑合併促性腺素對於控制卵巢刺激之作用
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | 154 poor responders who had undergone at least 1 previous IVF attempt with a poor response. Responses were assessed as poor when baseline follicle‐stimulating hormone concentration was > 15 mIU/mL, oestradiol concentration on the day of hCG injection was < 500 pg/mL, or the number of pre‐ovulatory follicles > 16 mm in diameter was fewer than 3. | |
| Interventions | 45 women went into the hMG group, 52 women into the GnRH agonist plus hMG group, and 34 women into the CC plus hMG group. | |
| Outcomes | Premature LH surges, cycles cancelled in the follicular phase, and the number of mature oocytes retrieved | |
| Notes | Authors were contacted for the missing data through email but they did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is an RCT, although method of random sequence generation was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes (there was no mention of whether they were opaque or not or whether serially numbered or not). |
| Blinding (performance bias and detection bias) | Low risk | Although blinding was not mentioned, we did not consider that blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up and missing data information was not mentioned. Information unclear to make a judgement. |
| Selective reporting (reporting bias) | High risk | Most of the reported outcomes were surrogate outcomes, and there was no mention of our primary and secondary outcomes (i.e. live birth and OHSS). |
| Other bias | Unclear risk | We contacted the authors for missing data but received no response. |
| Methods | RCT Country: Turkey Single‐centre | |
| Participants | Included poor responders by Bologna criteria (2 out of 3) ≥ 40 years or other risk factor for POR or abnormal ovarian test or previous ≤ 3 oocytes retrieved. Age 18 to 42; normal uterus by HSG or hysteroscopy; regular cycles; normal hormonal cycles; BMI 19.3 to 28.9; ejaculate sample; no endocrine abnormalities. Exclusion: history of gonadotoxic therapy; ovarian surgery; natural IVF; DHEA or testosterone supplement. | |
| Interventions | Group 1 (n = 31): gonadotropins 450 (hMG + recombinant) + antagonist Group 2 (n = 31): gonadotropins 300 (hMG + recombinant) + antagonist Group 3 (n = 33): mild stimulation: letrozole 5 days, 5 mg/day + hMG 150 IU + antagonist | |
| Outcomes | Clinical pregnancy rate; ongoing pregnancy rate; implantation rate; gonadotropins usage; mean number of oocytes; cycle cancellation rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation described "computer generated list". |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelope used"; does not mention whether opaque or numbered |
| Blinding (performance bias and detection bias) | Low risk | Clinician and embryologist blinded. Overall we did not consider that blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up reported, and cancellation across groups was balanced. An intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | High risk | Even though clinical and ongoing pregnancy rates were stated outcomes, in the results these outcomes were clubbed and presented as a single outcome. |
| Other bias | Unclear risk | Funding not mentioned. |
| Methods | RCT Country: Egypt Single‐centre | |
| Participants | Included normoresponders; unexplained infertility; AFC > 5, AMH > 1 ng/mL; BMI 18 to 29; age 20 to 35. Exclusion criteria: endometriosis; azoospermia; BMI > 29. | |
| Interventions | Group 1 (n = 40): letrozole 10 mg daily day 3 to 7 along with FSH 75 IU/day from day 5 along with antagonist. Group 2 (n = 40): long protocol with FSH 150 to 225 IU/day. | |
| Outcomes | Clinical pregnancy rate; total gonadotropins usage; mature oocytes retrieved | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly stated. |
| Allocation concealment (selection bias) | Unclear risk | Used "sealed envelopes" |
| Blinding (performance bias and detection bias) | Unclear risk | Not enough information to make a judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information to make a judgement. Intention‐to‐treat analysis or loss to follow‐up was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgement, event rates are not mentioned. |
| Other bias | Unclear risk | There is not enough information to make a judgement. This is a conference abstract publication. |
| Methods | RCT | |
| Participants | 30 women under age of 38 years with only tubal infertility and fertile semen samples from their partners | |
| Interventions | 3‐arm study: Group I: clomiphene + hMG Group II: triptorelin (Decapeptyl Depot) (3.5 mg) from day 22 of the preceding cycle + hMG after desensitisation (long protocol) Group III: both GnRHa and hMG from day 2 of the cycle until day of hCG administration (short protocol) | |
| Outcomes | Pregnancy rate, cancellation rate, premature LH surge, mean number of hMG ampoules, mean number of oocytes | |
| Notes | Article in French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This an RCT, however the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Although the authors stated in their study that "women were not aware of their allocation", the method of concealment of allocation was not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | The analysis was per cycle. Not enough information to make a judgement. |
| Selective reporting (reporting bias) | Low risk | The protocol of the study was not available, however most outcomes of interest in this review were reported. |
| Other bias | Low risk | We found no potential sources of within‐study bias. |
| Methods | RCT Country: Japan Recruitment: poor responder patients who visited the IVF Center of University of Tokyo Hospital for the purpose of ART | |
| Participants | 99 women undergoing ART | |
| Interventions | Group 1 (n = 44): controlled ovarian stimulation initiated on day 3 with 5 days of clomiphene citrate (2 tabs daily) followed by hMG administration. After leading follicle diameter reached 14 mm, GnRH antagonist Ganirelix was administered in addition to hMG. Group 2 (n = 45): hMG administration was started on day 3, followed by combination with Ganirelix as above. | |
| Outcomes | Cumulative live‐birth rate per woman; cancellation rate Other outcomes reported in study but not entered into review: fertilisation rate; oestradiol levels on day of trigger; number of growing follicles | |
| Notes | Number of events not reported. This was a conference absract. We could not contact the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) | Low risk | There was no description of blinding participants, personnel, or outcome assessment in this conference abstract. However, we did not consider that potential lack of blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information to make a judgement. Intention‐to‐treat analysis or loss to follow‐up was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgement, event rates are not mentioned. |
| Other bias | Unclear risk | There is not enough information to make a judgement. This is a conference abstract publication. |
| Methods | RCT Country: Egypt Single‐centre | |
| Participants | Women with PCOS and planned for ICSI | |
| Interventions | Mild stimulation (n = 20): letrozole 10 mg day 2 to 6 along with hMG (150 to 225 IU). Conventional stimulation (n = 20): hMG (150 to 225 IU) in antagonist protocol. | |
| Outcomes | Main outcomes were gonadotropins use, day of stimulation, mean oocytes retrieved, and clinical pregnancy rate. | |
| Notes | Number of events not reported. This was a conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described. |
| Blinding (performance bias and detection bias) | Unclear risk | There was no description of blinding participants, personnel, or outcome assessment in this conference abstract. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information to make a judgement. Intention‐to‐treat analysis or loss to follow‐up was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgement, event rates are not mentioned. |
| Other bias | Unclear risk | There is not enough information to make a judgement. This is a conference abstract publication. |
| Methods | RCT | |
| Participants | 116 good‐prognosis patients undergoing their first IVF cycle | |
| Interventions | Women were randomised into 2 groups. Group A participants received clomiphene citrate from day 2 to day 6 of cycle and rFSH (100 to 150 IU) on days 3 and 5 and then daily from day 7 onwards. GnRH antagonist (0.25 mg) was administered subcutaneously daily once lead follicle measured 13 to 14 mm until day of hCG. GnRHa protocol and ovarian stimulation with rFSH (200 to 225 IU starting dose) was started in Group B. | |
| Outcomes | Pregnancy rate, implantation rate, number of top‐quality embryos | |
