تاثیر تحریک عمقی مغز و کورتیکال برای درمان صرع
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind balanced cross‐over randomized controlled trial
| |
| Participants | n = 7, 42.9% male, mean age 28.0 years (range 16‐41 y), duration of epilepsy ranged from 14 to 29 years 2 patients with focal epilepsy (one with and one without secondary generalization), 5 patients with generalized epilepsy (2/5 had Lennox‐Gestaut syndrome); poor candidates for resective surgery mean baseline seizure frequency of 23.4 (SD 15.9) seizures per month | |
| Interventions | Active: bilateral stimulation of the centromedian thalamic nucleus
Control: sham stimulation (output voltage set at zero) | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (spontaneous reporting, postoperative CT scan) (5) Neuropsychological outcome [tests of general intelligence (WAIS‐R), speech and language functions (the Boston Naming Test, the Controlled Oral Word Association Test, a written description of the Cookie Theft Picture from the BDAE), visual and verbal memory functions (the Weschler Memory Scale, the Rey Auditory Verbal Learning Test with delayed recall and the Warrington Recongnition Memory Test (words and faces)), parietal lobe‐type functions (the Rey Osterreith Complex Figure Test with delayed recall), frontal lobe‐type functions (the Wisconsin Card Sorting Test) and psychomotor functions (the Trial Making Test (A and B) and the Perdue Grooved Pegboard)] | |
| Notes | The study was supported by Medtronic Inc. (Minneapolis, MN) who also donated hardware for the protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to either stimulation ON for A and OFF for B or to stimulation OFF for A and ON for B" Personal communication: "envelopes were chosen at random picking from a pile for each patient" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization order was provided in a sealed envelope" Personal communication: sealed and sequentially numbered envelopes, unclear if they were specific opaque envelopes (study was conducted more than 20 years ago); however, randomization was performed by a third person, not involved in selecting, treating or evaluating patients |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "neither patient, families, treating medical team nor data analysts knew whether the stimulator was ON or OFF during phases A and B"; "patients could not detect when stimulation was ON or OFF"; "stimulation was set to half the sensory threshold"; "a single unblinded individual was aware of treatment parameters and tested stimulator function at each monthly visit" Personal communication: the single unblinded individual was not involved in treating or evaluating patients |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above; seizure frequency was recorded in a seizure calendar |
| Incomplete outcome data (attrition bias) | High risk | One of the two patients who improved markedly with centromedian thalamic stimulation experienced several episodes of multiple daily seizures in the washout period and therefore was dropped from the blinded protocol and stimulation was reinstalled. As there were only seven patients, with only two responders, this one patient represents a significant proportion. |
| Selective reporting (reporting bias) | High risk | ‐ The results of a statistical analysis including all patients, to evaluate the efficacy of the intervention on seizure frequency, are not reported. Instead, only the results of an analysis including all patients with (primarily or secondarily) generalized seizures are presented (thus excluding one patient with only complex partial seizures). This was not prespecified in the Methods section. However, as all raw data are present in the article, all information necessary for this review is available. ‐ Concerning the neuropsychological outcome: "multivariate analysis with repeated measures showed no significant differences in any measure between baseline, placebo (OFF) and treatment (ON) conditions". Personal communication: exact figures no longer available Comment: no exact figures were reported, probably because there was too much data for a journal article (rather incomplete than selective reporting) |
| Outlasting effect due to prior stimulation | Low risk | Comment: cross‐over design, but with a 3‐month washout period |
| Anti‐epileptic drug policy | Low risk | Quote: "AED dosages were kept constant throughout the study" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Multicentre, double‐blind, parallel‐design, randomized controlled trial:
| |
| Participants | n = 109, 50.0% male, mean age 36.1 years (inclusion criterion:18‐65 y), mean duration of epilepsy was 22.3 (SD 13.3) years; all patients suffered from partial‐onset epilepsy (partial seizures and/or secondarily generalized seizures), IQ > 70 in all patients, 24.5% and 44.5% had prior resection and vagus nerve stimulation, respectively; median baseline seizure frequency of 19.5 seizures per month (inclusion criterion: ≥6 seizures) | |
| Interventions | Active (n = 55): bilateral anterior thalamic nucleus stimulation
Control (n = 54): sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (based on spontaneous reporting by patients, postoperative MRI) (5) Neuropsychological outcome (attention, executive function, verbal memory, visual memory, intelligence, expressive language, depression, tension / anxiety, total mood disturbance, confusion, subjective cognitive function) (6) Quality of life (QOLIE‐31) | |
| Notes | The study was supported by Medtronic Inc. (Minneapolis, MN) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done by a central statistical site, using random numbers tables, a one‐to‐one allocation to active stimulation versus control, balanced at each study site and with no weighting for any subject characteristics" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was done by a central statistical site" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "no care or assessment personnel knew the voltage settings" and "participants were unaware of their treatment group" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "no care or assessment personnel knew the voltage settings" |
| Incomplete outcome data (attrition bias) | Low risk | 108 out of 109 randomized patients completed the blinded phase. One patient (control group) developed an infection requiring explant, but was included in all analyses as randomized |
| Selective reporting (reporting bias) | High risk | Quote: "Changes in additional outcome measures did not show significant (...) differences during the double‐blind phase, including 50% responder rates, Liverpool Seizure Severity Scale and Qulatiy of Life in Epilepsy scores" Comment 1: not all available (as can be deducted from the protocol on clinicaltrials.gov or the online "Medtronic DBS therapy for epilepsy sponsor information", www.fda.gov) outcome measures (including seizure‐free days and seizure‐free intervals) were mentioned or reported in the paper in Epilepsia Comment 2: different analyses were performed; one patient of the treatment group who experienced a marked seizure frequency increase was excluded (not prespecified) and another patient with only 66 of 70 protocol‐required diary days was included (ITT analysis) in the analysis used to estimate the treatment effect for the entire BEP (and not per month). As there were good reasons to do so and the results of the other prespecified analysis were also reported, we do not consider this as a major source of selective reporting. |
| Outlasting effect due to prior stimulation | Low risk | Comment: parallel‐group design, no stimulation prior to the randomized phase |
| Anti‐epileptic drug policy | Low risk | Quote: "medication were kept constant during the 3‐month blinded phase and the 9‐month unblinded phase" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind cross‐over randomized controlled trial
| |
| Participants | n = 4, 25% male, mean age 36.7 years (range 28‐44 y), mean duration of epilepsy was 12.5 years (range 9‐15 years); all patients suffered from pharmaco‐resistant partial‐onset epilepsy, resection or further invasive assessment had been dismissed or surgery had been unsuccessful, patients preferred participation in the study above VNS or standard anterior thalamic DBS treatment, region of seizure onset was bilateral frontal in 2 patients and bilateral temporal in the 2 other patients mean baseline seizure frequency of 7.3, 4.3, 10.5 and 20.3 'disabling' seizures (complex partial or generalized tonic‐clonic seizure) per month (inclusion criterion: at least 3 'disabling' seizures every 4 weeks during the 12‐week baseline period), 1 of the patients also experienced 99.2 simple partial seizures per month | |
| Interventions | Active: bilateral nucleus accumbens stimulation
Control: sham stimulation Note: all patients had quadripolar electrodes implanted in both the nucleus accumbens and the anterior nucleus of the thalamus | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (5) Neuropsychological outcome (Test of Attentional Performance, Trail Making Test, Performance Evaluation System subtest 7 (Leistungspruefungssystem (LPS), subtest 7), d2–Attention Stress Test, 'Regensburger' Word Fluency Test, Hamasch 5‐Point Test, Verbal Learning and Memory Test, Wechsler Memory Scale–Revised, and the Boston Naming Test; during the visits (V1–V8) different tests were done; Beck‐Depression‐Inventory Version IA; Mini International Neuropsychiatric Interview) (6) Quality of life (QOLIE‐31‐P) | |
| Notes | Institutional budget, no external funding for this trial; several authors had previously received reimbursement for travelling expenses and/or speaker honoraria from Medtronic Inc. (Minneapolis, MN) and 1 author also served as consultant for Medtronic Inc. (Minneapolis, MN) and Sapiens Inc. (California, CA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the sequence was randomized using an internet‐randomizing tool (www.random.org)" |
| Allocation concealment (selection bias) | Low risk | Quote: "individuals not involved in the study performed allocation process" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "individuals not involved in the study performed allocation process and change of stimulation parameters. Patients and assessing epileptologists remained blinded until start of the open‐label phase"; "none of the patients reported to notice nucleus accumbens, anterior thalamic nucleus or combined nucleus accumbens / anterior thalamic nucleus stimulation" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "individuals not involved in the study performed allocation process and change of stimulation parameters. Patients and assessing epileptologists remained blinded until start of the open‐label phase"; "none of the patients reported to notice nucleus accumbens, anterior thalamic nucleus or combined nucleus accumbens / anterior thalamic nucleus stimulation" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4 patients underwent electrode implantation for DBS and all outcomes are reported for all patients |
| Selective reporting (reporting bias) | Low risk | Comment: selective reporting very unlikely. The study was registered in the German Trial Registry (http://www.drks.de/DRKS00003148). All outcomes mentioned in this protocol are reported on in the published paper (including online supporting information) in a very detailed and extensive way. The only shortcoming is the fact that specific details on the measurements that were planned to be used to assess the outcomes mentioned were not provided in the protocol. However, the published report includes all expected outcomes. |
| Outlasting effect due to prior stimulation | Unclear risk | Comment: cross‐over study with a 1‐month washout period after 3 months of stimulation which might be too short although we recognize that clear judgements on this issue are difficult to make and arbitrary |
