Supplémentation en vitamine K dans le traitement de la mucoviscidose
Résumé scientifique
Contexte
La mucoviscidose est une maladie génétique qui peut conduire au dysfonctionnement de plusieurs organes. Une mauvaise assimilation des graisses et des vitamines liposolubles (A, D, E, K) peut survenir et provoquer des carences subcliniques dans certaines de ces vitamines. On sait que la vitamine K joue un rôle important dans la coagulation du sang et dans la formation des os. Une supplémentation en vitamine K semble être une manière de traiter la carence, mais il n'existe pas véritablement de consensus quant à la dose et à la fréquence d'utilisation adéquates de ces compléments. Ceci est une version mise à jour de la revue.
Objectifs
Évaluer les effets d'une supplémentation en vitamine K chez les personnes atteintes de mucoviscidose et déterminer la dose et la voie d'administration optimales de vitamine K, tant pour une utilisation systématique que thérapeutique.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre d'essais cliniques du groupe Cochrane sur la mucoviscidose et autres maladies génétiques constitué des références identifiées lors de recherches exhaustives dans des bases de données électroniques et de recherches manuelles de revues pertinentes et de résumés d'actes de conférence.
La recherche la plus récente date du 30 janvier 2017.
Critères de sélection
Essais contrôlés randomisés et quasi randomisés comparant toutes les préparations de vitamine K utilisées dans le cadre d'une supplémentation par rapport à l'absence de supplémentation (ou à un placebo), à n'importe quelle dose, par n'importe quelle voie et pendant n'importe quelle durée, chez les enfants ou les adultes présentant un diagnostic de mucoviscidose (par des tests de transpiration ou des dépistages génétiques).
Recueil et analyse des données
Deux auteurs ont indépendamment passé au crible les articles, extrait les données des essais et évalué leur risque de biais.
Résultats principaux
Deux essais (pour un total de 32 participants) d'une durée d'un mois chacun ont été inclus dans la revue et ont été évalués comme présentant un risque de biais modéré. L'un était un essai en groupes parallèles sur le dosage chez des enfants (âgés de 8 à 18 ans) et l'autre (avec une cohorte plus âgée) avait un plan d'étude croisé comparant la supplémentation à l'absence de traitement, mais aucune donnée séparée n'a été rapportée pour la première période d'intervention. Aucun des essais n'a pris en compte les critères de jugement principaux (coagulation, formation des os et qualité de vie). Les deux essais ont signalé la restauration des taux sériques de vitamine K et d'ostéocalcine faiblement carboxylée dans la fourchette normale après un mois de supplémentation quotidienne avec 1 mg de vitamine K.
Conclusions des auteurs
Les preuves issues d'essais contrôlés randomisés concernant les bénéfices d'une supplémentation systématique en vitamine K pour les personnes atteintes de MV sont actuellement faibles et limitées à deux essais de petite taille et de courte durée. Cependant, aucun préjudice n'a été constaté et jusqu'à ce que de nouvelles preuves soient disponibles, les recommandations actuelles doivent être respectées.
PICO
Résumé simplifié
Supplémentation en vitamine K dans le traitement de la mucoviscidose
Question de la revue
Nous avons examiné les éléments de preuve afin de déterminer si une supplémentation en vitamine K permet de contrer les effets de la carence sur la coagulation, la résistance des os et la qualité de vie des personnes atteintes de mucoviscidose. Nous avons tenté de déterminer le dosage optimal nécessaire pour prévenir cette carence. Ceci est une mise à jour d'une revue précédente.
Contexte
La mucoviscidose est une affection héréditaire provoquant des maladies principalement dans les poumons, l'appareil digestif et le pancréas. Chez les personnes atteintes de mucoviscidose, le pancréas ne produit souvent pas suffisamment d'enzymes permettant à l'organisme d'absorber correctement les aliments digérés, et cela peut également être lié à des carences en vitamines liposolubles telles que la vitamine K. La vitamine K est nécessaire à la bonne coagulation du sang, à la formation des os et à certaines fonctions métaboliques.
Date de la recherche
Les preuves sont à jour en date du 30 janvier 2017.
Caractéristiques de l'étude
Deux essais (portant sur un total de 32 participants) ont été inclus dans cette revue. Dans un essai (14 enfants âgés de 8 à 18 ans), la moitié des participants a reçu des suppléments de vitamine K par voie orale à la dose de 1 mg/jour et l'autre moitié a reçu 5 mg/jour pendant un mois. Dans l'autre essai, les 18 volontaires (âgés de 13 à 35 ans) ont reçu un supplément de vitamine K par voie orale à 5 mg ou n'ont rien reçu pendant un mois, avant de changer de groupe pour un autre mois. Malheureusement, nous n'avons pas pu analyser les données de ce second essai, car les investigateurs n'ont pas rapporté de données sur la première partie de l'essai seulement (mais uniquement à la fin de l'essai, lorsque tous les volontaires avaient pris part aux deux groupes), nous n'avons donc pas pu déterminer si les effets étaient dus aux suppléments ou à leur absence.