| Notes | The abstract did not report the number of women assigned to each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No available protocol |
| Other bias | Unclear risk | The study was published as an abstract, and the authors did not reply to our emails. |
| Methods | RCT Country: India Single‐centre | |
| Participants | Women who had previous 1 to 3 IVF failures due to poor response were included. Women with severe endometriosis, FSH > 12 IU, and history of previous pelvic surgery were excluded. Women were randomised in a 1:2 ratio. | |
| Interventions | Mild stimulation (n = 13): letrozole 2.5 mg from day 3 to 7 along with recombinant FSH (75 IU) from day 3 to 8. Conventional protocol (n = 25): long agonist protocol with FSH. | |
| Outcomes | Main outcomes were total dose of gonadotropins, oocytes retrieved, endometrial thickness, and clinical pregnancy rates. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using "random number table" by study co‐ordinator. |
| Allocation concealment (selection bias) | Unclear risk | "Sequentially number sealed envelopes were used" for allocation concealment. However, there was no mention of whether the envelopes were opaque or not. |
| Blinding (performance bias and detection bias) | Low risk | Single‐blinding of clinician done. We did not consider that blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women were included in the analysis. No loss to follow‐up or missing data reported. |
| Selective reporting (reporting bias) | Low risk | All the outcomes mentioned in material and methods section were reported. |
| Other bias | Low risk | We did not find any other source of bias within the study. |
| Methods | RCT | |
| Participants | 324 infertile couples undergoing IVF/ICSI Inclusion criteria: women younger than 36 years of age, regularly menstruating, and cause of infertility indicates IVF/ICSI Exclusion criteria: not mentioned | |
| Interventions | 2 groups: Group A: clomiphene citrate + hMG Group B: GnRHa (long) + hMG | |
| Outcomes | Pregnancy rate, implantation rate, cancellation rate, multiple pregnancy, OHSS rate, mean number of oocytes retrieved, mean number of gonadotropins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is an RCT, however the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Serially numbered, closed envelopes, however there was no mention of whether they were opaque or not. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis done. No missing data or loss to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | A duplicate publication for this study was checked and the trial appears to be free from selective reporting. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT | |
| Participants | 150 women undergoing IVF for the first time | |
| Interventions | 150 women were randomised into 3 groups of 50 women each. Group A: triptorelin intramuscularly from day 1 of the cycle, and hMG was given daily when down regulation occurred. Group B: 100 mg clomiphene citrate from day 2 for 5 days with hMG daily from day 4 of the cycle. Group C: buserelin intranasally from day 1, and hMG was added when down regulation was confirmed. | |
| Outcomes | Live‐birth rate, pregnancy rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised code |
| Allocation concealment (selection bias) | Unclear risk | Although quoted "patient allocation was performed by a second party and clinicians were blinded to patient allocation", there was no description of how allocation concealment was performed. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Although intention‐to‐treat analysis was not mentioned in the study, we easily retrieved all data required from the published material with no need to contact the authors. |
| Selective reporting (reporting bias) | Low risk | The protocol of the study was not available, however most outcomes of interest were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT Country: India Single‐centre | |
| Participants | Women < 40 years, no further details | |
| Interventions | Mild stimulation (n = 173): clomiphene citrate or aromatase inhibitor for the first 5 days of cycle followed by gonadotropins and 0.25 mg antagonist (cetrorelix) injection daily until the day of hCG. Long GnRH analogue protocol (n = 173) | |
| Outcomes | gonadotropins usage, mean number of oocytes retrieved, clinical pregnancy rate Outcomes reported but not used in review: cost of the oral ovulation induction agents | |
| Notes | This was published as a conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using "computer generated list". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mention of any loss to follow‐up. Information insufficient to make a judgement. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in materials and methods section were reported. |
| Other bias | Unclear risk | The trial was not registered. Funding not mentioned. |
| Methods | RCT | |
| Participants | Women undergoing their first IVF cycle | |
| Interventions | CC + hMG versus gonadotropins in GnRH agonist short protocol | |
| Outcomes | Number of gonadotropins ampoules and midluteal progesterone | |
| Notes | This trial was published as an abstract. Number of participants was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | High risk | The total number of participants was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No important outcomes of interest were reported. Not enough information to make a judgement. |
| Other bias | Unclear risk | The study was published as an abstract and the authors did not reply to our emails. |
| Methods | RCT | |
| Participants | 243 women who were candidates for ART Inclusion criteria: women aged 18 to 35 years, presence of a regular and proven ovulatory menstruation cycle with a length of 26 to 35 days, basal FSH < 10 IU/L, BMI 18 to 30 kg/m2, and first IVF attempt. Indications for IVF were unexplained infertility, male factor, tubal factor, early‐stage endometriosis, and cervical factor. | |
| Interventions | Group A: GnRHa every day for menstrual cycle 21 until day of desensitisation, then ovarian stimulation would commence with rFSH. | |
| Outcomes | Pregnancy rate, implantation rate, cancellation rate, multiple pregnancy, OHSS rate, mean number of oocytes retrieved, mean number of gonadotropins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, however there was no mention of whether they were opaque or not. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis, however data for number of women who started treatment were provided and so could be calculated in meta‐analysis; besides percentage of dropouts was above 5%. |
| Selective reporting (reporting bias) | Low risk | The protocol of the study was not available, however most outcomes of interest were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT | |
| Participants | 308 women undergoing their first IVF cycle | |
| Interventions | 4‐arm study: Group A: hMG (alone) Group B: clomiphene citrate + hMG Group C: GnRH agonist from first day of the cycle and for 3 days only, then hMG was started (ultrashort the flare‐up protocol) Group D: GnRH agonist from day 21 of previous cycle and then hMG was added after desensitisation (long protocol) | |
| Outcomes | Live birth Pregnancy (not defined) rate per woman/cycle Cancellation rate Multiple pregnancy rate Mean number of oocytes retrieved | |
| Notes | Inclusion criteria and exclusion criteria not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is an RCT, however the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Sealed, serially numbered envelopes, however there was no mention of whether they were opaque or not. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not state that analysis was by intention‐to‐treat, however the outcomes were analysed for all participants. No missing data or loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Low risk | We checked a duplicate publication and there was no risk of selective reporting. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT Country: China Single‐centre | |
| Participants | Women < 40 years undergoing IVF. History of < 4 oocytes retrieved in previous cycle (poor responders) or < 5 AFC | |
| Interventions | Mild stimulation (n = 26): letrozole 2.5 mg from day 2 to 6 with hMG (225 IU) with antagonist. Conventional stimulation (n = 27): hMG (225 IU) with antagonist. | |
| Outcomes | Main outcomes were oocytes retrieved, clinical pregnancy rate, ongoing pregnancy rate, and live‐birth rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using "computer generated list". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment done using "opaque sealed envelopes"; not mentioned if envelopes were numbered. |