| Anti‐epileptic drug policy | Low risk | Quote: "antiepileptic drug dosages remained unchanged in all patients. Furthermore, serum concentrations of antiepileptic drugs (except retigabine/ezogabine) were determined at each visit and showed no clinically relevant variability" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind balanced cross‐over randomized controlled trial Total duration 15 months:
| |
| Participants | n = 2, 50% male, 45 and 54 years old, duration of epilepsy was 15 and 29 years; medically intractable focal epilepsy, poor candidates for resective surgery on the basis of independent bitemporal originating seizures, normal MRI in patient 1 and bilateral hippocampal sclerosis in patient 2; baseline seizure frequency of 32 and 16 seizures per month | |
| Interventions | Active: bilateral hippocampal stimulation
Control: sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (standard questionnaire) (5) Neuropsychological outcome (objective memory: Hopkins Verbal Learning Test‐Revised and the Brief visuospatial Memory Test‐Revised; subjective memory: Memory Assessment Clinic Self‐Rating Scale) | |
| Notes | No external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization of the first treatment" Personal communication: computer‐generated randomized sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization of the first treatment was determined independently by the research unit and placed in a sealed envelope" Personal communication: sealed, double‐opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "both the treating neurologist and patient were blind to the stimulator status"; "the voltage was decreased until it was subthreshold for conscious appreciation so that patients were unaware of the status of the stimulator"; "neither patient was able to accurately assess when the stimulator was ON or OFF"; "the envelope with the stimulation sequence was given to a neurosurgeon not involved in outcome assessment who turned the device ON or OFF at each 3‐month visit" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above, only one neurosurgeon, not involved in outcome assessment, knew the stimulator status |
| Incomplete outcome data (attrition bias) | Low risk | Comment: for the ON‐ and OFF‐period all data were available; only the objective memory data of one patient in the washout period were not available |
| Selective reporting (reporting bias) | High risk | Quote: in the Methods section: "differences in mean monthly seizure frequency were assessed using repeated measures ANOVA" ; in the Results section: "ANOVA revealed a significant difference in the median monthly seizure frequency between the four epochs (p<0.01)" Comment: unclear why (only) the median monthly seizure frequency was used in this analysis instead of all available data, i.e. total number of seizures (or mean monthly seizure frequency, as announced in the methods section and as was indeed reported as a descriptive variable to quantify the treatment effect); however, as all available individual patient data were provided to us by the author, this had no influence on this review. |
| Outlasting effect due to prior stimulation | Low risk | Comment: cross‐over study, but with a 3‐month washout phase |
| Anti‐epileptic drug policy | Low risk | Quote: "(...) antiseizure drugs, which remained unchanged during the study" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Multicentre, double‐blind, parallel‐design, randomized controlled trial:
| |
| Participants | n = 191, 52% male, mean age 34.9 years (range 18‐66 y), duration of epilepsy ranged from 2 to 57 years all patients suffered from medically intractable partial onset seizures, 45% had only one seizure focus and 55% had two seizure foci, 32 and 34% had prior therapeutic surgery and vagus nerve stimulation, respectively mean baseline seizure frequency of 1.2 (SD 2.2) seizures per day (inclusion criterion ≥3 seizures per month) | |
| Interventions | Active (n = 97): stimulation directly to the seizure focus in response to epileptiform electrographic events (device: RNS® System, NeuroPace, Mountain View, CA)
Control (n = 94): sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (as assessed by clinicians, additionally vital signs were collected and a neurological examination was conducted at every office appointment) (5) Neuropsychological outcome [visual motor speed (trailmaking part A and B), motor speed / dexterity (grooved pegboard, dominant and nondominant), auditory attention (Wechsler Adult Intelligence Scale (WAIS)‐III digit span), general verbal ability (WAIS‐III information), general visuospatial ability (WAIS‐III block design), verbal memory (Rey Auditory Verbal Learning Test (RAVLT) I‐V, VII (delayed recall) and memory recognition), visuospatial memory (Brief Visuospatial Memory Test‐Revised (BVMT‐R) total recall, delayed recall and recognition discrimination index), language (Boston Naming Test (60 items) spontaneous with semantic clue; Delis‐Kaplan Executive Function System (D‐KEFS) verbal fluency test, condition 1: letter fluency), design fluency (D‐KEFS design fluency, total composite); mood inventories included the Beck Depression Inventory II (BDI‐II) and the Center for Epidemiologic Studies Depression Scale (CES‐D)] (6) Quality of life (QOLIE‐89) | |
| Notes | The study was sponsored by NeuroPace Inc., Mountain View, California (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were assigned 1:1 to treatment or sham groups using an adaptive randomization algorithm controlling for investigational site, location and number of seizure onsets and prior epilepsy surgery" Personal communication: "computer based random sequence generation", "an adaptive randomization process was used to minimize the imbalance within the covariates listed above: imbalance was calculated for each covariate and each potential therapy allocation, the less‐imbalancing therapy allocation was selected with a 75% probability, and the more‐imbalancing therapy allocation was selected with a 25% probability" |
| Allocation concealment (selection bias) | Low risk | Personal communication: central allocation, "An adaptive randomization was performed to minimize imbalance (...). So that therapy allocation could not be guessed or determined for a given subject (even with knowledge of the therapy allocation of all other subjects), the final therapy allocation for a subject was selected with a 75% probability towards the less imbalancing allocation and 25% probability towards the more imbalancing allocation" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "a blinded physician gathered all outcome data and a nonblinded physician managed the neurostimulator"; "to maintain the subject blind, all subjects underwent actual or sham programming of the neurostimulator to ensure that time with the physician was similar"; "the blind was successfully maintained. At the end of the BEP 24% said that they did not know to which group they had been randomized, 33% guessed incorrectly and 43% guessed correctly" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) | Low risk | Active stimulation group: 95/97 participants completed the trial: one patient did not complete the stimulation optimization period (participant preference), one did not complete the BEP (emergent explant) Sham stimulation group: 92/94 participants completed the trial: one patient did not complete the stimulation optimization period (death), one did not complete the BEP (emergent explant) |
| Selective reporting (reporting bias) | Low risk | Comment: ‐ no evidence of selective reporting; study was registered on www.clinicaltrials.gov but outcome measures were not mentioned; ‐ concerning the neuropsychological outcome, quality of life and adverse events, no or not all exact figures per group (sham versus treatment group) were reported, they only mentioned that there were no significant differences. Probably this was due to the fact that there was too much data for publication (rather incomplete than selective reporting). Authors provided us these data upon our request |
| Outlasting effect due to prior stimulation | Low risk | Comment: parallel‐group design, no stimulation prior to the randomized phase |
| Anti‐epileptic drug policy | Low risk | Quote: "anti‐epileptic drugs were to be held constant through the BEP, and then could be adjusted as needed; benzodiazepines for seizure clusters or prolonged seizures were permitted" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, multiple cross‐over, constrained (paired) randomized controlled design
| |
| Participants | n = 4, 25% male, mean age 31.8 years (range 24‐37 y), duration of epilepsy ranged from 16 to 24 years the patients suffered from refractory left unilateral medial temporal lobe epilepsy whose risk to memory contraindicated temporal lobe resection, all patients showed mesial temporal sclerosis on MRI mean baseline seizure frequency of 4, 2.3, 25 and 4 seizures per month | |
| Interventions | Active: left hippocampal stimulation
Control: sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (open questions) (5) Neuropsychological outcome (this included alternate forms of the Boston Naming Test; alternate forms of the Digit Span Test; Hopkins Verbal Learning Test; the Brief Visual Memory Test; Memory Assessment Clinic Self‐Rating Scale; due to concerns with potential floor effects associated with standard neuropsychological memory tests, one patient underwent some alternative tests; the Center for Epidemiologic Studies Depression (CES‐D) scale was used to assess mood) (6) Quality of Life (QOLIE‐89) | |
| Notes | The authors reported no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization to one of the eight possible sequences was done independently by the research unit, each month's sequence was placed in sealed, double‐opaque, sequentially numbered envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "patients, treating clinicians and outcome assessors were blinded"; "stimulation was set subthreshold for conscious appreciation"; "the patients' ability to guess ON or OFF status was no better than chance"; "a neurosurgeon not involved in outcome assessment or medical therapy received one envelope each month and turned the stimulator ON or OFF" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: see above |
| Incomplete outcome data (attrition bias) | Low risk | Comment: one patient did not complete quality of life related assessments; however, this was the case both during active and sham stimulation, so no real risk of attrition bias; all other outcome data were complete |
| Selective reporting (reporting bias) | Low risk | ‐ Quote: "neuropsychological testing revealed no differences between ON, OFF or baseline periods in any of the patients on any of the formal measures, or in the subjective memory scale" Comment: exact figures were not reported for the subjective memory scores (the Memory Assessment Clinic Self‐Rating Scale) and for none of the test results measures of variance were provided. However, this seems more a case of incomplete rather than selective reporting. |
| Outlasting effect due to prior stimulation | Unclear risk | Comment: multiple cross‐over design without washout period |
| Anti‐epileptic drug policy | High risk | Comment: anti‐epileptic drugs remained unchanged in only one patient |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, multiple cross‐over, randomized controlled trial
| |
| Participants | n = 5, mean age 27.2 years (range 18‐34 y), duration of epilepsy ranged from 8 to 23 years the patients suffered from medically intractable seizures; seizures were not classified but described; presumably, four suffered from focal epilepsy with partial seizures (and secondarily generalized seizures in two patients) and one from generalized epilepsy (with myoclonic seizures and unresponsive episodes with prolonged bilateral jerking) mean baseline seizure frequency of 0.6 to 21.2 seizures per day (mean 5.1) | |
| Interventions | Active: bilateral stimulation of the superior surface of the cerebellum parallel to and about 1 cm from either side of the midline