Résultats principaux
Aucun des deux essais n'a examiné les principaux critères de jugement de la revue (coagulation sanguine, formation osseuse et qualité de vie). Les deux essais ont indiqué que, chez les patients aux faibles taux de vitamine K mesurés dans le sang ainsi que d'ostéocalcine faiblement carboxylée (un autre marqueur de laboratoire de la vitamine K), ces taux sont remontés dans la fourchette normale après un mois de supplémentation quotidienne avec 1 mg de vitamine K.
Qualité des preuves
Nous sommes convaincus que tous les critères de jugement prévus dans ces deux essais ont été rapportés et que les résultats ne sont pas à risque de biais en raison de volontaires ayant arrêté les essais. Nous n'avions pas suffisamment de détails pour décider si les résultats seraient affectés par la façon dont les essais avaient été mis en place ou si les volontaires pouvaient savoir le traitement qu'ils recevaient (ce qui aurait été évident dans l'essai comparant les suppléments à l'absence de traitement).
Authors' conclusions
Background
Description of the condition
Cystic fibrosis (CF) is a multisystem disorder that primarily affects the respiratory and gastrointestinal (GI) systems (Morgan 1999). It is caused by homozygous presence of a mutation in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein.
In the UK there are over 8000 people affected by CF and in the United States of America this figure is approximately 30,000; most are diagnosed by six months of age and the median survival has reached the fifth decade of life (Davis 2006; Goss 2004; Staab 2004). It is the most common, life‐threatening, autosomal‐recessively inherited disease in the white population, with a carrier rate of 1 in 25 and an incidence of 1 in 2500 live births (Ratjen 2003); CF is less common in other ethnic groups, approximately 1 in 46 Hispanics, 1 in 65 Africans and 1 in 90 Asians carry at least one abnormal CFTR gene (Bobadilla 2002).
The CFTR protein is a chloride ion channel, important in tissues that produce sweat, digestive juices and mucus. The dominant symptoms of CF relate to the respiratory and GI systems (Wagener 2003). In the GI system, liver dysfunction, intestinal obstruction and exocrine pancreatic insufficiency are the most common morbidities. Pancreatic insufficiency (PI) affects up to 90% of people with CF and causes fat malabsorption (Dodge 2006). Fat‐soluble vitamins (A, D, E and K) are co‐absorbed with fat and thus deficiency of these vitamins may occur (Dodge 2006). Hence ,vitamin K deficiency is well recognised in patients with CF and PI. While deficiencies may occur from the disease process of CF and from insufficient supplementation, another additional co‐factor is the long‐term use of certain types of antibiotics. These can put some individuals at additional risk of vitamin K deficiency by altering intestinal flora which produce vitamin K (Conway 2005). Long‐term effects of bowel resection, an intervention required for intestinal obstruction in some newborns with CF and varying degrees of liver dysfunction can pose a further additional risk for vitamin K deficiency (Fuchs 1998).
The manifestations of vitamin K deficiency can range from a mild subclinical identification (e.g. low levels of vitamin K in the blood) to widespread coagulopathy (defect in the body's mechanism for blood clotting). Such manifestations may include mucosal bleeding (e.g. in the nose, gastro‐intestinal system, and in the urine) and subcutaneous bleeding (e.g. oozing from venipuncture sites and susceptibility to bruising). Vitamin K is also involved in the calcium binding proteins in the bone and its deficiency is implicated in defective bone remineralization and thus osteoporosis (Conway 2005).
The majority of CF centres routinely administer vitamins A, D, and E as supplements from the neonatal period. Vitamin K administration is usually prescribed when clinical deficiencies are detected or following routine investigations. The limited storage capacity and rapid metabolic turnover of vitamin K (Olsen 1994) supports the recommendations for daily rather than weekly supplementation of vitamin K (Beker 1997).
Description of the intervention
Two forms of vitamin K (K1 and K2) occur naturally and synthetic forms of the vitamin (K3, K4, and K5) are also available. Naturally occurring vitamin K is found in green vegetables i.e. kale, collards, spinach and salads (K1 ‐phytonadione) and a small amount is made in human gut by bacteria (K2‐menaquinones). Therapeutic vitamin K is available in both water soluble and insoluble forms (Durie 1994). The enteral forms of vitamin K supplementation are commonly prescribed. These can be in tablet, ampoule (Borowitz 2002; Cystic Fibrosis Trust 2007) or multivitamin preparations (Durie 1994). Vitamin K can also be administered by intramuscular (Shearer 1995) or intravenous injections (Verghese 2003).