| Blinding (performance bias and detection bias) | Low risk | Although blinding was not mentioned, we did not consider that blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All the specified outcomes were reported. |
| Other bias | Low risk | We detected no other source of bias within the study. |
| Methods | RCT | |
| Participants | 120 women undergoing their first ICSI cycle Inclusion criteria: women aged 20 to 38 years with regular cycles, day 3 FSH < 10 mIU/mL, BMI between 18.5 and 24.9 kg/m2, male factor infertility. Exclusion criteria: other indications for infertility including endometriosis, anovulation, PCOS, and hydrosalpinx. | |
| Interventions | Clomiphene citrate + hMG + cetrorelix (antagonist) versus GnRHa (long) + hMG | |
| Outcomes | Live‐birth rate Clinical pregnancy (ultrasound viable foetus) rate Cancellation rate Implantation rate Severe OHSS rate Mean number of oocytes Mean number of gonadotropins | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This an RCT, however the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation concealment we performed through sealed envelopes and physicians were not aware of the allocation until the patients were about to start ovarian stimulation"; however, there was no mention of whether or not the envelopes were opaque or serially numbered. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women were analysed. No missing data or loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Low risk | Although there was no available published protocol, all outcomes of interest were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT | |
| Participants | 75 patients undergoing their first IVF cycle; women were between 25 and 45 years old | |
| Interventions | CC + hMG versus GnRHa + hMG (short protocol) | |
| Outcomes | Pregnancy rate per couple | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is an RCT, however the method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in study if allocation concealment was performed. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | All couples that participated in the study were analysed. |
| Selective reporting (reporting bias) | Low risk | The published protocol for this study was not available, however most outcomes of interest were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT Country: Egypt Single‐centre | |
| Participants | Women undergoing IVF with previous failed IVF due to poor response were included. Women with severe endometriosis, severe male factor, and history of previous pelvic or ovarian surgery were excluded. | |
| Interventions | Mild stimulation (n = 30): letrozole 2.5 mg from day 2 to 6 and hMG along with antagonist. Conventional (n = 30): microdose flare protocol with 300 IU hMG. | |
| Outcomes | Clinical pregnancy rate, cancellation rates; outcomes were not clearly mentioned. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment done by sealed envelopes. No other details. |
| Blinding (performance bias and detection bias) | Low risk | Although blinding was not mentioned, we did not consider that blinding was likely to influence findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women were included in the analysis with no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly stated, and protocol not available; not enough information to make a judgement. |
| Other bias | Low risk | We did not find any other bias in the study. |
| Methods | RCT Country: India Single‐centre | |
| Participants | Women between 25 and 35 years of age Normogonadotropic, without PCOS or endometriosis Undergoing IVF for male factor (azoospermia) | |
| Interventions | Group A (42 women): letrozole 5 mg from day 3 to 7 along with recombinant FSH (75 IU) and antagonist. Group B (52 women): recombinant FSH (150 to 225 IU) and antagonist protocol. | |
| Outcomes | Outcomes were total gonadotropins dose, oocytes retrieved, clinical pregnancy rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not described, so we are unable to judge. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment is not clearly described; only randomly divided by "sealed envelopes". |
| Blinding (performance bias and detection bias) | Unclear risk | Although trial is described as single‐blinded, it was unclear who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All couples that participated in the study were analysed. |
| Selective reporting (reporting bias) | Low risk | The published protocol for this study was not available, however most outcomes previously specified were reported. |
| Other bias | Unclear risk | We found no other potential sources of within‐study bias. |
| Methods | RCT Country: Iran Single‐centre | |
| Participants | Included women who were poor responders: FSH 10 to 15 IU/mL or oestradiol < 1500 pg/mL or ultrasound with 3 follicles in previous IVF or age > 40 years. Women were excluded for endometriosis, sustained hyperprolactinaemia, FSH > 15 IU/mL, male azoospermia, or single ovary. | |
| Interventions | Mild stimulation (n = 62): letrozole 5 mg twice daily from day 2 to 6 with gonadotropins 450 IU until trigger versus microdose flare protocol (n = 61) with gonadotropins 300 IU. | |
| Outcomes | gonadotropins consumption, number of days stimulation, number of oocytes retrieved, and clinical pregnancy rate per woman randomised | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Design stated in title and abstract, however randomisation method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Ultrasound personnel and embryologist blinded. However, we did not consider blinding to influence the primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not mentioned, however intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | Outcomes not clearly stated in methods section, however registered trial and all stated outcomes have been reported. |
| Other bias | Low risk | We identified no potential source of bias within the study. |
| Methods | RCT Country: Iran Single‐centre | |
| Participants | Poor responder according to Bologna criteria, 2 out of 3 criteria: advanced maternal age ≥ 40 years or previous poor response < 3 oocytes or AFC 5 to 7 or AMH < 0.5 ‐1.1 ng/mL. Exclusion criteria: use of any infertility medicine in the previous 3 months and "presence of any medical history". | |
| Interventions | Group 1 (n = 42): mild stimulation, clomiphene 100 mg from day 2 for 5 days with hMG 150 IU/day from day 5 with antagonist. Group 2 (n = 35): conventional protocol, gonadotropins (hMG/recombinant FSH) 300 IU/day with antagonist. | |
| Outcomes | Clinican pregnancy rate, days of stimulation, number of oocytes, cancellation rate Other outcomes not included in review: fertilisation rate, endometrial thickness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Although blinding was not mentioned, we did not consider that blinding was likely to influence the findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women were analysed. No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the outcomes mentioned in methods section were reported. |
| Other bias | Low risk | No funding. We identified no other potential source of bias. |
| Methods | RCT Country: Italy Recruitment: patients referring to 4 infertility units in Milan, Rozzano, and Monza in Italy and selected for IVF were evaluated for study entry. | |
| Participants | 304 women with day 3 serum FSH > 12 IU/mL on at least 2 occasions or previous poor response to hyperstimulation. | |
| Interventions | Group 1 (n = 148): clomiphene citrate oral tablets 150 mg/day from day 3 to 7 of the cycle Group 2 (n = 156): daily s.c. injections of triptorelin (GnRH agonist) started on day 1 or 2 of the menstrual cycle and 450 IU of s.c. recombinant FSH from day 3 of the cycle, short protocol | |
| Outcomes | Live birth per women randomised, clinical pregnancy rate, cycle cancellation rate, multiple pregnancy rate, rate of foetal abnormalities Other outcomes not included in review: number of follicles > 15 mm; number of follicles > 10 mm; number of oocytes retrieved; fertilisation rate; number of women who underwent embryo transfer; number of embryos transferred; implantation rate; any adverse events; costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by means of a computer‐generated list into two groups" |
| Allocation concealment (selection bias) | Unclear risk | "Sealed opaque envelopes containing treatment allocation were opened after inclusion"; not mentioned if envelopes were numbered |
| Blinding (performance bias and detection bias) | Low risk | Although this study was not blinded, we did not consider that lack of blinding was likely to influence the findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up was reported and reasons given. The numbers were balanced between groups. An intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | The proposed outcomes in the ClinicalTrials.gov registration (NCT01389713) were reported in the paper publication. |