Control: same procedure, but with inserting an adhesive pad that had a layer of aluminium foil within it, which blocked radiofrequency transmission and in this way prevented true stimulation (versus active group: adhesive pad which consisted solely of adhesive plaster) | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (5) Neuropsychological outcome (full scale intelligence quotients and memory quotients) | |
| Notes | No statement concerning external support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the pairs of pads (with or without an aluminium foil within it) were selected at random" Comment: probably completely random selection (picking one out of two) |
| Allocation concealment (selection bias) | Low risk | Quote: "the pairs of pads were marked with identifying letters"; "the pair containing the foil was identified in a sealed note, which was opened only after the patient's observation period" Comment: although it was not mentioned explicitly, one could expect that the pads (note: the pads were selected randomly, not the notes) had an identical appearance (foil was within it) and the identifying letters were non‐disclosing (as efforts were made to conceal their meaning) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind"; "the pairs of pads were marked with identifying letters"; "the pair containing the foil was identified in a sealed note, which was opened only after the patient's observation period" Comment 1: although it was not mentioned explicitly, one could expect that the pads had an identical appearance (foil was within it) and the identifying letters were non‐disclosing (as efforts were made to conceal their meaning); unclear if the sealed notes were double‐opaque and by whom they were handled Comment 2: not mentioned if neuropsychological testing was performed during the double‐blind or the unblinded evaluation period |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: see above |
| Incomplete outcome data (attrition bias) | Low risk | ‐ Although in two patients only three inpatient evaluations were performed (instead of the four planned), enough data are available to evaluate the effects of the intervention ‐ Neuropsychological testing was not performed in one patient (not testable due to myoclonus), but low risk of attrition bias as this was the case both during effective and sham stimulation; incomplete preoperative neuropsychological testing in two additional patients, however postoperative evaluations (most important ones) were complete |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective reporting, but no protocol available |
| Outlasting effect due to prior stimulation | Unclear risk | Comment: multiple cross‐over study without washout period; inpatient evaluations after 1 to 21 months of stimulation |
| Anti‐epileptic drug policy | Low risk | Quote: "serum levels of phenytoin, primidone and phenobarbital were verified several times during each admission"; "additional (to the above mentioned drugs) diazepam was given in two patients and ethosuximide in one patient, but the serum levels were not monitored" Comment: probably a policy to keep anti‐epileptic drugs / their serum levels unchanged |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, cross‐over randomized controlled trial
| |
| Participants | n = 13, 62% male, mean age 19.2 years (range 4‐31 y), duration of epilepsy ranged from 4 to 33 years there were 8 patients with Lennox‐Gastaut syndrome (suffering mainly from atypical absences and generalized tonic‐clonic seizures), and 5 with refractory localization‐related epilepsy (suffering mainly from complex partial and secondarily generalized seizures) mean baseline seizure frequency of 1051 (SD 1434) seizures per month (median 119, interquartile range 56, 2576) | |
| Interventions | Active: stimulation of the centromedian thalamic nucleus
Control: sham stimulation | |
| Outcomes | (1) Seizure frequency reduction (2) Adverse events (open questions (not systematically) and physical examination ‐ spontaneous reporting; postoperative MRI) | |
| Notes | Medtronic Inc. (Minneapolis, MN) donated the neurostimulators for the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients entered into a double‐blind protocol" Personal communication: random selection of a folded paper (with a number on it) out of a box by the patient, who did not know the meaning of the number |
| Allocation concealment (selection bias) | Low risk | Personal communication: the folded paper was randomly selected by the patient, who did not know the meaning of number (i.e. if it corresponded to switching stimulation OFF between months 6 and 9 or between months 9 and 12) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "patients entered into a double‐blind protocol"; "because neither the patient nor the examiner could determine when the stimulator was OFF, the double‐blind protocol was considered valid" Personal communication: only an EEG technician who was not involved in treating or evaluating the patients knew the stimulation status Comment: although the blinding procedure seems adequate, performance bias may exist as the double‐blind stimulation OFF periods were compared to the 3‐month periods preceding them (stimulation ON in all patients, but double‐blind in only half of patients!) instead of consistently comparing to the double‐blind stimulation ON periods |
| Blinding of outcome assessment (detection bias) | High risk | Comment: see above, as outcome was assessed by the patient and the treating physician |
| Incomplete outcome data (attrition bias) | Low risk | Comment: despite good initial seizure control, neurostimulators were explanted in 2/15 patients originally included in the study due to skin erosions along the internalized stimulation system; however, this occurred before the patients entered the randomized phase |
| Selective reporting (reporting bias) | Low risk | Comment 1: no evidence of selective reporting, but no protocol available Comment 2: although there is no evidence of selective reporting, authors reported their findings incompletely: exact figures of seizure frequency (reduction) were not reported and are no longer readily available (personal communication), which prevents inclusion into the meta‐analysis (the results were only presented in graphs in the original article) |
| Outlasting effect due to prior stimulation | Unclear risk | Comment: cross‐over protocol with 6 to 9 months of stimulation before the randomized phase and without washout period |
| Anti‐epileptic drug policy | Low risk | Quote: "anticonvulsive medication remained unchanged and anticonvulsive blood levels were repeated every 3 to 6 months throughout the study" |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, parallel‐group randomized controlled trial
| |
| Participants | n = 5, 80% male, mean age 26.0 years (range 16‐35 y), duration of epilepsy ranged from 11 to 27 years three patients had generalized epilepsy and two patients (multi)focal epilepsy of frontal origin; all patients suffered from generalized tonic‐clonic seizures, 4/5 patients also had tonic seizures, 2/5 had drop attacks and 1/5 had myoclonic seizures / atypical absences mean baseline seizure frequency of 14.1 (SD 6.2) seizures per month (generalized tonic‐clonic seizures 6.3 (SD 3.1)) | |
| Interventions | Active (n = 3): bilateral stimulation of the superomedial surface of the cerebellum
Control (n = 2): sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (standard open questions, postoperative CT scan or MRI) | |
| Notes | Medtronic Inc. (Minneapolis, MN) supported the study by providing the cerebellar stimulation systems | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the procedure used for randomisation was to assign patients a lottery number" Personal communication: random selection of a folded paper (with a number on it) out of a box by the patient, who did not know the meaning of the number |
| Allocation concealment (selection bias) | Low risk | Personal communication: the folded paper was randomly selected by the patient, who did not know the meaning of number (i.e. if it corresponded to ON or OFF) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "both patients and the evaluator were blinded with regard to whether the stimulator was ON or OFF, a different investigator manipulated the stimulation code" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all patients completed the double‐blind randomized phase and all data were available |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective reporting, but no protocol available |
| Outlasting effect due to prior stimulation | Low risk | Comment: parallel‐group design, no stimulation prior to the randomized double‐blind phase |
| Anti‐epileptic drug policy | Low risk | Quote: "All patients but one continued baseline AEDs throughout the study. Phenytoin was reduced from 300 to 200 mg per day in case 5 because of drug intolerance. Seizure decreases were not likely to be due to AEDs, because they were not modified." Personal communication: phenytoin dose reduction in case 5 was at the seventh month of the study Comment: AEDs were not changed during the randomized double‐blind phase of the trial |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, parallel‐group, randomized controlled trial
| |
| Participants | n = 9, 66% male, mean age 29.1 years (range 14‐43 y), duration of epilepsy ranged from 3 to 37 years intractable temporal lobe epilepsy patients, poor surgery candidates (bilateral independent foci (n = 4), unilateral focus (n = 3), lateralization not completely clear (n = 2)); neuroimaging: normal MRI (n = 5), left (n = 3) or bilateral (n = 1) hippocampal sclerosis; 6 patients had mild memory impairment in neuropsychological tests, three had severe abnormalities | |
| Interventions | Active (n = 4): uni‐ or bilateral hippocampal stimulation (according to seizure focus)
Control (n = 5): sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (open questions (not systematically) ‐ spontaneous reporting; postoperative MRI) | |
| Notes | No statement concerning external support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an aleatory (randomized by lottery number) double‐blind maneuver" Personal communication: a non see‐through box with small folded pieces of paper (with a code on it) within it, out of which one was randomly taken by the patient who did not know the meaning of the code |
| Allocation concealment (selection bias) | Low risk | Personal communication: "folded papers in a non see‐through box" and the aleatory manoeuvre was performed by the patient who did not know the meaning of the code |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind"; "because the stimulation at the therapeutic stimulation parameters induced no subjective or objective sensation, the double‐blind maneuver was considered valid" Personal communication: the only person who knew if the stimulation was ON or OFF was an EEG technician who was not involved in other parts of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no data missing or patients excluded from analyses |
| Selective reporting (reporting bias) | Low risk | Comment: ‐ exact figures of seizure frequency with stimulation ON during the blinded period were not reported (only graphs of individual patient data, from which one could estimate these exact figures). We consider this more as incomplete rather than selective reporting. The authors provided us these data upon our request. ‐ no evidence of selective reporting, but no protocol available |
| Outlasting effect due to prior stimulation | Low risk | Parallel‐group design, no stimulation prior to the randomized phase |
| Anti‐epileptic drug policy | Low risk | Quote: anti‐epileptic drug therapy was maintained with no modifications during follow‐up |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Five‐centre parallel‐group, double‐blind (participant, caregiver, investigator and outcome assessor) randomized controlled trial:
| |