How the intervention might work
Vitamin K functions as the cofactor of the enzyme vitamin K‐dependent carboxylase. This enzyme catalyses the post‐translation formation of gamma‐carboxyglutamyl (Gla) residues in specific proteins. Vitamin K‐dependent proteins are: blood coagulation factors (prothrombin and Factors VII, IX and X); other plasma proteins (protein C, protein S and protein Z); two proteins from bone (osteocalcin and matrix Gla‐protein); and proteins from lung, kidney, spleen, testis, placenta and other tissues (Uotila 1990).
Blood coagulation requires activation of inactive proenzymes, hence vitamin K is a vital factor in synthesis of clotting factors and causes haemostasis in vitamin K‐dependant bleeding manifestations.
Vitamin K‐related carboxylation allows the activation of the bone matrix protein, osteocalcin, resulting in osteoblast function and bone formation; vitamin‐K deficiency impairs this process and thus impairs bone formation (Okano 2005).
Why it is important to do this review
Vitamin K deficiency is common in people with CF with pancreatic insufficiency. In a recent study of people with CF, the pathological PIVKA‐II concentration (≥ 2 ng/ml) was found in 42.8% of the population studied and an abnormal percentage of osteocalcin (≥ 20%) in 35.7% of people studied (Krzyzanowska 2015). Supplementation (often oral and occasionally parenteral) appears to the most immediate measure to address the deficiency, although there is limited consensus on the dose and frequency of supplements for routine or therapeutic use (Rashid 1999). In 1992 the Consensus Committee of the Cystic Fibrosis Foundation (CFF) suggested a particular dosage (Ramsey 1992); but the recommendations were later proved ineffectual by another study (Beker 1997). The 2002 American Consensus Committee recommended a low‐dose supplementation of vitamin K for all ages (0.3 to 0.5 mg/day), but emphasized that no adverse effects had been reported at any dosage level of vitamin K (Borowitz 2002). Recent recommendations from Europe and the UK have suggested varying dose regimens ranging from 0.3 to 1 mg/day to 10 mg/week (Cystic Fibrosis Trust 2002; Sinaasappel 2002). There is a suggestion from one study that only a higher level of supplementation can normalise vitamin K levels in people with CF (Dougherty 2010). Another study found that vitamin K deficiency occurs in people with CF despite applied supplementation (Krzyzanowska 2010). The study authors suggested that an accurate supplementation dose should be estimated individually and the assessment of its effectiveness requires studies allowing investigators to determine the real body resources of vitamin K.
There is no uniform consensus on routine or at‐risk vitamin K supplementation in individuals with CF. There is increasing evidence that the prevalence of vitamin K deficiency in CF is associated with an increased morbidity (Rashid 1999). Thus, a systematic review on vitamin K supplementation in people with CF could provide evidence to guide clinical practice.
This is an update of previous review versions (Jagannath 2010; Jagannath 2011; Jagannath 2013; Jagannath 2015).
Objectives
To determine the effects of vitamin K supplementation on the morbidities in people with CF.
To investigate the hypotheses that vitamin K will decrease deficiency‐related coagulopathy, increase bone mineral density, decrease risk of fractures and improve quality of life in people with CF.
To determine the optimal dose and route of administration of vitamin K for people with CF (for both routine and therapeutic use).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised controlled trials.
Types of participants
Children and adults with a diagnosis of CF (defined by sweat test or genetic testing or both).
Exclusion criteria: Any intervention which may affect the interpretation of the effects of vitamin K or any allergy to vitamin K:
-
any anticoagulation in the past three months (will make interpretation of vitamin K effects on blood coagulation difficult);
-
bisphosphonates in the past six months (will make interpretation of vitamin K effects on bone metabolism difficult);
-
allergy to vitamin K.
Types of interventions
All preparations of vitamin K used as a supplement compared to placebo or no supplementation at any dose and for any duration. Studies comparing different doses and regimens of vitamin K were considered.