| Other bias | High risk | The study was interrupted after the scheduled 2 years of recruitment before reaching the sample size, leaving the study power at 60% instead of the planned 80%. One of the reasons for premature closure of the trial was slow recruitment. |
| Methods | RCT Country: Italy Recruitment: participants were recruited from those undergoing IVF who were classified as expectant poor responders. | |
| Participants | 695 women with clinical, endocrine, and ultrasound characteristics suggesting a low ovarian reserve and a poor responsiveness to COH. Each woman was included in the study for only 1 IVF cycle. Inclusion criteria: 1) circulating menstrual cycle day 3 FSH between 10 and 20 IU/L in the presence of oestradiol (E2) serum level < 80 pg/mL; 2) circulating AMH between 0.14 and 1.0 ng/mL; 3) antral follicle count assessed by transvaginal ultrasound of between 4 and 10. Exclusion criteria: women with basal FSH > 20 IU/L; undetectable AMH levels; AFC < 3; and age over 43 years. | |
| Interventions | “Mild” protocol (n = 355): clomiphene citrate 100 mg/day for 5 days from the 2nd to 6th day of the menstrual cycle + low‐dose 150 IU/day of subcutaneously injected gonadotropins + GnRH antagonist from the 8th day of the cycle until the day of hCG administration. “Long” protocol (n = 340): 0.8 mg/day GnRH agonist given intranasally from the 21st day of the run‐in cycle for 14 days and at the beginning of gonadotropins administration; the dose was reduced to 0.4 mg/day and continued during ovarian stimulation. Exogenous gonadotropins were administered at a starting daily dose of 300 IU, which was eventually increased up to a maximum of 450 IU/day after 1 week. | |
| Outcomes | Mean number of oocytes retrieved, cycle cancellation rate, total administered gonadotropins dose; length of ovarian stimulation, clinical (ultrasound‐confirmed) pregnancy rate per started cycle, miscarriage rate, ongoing pregnancy rate at 12 weeks' gestational age Other outcomes reported in study but not entered into review: fertilisation rate, implantation rate, pregnancy rate per oocyte pick‐up and per embryo transfer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computerized algorithm without any restriction. No blocks were used since the size of the study group was estimated to be large enough to ensure a balanced distribution of patients between groups" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was obtained using sequentially numbered dark envelopes: until they were opened at the time of allocation, both physicians and patients were blinded to the study." |
| Blinding (performance bias and detection bias) | Low risk | There was no description in the trial report of blinding participants or personnel after allocation was completed. However, we did not consider that potential lack of blinding was likely to influence the findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up after randomisation was reported. The "loss to follow up" term used in the report indicated the cancelled cycle due to poor response, which is expected in poor responder population. |
| Selective reporting (reporting bias) | Low risk | Every outcome proposed in the methods was explored. However, the study protocol was not available. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT Country: Italy Single‐centre | |
| Participants | Women meeting at least 2 of the following criteria were defined as poor responders: 1) age > 40 years; 2) basal FSH > 12 mIU/ mL; 3) 3 or fewer oocytes retrieved in the previous IVF cycle; 4) low oestradiol levels on the day of hCG administration (< 1500 pmol/mL). Exclusion criteria: women with a BMI > 30; biochemical and ultrasound evidence of polycystic ovary syndrome; stage III‐IV endometriosis; inflammatory, autoimmune, or metabolic disorders; infertility medications (gonadotropins, clomiphene citrate) taken within the past 2 months. | |
| Interventions | Group 1 (n = 78) mild stimulation: clomiphene citrate 100 mg from day 2 for 5 days and FSH 450 IU/day from day 5 with antagonist. Group 2 (n = 78): FSH 450 IU/day with antagonist. Group 3 (n = 78): FSH 450 IU/day with short agonist protocol. | |
| Outcomes | Clinical pregnancy rate, implantation rate, days of stimulation, mature oocytes retrieved | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation; block randomisation done |
| Allocation concealment (selection bias) | Unclear risk | Study mentions blinding of study team to allotted group, but does not describe actual method used. |
| Blinding (performance bias and detection bias) | Low risk | Blinding not mentioned, however we did not consider that potential lack of blinding was likely to influence the findings for our primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | All participants randomised and loss to follow‐up were mentioned and appeared to be balanced. However, intention‐to‐treat analysis not done. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Funding not mentioned. We identified no other potential source of bias. |
| Methods | RCT | |
| Participants | 508 couples undergoing their first IVF cycle were randomised into 2 groups. However, only 408 couples initiated treatment. Inclusion criteria: any type of infertility that indicates IVF. Exclusion criteria: couples in whom the sperm count was less than 100,000 motile spermatozoa. | |
| Interventions | Group A: clomiphene citrate + hMG Group B: GnRHa + hMG (long protocol) | |
| Outcomes | Pregnancy rate Implantation rate Cancellation rate Mean number of oocytes Mean number of gonadotropins | |
| Notes | 17% of couples assigned to Group A dropped out after randomisation and before start of treatment, while 23% of couples in Group B dropped out after randomisation and before start of treatment. Reasons were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A sequence of randomisation numbers |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not mentioned. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned. |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis and the analysis was per cycle. Loss to follow‐up and dropout rates were large and reasons were not clearly specified. |
| Selective reporting (reporting bias) | Low risk | Although live‐birth rate was not reported, most secondary outcomes were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT | |
| Participants | 294 infertile women undergoing IVF‐embryo transfer Inclusion criteria: first IVF cycle; women between 20 and 39 years of age; normal ovulatory cycles; tubal infertility, male factor, or unexplained infertility; early stage endometriosis. Exclusion criteria: women with chronic medical diseases, contraindication or allergy to the study medications, irregular cycles, low or high BMI (< 20 or > 30 kg/m2), or baseline FSH level > 15 IU/L. | |
| Interventions | Clomiphene citrate + rFSH + rLH + prednisolone (Group A) versus long GnRH agonist suppression + rFSH (long protocol) (Group B) | |
| Outcomes | Pregnancy rate, cancellation rate, OHSS rate, fertilisation rate, implantation rate, mean number of gonadotropins, mean number of oocytes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not mentioned. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding was not mentioned; not enough information to make a judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | No intention‐to‐treat analysis and the analysis was per cycle. No clear mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although live‐birth rate was not reported, most secondary outcomes were reported. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
| Methods | RCT | |
| Participants | 70 women undergoing IVF treatment Inclusion criteria: women aged 20 to 42 years with a history of 1‐ or 2‐year infertility were included. Poor response was defined by the number of dominant follicles on hCG day and number of mature oocytes < 3 or cycle cancellation due to poor ovarian response. | |
| Interventions | Study group (35 women): clomiphene citrate + hMG + midcycle antagonist Control group (35 women): GnRH agonist + hMG (long protocol) | |
| Outcomes | Pregnancy rate Cancellation rate Mean number of oocytes Mean number of gonadotropins | |
| Notes | We have categorised this study as poor responders as mentioned in the abstract after analysing the data and outcomes (e.g. mean oocytes retrieved). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This is an RCT in which random sequence was computer generated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes (there was no mention of whether they were opaque or not). |
