Administración de suplementos de vitamina K para la fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008482.pub5Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
VJ, ZF and VT were responsible for:
-
organising the retrieval of papers;
-
writing to authors of papers for additional information;
-
screening search results;
-
screening retrieved papers against inclusion criteria;
-
appraising the quality of papers;
-
data collection for the review;
-
extracting data from papers; and
-
obtaining and screening data on unpublished trials;
-
the analysis and interpretation of data.
VJ and VT were responsible for :
-
designing the review;
-
co‐ordinating the review;and
-
data extraction and management for the review.
All review authors contributed to writing the review.
VJ conceived the idea for the review and is the guarantor of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Health and Medical Research Council, Australia.
Support for AC (Practitioner Fellowship grant number 545216)
-
NIH training grant, USA.
Training grant T32DK007699/DK/NIDDK awarded to Vidhu Thaker.
Declarations of interest
Anne Chang declares she has received a grant provided by GSK which is unrelated to this topic. She is also the principal investigator on a study examining azithromycin for bronchiolitis in Indigenous children.
The remaining authors declare no financial conflicts of interest and that they do not have any associations with any parties who may have vested interests in the results of this review.
Acknowledgements
The authors would like to thank Nikki Jahnke of the Cochrane Cystic Fibrosis and Genetic Disorders Group for her support throughout this review.
We would also like to thank Professor Zbys Fedorowicz for his contributions to the review from its inception up to the update in 2017.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jun 04 | Vitamin K supplementation for cystic fibrosis | Review | Vanitha A Jagannath, Vidhu Thaker, Anne B Chang, Amy I Price | |
| 2017 Aug 22 | Vitamin K supplementation for cystic fibrosis | Review | Vanitha A Jagannath, Vidhu Thaker, Anne B Chang, Amy I Price | |
| 2015 Jan 18 | Vitamin K supplementation for cystic fibrosis | Review | Vanitha A Jagannath, Zbys Fedorowicz, Vidhu Thaker, Anne B Chang | |
| 2013 Apr 30 | Vitamin K supplementation for cystic fibrosis | Review | Vanitha A Jagannath, Zbys Fedorowicz, Vidhu Thaker, Anne B Chang | |
| 2011 Jan 19 | Vitamin K supplementation for cystic fibrosis | Review | Vanitha A Jagannath, Zbys Fedorowicz, Vidhu Thaker, Anne B Chang | |
| 2010 Apr 14 | Vitamin K supplementation for cystic fibrosis | Protocol | Vanitha A Jagannath, Zbys Fedorowicz, Vidhu Thaker, Anne B Chang, Nutayla Al‐Harthy | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Blood Coagulation [drug effects];
- Cystic Fibrosis [*blood, complications];
- Dietary Supplements;
- Osteogenesis [drug effects];
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Vitamin K [*administration & dosage];
- Vitamin K Deficiency [complications, *drug therapy];
- Vitamins [*administration & dosage];
Medical Subject Headings Check Words
Adolescent; Adult; Child; Humans;
PICO
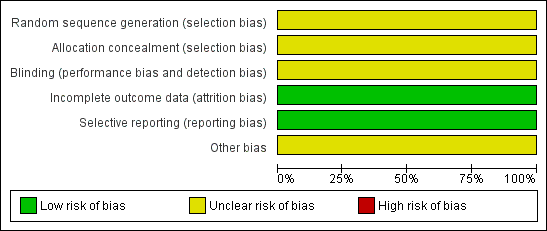
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
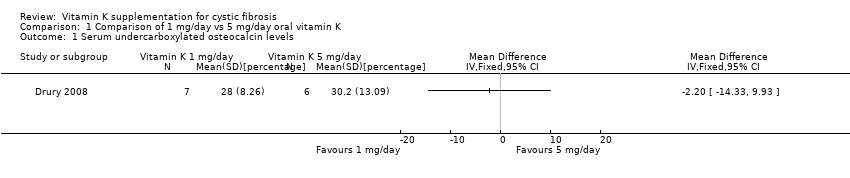
Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 1 Serum undercarboxylated osteocalcin levels.

Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 2 Serum vitamin K levels.
| Core elements | Issues to consider | Status of research for this review |
| Evidence | What is the current state of evidence? | A systematic review found only limited high quality evidence in relation to the effectiveness or otherwise of vitamin K supplementation for people with CF. |
| Population | Diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Any age group with a diagnosis of CF (defined by sweat test or genetic testing or both). Pancreatic insufficient. |
| Intervention | Type, prognostic | All preparations of vitamin K used as a supplement at any dose and for any duration. |
| Comparison | Type, prognostic factor | Placebo with a dose, frequency, duration comparable to the intervention, or no supplementation. |
| Outcome | Which clinical or patient related outcomes will the researcher need to measure, improve, influence or accomplish? | Clinical outcomes related to:
Biochemical analysis:
Quality of life:
Adverse events Data type: continuous and dichotomous |
| Time stamp | Date of literature search or recommendation | 15 April 2010. |
| Study type | What is the most appropriate study design to address the proposed question? | RCT (adequately powered/large sample size, sufficient duration) |
| BMI: body mass index | ||
| Dose | n | UcOC % | UcOC % |
| 1 mg/day | 7 | 46 (14.4) | 28 (8.26) |
| 5 mg/day | 6 | 47.6 (9.45) | 30.2 (13.09) |
| SD: standard deviation | |||
| Dose | n | Serum vitamin K levels (nmol/L) | Serum vitamin K levels (nmol/L) |
| 1 mg/day | 7 | 0.28 (0.25) | 2.52 (2.61) |
| 5 mg/day | 6 | 0.15 (0.19) | 6.98 (9.95) |
| SD: standard deviation | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum undercarboxylated osteocalcin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Serum vitamin K levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