Types of outcome measures
Primary outcomes
-
Clinical outcomes related to coagulopathy (Sutor 1995)
-
time to cessation of bleeding manifestations (symptomatic coagulopathy)
-
time to normalisation of sub‐therapeutic international normalized ratio (INR) (asymptomatic or sub‐clinical coagulopathy)
-
-
Bone formation outcome measures
-
bone mineral density at the spine (L1‐L4) and the total hip (measured by dual energy X‐ray absorptiometry (DEXA) scans with z score compared to reference population) (Borowitz 2002)
-
reduction in risk of bone fractures
-
-
Quality of life (e.g. the CFQ‐R (Quittner 2000) and the CFQoL (Gee 2000))
Secondary outcomes
-
Nutritional parameters (including z scores or centiles)
-
weight
-
height
-
body mass index (BMI)
-
-
Adverse events
-
mild (not requiring intervention)
-
moderate (requiring treatment)
-
severe (life threatening or requiring hospitalisation)
-
-
Serum levels
-
serum undercarboxylated osteocalcin (ucOC)
-
ucOC/cOC (carboxylated osteocalcin) ratio (UCR)
-
-
Vitamin K‐specific laboratory outcomes
-
plasma level of vitamin K1 (measured by high performance liquid chromatography (HPLC) and fluorescence detection (Wang 2004)
-
proteins induced by vitamin K absence or antagonism factor II (PIVKA II) levels (measured by enzyme‐linked immunosorbent assay (ELISA)) (Belle 1991; Belle 1995)
-
Search methods for identification of studies
There are no restrictions regarding language or publication status.
Electronic searches
We identified relevant trials from the Group's Cystic Fibrosis Trials Register using the term: 'vitamin K'.
A systematic search without language restrictions was conducted using the optimally sensitive strategy developed for the Cochrane Collaboration to identify all relevant published and unpublished randomised controlled trials (Lefebvre 2009) in the Cystic Fibrosis Trials Register which is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the latest search: 30 January 2017.
We also searched the clinical trials registries: clinicaltrials.gov; International Standard Randomised Controlled Trials Number (ISRCTN) Registry and WHO ICTRP using the search terms "vitamin K" and "cystic fibrosis".
Date of the latest search: 01 April 2017.
Searching other resources
The following additional resources were used:
-
the bibliographical references of identified studies were searched for citations to additional studies;
-
personal contact with corresponding authors of relevant trials or reviewers and other experts.
Data collection and analysis
Selection of studies
Up to the 2017 update, two review authors (Vanitha Jagannath (VJ) and Zbys Fedorowicz (ZF)) independently assessed the abstracts of trials resulting from the searches. We obtained full text copies of all relevant and potentially relevant trials, those appearing to meet the inclusion criteria, and those for which there was insufficient detail in the title and abstract to make a clear decision. The two authors then independently assessed the full text papers. There were no disagreements; however, if there are any disagreements on the eligibility of trials in the future, we will resolve these through discussion and consensus, or through a third party (Vidhu Thaker (VT)). All irrelevant records were excluded and details of the trials and the reasons for their exclusion were noted in the tables (Characteristics of excluded studies) in RevMan (RevMan 2014).
Data extraction and management
We entered details for the included trials into the tables (Characteristics of included studies) in RevMan (RevMan 2014) and collected outcome data using a pre‐determined form designed for this purpose. For the original review, two review authors (VJ, ZF) extracted data independently and in duplicate and only included these if there was a consensus; there were no disagreements; if these occur in the future, we will resolve them by consulting with a third review author (VT).
The authors extracted the following details.
-
Trial methods
-
method of allocation
-
masking of participants, trialists and outcome assessors
-
exclusion of participants after randomisation and proportion and reasons for losses at follow‐up
-
-
Participants
-
country of origin and study setting
-
sample size
-
age
-
gender
-
inclusion and exclusion criteria
-
-
Intervention
-
type
-
dose and frequency
-
duration of intervention in follow‐up
-
-
Control
-
type
-
dose and frequency
-
duration of intervention in follow‐up
-
-
Outcomes: primary and secondary outcomes mentioned in the Types of outcome measures section of this review and categorised and grouped accordingly: short term (less than 12 months) data at 3, 6 and 12 months and medium to long term (over 12 months)
If stated in the trial reports, we recorded the sources of funding of any of the included trials.
The review authors used this information to help them assess clinical heterogeneity and the external validity of any included trials.
Assessment of risk of bias in included studies
For the original review, two authors (VJ, ZF) independently graded the selected trials using a simple contingency form and followed the domain‐based evaluation described in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The authors compared evaluations and discussed and resolved any inconsistencies in these evaluations.
The authors assessed the following domains as 'Yes' (i.e. low risk of bias), 'Unclear' (uncertain risk of bias) or 'No' (i.e. high risk of bias):
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, personnel and outcome assessors);
-
incomplete outcome data addressed;
-
free of selective outcome reporting;
-
free of other bias.
The authors categorised the risk of bias in any included studies according to the following:
-
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria met;
-
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria assessed as unclear; or
-
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria not met.
We report these assessments for each trial in the tables (Risk of bias in included studies) in the review.
Measures of treatment effect
For dichotomous outcomes, we planned to express results as odds ratios (OR) with 95% confidence intervals (CI). For continuous outcomes, we calculated the mean difference (MD); we would have calculated the standardized mean difference (SMD) if different measurement scales had been used. We planned to express any time‐to‐event outcomes data as ORs or hazards ratios.
