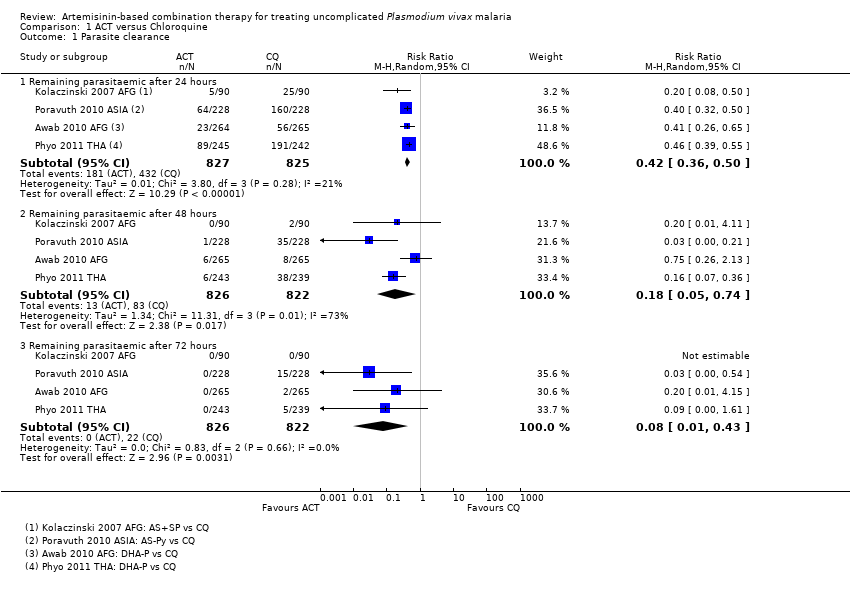
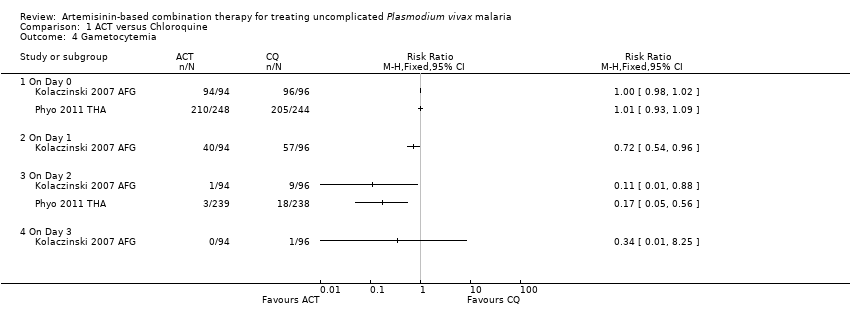
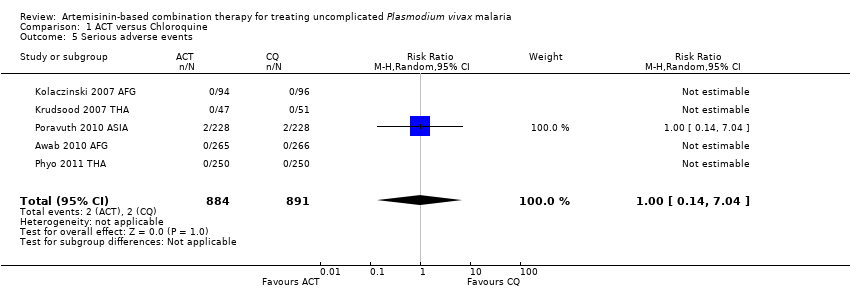
| 1 Remaining parasitemic after 24 hours Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 1.1 DHA‐P versus AS+MQ | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.28, 4.92] |
| 1.2 DHA‐P versus AL6 | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.94] |
| 1.3 AS+MQ versus AL6 | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.60, 1.72] |
| 1.4 DHA‐P versus AS+SP | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.49, 3.72] |
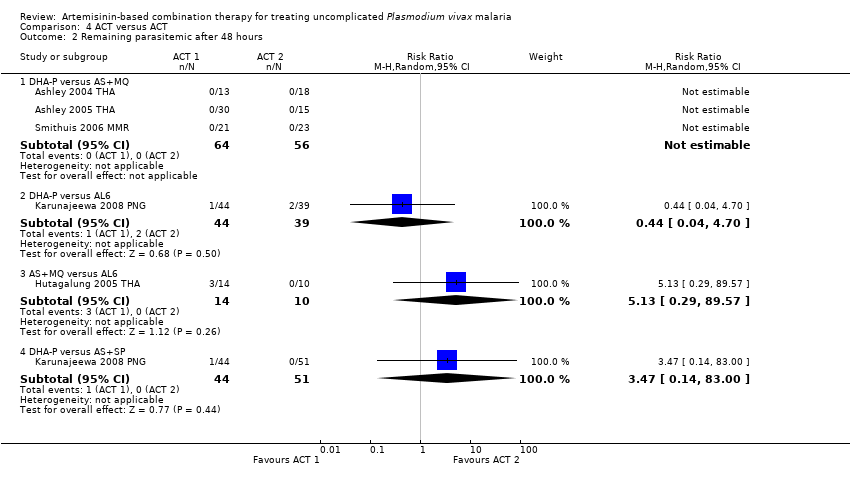
| 2 Remaining parasitemic after 48 hours Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 2.1 DHA‐P versus AS+MQ | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 DHA‐P versus AL6 | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.04, 4.70] |
