Davanje nadomjestaka vitamina K kod cistične fibroze
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised cross‐over trial (2 periods of 4 weeks). | |
| Participants | Randomised: N = 18 (8 male, 10 female); mean age 20 years (range 13 ‐ 35 years). Inclusion criteria
Exclusion criteria
Withdrawal or loss to follow‐up: none reported. | |
| Interventions | Intervention: 5 mg oral vitamin K1 supplementation per week. Control: no supplementation. 4 weeks of first treatment then crossed over to the other treatment for a second 4‐week period. Concomitant medications permitted: cephalosporin (13); sulfamethoxazole (3); erythromycin (1); bronchodilators; standard multivitamins and 200 ‐ 400 IU vitamin E. | |
| Outcomes | Primary outcomes: none reported Secondary outcomes (assessments at entry and end of each trial period)
3‐day dietary intake records were completed during each treatment period, but these did not correspond with the nutritional parameters sought as secondary outcomes for this review. Patient compliance was verified by the trial coordinator at each visit. | |
| Notes | Randomised cross‐over trial, vitamin K supplementation compared with no treatment. No wash‐out period with a potential carry‐over of treatment effect. No first‐period data available. "Supported in part by grants from the Board of Lady Visitors, Children's National Medical Center, Washington, DC, and the University of Maryland, College Park, Maryland." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" Page 512 |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Participants: not possible; control was 'no treatment'. Healthcare providers: not possible; control was 'no treatment'. Outcomes assessors and data analysts: unclear. Comment: overall judgement unclear. |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals and no missing or incomplete data. |
| Selective reporting (reporting bias) | Low risk | Although the protocol was not available all relevant outcomes appear to have been addressed. |
| Other bias | Unclear risk | Quote: "Supported in part by grants from the Board of Lady Visitors, Children's National Medical Center, Washington, DC, and the University of Maryland, College Park, Maryland." |
| Methods | Randomised control trial over 1‐month period. | |
| Participants | Randomised: N = 14; 8 to 18 years, gender unspecified. Inclusion criteria
Exclusion criteria:
Withdrawal or loss to follow‐up: missing data (1) from 5 mg group at final assessment. | |
| Interventions | Intervention: oral administration of injectable formulation of vitamin K1 phytonadione (Sandoz Canada, Boucherville, Qc) diluted 1 mg/1 ml. Dose 1 mg/day for 1 month. | |
| Outcomes | Primary outcomes: none reported Secondary outcomes
Measured at the beginning of the trial and at the end of 1 month. | |
| Notes | This project was funded by the Canadian CF Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomised to receive either 1 mg/day, or 5 mg/day" Page 458. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Participants: not reported. Healthcare providers: unclear. Outcomes assessors and data analysts: unclear. Comment: overall judgement unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote "One subject in the 5 mg group lost consciousness at the time of the second blood procurement". Page 458 |
| Selective reporting (reporting bias) | Low risk | The stated objectives of the trial appear to match the listed outcomes. There was no evidence of selective reporting of outcomes. |
| Other bias | Unclear risk | Quote: "This project was funded by the Canadian CF Foundation". |
ALT: alanine aminotransferase
AST: aspartate aminotransferase
BMI: body mass index
CF: cystic fibrosis
IU: international units
NCHS: National Center for Health Statistics
PIVKA‐II: proteins induced by vitamin K absence or antagonism factor II
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐RCT. | |
| Non‐RCT. | |
| Non‐RCT. | |
| Non‐RCT, non‐CF control group. | |
| Uncontrolled study, non‐RCT. |
CF: cystic fibrosis
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Case controlled study |
| Participants | People with CF |