Unit of analysis issues
We included trials with a parallel group design, such that participants were randomised to either intervention or control with subsequent analysis at individual allocation level. Unit of analysis issues can arise with cross‐over trials and therefore we decided not to include end‐of‐trial data from these trials because the effects of vitamin K on bone metabolism are likely to be long‐term and an appropriate wash‐out period cannot be defined. However, we planned to include any data reported from the first intervention period.
Dealing with missing data
We attempted to contact the authors of any trials where we needed clarification of trial design or results (at all stages of the review and updates). We have only received a response the investigators from one of the trials (Drury 2008). We obtained individual patient data which we have included in the analysis.
Assessment of heterogeneity
As we were only able to include two trials in this review, we did not assess heterogeneity; but in future updates of this review, if we are able to include further trials, we will apply the following methods of assessment.
We will assess clinical diversity between the trials by examining the trial characteristics, the similarity between the types of participants, the interventions and the outcomes as specified in the inclusion criteria.
We will assess statistical heterogeneity using a Chi² test and the I² statistic, where I² values of 30% to 60% indicate moderate to high, 50% to 90% substantial and 75% to 100% considerable heterogeneity. We will consider heterogeneity to be significant when the P value is less than 0.10 (Higgins 2003).
Assessment of reporting biases
The paucity of trials included in this review did not permit an assessment of publication bias. If we had identified a sufficient number of trials for inclusion in this review (at least 10), we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry as described in chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If we had then identified asymmetry, we would have tried to assess other possible causes and explored these further in the Discussion section of the review, if appropriate.
Data synthesis
For the original review, two review authors (VJ and ZF) analysed the data in Review Manager (RevMan 2014) and reported them as specified in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Although we included two trials in this review, we were only able to report reliable data from one of them (Drury 2008) and therefore did not carry out a meta‐analysis. If at a later date we include further trials in this review, we will apply the following methods of data synthesis. When we include sufficient numbers of trials investigating similar interventions, we will conduct the analysis in RevMan (RevMan 2014). In general, we will use the fixed‐effect model; but if there is substantial clinical diversity, we will use the random‐effects model with trials grouped by action.
Subgroup analysis and investigation of heterogeneity
Lack of data did not permit a subgroup analysis, but in future updates and if further data become available we plan to carry out the following subgroup analyses:
-
cumulative dose (as per kg body weight per day) of vitamin K2;
-
route of administration (oral or parenteral);
-
baseline plasma level of vitamin K1 (subnormal versus normal);
-
presence or absence of any of the following: pancreatic insufficiency, liver dysfunction, bowel resection or prolonged usage of antibiotics and chronic steroid administration;
-
mean 25OH vitamin D level.
Sensitivity analysis
In view of the low number of trials included in this review, a sensitivity analysis was not possible. For future updates and if we are able to include sufficient trials, we will undertake sensitivity analyses to assess the robustness of our review results by repeating the analysis with the following adjustments:
-
exclusion of trials with unclear or inadequate allocation concealment;
-
exclusion of trials with unclear or inadequate blinding of outcomes assessment;
-
exclusion of trials with unclear or inadequate completeness of follow‐up;
-
exclusion of quasi‐randomised trials.
Results
Description of studies
Results of the search
The electronic searches retrieved references to nine trials. After examination of the titles and abstracts of these references, all of those which did not match our inclusion criteria and were clearly ineligible were eliminated from the review. Full text copies of the remaining potentially eligible trials were obtained and subjected to further evaluation. The review authors discussed the eligibility of these trials, resolved any remaining uncertainties by consensus. Two trials were included (Beker 1997; Drury 2008), five trials were excluded (Cornelissen 1992; Grey 2008; Mosler 2003; Nicolaidou 2006; Wilson 2001), and two trials are awaiting classification until we obtain further information from the trialists (van Hoorn 2003; van Hoorn 2008).
The search of the Cystic Fibrosis Trials Register in 2012 retrieved one reference, which is an abstract of the ongoing trial in the review which will be assessed for inclusion as soon as it has been published (Kuitert 2010).
Included studies
Two trials, neither of which addressed any of our primary outcomes and only reported on two of the secondary outcomes were included in this review. Albeit these two trials were assessed as having a moderate risk of bias, and provided limited data, it was considered that their inclusion and the reporting of their results would provide at least some evidence towards answering this research question.
One of the trials was cross‐over in design, but did not include a wash‐out period and thus the potential risk of bias as a consequence of the carry‐over of treatment effect could not be ruled out (Beker 1997). Although trials with a cross‐over design were eligible for inclusion in this review, we had specified that only data from the first intervention period would be used. Unfortunately, the trial investigators only provided an analysis across both treatment periods and, even though we were unable to contact the authors to obtain the first period data, this trial has been included in the review but it has not been possible to enter the data into a meta‐analysis.