| Blinding (performance bias and detection bias) | Low risk | Although blinding was not mentioned, we acknowledge that participant blinding is not possible for this type of comparison. |
| Incomplete outcome data (attrition bias) | Low risk | Data were analysed per woman randomised. No loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Low risk | The reported outcomes were similar to those published in the protocol. |
| Other bias | Low risk | We found no other potential sources of within‐study bias. |
AFC: antral follicle count
AMH: anti‐Müllerian hormone
ART: assisted reproductive technology
BMI: body mass index
CC: clomiphene citrate
COH: controlled ovarian stimulation
DHEA: dehydroepiandrosterone
FSH: follicle‐stimulating hormone
GnRH: gonadotropin‐releasing hormone
GnRHa: gonadotropin‐releasing hormone agonist
hCG: human chorionic gonadotropin
hMG: human menopausal gonadotropin
HSG: hysterosalpingogram
LH: luteinising hormone
ICSI: intracytoplasmic sperm injection
IVF: in vitro fertilisation
MESA‐TESE: microsurgical epididymal sperm aspiration‐testicular excisional sperm extraction
OHSS: ovarian hyperstimulation syndrome
PCOS: polycystic ovary syndrome
POR: poor ovarian reserve
RCT: randomised controlled trial
rFSH: recombinant follicle‐stimulating hormone
rLH: recombinant luteinising hormone
s.c.: subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A quasi‐randomised trial, as participants were randomised into 1 of 2 groups according to the day of their first consultation. | |
| The population was not infertile women but women undergoing ovarian hyperstimulation for the sole purpose of oocytes donation. | |
| Participants may have had either GIFT or IVF, and it was not possible to separate the outcomes of the 2 forms of assisted reproduction. | |
| Non‐randomised trial | |
| A cohort study | |
| Participants may have had either GIFT or IVF and the results were analysed per cycle, and it was not possible to obtain the results per woman randomised. | |
| Inappropriate comparison: both arms compared CC + hMG with and without antagonist. | |
| Participants were not undergoing IVF or ICSI. | |
| Inappropriate comparison: included IUI versus IVF treatments. | |
| Unclear whether study had a randomised trial design | |
| Participants were not undergoing IVF or ICSI. | |
| Inappropriate comparison: all 3 groups used CC initially. | |
| Control arm inappropriate. | |
| Unclear whether study had a randomised trial design. We could not contact author due to lack of contact information. | |
| A quasi‐randomised method (alternating method). This study was included in a previous meta‐analysis by Hughes 1992, and the author of the meta‐analysis obtained information about the randomisation method from the authors of the trial. | |
| Participants were not undergoing IVF or ICSI. | |
| Did not have fresh embryo transfer | |
| A quasi‐randomised trial | |
| Inappropriate comparison: comparing short versus antagonist protocol. | |
| Inappropriate comparison: study compared clomiphene with gonadotropins versus letrozole with gonadotropins. | |
| Participants were not undergoing IVF or ICSI. | |
| Inappropriate comparison: study compared clomiphene with gonadotropins versus letrozole with gonadotropins. | |
| Protocol was withdrawn before recruitment. | |
| Participants were not undergoing IVF or ICSI. | |
| Participants were not undergoing IVF or ICSI. | |
| Participants were not undergoing IVF or ICSI. | |
| Study evaluated use of CC in luteal phase on LH levels. | |
| Participants were not undergoing IVF or ICSI. | |
| Participants were not undergoing IVF or ICSI. | |
| Not randomised | |
| Not randomised | |
| Inappropriate comparison: compared CC versus CC with gonadotropins. | |
| Not all participants were undergoing IVF or ICSI. | |
| Not randomised | |
| Participants were not undergoing IVF or ICSI. | |
| Not randomised | |
| Non‐randomised trial, as allocation was intentionally by 2 clinicians acting independently without randomisation | |
| Quasi‐randomised trial | |
| Participants were not undergoing IVF or ICSI. | |
| Did not have fresh embryo transfer | |
| Did not have fresh embryo transfer in the minimal‐stimulation group |
CC: clomiphene citrate
GIFT: gamete intrafallopian transfer
hMG: human menopausal gonadotropin
ICSI: intracytoplasmic sperm injection
IUI: intrauterine insemination
IVF: in vitro fertilisation
LH: luteinising hormone
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Maximal stimulation and delayed fertilization for diminished ovarian reserve: a randomized pilot study |
| Methods | Open‐label RCT |
| Participants | Women with a poor prognosis due to diminished ovarian reserve. Inclusion criteria: basal FSH 17 IU/mL (highest ever); basal FSH 15 to 17 (highest ever), and failed EFORT test; age > 43 at the time of expected retrieval; failure to conceive with a prior "poor prognosis" IVF stimulation protocol (microdose leuprolide flare or GnRH antagonist cycle) if administered because of evidence of diminished ovarian reserve; failure to conceive with 3 or more IVF cycles at Carolinas Medical Centre (CMC). Exclusion criteria: contraindications to IVF; contraindication to pregnancy; allergy or contraindication to medications used for IVF or embryo transfer; use for a gestational carrier; uncorrected or untreatable uterine infertility; smoking or substance abuse within 3 months of initiating stimulation for IVF. |
| Interventions | Clomiphene plus gonadotropins Leuprolide flare |
| Outcomes | Number of oocytes retrieved; number of oocytes vitrified; number of embryos from vitrified oocytes per ovarian stimulation treatment protocol |
| Starting date | January 2011 |
| Contact information | Brad Hurst, Director, Assisted Reproductive Therapies, Carolinas Healthcare System |
| Notes | The status of the study in the registry is completed. We emailed contact person; authors responded with incomplete data that could not be pooled. |
| Trial name or title | The comparison of effect of four different treatment protocols on IVF outcomes in poor responders undergoing in vitro fertilization |
| Methods | Double‐blind RCT |
| Participants | Poor responders undergoing in vitro fertilisation Inclusion criteria: at least 1 of the following:
|
| Interventions | GnRH antagonist/letrozole protocol Microdose flare‐up protocol Antagonist/clomiphene protocol GnRH antagonist protocol |
| Outcomes | Clinical pregnancy rates; total number of oocytes retrieved |
| Starting date | January 2014 |
| Contact information | Pınar Özcan Cenksoy, Medical Doctor, Yeditepe University Hospital |
| Notes | We emailed contact person, have as yet received no response. |
| Trial name or title | Clomiphene citrate in combination with gonadotropins for ovarian stimulation in women with poor ovarian response |
| Methods | Single‐blind RCT |
| Participants | Women with poor response to ovarian stimulation. The definition of poor response was based on the presence of at least 1 of the following criteria:
|
| Interventions | Clomiphene citrate: clomiphene citrate (100 mg/day) in combination with gonadotropins according to a short stimulation GnRH antagonists protocol. Gonadotropins: short stimulation protocol with gonadotropins and GnRH antagonists. All women will be stimulated with a fixed GnRH antagonist protocol. Ovarian stimulation will be initiated with 450 IU of gonadotropins either in the form of a combination of highly purified urinary FSH and LH or with a combination of rFSH and rLH. |
| Outcomes | Clinical pregnancy |
| Starting date | October 2014 |
| Contact information | Nikos Vlahos, MD, University of Athens, 2nd Department of Obstetrics and Gynecology, [email protected] |
| Notes | We contacted the author but have received no response. |
| Trial name or title | Letrozole in stimulated IVF cycles (A randomized trial of letrozole as an adjunct to follicle stimulating hormone in stimulated in vitro fertilization cycles) |
| Methods | RCT |
| Participants | 900 |
| Interventions | Experimental: Letrozole group: letrozole + standard treatment: daily 150 to 300 IU hMG/FSH from cycle day 2 to 4 (at least 5 days after stopping the oral contraceptive pill) and cotreatment with letrozole 2.5 mg daily from stimulation day 5 until the day before hCG administration. GnRH antagonist (cetrorelix (Cetrotide) or ganirelix (Orgalutran)) 0.25 mg daily from stimulation day 5 until the day of hCG administration. Control group: Standard treatment: daily 150 to 300 IU hMG/FSH cycle day 2 to 4 (at least 5 days after stopping the oral contraceptive pill) until the day before hCG administration. GnRH antagonist 0.25 mg daily from stimulation day 5 until the day of hCG administration. |
| Outcomes |