| 2.3 AS+MQ versus AL6 | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 5.13 [0.29, 89.57] |
| 2.4 DHA‐P versus AS+SP | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 3.47 [0.14, 83.00] |
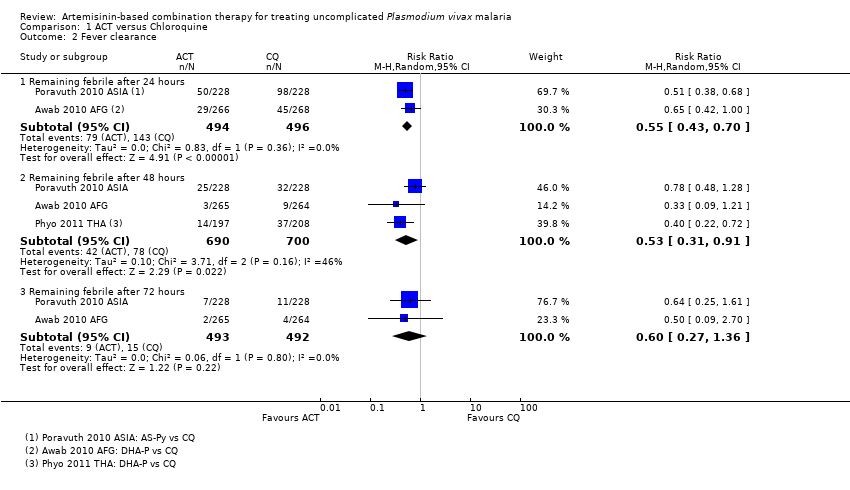
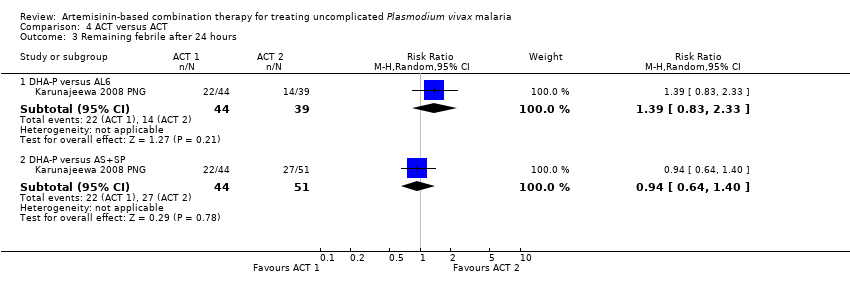
| 3 Remaining febrile after 24 hours Show forest plot | 1 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 3.1 DHA‐P versus AL6 | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.83, 2.33] |
| 3.2 DHA‐P versus AS+SP | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.64, 1.40] |
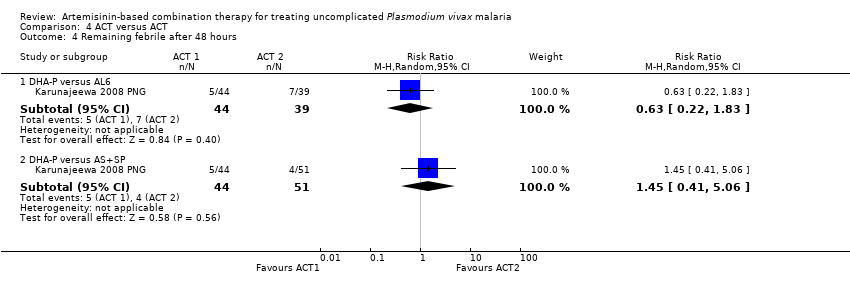
| 4 Remaining febrile after 48 hours Show forest plot | 1 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 4.1 DHA‐P versus AL6 | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.83] |
| 4.2 DHA‐P versus AS+SP | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.41, 5.06] |