| Participants | n = 6 (sham stimulation: n = 4; active stimulation: n = 2), age 30‐46 years, IQ ≥70 adults with refractory uni‐ (n = 4) or bilateral (n = 2) mesial temporal lobe epilepsy (failure of ≥ 2 AEDs), preference for non‐resective surgery, or not a candidate for mesial temporal resection median baseline monthly seizure frequency of 10 (all seizures; CPS + GTCS = 1) in the sham group and 12 (CPS + GTCS = 2) in the stimulation group | |
| Interventions | Active (n = 2): uni‐ or bilateral hippocampal stimulation for 6 months
Control (n = 4): sham stimulation for 6 months | |
| Outcomes | (1) Seizure freedom (2) Responder rate (3) Seizure frequency reduction (4) Adverse events (5) Neuropsychological outcome (6) Quality of life | |
| Notes | The study has been preliminary terminated in March 2012 after recruitment of only 6 participants (target sample = 57) due to difficulties in patient recruitment despite the multicentre participation; the results collected in those 6 patients were published as an abstract. However, many details on the methodology, participants, interventions and outcomes are missing for a complete judgement of the methodology used or for full incorporation into this review. We tried to contact the authors but could not obtain additional information or data yet. Another attempt will be made by the next update of this review. The trial was sponsored by the University of Calgary, no evidence for external funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'randomized' Comment: additional information on the methods used for random sequence generation could not be obtained |
| Allocation concealment (selection bias) | Unclear risk | Quote: 'randomized' Comment: additional information on the methods used for concealment of treatment allocation could not be obtained |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: 'double‐blind (subject, caregiver, investigator and outcome assessor)' Comment: additional information on the methods used for blinding could not be obtained |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: 'double‐blind (subject, caregiver, investigator and outcome assessor)' Comment: additional information on the methods used for blinding could not be obtained |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no evidence for incomplete outcome data leading to attrition bias but insufficient details available for full appreciation |
| Selective reporting (reporting bias) | Low risk | Comment 1: no clear evidence for selective reporting, all outcome measures mentioned in the protocol were briefly discussed in the abstract although many details are missing for full appreciation (see comment 2); Comment 2: although there was no evidence for selective reporting, the authors reported their results incompletely as these were only published as an abstract and many details on the collected outcomes are missing for full incorporation of this trial into the review (e.g. results after 3 months, detailed neuropsychological outcomes, variance between participants...) |
| Outlasting effect due to prior stimulation | Low risk | Quote: parallel‐group randomized controlled trial |
| Anti‐epileptic drug policy | Unclear risk | Comment: AED policy not specified |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
| Methods | Double‐blind, cross‐over randomized controlled study
| |
| Participants | n = 12, 83% male, mean age 30 years (range 20‐38 y), duration of epilepsy ranged from 10 to 32 years type of epilepsy not reported, 5/12 patients had only generalized seizures, 1/12 only partial seizures, 4/12 partial and generalized seizures, 2/12 dd complex partial seizures versus complex absences; in addition it was reported that the EEG in each case contained quantifiable generalized paroxysmal activity, but six patients showed additional focal activity in the frontal or temporal regions, all patients had an IQ of ≥ 80 mean seizure frequency during sham stimulation: 61.7 (SD 53.3) seizures per month | |
| Interventions | Electrode pads were placed on the upper surface of the cerebellum, positioned parasagittally approximately 2 cm from the midline on each side; stimulation parameters were:
Treatment 1: continuous stimulation
Treatment 2: contingent (responsive) stimulation
Control: sham stimulation | |
| Outcomes | (1) Proportion of participants who were seizure‐free (2) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (3) Seizure frequency reduction (4) Adverse events (5) Neuropsychological outcome ('psychometry') (6) 'Proxy' of quality of life (patients' impressions on cerebellar stimulation) | |
| Notes | Baseline seizure frequency was not reported, changes in seizure frequency are therefore expressed relative to the sham stimulation phase; no statement concerning external support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the sequence of the phases was randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the sequence of the phases was randomly allocated" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind"; "the sequence of the phases was randomly allocated and the code was not broken until the trial had been completed"; "stimulation was set at stimulation parameters that couldn't be detected by the patients"; "before surgery and at the end of each phase of the trial, each patient was assessed clinically by two independent consultant neurologists who were not involved in the trial or the patient's routine management" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) | Low risk | Comment: seizure frequency during the three phases was not fully quantifiable in 3/12 patients (reasons: 1) one patient became uncooperative; 2) one patient mislaid some of his records; 3) one patient suffered prolonged periods of confusion associated with absence attacks and myoclonic jerks which were difficult to quantify); however, this was the case for each phase of the study; moreover, the evolution of the seizure frequency during the three phases of the trial was qualitatively described |
| Selective reporting (reporting bias) | Low risk | Quote: "psychometry did not reveal any major changes in any patients in any of the phases of the trial" Comment: no exact figures were provided, probably because there was too much data for publication in the journal article (rather incomplete than selective reporting). Comment: no evidence of selective reporting concerning the other outcomes, but no protocol available |
| Outlasting effect due to prior stimulation | Unclear risk | Comment: cross‐over design without a washout period between the different treatment phases |
| Anti‐epileptic drug policy | Low risk | Quote: "at the time of admission to the trial they were considered to be on the best combination of anticonvulsants at optimum dosage and this dosage had not been changed during the previous six months" Comment: although it was not stated explicitly, it seems unlikely that the antiepileptic drug regimen was changed during the trial |
| Other bias | Low risk | Comment: there is no clear evidence for a risk of 'other bias' |
AED: antiepileptic drug
BEP: blinded evaluation period
CT: computed tomography
DBS: deep brain stimulation
ITT: intention‐to‐treat
MRI: magnetic resonance imaging
SD: standard deviation
VNS: Vagus Nerve Stimulation
WAIS: Wechsler Adult Intelligence Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| not a randomized controlled trial | |
| 4/7 patients not in a randomized controlled trial; 3/7 patients participated in a randomized trial but no information about outcomes relevant to this study; additionally patients were also included in a large randomized controlled trial already included in this review (Morrell 2011) | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not intracranial stimulation | |
| not intracranial stimulation | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial / no new randomized controlled trials included | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| intracranial stimulation for other purposes / not to treat refractory epilepsy patients | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial | |
| not a randomized controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double‐blind (participant, investigator, outcome assessor), randomized controlled clinical trial with two cross‐over groups |
| Participants | Epilepsy resistant to AEDs and dopaminergic D2‐agonist Curative resective surgery not possible Metabolism deficiency of DOPA above 1 DS, evaluated by Positron Emission Tomography (PET) using fluorodopa Age ranging from 18 to 50 |
| Interventions | Group 1: 3 months high‐frequency stimulation of the subthalamic nucleus followed by 3 months SHAM stimulation Group 2: 3 months SHAM stimulation followed by 3 months high‐frequency stimulation of the subthalamic nucleus |
| Outcomes | (1) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (2) Seizure frequency reduction (3) Adverse events (4) Neuropsychological outcome (WAIS, GROBER and Busckhe, Wisconsin Card Sorting Test, TRAIL test, LURIA test, Beck Depression Inventory, verbal flow test, empathy test) (5) Quality of life (SEALS, QOLIE‐31 and NHP scales) |
| Notes | The study has been preliminary terminated in March 2010 due to insufficient patient recruitment. Four participants were recruited. Results have not been published yet. We tried to contact the authors but could not obtain any results yet. Further efforts will be made. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | A randomized controlled trial evaluating the efficacy and safety of DBS of the mammillary bodies and mammillothalamic tracts was announced but results have not been published yet; authors were contacted but results could not be provided yet. Further efforts will be made. |
AED: antiepileptic drug
DBS: deep brain stimulation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Prospective randomized controlled study of neurostimulation in the medial temporal lobe for patients with medically refractory medial temporal lobe epilepsy;: Controlled Randomized Stimulation Versus Resection (CoRaStiR) |
| Methods | Prospective, multicentre, parallel‐group, single‐blind (participant) randomized controlled trial |
| Participants | Presurgical candidates with pharmacoresistant partial seizures despite optimal medical treatment and history of temporal lobe epilepsy Video‐EEG characteristics showing temporal lobe seizure onset (left‐sided or right‐sided seizure onset) in at least one recorded habitual seizure Presence of a structural abnormality in the medial temporal lobe, suggestive of hippocampal sclerosis as evidenced by optimum MRI Age ≥ 18 years Total IQ > 80 |
| Interventions | Group 1: electrode implantation in the medial temporal lobe and immediate unilateral hippocampal neurostimulation (12 months) Group 3: amygdalohippocampectomy |
| Outcomes | (1) Proportion of participants with a ≥ 50% seizure frequency reduction (responder rate) (2) Seizure frequency reduction (3) Adverse events |
| Starting date | June 2007 |
| Contact information | Kristl Vonck, MD, PhD ‐ Ghent University, Belgium ‐ [email protected] |
| Notes | Currently still recruiting participants (December 2014) Sponsored by Medtronics |
| Trial name or title | Clinical and medico‐economical assessment of deep brain stimulation of the anterior nucleus of the thalamus for the treatment of pharmacoresistant partial epilepsy |
| Methods | Open‐label parallel‐group randomized controlled trial |
| Participants | Pharmacoresistant (≥ 2 AEDS) focal or multifocal epilepsy patients Epilepsy inoperable at the time of inclusion Failure of vagus nerve stimulation Age 16‐60 years IQ > 55 |
| Interventions | Group 1: anterior thalamic nucleus deep brain stimulation Group 2: maintaining 'usual' treatment, including vagus nerve stimulation |
| Outcomes | (1) Seizure severity (2) Adverse events (special focus on depression) (3) Neuropyschological outcome (4) Quality of life |
| Starting date | March 2014 |
| Contact information | Sandra David‐Tchouda, MD ‐ University Hospital of Grenoble Michallon, France ‐ SDavidTchouda@chu‐grenoble.fr Sandrine Massicot, CRA ‐ University Hospital of Grenoble Michallon, France ‐ [email protected] |