|
| Starting date | November 2016 |
| Contact information | Ernest HY Ng, MD, [email protected] |
| Notes | Multicentre trial:
|
AMH: anti‐Müllerian hormone
BMI: body mass index
COH: controlled ovarian stimulation
EFORT: exogenous follicle‐stimulating hormone ovarian reserve
FSH: follicle‐stimulating hormone
GnRH: gonadotropin‐releasing hormone
hCG: human chorionic gonadotropin
hMG: human menopausal gonadotropin
IVF: in vitro fertilisation
LH: luteinising hormone
RCT: randomised controlled trial
rFSH: recombinant follicle‐stimulating hormone
rLH: recombinant luteinising hormone
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| Analysis 1.1  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 1 Live birth. | ||||
| 1.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol. | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| 1.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Ovarian hyperstimulation syndrome Show forest plot | 5 | 1067 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.11, 0.41] |
| Analysis 1.2  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 2 Ovarian hyperstimulation syndrome. | ||||
| 2.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 973 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.11, 0.47] |
| 2.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.03, 0.68] |
| 3 Ongoing pregnancy rate Show forest plot | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| Analysis 1.3 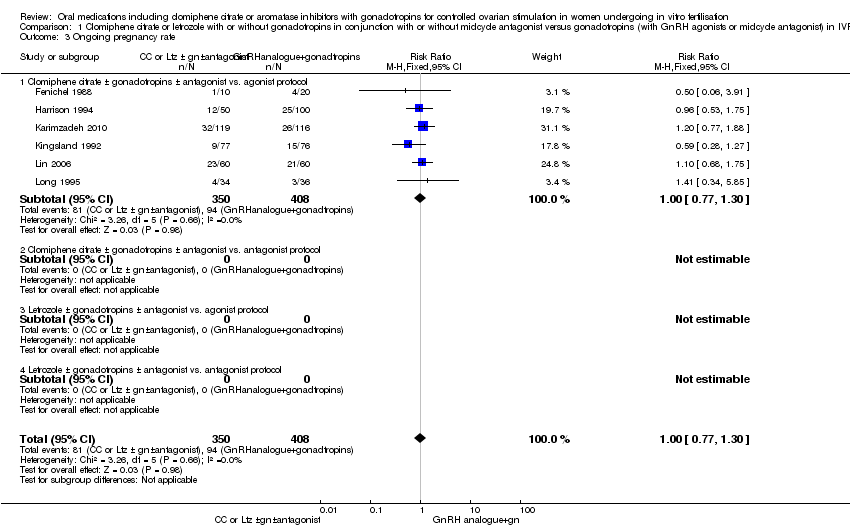 Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 3 Ongoing pregnancy rate. | ||||
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy rate Show forest plot | 12 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.16] |
| Analysis 1.4  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 4 Clinical pregnancy rate. | ||||
| 4.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 4.2 Clomiphene citrate± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.72] |
| 4.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.94] |
| 5 Cancellation rate Show forest plot | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| Analysis 1.5  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 5 Cancellation rate. | ||||
| 5.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| 5.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of ampoules used Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 6 Mean number of ampoules used. | ||||
| 6.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 7 Mean number of oocytes retrieved. | ||||
| 7.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Letrozole ± gonadotropins ± antagonists vs agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Multiple pregnancy rate Show forest plot | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.43] |
| Analysis 1.8  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 8 Multiple pregnancy rate. | ||||
| 8.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.57] |
| 8.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 3.82] |
| 9 Rate of miscarriage Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.47] |
| Analysis 1.9  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 9 Rate of miscarriage. | ||||
| 9.1 Clomiphene citrate ± gonadotropins ± antagonists vs. agonist protocol | 6 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.61, 1.75] |
| 9.2 Clomiphene citrate ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Letrozole ± gonadotropins ± antagonists vs. antagonists protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.66] |
| 10 Rate of ectopic pregnancy Show forest plot | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| Analysis 1.10  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 10 Rate of ectopic pregnancy. | ||||
| 10.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| 10.2 Clomiphene citrate ± gonadotrophins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Letrozole ± gonadotropins ± antagonists vs. agonists protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Rate of foetal abnormalities Show forest plot | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.11  Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 11 Rate of foetal abnormalities. | ||||
| 11.1 Clomiphene citrate ± gonadotropins ± antagonists vs. GnRHagonists or antagonist protocol | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Letrozole ± gonadotropins ± antagonists vs. GnRH agonist or antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.49, 2.79] |
| Analysis 2.1 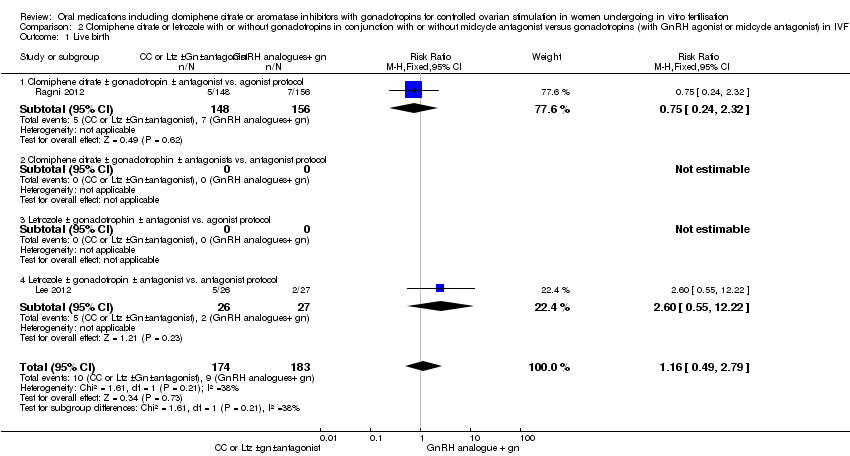 Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 1 Live birth. | ||||
| 1.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.24, 2.32] |
| 1.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropin ± antagonist vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 2 Ongoing pregnancy rate Show forest plot | 2 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| Analysis 2.2  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 2 Ongoing pregnancy rate. | ||||
| 2.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.19] |
| 2.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotrophin ± antagonists vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 3 Clinical pregnancy rate Show forest plot | 8 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| Analysis 2.3 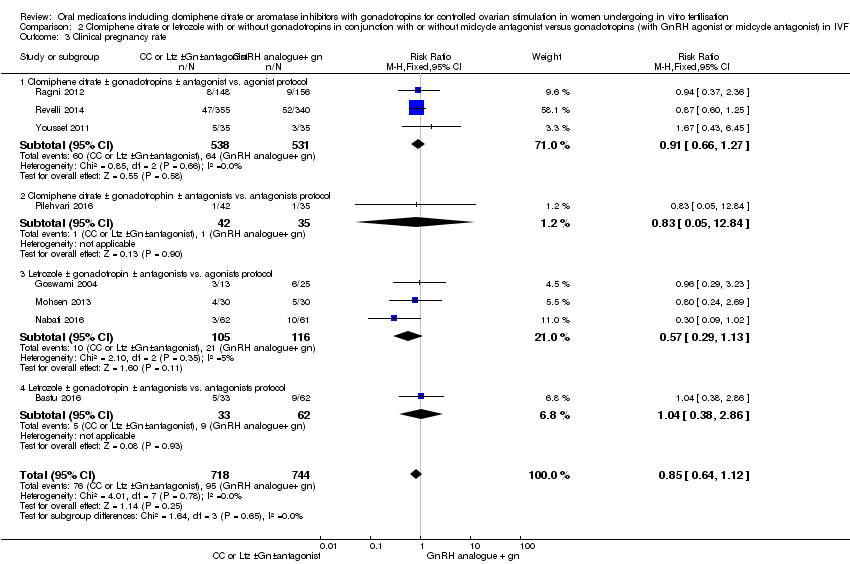 Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 3 Clinical pregnancy rate. | ||||
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 3 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.27] |
| 3.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.84] |
| 3.3 Letrozole ± gonadotropin ± antagonists vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.13] |
| 3.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.38, 2.86] |
| 4 Cancellation rate Show forest plot | 10 | 1601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.18, 1.81] |
| Analysis 2.4  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 4 Cancellation rate. | ||||
| 4.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 4 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.20, 2.10] |
| 4.2 Clomiphene citrate ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.39, 1.53] |
| 4.3 Letrozole ± gonadotropin ± antagonist vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.10, 3.13] |
| 4.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.67, 2.01] |
| 5 Mean number of ampoules used Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 5 Mean number of ampoules used. | ||||
| 5.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐23.98 [‐27.41, ‐20.56] |
| 5.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropin ± antagonist vs. agonist protocol | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐46.24 [‐50.93, ‐41.55] |
| 5.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of oocytes retrieved. Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 6 Mean number of oocytes retrieved.. | ||||
| 6.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropin ± antagonist vs. antagonist protocol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropin ± antagonists vs. agonist protocol | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Multiple pregnancy rate Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| Analysis 2.7  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 7 Multiple pregnancy rate. | ||||
| 7.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| 7.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Rate of miscarriage Show forest plot | 3 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.45, 2.12] |
| Analysis 2.8  Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 8 Rate of miscarriage. | ||||
| 8.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.01] |
| 8.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.73] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
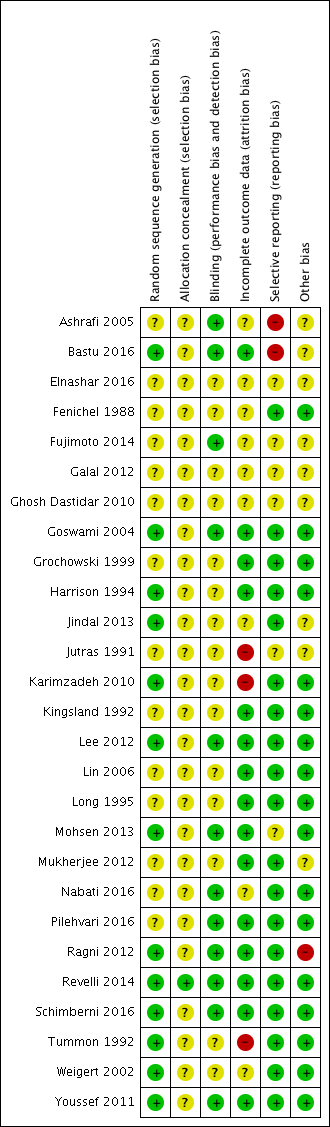
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, outcome: 1.1 Live birth.

Forest plot of comparison: 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, outcome: 1.2 Ovarian hyperstimulation syndrome.
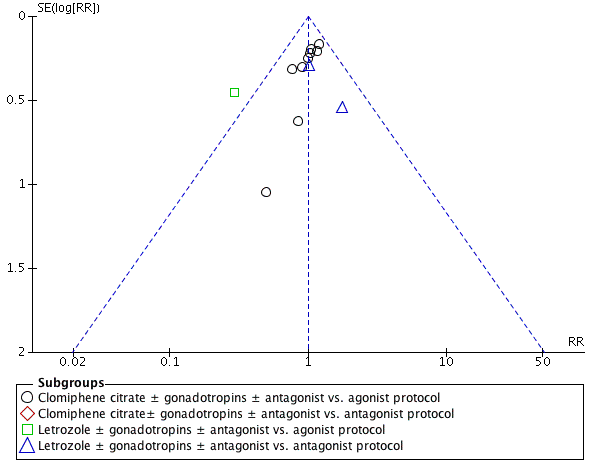
Funnel plot of comparison: 1 Clomiphene citrate or letrozole with gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins with GnRH protocols in IVF and ICSI cycles in general population, outcome: 1.4 Clinical pregnancy rate.
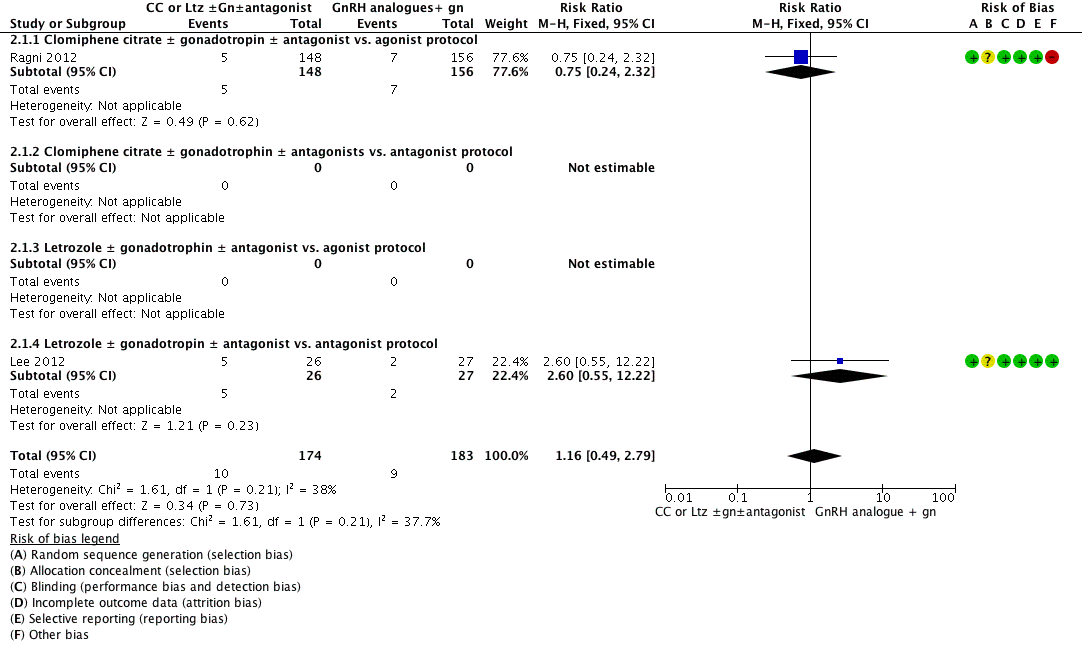
Forest plot of comparison: 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, outcome: 2.1 Live birth.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 1 Live birth.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 2 Ovarian hyperstimulation syndrome.