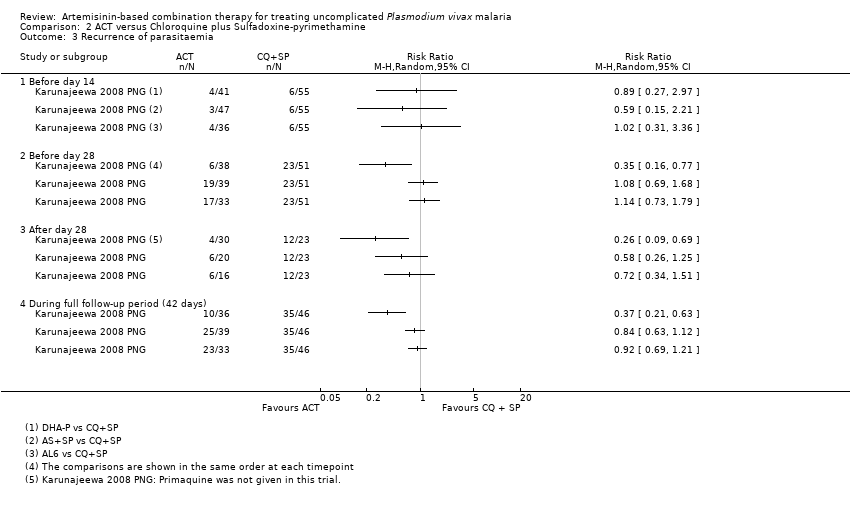
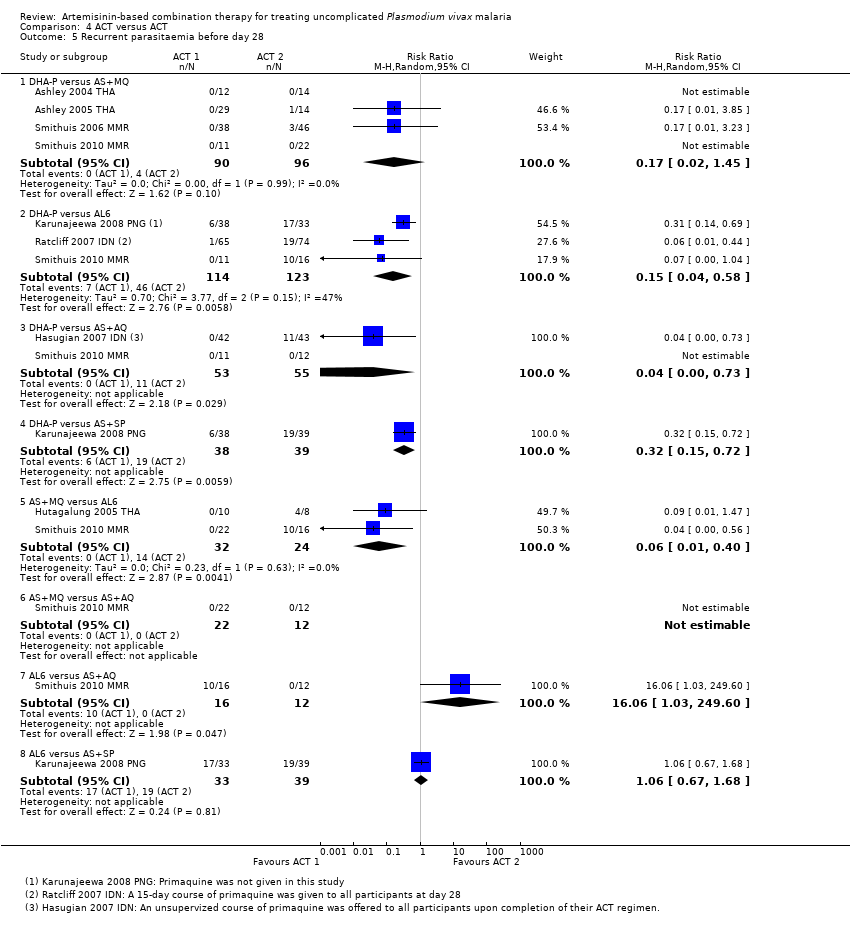
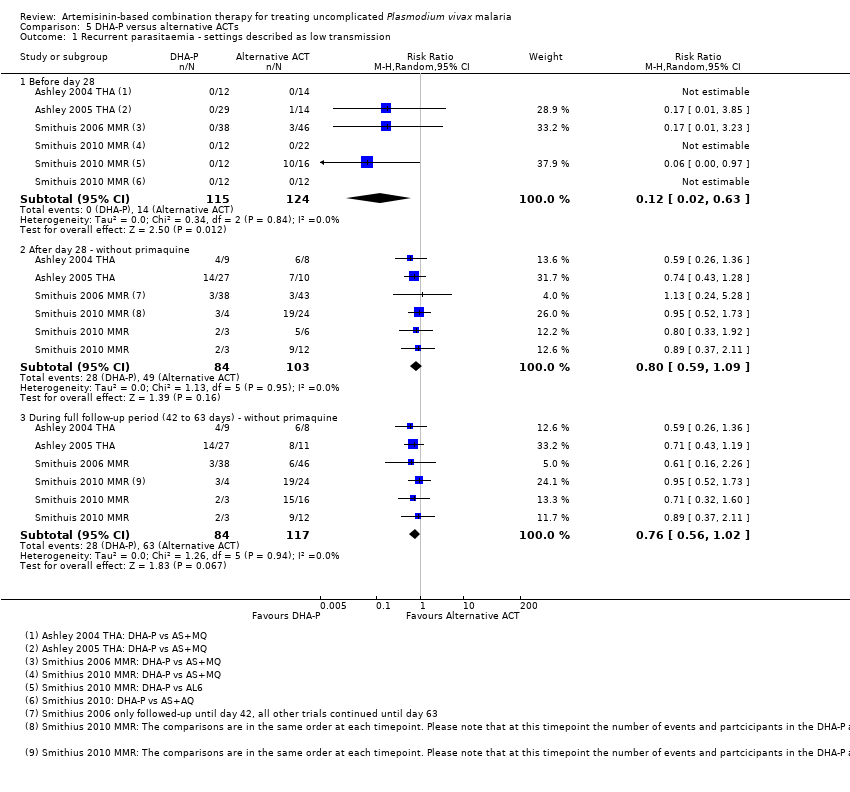
| 5 Recurrent parasitaemia before day 28 Show forest plot | 8 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 5.1 DHA‐P versus AS+MQ | 4 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.45] |
| 5.2 DHA‐P versus AL6 | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.58] |
| 5.3 DHA‐P versus AS+AQ | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 5.4 DHA‐P versus AS+SP | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.72] |
| 5.5 AS+MQ versus AL6 | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.40] |
| 5.6 AS+MQ versus AS+AQ | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 AL6 versus AS+AQ | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 16.06 [1.03, 249.60] |