| Notes | Currently still recruiting patients (December 2015) Sponsored by Grenoble University Hospital |
| Trial name or title | Low frequency electrical stimulation of the fornix in intractable Mesial Temporal Lobe Epilepsy (MTLE) |
| Methods | Parallel‐group single‐blind (participant) randomized controlled trial |
| Participants | Patients with intractable (failure of ≥ 2 AEDs) uni‐ or bilateral medial temporal lobe epilepsy (based on non‐invasive video‐EEG monitoring; lesional or non‐lesional hippocampus) Demonstration that the hippocampus ipsilateral to seizure onset is contributing to memory function Age 18‐65 years IQ ≥ 70 |
| Interventions | Group 1: 1 Hz low‐frequency electrical stimulation of the fornix using a Medtronic deep brain stimulation device Group 2: 5 Hz low‐frequency electrical stimulation of the fornix using a Medtronic deep brain stimulation device |
| Outcomes | (1) Seizure frequency (2) Adverse events, especially safety and tolerability with regards to memory function ‐ Psychiatriac Health (3) Quality of life (QOLIE‐31 and SF‐36) |
| Starting date | December 2013 |
| Contact information | Mohamad Z Koubeissi, MD ‐ George Washington University, Washington DC, USA ‐ [email protected] |
| Notes | Currently still recruiting participants (March 2015) Sponsored by George Washington University |
| Trial name or title | Prospective randomized trial comparing vagus nerve stimulation and deep brain stimulation of the anterior nucleus of the thalamus in patient with pharmacoresistant epilepsy |
| Methods | Parallel‐group randomized controlled clinical trial |
| Participants | Patients with diagnosis of pharmacoresistant partial‐onset seizures (persistent seizures despite at least 3 AEDs) Prior electroencephalography and magnetic resonance imaging studies are consistent with the diagnosis Age 12‐60 years |
| Interventions | Group 1: vagus nerve stimulation Group 2: anterior thalamic nucleus deep brain stimulation |
| Outcomes | (1) Seizure frequency reduction (2) Adverse events including depression and anxiety (3) Quality of life |
| Starting date | January 2015 |
| Contact information | Zhang K ‐ Beijing Neurosurgical Institute, China ‐ [email protected] |
| Notes | Currently still recruiting participants (May 2015) Sponsored by Beijing Tiantan Hospital, Capital Medical University |
AED: antiepileptic drug
MRI: magnetic resonance imaging
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure freedom Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Stimulation versus sham stimulation, Outcome 1 Seizure freedom. | ||||
| 1.1 Anterior thalamic nucleus | 1 | 109 | Odds Ratio (Fixed, 95% CI) | 0.33 [0.01, 8.36] |
| 1.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.11, 9.39] |
| 1.3 Cerebellar stimulation | 3 | 39 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.22, 4.12] |
| 1.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.03 [0.21, 5.15] |
| 1.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 1.80 [0.03, 121.68] |
| 1.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.07, 13.64] |
| 1.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 4.95 [0.23, 104.44] |
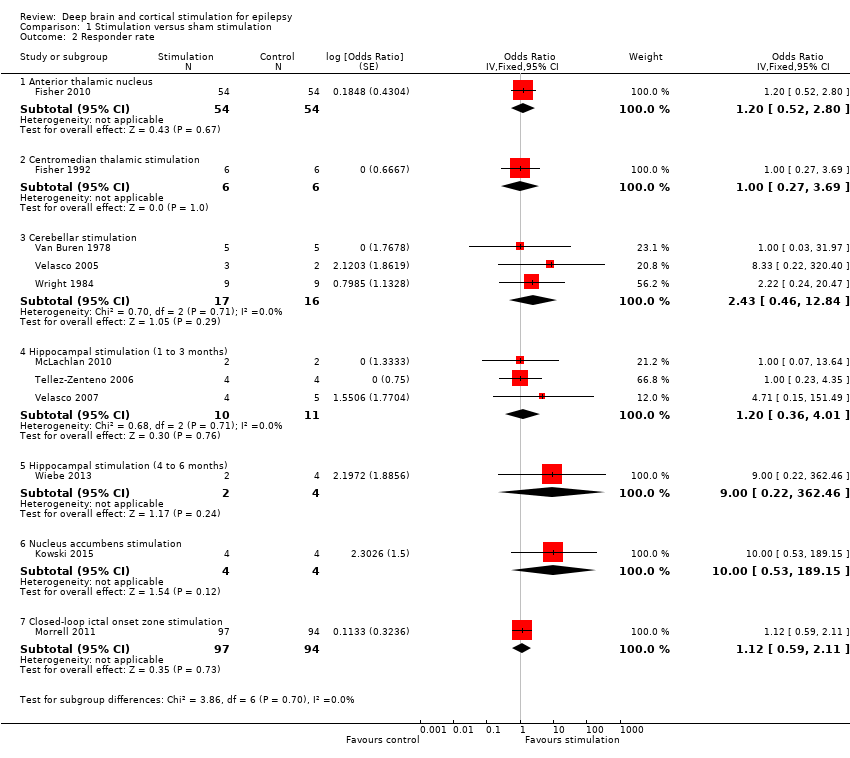
| 2 Responder rate Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 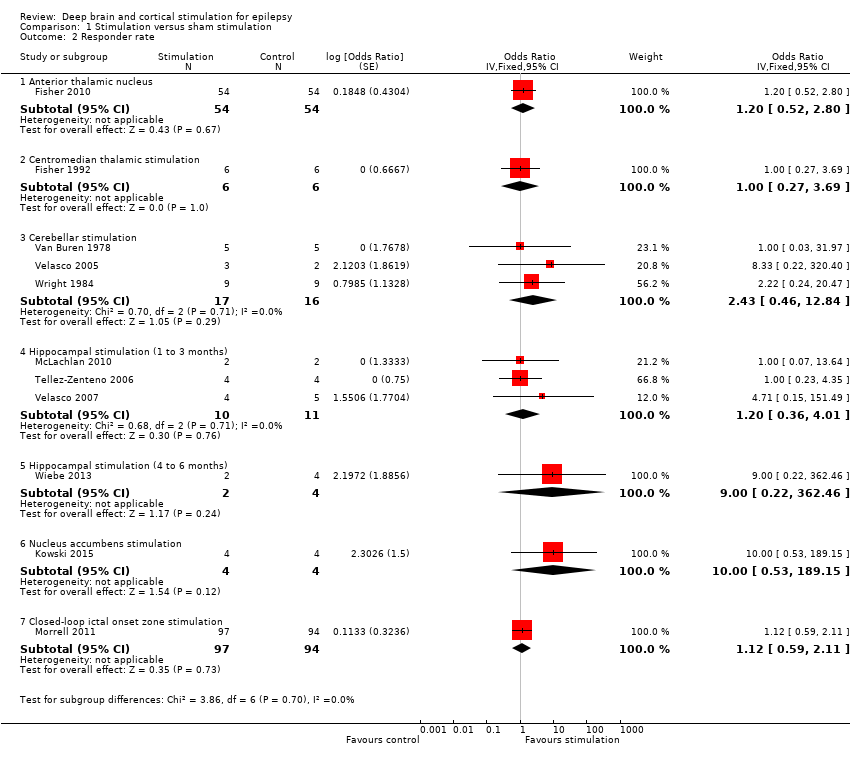 Comparison 1 Stimulation versus sham stimulation, Outcome 2 Responder rate. | ||||
| 2.1 Anterior thalamic nucleus | 1 | 108 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.52, 2.80] |
| 2.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.27, 3.69] |
| 2.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 2.43 [0.46, 12.84] |
| 2.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.36, 4.01] |
| 2.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 9.00 [0.22, 362.46] |
| 2.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 10.00 [0.53, 189.15] |
| 2.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 3 Seizure frequency reduction Show forest plot | 10 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 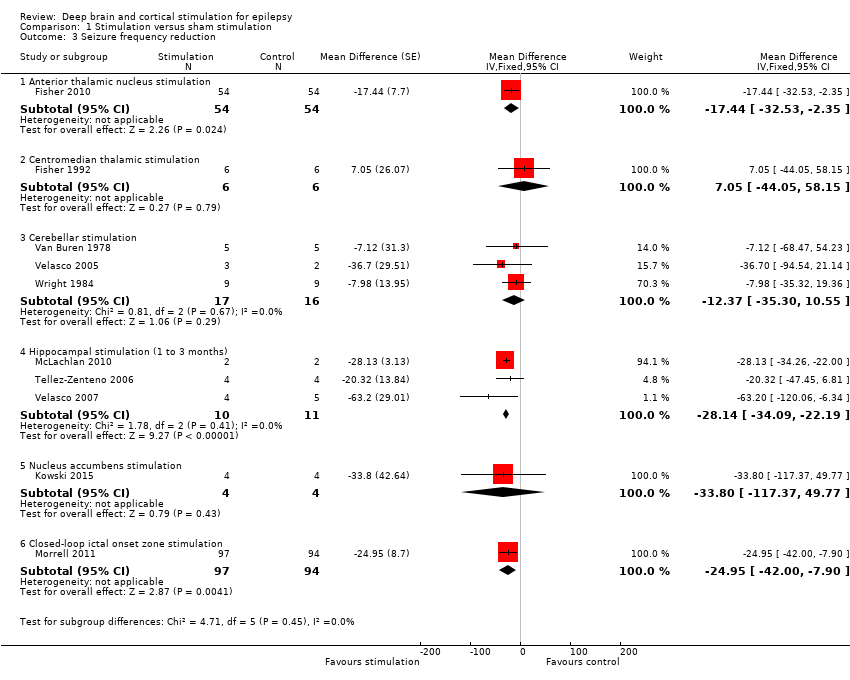 Comparison 1 Stimulation versus sham stimulation, Outcome 3 Seizure frequency reduction. | ||||
| 3.1 Anterior thalamic nucleus stimulation | 1 | 108 | Mean Difference (Fixed, 95% CI) | ‐17.44 [‐32.53, ‐2.35] |
| 3.2 Centromedian thalamic stimulation | 1 | 12 | Mean Difference (Fixed, 95% CI) | 7.05 [‐44.05, 58.15] |
| 3.3 Cerebellar stimulation | 3 | 33 | Mean Difference (Fixed, 95% CI) | ‐12.37 [‐35.30, 10.55] |
| 3.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Mean Difference (Fixed, 95% CI) | ‐28.14 [‐34.09, ‐22.19] |
| 3.5 Nucleus accumbens stimulation | 1 | 8 | Mean Difference (Fixed, 95% CI) | ‐33.8 [‐117.37, 49.77] |
| 3.6 Closed‐loop ictal onset zone stimulation | 1 | 191 | Mean Difference (Fixed, 95% CI) | ‐24.95 [‐42.00, ‐7.90] |
| 4 Quality of Life Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 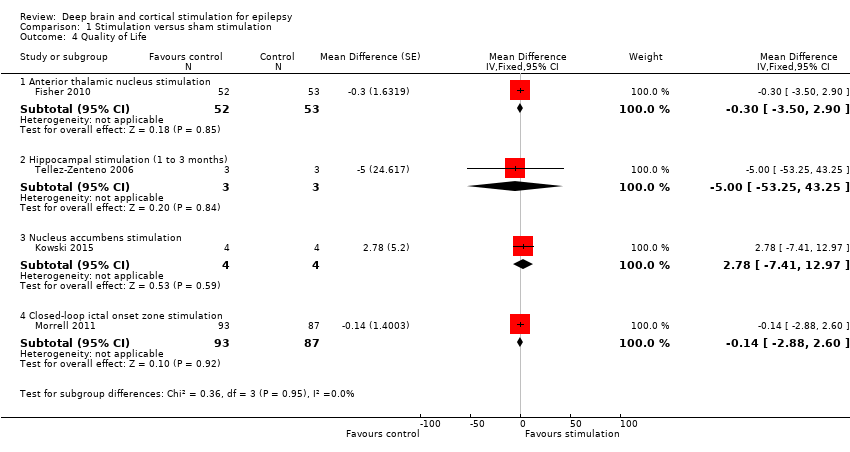 Comparison 1 Stimulation versus sham stimulation, Outcome 4 Quality of Life. | ||||
| 4.1 Anterior thalamic nucleus stimulation | 1 | 105 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐3.50, 2.90] |
| 4.2 Hippocampal stimulation (1 to 3 months) | 1 | 6 | Mean Difference (Fixed, 95% CI) | ‐5.0 [‐53.25, 43.25] |
| 4.3 Nucleus accumbens stimulation | 1 | 8 | Mean Difference (Fixed, 95% CI) | 2.78 [‐7.41, 12.97] |
| 4.4 Closed‐loop ictal onset zone stimulation | 1 | 180 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐2.88, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure freedom RR Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 1 Seizure freedom RR. | ||||
| 1.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.34 [0.01, 8.15] |
| 1.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.26, 3.52] |
| 1.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.25, 4.19] |
| 1.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.67 [0.04, 64.08] |
| 1.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 4.85 [0.24, 99.64] |
| 2 Responder rate RR Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 2 Responder rate RR. | ||||
| 2.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 2.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.38, 2.66] |
| 2.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.00 [0.51, 7.86] |
| 2.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.47, 2.66] |
| 2.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 5.00 [0.29, 87.54] |
| 2.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 4.00 [0.56, 28.40] |
| 2.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |
| 3 Seizure freedom OR 0.25 Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3 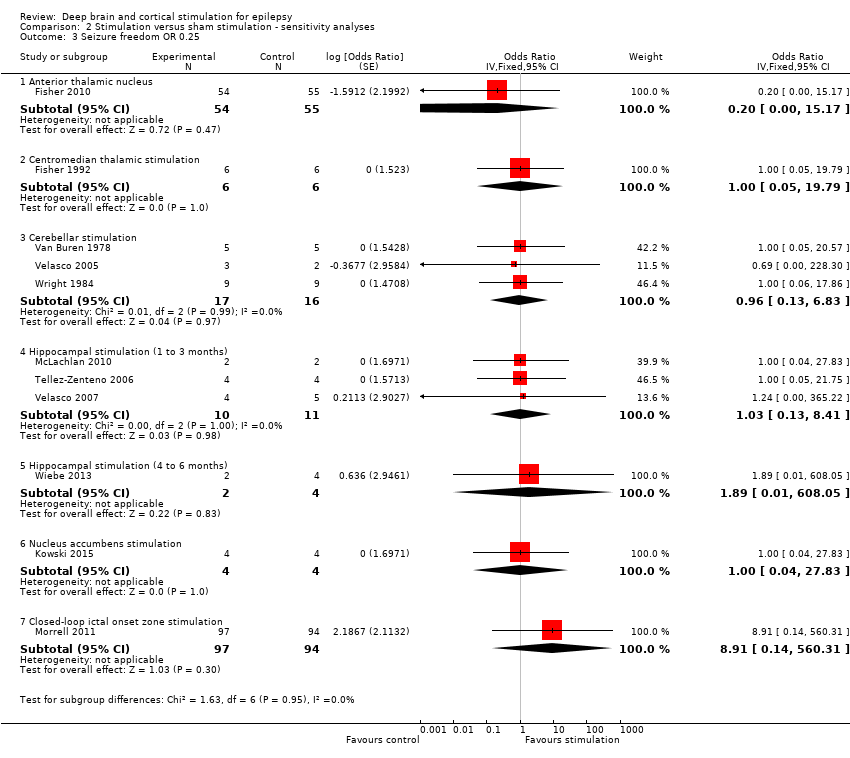 Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 3 Seizure freedom OR 0.25. | ||||
| 3.1 Anterior thalamic nucleus | 1 | 109 | Odds Ratio (Fixed, 95% CI) | 0.20 [0.00, 15.17] |
| 3.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.05, 19.79] |
| 3.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.13, 6.83] |
| 3.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.03 [0.13, 8.41] |
| 3.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 1.89 [0.01, 608.05] |
| 3.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.04, 27.83] |
| 3.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 8.91 [0.14, 560.31] |
| 4 Responder rate OR 0.25 Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 4 Responder rate OR 0.25. | ||||
| 4.1 Anterior thalamic nucleus | 1 | 108 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.52, 2.80] |
| 4.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.31, 3.24] |
| 4.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 2.98 [0.39, 22.77] |
| 4.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.15 [0.35, 3.77] |
| 4.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 17.00 [0.15, 1934.66] |
| 4.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 21.00 [0.51, 864.51] |
| 4.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 5 Seizure freedom RR 0.25 Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 5 Seizure freedom RR 0.25. | ||||
| 5.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.21 [0.00, 14.95] |
| 5.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.15, 6.04] |
| 5.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.16, 6.46] |
| 5.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.80 [0.01, 369.24] |
| 5.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 8.72 [0.14, 538.18] |
| 6 Responder rate RR 0.25 Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 6 Responder rate RR 0.25. | ||||
| 6.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 6.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.40, 2.52] |
| 6.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.28 [0.40, 13.02] |
| 6.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.08 [0.46, 2.55] |
| 6.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 9.00 [0.16, 494.41] |
| 6.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 7.00 [0.44, 111.91] |
| 6.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |

Study flow diagram.
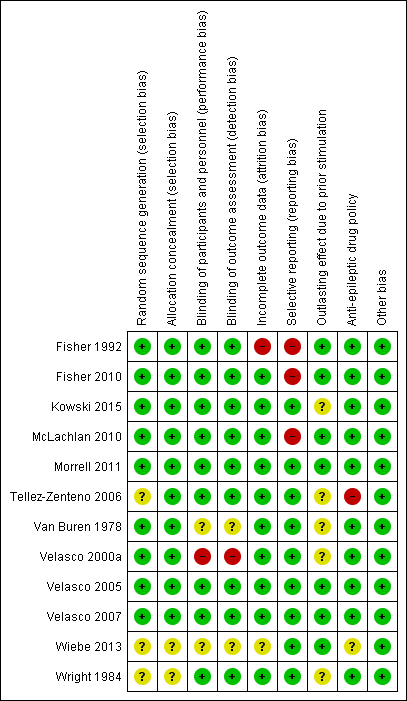
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.1 Seizure freedom.

Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.2 Responder rate.
![Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.3 Seizure frequency reduction.Note: Fisher 2010 (anterior thalamic nucleus stimulation) and Morrell 2011 (closed‐loop ictal onset zone stimulation) estimated the treatment effect and its standard error on a logarithmic scale, using the generalized estimating equation (GEE) model. As in this figure standard errors could not be inputted on the logarithmic scale, the values for the 95% confidence interval presented here differ slightly from the (more correct) values mentioned in the text. These correct values are ‐17.4% with 95% CI [‐31.2;‐1.0] for Fisher 2010 and ‐24.9% with 95% CI [‐40.1;‐6.0] for Morrell 2011.](/es/cdsr/doi/10.1002/14651858.CD008497.pub3/media/CDSR/CD008497/image_n/nCD008497-AFig-FIG05.png)
Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.3 Seizure frequency reduction.
Note: Fisher 2010 (anterior thalamic nucleus stimulation) and Morrell 2011 (closed‐loop ictal onset zone stimulation) estimated the treatment effect and its standard error on a logarithmic scale, using the generalized estimating equation (GEE) model. As in this figure standard errors could not be inputted on the logarithmic scale, the values for the 95% confidence interval presented here differ slightly from the (more correct) values mentioned in the text. These correct values are ‐17.4% with 95% CI [‐31.2;‐1.0] for Fisher 2010 and ‐24.9% with 95% CI [‐40.1;‐6.0] for Morrell 2011.

Forest plot of comparison: 1 Stimulation versus sham stimulation, outcome: 1.4 Quality of Life. To measure quality of life, Tellez‐Zenteno 2006 and Morrell 2011 used the QOLIE‐89 questionnaire, Fisher 2010 used the QOLIE‐31 questionnaire (= abbreviated form of the QOLIE‐89 questionnaire) and Kowski 2015 usde the QOLIE‐31‐P questionnaire (slightly modified version of the QOLIE‐31 questionnaire). These questionnaires have the same range and for the QOLIE‐89 and QOLIE‐31 questionnaires very similar means, standard deviations and minimum clinically important change values in the same population have been reported (Cramer 1998; Devinsky 1995; Wiebe 2002). For this reason results from the different trials are presented in one forest plot (see also Methods section). For the QOLIE‐89 and QOLIE‐31 questionnaires, improvements of 5‐11.7 have been defined in literature (Borghs 2012; Cramer 2004; Wiebe 2002) as being clinically meaningful, positive is better.

Comparison 1 Stimulation versus sham stimulation, Outcome 1 Seizure freedom.
Comparison 1 Stimulation versus sham stimulation, Outcome 2 Responder rate.
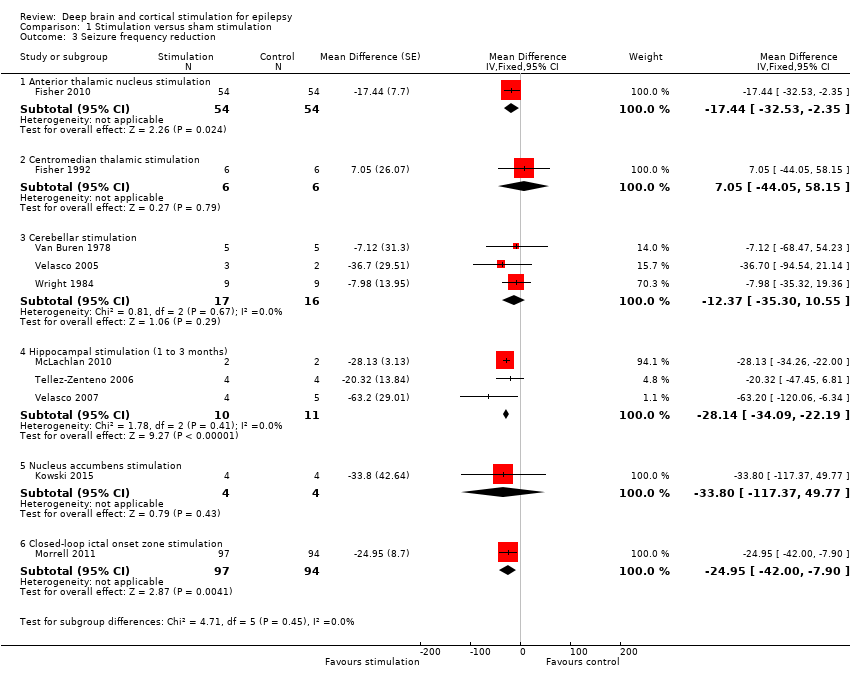
Comparison 1 Stimulation versus sham stimulation, Outcome 3 Seizure frequency reduction.