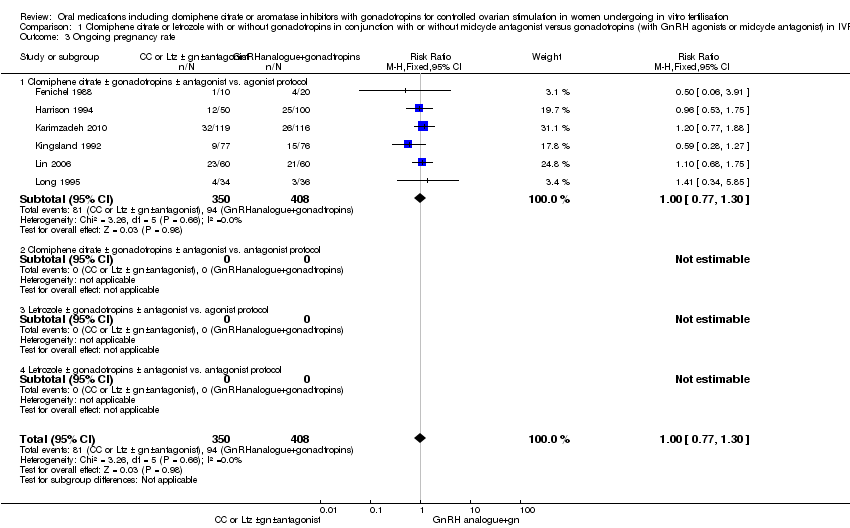
Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 3 Ongoing pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 4 Clinical pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 5 Cancellation rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 6 Mean number of ampoules used.
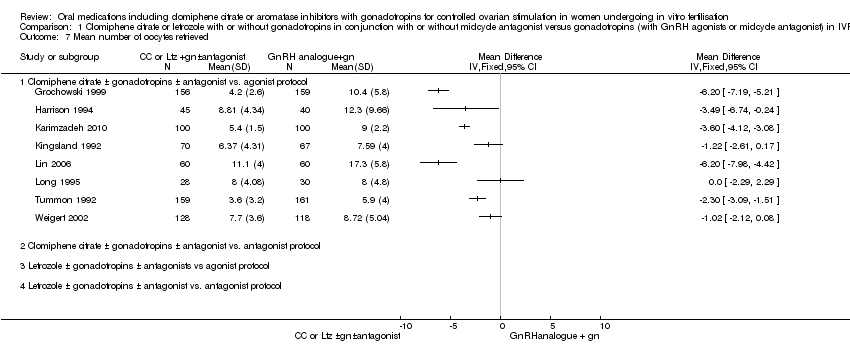
Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 7 Mean number of oocytes retrieved.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 8 Multiple pregnancy rate.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 9 Rate of miscarriage.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 10 Rate of ectopic pregnancy.

Comparison 1 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population, Outcome 11 Rate of foetal abnormalities.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 1 Live birth.
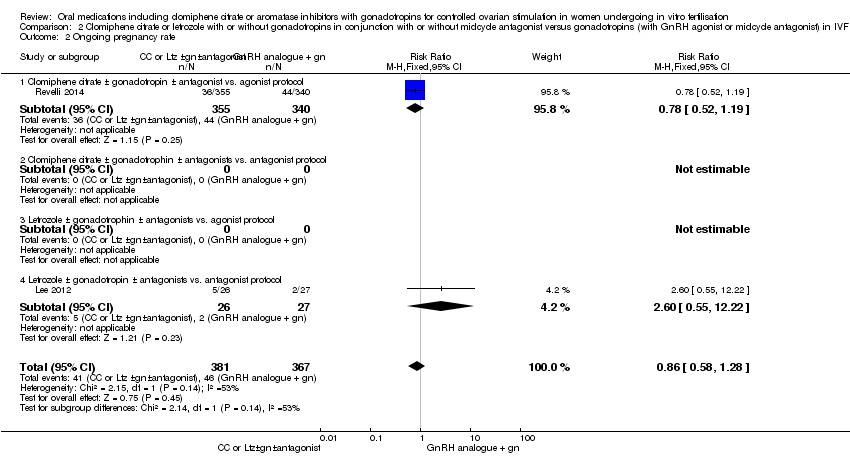
Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 2 Ongoing pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 3 Clinical pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 4 Cancellation rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 5 Mean number of ampoules used.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 6 Mean number of oocytes retrieved..

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 7 Multiple pregnancy rate.

Comparison 2 Clomiphene citrate or letrozole with or without gonadotropins in conjunction with or without midcycle antagonist versus gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders, Outcome 8 Rate of miscarriage.
| Clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) compared to gonadotropins (with GnRH agonists or midcycle antagonist) in IVF and ICSI cycles in general population for controlled ovarian stimulation | ||||||
| Patient or population: Women undergoing controlled ovarian stimulation in IVF and ICSI cycles (general population) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotropins (with GnRH agonists or midcycle antagonist) | Risk with clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) | |||||
| Live birth per woman | 235 per 1000 | 216 per 1000 | RR 0.92 | 493 | ⊕⊕⊝⊝ | |
| Ovarian hyperstimulation syndrome per woman | 63 per 1000 | 14 per 1000 | Peto OR 0.21 | 1067 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate per woman | 248 per 1000 | 248 per 1000 | RR 1.00 | 1998 | ⊕⊕⊕⊝ | |
| Cancellation rate per woman | 80 per 1000 | 150 per 1000 | RR 1.87 | 1784 | ⊕⊕⊝⊝ | |
| Mean number of gonadotropin ampoules used per woman | The mean number of ampoules used in the control group ranged from 18 to 50. | In all studies CC plus gonadotropins was associated with use of fewer ampoules. The mean difference ranged from 5.6 to 24.6 ampoules | ‐ | 1098 | ⊕⊕⊕⊝ | |
| Mean number of oocytes retrieved per woman | The mean number of oocytes retrieved in the control group ranged from 5 to 17. | In seven studies CC plus gonadotropins was associated with retrieval of fewer oocytes, with the mean difference ranging from 1.02 to 6.20 oocytes. The difference was statistically significant in five of these studies. The eighth study found no evidence of a difference between the groups | ‐ | 1481 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level (serious risk of bias). All included studies had unclear risk of bias for allocation concealment. | ||||||
| Clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) compared to gonadotropins (with GnRH agonist or midcycle antagonist) in IVF and ICSI cycles in poor responders for controlled ovarian stimulation | ||||||
| Patient or population: Women undergoing controlled ovarian stimulation in IVF and ICSI cycles (poor responders) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotropins (with GnRH agonist or midcycle antagonist) | Risk with clomiphene citrate or letrozole with or without gonadotropins (with or without midcycle antagonist) | |||||
| Live birth per woman | 49 per 1000 | 57 per 1000 | RR 1.16 | 357 | ⊕⊕⊝⊝ | |
| Clinical pregnancy rate per woman | 128 per 1000 | 109 per 1000 | RR 0.85 | 1462 | ⊕⊕⊝⊝ | |
| Cancellation rate per woman | 145 per 1000 | 212 per 1000 | RR 1.46 | 1601 | ⊕⊕⊝⊝ | |
| Mean number of gonadotropin ampoules used per woman | The mean number of ampoules used in the control group ranged from 39 to 71. | There were fewer ampoules used in the intervention groups (CC plus gonadotropins: MD ‐23.98, 95% CI ‐27.41 to ‐20.56; participants = 87; studies = 2); letrozole plus gonadotropins: MD ‐46.24, 95% CI ‐50.93 to ‐41.55; participants = 49; studies = 1). | ‐ | 136 | ⊕⊕⊕⊝ | |
| Mean number of oocytes retrieved per woman | The mean number oocytes retrieved in the control group ranged from 2 to 5. | In three of four studies CC plus gonadotropins versus gonadotropins in an agonist protocol was associated with retrieval of fewer oocytes, with the mean difference ranging from 0.75 to 2.10 oocytes. The difference was statistically significant in two of these studies. One study found no evidence of difference between CC plus gonadotropin versus gonadotropin in an antagonist protocol. Of three studies comparing letrozole plus gonadotrophins, one study reported significantly lower oocyte retrieval while the other two studies found no clear evidence of a difference. | ‐ | 1203 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level (serious risk of bias). Many of the included studies had unclear risk of bias for allocation concealment. | ||||||
| Study ID | Downregulation used | Type of FSH used | Starting dose of FSH | Dose of clomiphene citrateor letrozole | Cycle monitoring | Luteal support | Timing of hCG |
| Buserelin | hMG | 150 to 225 IU/day | 100 mg CC | Ultrasound | Progesterone | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG and recombinant FSH | hMG and recombinant FSH but in different doses: Group 1: 225 IU hMG + 225 IU rFSH Group 2: 150 IU hMG + 150 IU rFSH Group 3: 5mg Ltz + 150 IU rFSH | 5 mg Ltz | Ultrasound | Progesterone | Leading follicle > 17 mm | |
| Antagonist (ganirelix (Orgalutran) subcutaneous) for the Ltz group and triptorelin subcutaneous in the agonist control group | FSH | 75 IU for the Ltz group versus 150 to 225 IU for the control FSH/agonist group | 10 mg Ltz | Not mentioned | Not mentioned | Not mentioned | |
| Triptorelin intramuscular | hMG | hMG 125 to 300 IU/day | 200 mg CC | Ultrasound and oestradiol | hCG | Leading follicle 17 mm | |
| Ganirelix | hMG | Not mentioned | 100 mg CC | Not mentioned | Not mentioned | Not mentioned | |
| Not mentioned | hMG | 150 to 225 IU | 10 mg Ltz | Not mentioned | Not mentioned | Not mentioned | |
| Not mentioned | Recombinant FSH | 100 to 150 IU in the CC + gonadotropins group; 200 to 225 IU in the gonadotropins + GnRH agonist group) | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Triptorelin intramuscular depot | hMG | 150 to 225 IU/day | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 18 mm | |
| Triptorelin intramuscular and buserelin intranasal | hMG | 150 IU/day | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) for the Ltz or CC group and GnRH agonist for the control group, type not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | |
| Leuprorelin | hMG | 150 IU/day | 50 mg CC | Ultrasound and oestradiol | Not mentioned | Leading follicle 15 mm | |
| Buserelin | Recombinant FSH | 150 to 225 IU/day | 100 mg CC | Ultrasound | Progesterone | Leading follicle 18 mm | |
| Buserelin nasal spray | hMG | According to age (225 IU for women < 35 years and 300 IU for women > 35 years) | 100 mg CC | Ultrasound and oestradiol | hCG | Leading follicle 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 225 IU | 2.5 mg Ltz | Ultrasound and oestradiol | Progesterone | Leading follicle 18 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 150 to 300 IU/day | 100 mg CC | Ultrasound, serum oestradiol, LH, and progesterone | Progesterone | Leading follicle 18 mm | |
| Leuprorelin (Lupron) | hMG | 150 IU/day | 50 mg CC | Ultrasound and oestradiol | None | Leading follicle 15 mm | |
| Antagonist (cetrorelix subcutaneous) for the Ltz group and agonist (leuprorelin) for the conventional agonist group | hMG | 150 IU for the Ltz group versus 300 IU for the control hMG/agonist group | 2.5 mg Ltz | Ultrasound and oestradiol | Progesterone | 18 mm | |