The second trial included in this review was a dose‐ranging randomised controlled trial which reported some data for two of our secondary outcomes, but these were presented as graph‐plots from which we were unable to obtain precise data (Drury 2008). After successful contact with the principal investigator via electronic mail we received the relevant individual patient data for these two outcomes and have presented them in this review.
Characteristics of the trial setting and investigators
Both were single centre trials, one was conducted at the Montreal Children's Hospital CF Clinic in Canada (Drury 2008), and the other was carried out at the CF Clinic of the Children's National Medical Center (CNMC), Washington DC, USA (Beker 1997).
Although the overall duration of the two trials differed, the active treatment period was the same (i.e. one month). Thus, in the cross‐over trial, the participants were allocated to either active intervention or no‐treatment control for a four‐week period and then crossed over for a further four weeks but without undergoing a wash‐out period (Beker 1997).
The providers of care in both trials were hospital staff and the assessors of outcomes were the investigators and other healthcare providers (Beker 1997; Drury 2008).
Characteristics of the participants
The total sample size comprised of 32 participants between the ages of 8 and 35 years. In the Beker study, the diagnosis of CF was confirmed by duplicate sweat test (Beker 1997). Although Drury did not report the method used to confirm the diagnosis of CF, it is most probable that participants with CF were enrolled in this trial (Drury 2008).
All of the participants in the Beker trial were pancreatic insufficient, as documented by previous fecal fat measurement, and they received replacement therapy of 750 to 200 units of lipase/kg of body weight at meal times during the course of the trial (Beker 1997). The investigators in the Drury trial reported that only pancreatic insufficient participants were included, but provided no further details (Drury 2008). Participants with liver disease (diagnosed by ultrasound, liver function tests or hepatomegaly or both) and those who were taking supplemental therapeutic vitamin K to treat coagulopathies at enrolment were excluded from both trials (Beker 1997; Drury 2008).
Characteristics of the interventions
The active intervention in the Beker trial consisted of 5 mg oral vitamin K1 supplementation per week and the control was no supplementation for four weeks; participants then crossed over for a second four‐week period (Beker 1997). Compliance with the intervention by participants was verified by the trial coordinator at each visit. Oral antibiotic medications of cephalosporin; sulfamethoxazole; erythromycin as well as concomitant usage of bronchodilators and standard multivitamins and 200 to 400 IU vitamin E were allowed during the course of the trial.
The participants in the Drury trial were randomised to either the orally administered injectable formulation of vitamin K1 phytonadione of 1 mg/day (diluted to 1 mg/ml) or the 5 mg/day dose (Drury 2008).
Characteristics of the outcome measures
None of the primary outcomes specified in the protocol for the review were considered in either of the included trials (Beker 1997; Drury 2008). Both trials carried out assessments of plasma Vitamin K1 levels and serum undercarboxylated osteocalcin levels; these were measured at entry and at the completion of the trial by Drury (Drury 2008), and at the end of each period by Beker (Beker 1997). Dietary intake records to estimate the extent of dietary contribution of Vitamin K1 were maintained by the participants in one trial (Beker 1997). The vitamin K1 data of foods were analysed using Nutritionist III software, the database, however, did not have complete vitamin K1 data for many foods.
Excluded studies
Five trials were excluded from the review since these were not randomised controlled trials. Further information about the reasons for exclusion of trials is available in the Excluded studies table.
Risk of bias in included studies
The risks of bias for the two included trials in this review were classified as previously described (Assessment of risk of bias in included studies).
Both included trials were judged as having an 'unclear' risk of bias overall (Beker 1997; Drury 2008). These assessments were to a certain extent based on the inadequate reporting of several of the criteria that are considered to be important in the evaluation of methodological rigour in terms of trial design and conduct.
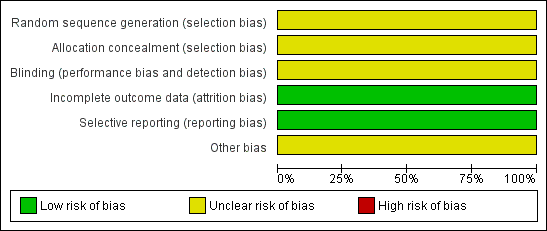
For further details see the risk of bias tables in Characteristics of included studies, the risk of bias graph (Figure 1) and the risk of bias summary (Figure 2).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
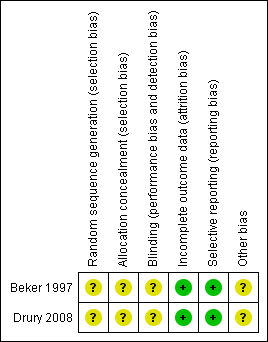
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods used to generate the allocation sequence were not reported in either trial. Moreover, neither of the trials described how the allocation sequence was concealed, which did not allow us to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. Inadequate reporting quality did not permit a clear judgement to be made for both domains in either of the included trials (Beker 1997; Drury 2008).