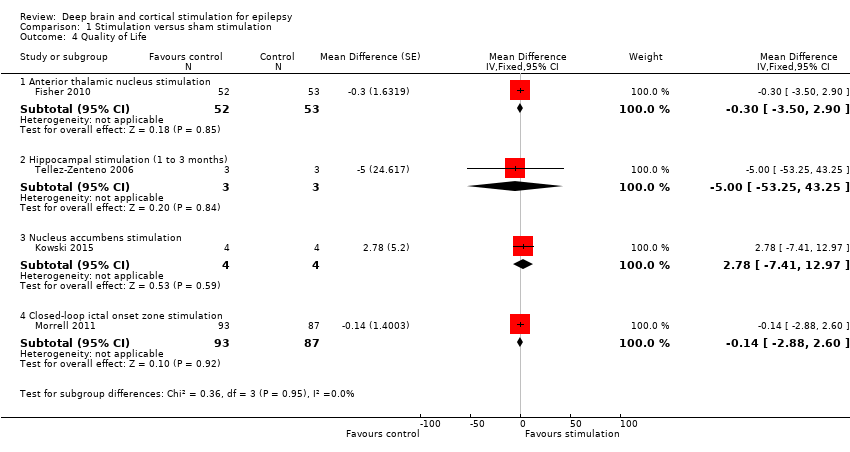
Comparison 1 Stimulation versus sham stimulation, Outcome 4 Quality of Life.
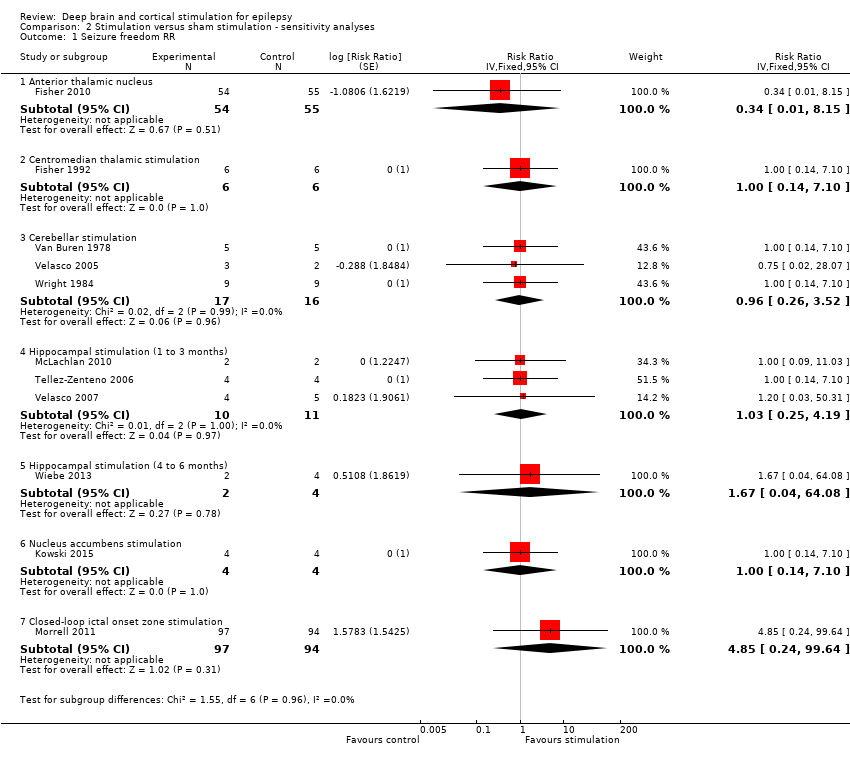
Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 1 Seizure freedom RR.

Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 2 Responder rate RR.

Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 3 Seizure freedom OR 0.25.
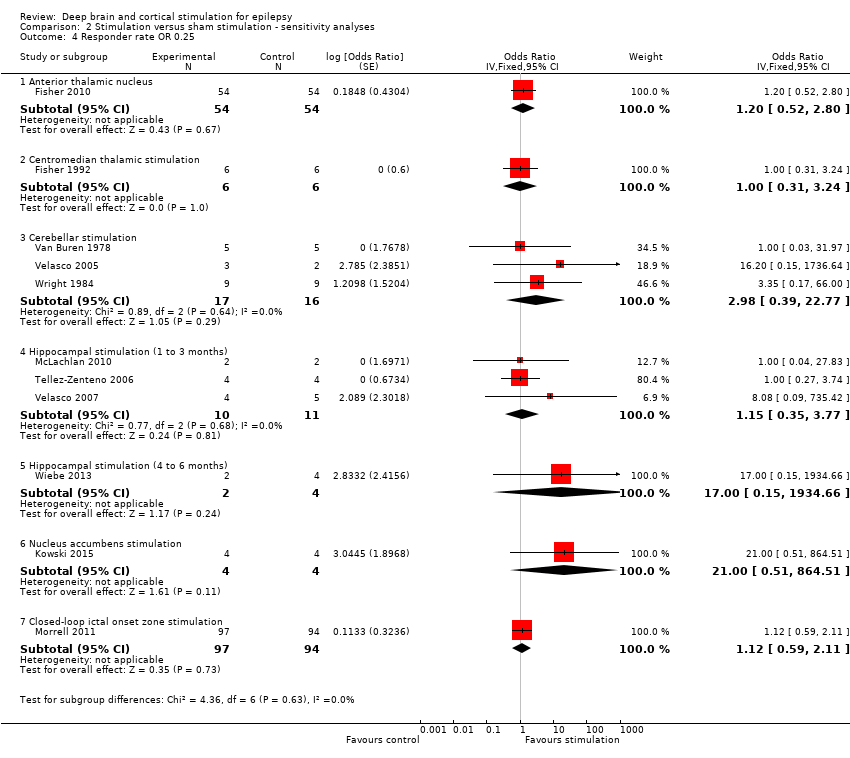
Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 4 Responder rate OR 0.25.

Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 5 Seizure freedom RR 0.25.
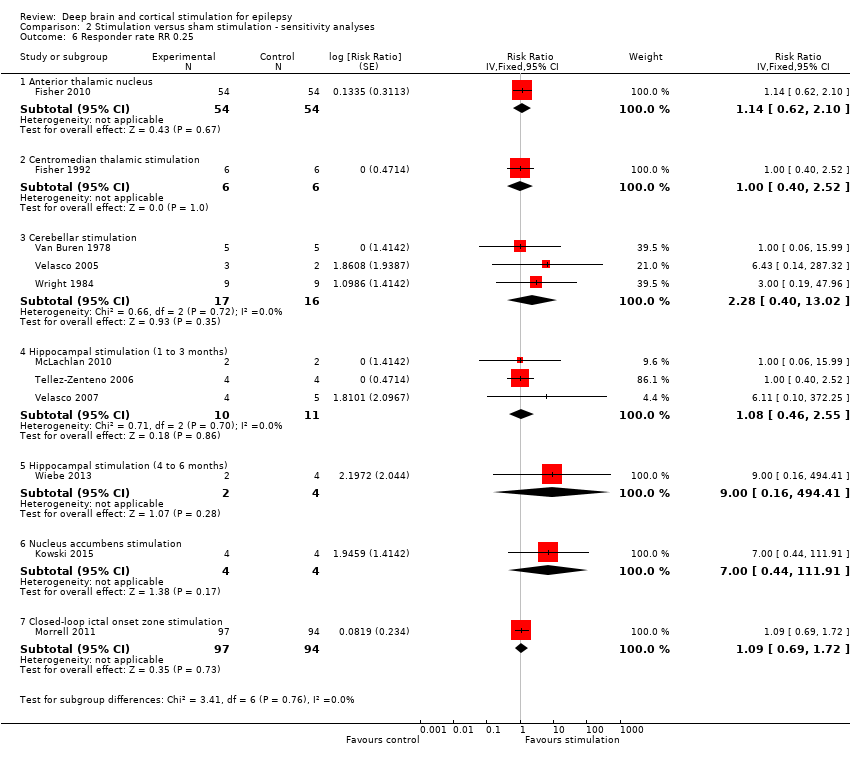
Comparison 2 Stimulation versus sham stimulation ‐ sensitivity analyses, Outcome 6 Responder rate RR 0.25.