| Antagonist (ganirelix (Orgalutran) subcutaneous) | Recombinant FSH | 75 IU for the Ltz group versus 150 to 225 IU for the control FSH/antagonist group | 5 mg Ltz | Ultrasound and oestradiol | Progesterone | 18 mm | |
| The type of antagonist used was not mentioned in the study while the agonist used in the control group was buserelin | Recombinant FSH | 300 IU for the Ltz group versus 450 IU for the control FSH/agonist group | 5 mg Ltz | Ultrasound | Progesterone | 17 mm | |
| Antagonist (cetrorelix subcutaneous) | hMG | 150 IU for the CC group versus 300 IU for the control hMG/antagonist group | 100 mg CC | Ultrasound | Progesterone | 17 to 18 mm | |
| Buserelin | Recombinant FSH | 450 IU | 150 mg CC | Ultrasound and oestradiol | Progesterone | 18 to 20 mm | |
| Antagonist (cetrorelix or ganirelix (Orgalutran) subcutaneous); agonist was leuprorelin | hMG | 150 IU for the CC group versus 300 to 450 IU for the control hMG/antagonist group | 100 mg CC | Ultrasound and oestradiol | Progesterone | 18 to 20 mm | |
| Antagonist (cetrorelix subcutaneous) | Recombinant FSH | 450 IU for both groups | 100 mg CC | Ultrasound and oestradiol | Progesterone | 18 mm | |
| Leuprorelin subcutaneous | hMG | According to body weight (less than 52 kg would start with 75 IU/day, 52 to 75 kg would start with 112.5 IU/day, and 150 IU/day for women who weighed more than 75 kg) | 100 mg CC | Ultrasound and oestradiol | Progesterone | Leading follicle 16 mm | |
| Buserelin | Recombinant FSH | 150 IU/day | 100 mg | Ultrasound | Progesterone | Leading follicle 18 mm | |
| Buserelin | hMG | 225 to 300 IU/day | 100 mg | Ultrasound | Progesterone | Not mentioned | |
| CC: clomiphene citrate | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| 1.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol. | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.27] |
| 1.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Ovarian hyperstimulation syndrome Show forest plot | 5 | 1067 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.11, 0.41] |
| 2.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 973 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.11, 0.47] |
| 2.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.03, 0.68] |
| 3 Ongoing pregnancy rate Show forest plot | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy rate Show forest plot | 12 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.16] |
| 4.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.23] |
| 4.2 Clomiphene citrate± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.72] |
| 4.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.94] |
| 5 Cancellation rate Show forest plot | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| 5.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.43, 2.45] |
| 5.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of ampoules used Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean number of oocytes retrieved Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Letrozole ± gonadotropins ± antagonists vs agonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Multiple pregnancy rate Show forest plot | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.43] |
| 8.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 4 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.57] |
| 8.2 Clomiphene citrate ± gonadotropins ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropins ± antagonist vs. antagonist protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.04, 3.82] |
| 9 Rate of miscarriage Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.47] |
| 9.1 Clomiphene citrate ± gonadotropins ± antagonists vs. agonist protocol | 6 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.61, 1.75] |
| 9.2 Clomiphene citrate ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Letrozole ± gonadotropins ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Letrozole ± gonadotropins ± antagonists vs. antagonists protocol | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.66] |
| 10 Rate of ectopic pregnancy Show forest plot | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| 10.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 2 | 223 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.47, 120.94] |
| 10.2 Clomiphene citrate ± gonadotrophins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Letrozole ± gonadotropins ± antagonists vs. agonists protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Letrozole ± gonadotropins ± antagonists vs. antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Rate of foetal abnormalities Show forest plot | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Clomiphene citrate ± gonadotropins ± antagonists vs. GnRHagonists or antagonist protocol | 1 | 74 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Letrozole ± gonadotropins ± antagonists vs. GnRH agonist or antagonist protocol | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.49, 2.79] |
| 1.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.24, 2.32] |
| 1.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Letrozole ± gonadotropin ± antagonist vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 2 Ongoing pregnancy rate Show forest plot | 2 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 2.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.19] |
| 2.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Letrozole ± gonadotrophin ± antagonists vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.55, 12.22] |
| 3 Clinical pregnancy rate Show forest plot | 8 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.12] |
| 3.1 Clomiphene citrate ± gonadotropins ± antagonist vs. agonist protocol | 3 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.27] |
| 3.2 Clomiphene citrate ± gonadotrophin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.05, 12.84] |
| 3.3 Letrozole ± gonadotropin ± antagonists vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.13] |
| 3.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.38, 2.86] |
| 4 Cancellation rate Show forest plot | 10 | 1601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.18, 1.81] |
| 4.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 4 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.20, 2.10] |
| 4.2 Clomiphene citrate ± gonadotropin ± antagonists vs. antagonists protocol | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.39, 1.53] |
| 4.3 Letrozole ± gonadotropin ± antagonist vs. agonists protocol | 3 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.10, 3.13] |
| 4.4 Letrozole ± gonadotropin ± antagonists vs. antagonists protocol | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.67, 2.01] |
| 5 Mean number of ampoules used Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐23.98 [‐27.41, ‐20.56] |
| 5.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Letrozole ± gonadotropin ± antagonist vs. agonist protocol | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐46.24 [‐50.93, ‐41.55] |
| 5.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Mean number of oocytes retrieved. Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clomiphene citrate ± gonadotropin ± antagonist vs. antagonist protocol | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Letrozole ± gonadotropin ± antagonists vs. agonist protocol | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Multiple pregnancy rate Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| 7.1 Clomiphene citrate ± gonadotropin ± antagonist vs. agonist protocol | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.75] |
| 7.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Letrozole ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Rate of miscarriage Show forest plot | 3 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.45, 2.12] |
| 8.1 Clomiphene citrate ± gonadotropin ± antagonists vs. agonist protocol | 2 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.01] |
| 8.2 Clomiphene citrate ± gonadotrophin ± antagonist vs. antagonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Letrozole ± gonadotrophin ± antagonist vs. agonist protocol | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Letrozole ± gonadotropin ± antagonists vs. antagonist protocol | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.73] |