Blinding
The measures used to blind trial participants and personnel from knowledge of which intervention a participant received or any information relating to whether the intended blinding was effective were not reported in either trial (Beker 1997; Drury 2008). However, whilst it may be accepted that blinding of participants and investigators in the Beker trial may not have been feasible, it was unclear if the assessors of the outcomes were adequately blinded (Beker 1997). Therefore the judgement given for this domain in both trials was 'unclear'.
Incomplete outcome data
There were no withdrawals and no missing or incomplete data in the Beker trial (Beker 1997); and the only missing data in the Drury trial was as a result of the inability of the investigators to make the final outcome assessment for one participant (Drury 2008). Therefore, we judge there to be a low risk of bias from incomplete outcome data in both trials.
Selective reporting
Although neither trial protocols were available, based on information presented in the methods sections of each of the reports, the investigators appear to have reported on all of their stated objectives and expected outcomes, a number of which were pre‐specified inclusion criteria for this systematic review (Beker 1997; Drury 2008). We therefore judge there to be a low risk of bias from selective reporting for these trials.
Other potential sources of bias
Insufficient information was provided in the reports to assess whether any other important risk of bias exists, and therefore the judgement for this domain in both trials was 'unclear' (Beker 1997; Drury 2008).
Effects of interventions
Primary outcomes
None of the primary outcomes specified in the protocol for this review were considered in either of the included trials (Beker 1997; Drury 2008).
Secondary outcomes
1. Nutritional parameters
These were not considered in either of the included trials (Beker 1997; Drury 2008).
2. Adverse events
No adverse events were reported in either of the trials (Beker 1997; Drury 2008).
3. Serum levels
The Drury trial was under‐powered (Drury 2008). Even though we managed to obtain individual patient data, the small number of participants and the degree of baseline imbalance in vitamin K and undercarboxylated osteocalcin levels (both within and between the groups) did not support analysis of the magnitude of individual response to treatment or control, i.e. the measure of change from baseline. Although it is likely that baseline imbalance may reflect the clinical variability of CF, neither report provided sufficient clinical detail of the participants to enable any assessment of the degree of variability in presentation of CF (Beker 1997; Drury 2008). Therefore, the data for both of these outcomes have been presented in the analysis as the end of trial mean response for the total number of participants in each of the treatment and control groups. We also report the baseline means and SDs alongside the end of trial values in the additional tables (Table 1; Table 2).
| Dose | n | UcOC % | UcOC % |
| 1 mg/day | 7 | 46 (14.4) | 28 (8.26) |
| 5 mg/day | 6 | 47.6 (9.45) | 30.2 (13.09) |
SD: standard deviation
ucOC: undercarboxylated osteocalcin
| Dose | n | Serum vitamin K levels (nmol/L) | Serum vitamin K levels (nmol/L) |
| 1 mg/day | 7 | 0.28 (0.25) | 2.52 (2.61) |
| 5 mg/day | 6 | 0.15 (0.19) | 6.98 (9.95) |
SD: standard deviation
In the Beker trial, the data for this outcome were analysed across both treatment periods and therefore we only report the end of trial data narratively (Beker 1997). We advise caution in its interpretation and in any comparison with data from the other trial included in this review.
a. Serum undercarboxylated osteocalcin (ucOC)
In the Drury trial, all of the participants had elevated (in excess of 21%) concentrations of ucOC before supplementation, but these levels were reduced after one month of vitamin K1 supplementation (Drury 2008). The overall level of ucOC decreased from a median of 46.8% to 29.1%, and the undercarboxylated osteocalcin levels decreased and returned to levels within the normal range in three (one in the 5 mg/day group, two in the 1 mg/day group) out of the 13 participants by the end of the trial. The mean end of trial difference in ucOC between the two intervention groups was ‐2.20 (95% CI ‐14.33 to 9.93) (Analysis 1.1).
There was no evidence of any difference between the 5 mg/day and the 1 mg/day vitamin K dosage in terms of statistically significant effect on ucOC levels at the end of the one‐month trial period (Drury 2008).
In the Beker trial, all of the participants had increased serum undercarboxylated osteocalcin concentrations (ucOC) at enrolment and prior to supplementation (Beker 1997). It was reported that following supplementation and by the end of the trial the majority had successfully achieved the normal reference mean levels (21%) of ucOC (Beker 1997).
b. ucOC/cOC (carboxylated osteocalcin) ratio (UCR)
This was not evaluated in either of the trials (Beker 1997; Drury 2008).