| 5.8 AL6 versus AS+SP | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.68] |
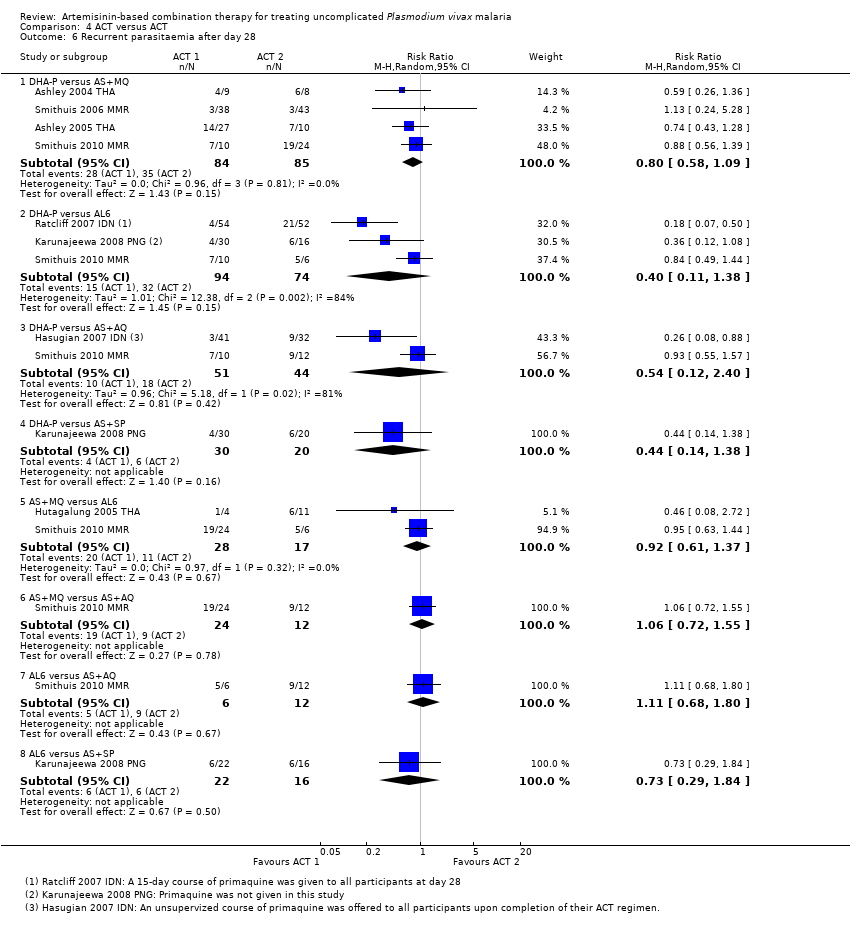
| 6 Recurrent parasitaemia after day 28 Show forest plot | 8 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 6.1 DHA‐P versus AS+MQ | 4 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.09] |
| 6.2 DHA‐P versus AL6 | 3 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.11, 1.38] |
| 6.3 DHA‐P versus AS+AQ | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.12, 2.40] |
| 6.4 DHA‐P versus AS+SP | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.14, 1.38] |
| 6.5 AS+MQ versus AL6 | 2 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.37] |
| 6.6 AS+MQ versus AS+AQ | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.72, 1.55] |
| 6.7 AL6 versus AS+AQ | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.68, 1.80] |
| 6.8 AL6 versus AS+SP | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.29, 1.84] |
| 7 Recurrent parasitaemia during full follow‐up period (0 to 42 or 63 days) Show forest plot | 8 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 7.1 DHA‐P versus AS+MQ | 4 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.45] |
| 7.2 DHA‐P versus AL6 | 3 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.58] |
| 7.3 DHA‐P versus AS+AQ | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 7.4 DHA‐P versus AS+SP | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.15, 0.72] |
| 7.5 AS+MQ versus AL6 | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.40] |
| 7.6 AS+MQ versus AS+AQ | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 AL6 versus AS+AQ | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 16.06 [1.03, 249.60] |
| 7.8 AL6 versus AS+SP | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.68] |