| Anterior thalamic nucleus stimulation for refractory epilepsy | ||||||
| Patient or population: adults with IQ > 70 with refractory focal epilepsy Settings: epilepsy centres in the USA Intervention: anterior thalamic nucleus stimulation Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Anterior Thalamic Nucleus stimulation | |||||
| Seizure freedom (3‐month blinded evaluation period) | Observed inFisher 2010 | OR 0.33 (0.01 to 8.36) | 109 | ⊕⊕⊕⊝ | ||
| 1 per 55 | 0 per 54 | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 0 per 1000 | |||||
| High risk population1 | ||||||
| 15 per 1000 | 5 per 1000 | |||||
| Responder rate (3‐month blinded evaluation period) | 26 per 100 | 30 per 100 (15 to 49) | OR 1.20 (0.52 to 2.80) | 108 | ⊕⊕⊕⊝ | |
| Seizure frequency reduction (%) (3‐month blinded evaluation period) | Median monthly seizure frequency reductions ranged from ‐14.5 to ‐28.7% | The mean seizure frequency in the intervention group was | 108 (1) | ⊕⊕⊕⊕ | A trend for increasing efficacy over time was observed during the blinded evaluation period and could result into an underestimation of the treatment effect (treatment effect of month 3: ‐29%). | |
| Adverse events | See comment | See comment | 109 (1) | ⊕⊕⊕⊝ | Stimulation‐related adverse events during the blinded evaluation period include (stimulation versus control): depression (14.8 versus 1.8%, P = 0.02), subjective memory impairment (13.8 versus 1.8%, P = 0.03) and epilepsy‐related injuries (7.4 versus 25.5%, P = 0.01). Standard stimulation parameters could be inappropriate and increase seizure frequency in a small minority of patients.4 Asymptomatic intracranial haemorrhages occurred in 3.7% of participants after the initial implant procedure. In 8.2% of participants leads had to be replaced after initial implantation outside the target. Postoperative implant site infections occurred in 4.5% of participants, increasing to 12.7% after 5 years of follow‐up urging (temporary) hardware removal in 8.2% of participants. Implant site pain was not uncommon (year 1: 10.9%, year 5: 20.9%). SUDEP rate during long‐term (including open‐label) follow‐up was 2.9 per 1000 p‐y which is comparable to rates reported in refractory epilepsy populations (2.2‐10 per 1000 p‐y) (Tellez‐Zenteno 2005; Tomson 2008). | |
| Neuropsychological outcome (3 months) | See comment | See comment | 96‐100 (1) | ⊕⊕⊕⊝ | Changes in neuropsychological test scores for cognition and mood were very similar in the treatment and control group and not significantly different. Individual patient data show worsening (> 1 SD) of Profile of Mood States Depression subscale (POMS‐D) in 3/8 stimulated participants with self‐reported depression and 0/7 patients with subjective memory impairment showed worsening (> 1 SD) of verbal or visual memory scores. | |
| Quality of life (QOLIE‐31) (3 months) | The mean improvement of the QOLIE‐31 score in the control group was +2.8 higher | The mean improvement in QOLIE‐31 score in the intervention group was | 105 (1) | ⊕⊕⊕⊕ | Positive changes in QOLIE‐31 (quality of life in epilepsy 31) scores indicate improvement. Changes of 5‐11.7 have been defined in literature as being clinically meaningful (Borghs 2012; Cramer 2004; Wiebe 2002). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 More trials and patients are needed to allow more precise estimation of stimulation effects (including more rare adverse effects) (GRADE ‐1). 3 The confidence interval includes clinically non‐significant changes (GRADE ‐1), however, the observed trend for increasing efficacy over time probably underestimates the treatment effect (GRADE +1). 4 One participant experienced a spectacular seizure frequency increase after initiation of stimulation, which was reversible after lowering output voltage. New or worse seizures occurred more frequently in the stimulation group compared to the control group but differences did not reach statistical significance. 5 Although clinically meaningful differences in formal neuropsychological testing results seem unlikely on the group level, the discrepancy between objective and subjective measures needs further clarification (GRADE ‐1). | ||||||
| Centromedian thalamic nucleus stimulation for refractory epilepsy | ||||||
| Patient or population: patients with refractory (multi)focal or generalized epilepsy Settings: epilepsy centres in the USA and in Mexico Intervention: centromedian thalamic nucleus stimulation Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Centromedian thalamic nucleus stimulation | |||||
| Seizure freedom (3‐month blinded evaluation period) | Observed inFisher 1992 | OR 1.00 (0.11 to 9.39) | 6 (1)2 | ⊕⊝⊝⊝ | ||
| 0 per 6 | 0 per 6 | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| High risk population1 | ||||||
| 15 per 1000 | 15 per 1000 | |||||
| Responder rate (3‐month blinded evaluation period) | Low risk population1 | OR 1.00 (0.27 to 3.69) | 6 (1)2 | ⊕⊝⊝⊝ | ||
| 10 per 100 | 10 per 1000 | |||||
| Medium‐high risk population1 | ||||||
| 25 per 100 | 25 per 1000 | |||||
| Seizure frequency reduction (3‐month blinded evaluation period) | The mean seizure frequency reduction in the control group was ‐0.4% | The mean seizure frequency in the intervention groups was | 6 (1)2 | ⊕⊝⊝⊝ | Also another trial (Velasco 2000a) (n = 13) could not demonstrate significant differences between stimulation ON and OFF periods. However, its cross‐over design without any washout period could mask a possible treatment effect. | |
| Adverse events | See comment | See comment | 19 (2)2 21 (2)2 | ⊕⊕⊝⊝ | Stimulation‐related adverse events did not occur. Postoperative CT revealed an asymptomatic and minimal haemorrhage in one patient, 1 patient required repair of the connection to the pulse generator and skin erosion urged device explantation in 3 other patients (including 2 young children). | |
| Neuropsychological outcome (3 months) | See comment | See comment | 6 (1)2 | ⊕⊝⊝⊝ | There were no significant differences in any of the neuropsychological tests between baseline, stimulation ON and OFF periods. | |
| Quality of life | See comment | See comment | 0 (0) | See comment | Impact of centromedian thalamic nucleus stimulation on quality of life has not been studied yet. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low, medium and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 Cross‐over trial(s). 3 No more than one small RCT was identified, resulting into wide 95% confidence intervals (GRADE score ‐2). This is of particular concern for neuropsychological outcome, as no exact figures were reported or could be provided, so evaluation of certain statistically non‐significant trends is not possible. 4 Only 2 hours of intermittent stimulation per day in Fisher 1992 (GRADE score ‐1). 5 Incomplete outcome data may introduce bias (GRADE score ‐1). 6 Number of participants too low to identify less frequent adverse events (GRADE score ‐1) | ||||||
| Cerebellar stimulation for refractory epilepsy | ||||||
| Patient or population: patients with refractory (multi)focal or generalized epilepsy Settings: epilepsy centres in the USA and in Mexico Intervention: stimulation of the superomedial surface of the cerebellum Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Cerebellar stimulation | |||||
| Seizure freedom (1‐ to 3‐month blinded evaluation period) | Observed | OR 0.96 (0.22 to 4.12) | 22 (3)2 | ⊕⊕⊕⊝ | ||
| 0 per 19 | 0 per 20 (not estimable) | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| High risk population1 | ||||||
| 15 per 1000 | 14 per 1000 | |||||
| Responder rate (1‐ to 3‐month blinded evaluation period) | Low risk population1 | OR 2.43 (0.46 to 12.84) | 19 (3)2 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 21 per 100 | |||||
| Medium‐high risk population1 | ||||||
| 25 per 100 | 45 per 100 | |||||
| Seizure frequency reduction (1‐ to 3‐month blinded evaluation period) | The mean seizure frequency reduction ranged across control groups from 0 to ‐18.8% | The mean seizure frequency in the intervention groups was | 19 (3)2 | ⊕⊕⊝⊝ | ||
| Adverse events | See comment | See comment | 22 (3)2 | ⊕⊕⊝⊝ | Stimulation‐related adverse events were not reported in any of the trials. In contrast, about half of the patients in every trial required repeated surgery due to electrode migration (n = 6), leakage of cerebrospinal fluid (n = 3), wound infection (n = 1), skin erosion (n = 2), lead problems (n = 1), subcutaneous seroma drainage (n = 1) and defective hardware (n = 1). Wound infections were solved with antibiotics only in 2 additional patients. In particular, electrode migration remains of specific concern, even in the most recent trial (Velasco 2005) (occurring in 3/5 patients). | |
| Neuropsychological outcome (1 to 2 months) | See comment | See comment | 16 (2)2 | ⊕⊝⊝⊝ | 'Psychometry' did not reveal any major change in any patient in any phase of the Wright 1984 trial. Comparing ON to OFF stimulation full scale intelligence and memory scores in Van Buren 1978 showed very similar results in two participants, a moderate increase in one patient and a moderate decrease in another. | |
| Quality of life (2 months) | See comment | See comment | 12 (1)7 | ⊕⊝⊝⊝ | Eleven out of 12 patients in Wright 1984 felt better for cerebellar stimulation, but only 5 chose one phase as being different from the others, being either the continuous (n = 2), contingent (n = 1) or no‐stimulation (n = 2) phase. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low, medium and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 Including 2 cross‐over trials: Van Buren 1978 (n = 4‐5) and Wright 1984 (n = 9‐12) 3 The small number of patients leave a considerable amount of uncertainty with regards to stimulation effects (GRADE ‐1). 4Wright 1984 and Van Buren 1978 are cross‐over trials without any washout period which could mask or reduce potential benefits of cerebellar stimulation (and explain some heterogeneity) (GRADE ‐1). 5 Unclear if, how and to what extent stimulation‐related side effects were evaluated in Van Buren 1978 and Wright 1984 (GRADE ‐1). 6 Unclear what neuropsychological tests were performed in Wright 1984 ('psychometry'). Moreover, as testing scores were not published and could not be provided, evaluation of certain statistically non‐significant trends is not possible. Unclear if neuropsychological testing in Van Buren 1978 was done in blinded or unblinded evaluation periods (GRADE‐1). 7 Cross‐over trial: Wright 1984 (n = 12). 8 No formal scoring of quality of life but evaluation of patients' impressions on cerebellar stimulation (GRADE ‐1). | ||||||
| Hippocampal stimulation for refractory epilepsy | ||||||
| Patient or population: patients with refractory medial temporal lobe epilepsy Settings: epilepsy centres in Canada and in Mexico Intervention: hippocampal deep brain stimulation Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Hippocampal stimulation | |||||
| Seizure freedom (1‐ to 3‐month blinded evaluation periods) | Observed | OR 1.03 | 15 (3)2 | ⊕⊕⊕⊝ | Also in Wiebe 20134 no single patient achieved seizure freedom after six months of hippocampal active or sham stimulation. | |
| 0 per 11 | 0 per 10 | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| High risk population1 | ||||||
| 15 per 1000 | 15 per 1000 | |||||
| Responder rate (1‐ to 3‐month blinded evaluation periods) | Low risk population1 | OR 1.20 (0.36 to 4.01) | 15 (3)2 | ⊕⊕⊝⊝ | In Wiebe 20134there was one responder in the stimulation group (n = 2) compared to none in the sham group (n = 4) after six months of follow‐up. | |
| 10 per 100 | 12 per 100 | |||||
| Medium‐high risk population1 | ||||||
| 25 per 100 | 29 per 100 | |||||
| Seizure frequency (1‐ to 3‐month blinded evaluation periods) | The mean change in seizure frequency ranged across control groups from ‐4.7% to +33.7% | The mean seizure frequency in the intervention groups was | 15 (3)2 | ⊕⊕⊕⊝ | One trial (Tellez‐Zenteno 2006) has a cross‐over design without any washout period which could result into an underestimation of the true treatment effect. In Wiebe 20134 the sham stimulation group reported a median seizure frequency increase of 60% compared to a 45% decrease in the stimulation group after 6 months of follow‐up. | |
| Adverse events | See comment | See comment | 15 (3)2 | ⊕⊕⊝⊝ | There were neither stimulation‐related adverse events, nor early surgical complications. Skin erosion and local infection required explantation after >2 years in 3/9 patients in Velasco 2007. Wiebe 20134 also did not report any adverse event after 6 months of follow‐up. | |
| Neuropsychological outcome (1‐ to 3‐month periods) | See comment | See comment | 6 (2)2 | ⊕⊝⊝⊝ | Neuropsychological test results were the same or very similar during stimulation ON and OFF periods in Tellez‐Zenteno 2006 (n = 4) and in one patient in McLachlan 2010. The other patient in McLachlan 2010 showed worse verbal and visuospatial memory scores when stimulated, notwithstanding that he reported subjective memory improvement during the same period. At seven months in Wiebe 20134, scores of cognitive scales assessing recall (Rey Auditory Verbal Learning Test, Rey Complex Figure Test) were generally lower in the active stimulation compared to the sham group (p>0.05). | |
| Quality of life (QOLIE‐89) (1‐ to 3‐month periods) | The mean QOLIE‐89 score in the control group was 60 | The mean QOLIE‐89 in the intervention group was ‐5 lower (‐53 lower to +43 higher). | 3 (1)7 | ⊕⊝⊝⊝ | Positive changes in QOLIE‐89 (quality of life in epilepsy 89) scores indicate improvement. Changes of 5‐11.7 have been defined in literature as being clinically meaningful (Borghs 2012; Cramer 2004; Wiebe 2002). The overall QOLIE‐89 score at seven months in Wiebe 20134 worsened by 13 points with sham stimulation compared to an improvement of 3 points with active stimulation (p>0.05), and there was a trend for increased QOLIE‐89 subjective memory and attention/concentration scores. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low, medium and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 Including two cross‐over trials: McLachlan 2010 (n = 2) and Tellez‐Zenteno 2006 (n = 4) 3 The small number of patients preclude more definitive judgements on effects of hippocampal stimulation (GRADE ‐1). 4Wiebe 2013 is a small parallel‐group RCT (n = 6) with a 6‐month blinded evaluation period. As there were no more than 2 participants in the active stimulation group and details needed for full methodological assessment are missing, the quality of the evidence is very low and we decided not to create separate 6‐month outcomes or a separate summary of findings table but only to describe the results. As the results of the first 3‐month epoch were not reported, the data of this trial could not be combined with the other trials evaluating one to three months of hippocampal stimulation. However, the reported six‐month results are generally compatible and in line with the estimated three‐month results. For more details and a sensitivity analysis combining all trials on hippocampal stimulation irrespective of the BEP duration, see text. 5 One trial (Tellez‐Zenteno 2006) had a cross‐over design without any washout period and allowed important changes in antiepileptic drugs, both of which could reduce or mask more important treatment effects. See also 'Sensitivity analyses' (GRADE ‐1). 6 Number of patients is too low to identify less frequent adverse events or changes in neuropsychological outcome or quality of life (GRADE‐score ‐2). 7 One cross‐over trial: Tellez‐Zenteno 2006 (n = 3) | ||||||