| Interventions | Vitamin K in 3 different groups ‐ no supplements, low supplements or high supplements |
| Outcomes | serum ucOC level |
| Notes | Awaiting inclusion until the response form the authors about randomisation in the study |
| Methods | Randomised controlled study |
| Participants | 26 participants not receiving vitamin K supplementation before |
| Interventions | 0.1 mg and 1 mg vitamin K supplementation for 2 years |
| Outcomes | ucOC levels and BMD |
| Notes | Only abstract is available now, likely to be included, but we will consider after we get further information from the investigators |
BMD: bone mineral density
CF: cystic fibrosis
ucOC: undercarboxylated osteocalcin
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Investigating the effect of vitamin K supplementation on markers of bone turnover and bone density in adolescents and adults with CF |
| Methods | RCT |
| Participants | Patients with a diagnosis of CF (positive sweat test or genotype testing) aged over 16 years (post pubertal‐stage IV Tanner), either sex, pancreatic insufficient (i.e. with a positive faecal elastase test, and requiring pancreatic enzyme supplementation) and no overt liver disease. |
| Interventions | 10 mg of menadiol phosphate (water soluble form of vitamin K) once daily orally for 12 months versus placebo. |
| Outcomes |
|
| Starting date | 09/05/2008 |
| Contact information | Lieske Kuitert, Department of Respiratory Medicine, London Chest Hospital, Bonner Road, E2 9JX, London, United Kingdom |
| Notes | Stated on www.controlled‐trials.com that trial completed, await publication of results. |
CF: cystic fibrosis
DEXA: dual energy x‐ray absorptiometry
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum undercarboxylated osteocalcin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 1 Serum undercarboxylated osteocalcin levels. | ||||
| 2 Serum vitamin K levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 2 Serum vitamin K levels. | ||||
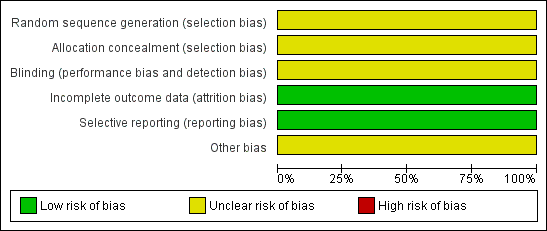
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
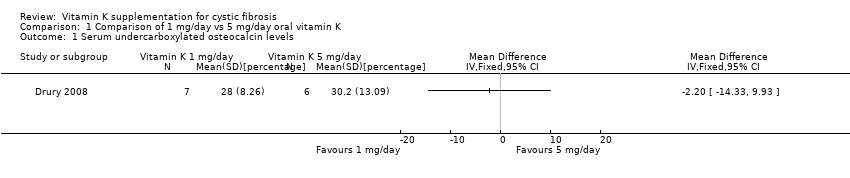
Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 1 Serum undercarboxylated osteocalcin levels.

Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 2 Serum vitamin K levels.
| Core elements | Issues to consider | Status of research for this review |
| Evidence | What is the current state of evidence? | A systematic review found only limited high quality evidence in relation to the effectiveness or otherwise of vitamin K supplementation for people with CF. |
| Population | Diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Any age group with a diagnosis of CF (defined by sweat test or genetic testing or both). Pancreatic insufficient. |
| Intervention | Type, prognostic | All preparations of vitamin K used as a supplement at any dose and for any duration. |
| Comparison | Type, prognostic factor | Placebo with a dose, frequency, duration comparable to the intervention, or no supplementation. |
| Outcome | Which clinical or patient related outcomes will the researcher need to measure, improve, influence or accomplish? | Clinical outcomes related to:
Biochemical analysis:
Quality of life:
Adverse events Data type: continuous and dichotomous |
| Time stamp | Date of literature search or recommendation | 15 April 2010. |
| Study type | What is the most appropriate study design to address the proposed question? | RCT (adequately powered/large sample size, sufficient duration) |
| BMI: body mass index | ||
| Dose | n | UcOC % | UcOC % |
| 1 mg/day | 7 | 46 (14.4) | 28 (8.26) |
| 5 mg/day | 6 | 47.6 (9.45) | 30.2 (13.09) |
| SD: standard deviation | |||
| Dose | n | Serum vitamin K levels (nmol/L) | Serum vitamin K levels (nmol/L) |
| 1 mg/day | 7 | 0.28 (0.25) | 2.52 (2.61) |
| 5 mg/day | 6 | 0.15 (0.19) | 6.98 (9.95) |
| SD: standard deviation | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum undercarboxylated osteocalcin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Serum vitamin K levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