| Nucleus accumbens stimulation for refractory epilepsy | ||||||
| Patient or population: adults with IQ >70 with refractory focal epilepsy Settings: epilepsy centre in Germany Intervention: nucleus accumbens stimulation Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Nucleus accumbens stimulation | |||||
| Seizure freedom (3‐month blinded evaluation period) | Observed inKowski 2015 | OR 1.00 (0.07 to 13.64) | 4 (1)2 | ⊕⊕⊝⊝ | ||
| 0 per 4 | 0 per 4 (not estimable) | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| High risk population1 | ||||||
| 15 per 1000 | 15 per 1000 (0 to 172) | |||||
| Responder rate (3‐month blinded evaluation period) | Low risk population1 | OR 10.0 (0.53 to 189.15) | 4 (1)2 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 53 per 100 | |||||
| Medium risk population1 | ||||||
| 25 per 100 | 77 per 100 | |||||
| Seizure frequency reduction (3‐month blinded evaluation period) | The mean change in seizure frequency in the control group was ‐13.8% | The mean seizure frequency in the intervention group was (‐100% lower to +49.8% higher) | 4 (1)2 | ⊕⊕⊝⊝ | When focusing on 'disabling seizures' only and excluding simple partial seizures (occurring in one patient), the mean change in seizure frequency in the control group was +8.2% with a ‐22.9% lower seizure frequency in the intervention group (‐100 lower to +94.0 higher) | |
| Adverse events | See comment | See comment | 4 (1)2 | ⊕⊕⊝⊝ | Except for one patient feeling sad for two weeks during the active stimulation period after a close relative had died, there were no adverse events that were exclusively linked to the active stimulation period (although various adverse events were reported in the sham and the active stimulation group, see text). One patient developed a local subcutaneous infection with colonization of the pulse generator and the leads 2 weeks post‐surgery urging antibiotic therapy and temporary hardware removal. | |
| Neuropsychological outcome (3 months) | See comment | See comment | 4 (1)2 | ⊕⊕⊝⊝ | Neurocognitive test scores were similar and not statistically significantly different during sham and active stimulation in this small trial. There were no categorical changes in Beck‐Depression‐Inventory scores during the BEP. However, the Mini International Neuropsychiatric Interview revealed a new‐onset major depression under nucleus accumbens stimulation in one patient, besides an ongoing low suicidal risk following one suicide attempt 10 years before the trial in another patient. | |
| Quality of Life (QOLIE‐31‐P) (3 months) | The mean change in the QOLIE‐31‐P score in the control group was ‐4.9 lower | The mean change in the QOLIE‐31‐P score in the intervention group was +2.8 higher (‐7.4 lower to +13.0 higher) | 4 (1)2 | ⊕⊕⊝⊝ | The QOLIE‐31‐P is a (slightly) modified version of the QOLIE‐31 questionnaire for which changes of 5 to 11.7 have been defined in the literature (Cramer 2004; Wiebe 2002; Borghs 2012) as being clinically meaningful; positive scores indicate improvement. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low, medium and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 Cross‐over trial 3No more than one small RCT was identified which leaves a considerable amount of uncertainty with regards to stimulation effects (GRADE score ‐2). | ||||||
| Closed‐loop stimulation of the ictal onset zone for refractory epilepsy | ||||||
| Patient or population: adults with refractory focal epilepsy (1 or 2 epileptogenic regions) Settings: epilepsy centres in the USA Intervention: responsive stimulation of the ictal onset zone(s) Comparison: sham stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham stimulation | Responsive ictal onset zone stimulation | |||||
| Seizure freedom (3‐month blinded evaluation period) | Observed inMorrell 2011 | OR 4.95 (0.23 to 104.44) | 191 (1) | ⊕⊕⊕⊝ | ||
| 0 per 94 | 2 per 97 (not estimable) | |||||
| Low risk population1 | ||||||
| 1 per 1000 | 5 per 1000 (0 to 95) | |||||
| High risk population1 | ||||||
| 15 per 1000 | 70 per 1000 | |||||
| Responder rate (3‐month blinded evaluation period) | 27 per 100 | 29 per 100 | OR 1.12 (0.59 to 2.11) | 191 (1) | ⊕⊕⊕⊝ | |
| Seizure frequency reduction (3‐month blinded evaluation period) | The mean estimated seizure frequency reduction in the control group was ‐17.3% | The mean seizure frequency in the intervention group was | 191 (1) | ⊕⊕⊕⊕ | A trend for increasing efficacy over time was observed during the blinded evaluation period and could result into an underestimation of the treatment effect (treatment effect of month 3: ‐32%). | |
| Adverse events | See comment | See comment | 191 (1) 256 (2) | ⊕⊕⊕⊝ | Adverse events during the blinded evaluation period were rare and there were no significant differences between the treatment and control group. Asymptomatic intracranial haemorrhages considered as serious adverse event were found postoperatively in 1.6% of participants. Postoperative implant or incision site infection occurred in 2.0% of participants, increasing to 9.4% of participants after 5 years of follow‐up (additional cases mainly upon battery replacement; urge for (temporary) explantation in the majority of cases). Cranial implantation of the neurostimulator was the probable cause of most adverse events, which include: implant site pain (16% during the first year of the trial), headache (11%), procedural headache (9%) and dysaesthesia (6%). Although the SUDEP rate (4 SUDEPs over 340 patient‐years = 11.8 per 1000 patient‐years) reported in the initial manuscript was slightly higher than those usually reported in refractory epilepsy patients (2.2 to 10 per 1000 p‐y) (Tellez‐Zenteno 2005; Tomson 2008), long‐term open‐label follow‐up has now reported reassuring figures (SUDEP rates of 3.5 per 1000 implant p‐y or 2.6 per 1000 stimulation p‐y). | |
| Neuropsychological outcome (3 months) | See comment | See comment | 160‐177 | ⊕⊕⊕⊕ | Changes in neuropsychological testing results were very similar in both groups and 95% confidence intervals did not include clinically meaningful differences. | |
| Quality of life (QOLIE‐89) (3 months) | The mean improvement of the QOLIE‐31 score in the control group was +2.18 higher | The mean improvement in QOLIE‐31 score in the intervention group was | 180 | ⊕⊕⊕⊕ | Positive changes in QOLIE‐89 (quality of life in epilepsy 89) scores indicate improvement. Changes of 5‐11.7 have been defined in literature as being clinically meaningful (Borghs 2012; Cramer 2004; Wiebe 2002). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risks (low and high) are based on the range of the number of events observed in the sham stimulation control groups of all RCTs evaluating deep brain and cortical stimulation in refractory epilepsy patients 2 More trials and patients are needed to allow more precise estimation of stimulation effects (GRADE ‐1). 3 The confidence interval includes clinically non‐significant changes (GRADE ‐1), however, the observed trend for increasing efficacy over time probably underestimates the treatment effect (GRADE +1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure freedom Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Anterior thalamic nucleus | 1 | 109 | Odds Ratio (Fixed, 95% CI) | 0.33 [0.01, 8.36] |
| 1.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.11, 9.39] |
| 1.3 Cerebellar stimulation | 3 | 39 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.22, 4.12] |
| 1.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.03 [0.21, 5.15] |
| 1.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 1.80 [0.03, 121.68] |
| 1.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.07, 13.64] |
| 1.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 4.95 [0.23, 104.44] |
| 2 Responder rate Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Anterior thalamic nucleus | 1 | 108 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.52, 2.80] |
| 2.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.27, 3.69] |
| 2.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 2.43 [0.46, 12.84] |
| 2.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.36, 4.01] |
| 2.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 9.00 [0.22, 362.46] |
| 2.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 10.00 [0.53, 189.15] |
| 2.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 3 Seizure frequency reduction Show forest plot | 10 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior thalamic nucleus stimulation | 1 | 108 | Mean Difference (Fixed, 95% CI) | ‐17.44 [‐32.53, ‐2.35] |
| 3.2 Centromedian thalamic stimulation | 1 | 12 | Mean Difference (Fixed, 95% CI) | 7.05 [‐44.05, 58.15] |
| 3.3 Cerebellar stimulation | 3 | 33 | Mean Difference (Fixed, 95% CI) | ‐12.37 [‐35.30, 10.55] |
| 3.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Mean Difference (Fixed, 95% CI) | ‐28.14 [‐34.09, ‐22.19] |
| 3.5 Nucleus accumbens stimulation | 1 | 8 | Mean Difference (Fixed, 95% CI) | ‐33.8 [‐117.37, 49.77] |
| 3.6 Closed‐loop ictal onset zone stimulation | 1 | 191 | Mean Difference (Fixed, 95% CI) | ‐24.95 [‐42.00, ‐7.90] |
| 4 Quality of Life Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Anterior thalamic nucleus stimulation | 1 | 105 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐3.50, 2.90] |
| 4.2 Hippocampal stimulation (1 to 3 months) | 1 | 6 | Mean Difference (Fixed, 95% CI) | ‐5.0 [‐53.25, 43.25] |
| 4.3 Nucleus accumbens stimulation | 1 | 8 | Mean Difference (Fixed, 95% CI) | 2.78 [‐7.41, 12.97] |
| 4.4 Closed‐loop ictal onset zone stimulation | 1 | 180 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐2.88, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure freedom RR Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.34 [0.01, 8.15] |
| 1.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.26, 3.52] |
| 1.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.25, 4.19] |
| 1.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.67 [0.04, 64.08] |
| 1.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] |
| 1.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 4.85 [0.24, 99.64] |
| 2 Responder rate RR Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 2.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.38, 2.66] |
| 2.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.00 [0.51, 7.86] |
| 2.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.12 [0.47, 2.66] |
| 2.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 5.00 [0.29, 87.54] |
| 2.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 4.00 [0.56, 28.40] |
| 2.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |
| 3 Seizure freedom OR 0.25 Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior thalamic nucleus | 1 | 109 | Odds Ratio (Fixed, 95% CI) | 0.20 [0.00, 15.17] |
| 3.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.05, 19.79] |
| 3.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.13, 6.83] |
| 3.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.03 [0.13, 8.41] |
| 3.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 1.89 [0.01, 608.05] |
| 3.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.04, 27.83] |
| 3.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 8.91 [0.14, 560.31] |
| 4 Responder rate OR 0.25 Show forest plot | 11 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Anterior thalamic nucleus | 1 | 108 | Odds Ratio (Fixed, 95% CI) | 1.20 [0.52, 2.80] |
| 4.2 Centromedian thalamic stimulation | 1 | 12 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.31, 3.24] |
| 4.3 Cerebellar stimulation | 3 | 33 | Odds Ratio (Fixed, 95% CI) | 2.98 [0.39, 22.77] |
| 4.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Odds Ratio (Fixed, 95% CI) | 1.15 [0.35, 3.77] |
| 4.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Odds Ratio (Fixed, 95% CI) | 17.00 [0.15, 1934.66] |
| 4.6 Nucleus accumbens stimulation | 1 | 8 | Odds Ratio (Fixed, 95% CI) | 21.00 [0.51, 864.51] |
| 4.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Odds Ratio (Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 5 Seizure freedom RR 0.25 Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Anterior thalamic nucleus | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.21 [0.00, 14.95] |
| 5.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.15, 6.04] |
| 5.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.16, 6.46] |
| 5.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 1.80 [0.01, 369.24] |
| 5.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.06, 15.99] |
| 5.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 8.72 [0.14, 538.18] |
| 6 Responder rate RR 0.25 Show forest plot | 11 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 6.1 Anterior thalamic nucleus | 1 | 108 | Risk Ratio (Fixed, 95% CI) | 1.14 [0.62, 2.10] |
| 6.2 Centromedian thalamic stimulation | 1 | 12 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.40, 2.52] |
| 6.3 Cerebellar stimulation | 3 | 33 | Risk Ratio (Fixed, 95% CI) | 2.28 [0.40, 13.02] |
| 6.4 Hippocampal stimulation (1 to 3 months) | 3 | 21 | Risk Ratio (Fixed, 95% CI) | 1.08 [0.46, 2.55] |
| 6.5 Hippocampal stimulation (4 to 6 months) | 1 | 6 | Risk Ratio (Fixed, 95% CI) | 9.00 [0.16, 494.41] |
| 6.6 Nucleus accumbens stimulation | 1 | 8 | Risk Ratio (Fixed, 95% CI) | 7.00 [0.44, 111.91] |
| 6.7 Closed‐loop ictal onset zone stimulation | 1 | 191 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.69, 1.72] |