4. Vitamin K‐specific laboratory outcomes
a. Plasma level of vitamin K1
In the Drury trial, the baseline vitamin K levels in seven out of the 14 participants were sub‐optimal (defined as less than 0.3 nmol/L) (Drury 2008). The paper reported that serum vitamin K levels appeared to improve significantly (P < 0.001) with supplementation, rising into the normal range in all of the participants who were below the optimum level. There was no statistically significant difference in effect between the 5 mg/day and the 1 mg/day vitamin K dose; MD ‐4.46 (95% CI ‐12.65 to 3.73) (Analysis 1.2).
In the Beker trial, a substantial number of the participants had below normal serum vitamin K levels at trial entry (Beker 1997). Although the mean concentration of plasma vitamin K was higher in the supplemented group, in only less than half of the total number of participants were these levels reportedly brought into the normal range after supplementation with the 5 mg dose (Beker 1997).
b. Proteins induced by vitamin K absence or antagonism factor II (PIVKA II) levels
Drury did not consider or report on this outcome (Drury 2008). In the Beker trial PIVKA‐II concentrations were elevated prior to supplementation and almost one third of the participants had PIVKA‐II levels within the normal range (≤ 2 ng/ml) following supplementation (Beker 1997).
Discussion
Summary of main results
The results from both of the included trials could not be pooled and entered into a meta‐analysis; however, both trials reported an increase in serum vitamin K levels, and a decrease in the undercarboxylated osteocalcin levels which returned to normal following supplementation with oral vitamin K 1 mg/day for one month. There did not appear to be any significant difference in these outcomes when a dose of 1 mg/day was compared with 5 mg/day in the Drury trial (Drury 2008). The PIVKA levels also showed a decrease and a return to normal in the Beker trial following supplementation (Beker 1997).
Overall completeness and applicability of evidence
The noticeable absence in the included trials of any assessments of important clinical outcomes related to coagulopathy or growth and improvement in quality of life as a result of vitamin K supplementation does somewhat limit the overall completeness and ultimately the generalisability of the evidence to the wider CF population. Equally, the short duration and follow‐up of the included trials does not permit any conclusions to be made about the longer‐term benefits and any potential harms of vitamin K supplementation.
Future research should aim to close this gap in the evidence by focusing more closely on some of the patient‐relevant and preferred outcomes rather than solely on biochemical laboratory analyses.
Quality of the evidence
The two trials included in this review were underpowered and of short duration which was illustrated by the wide confidence intervals in the comparisons of treatment effect and reflected the degree of imprecision in these results (Beker 1997; Drury 2008). Some of the practical and methodological difficulties faced by investigators of this research question were highlighted in these trials. The key factors which are likely to have had a degree of impact on the quality level of the evidence for the outcomes sought in this review can be linked to the design and implementation of the included trials, and in particular to the effective concealment of the allocation sequence and adequate blinding of investigators and outcome assessors.
Potential biases in the review process
Although it would be not unreasonable to assume that the comprehensive electronic searches employed in this review will have identified all existing randomised controlled trials and thereby helped to limit bias in the conduct of this review, the absence of any other published randomised trials over the intervening ten years between the two included trials and their scant contribution to the outcomes specified for this review, might presuppose an indication of publication bias.
Agreements and disagreements with other studies or reviews
Several trials have indicated a degree of support for the requirement of supplementation in addition to emphasising the comparative safety of oral supplementation with vitamin K in people with CF (Beker 1997; Borowitz 2002; Ramsey 1992; Sinaasappel 2002); but there is no agreement amongst the trials about the dosage of supplementation. However, there is some concern that the adequacy of dosing based on measurement of vitamin K levels may be inaccurate and that PIVKA levels and ucOC levels may be better indicators of effectiveness of supplementation. Some trials suggest a complimentary role for quantified dietary intake of vitamin K in people with CF (Dougherty 2010). This review did not show anything conclusively to agree or disagree with these trials.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
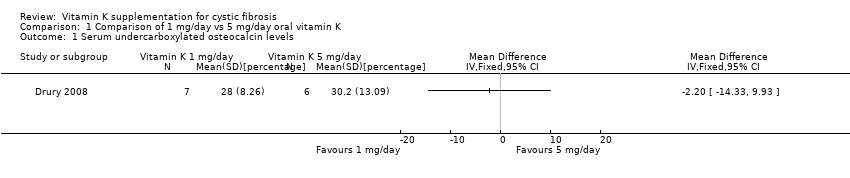
Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 1 Serum undercarboxylated osteocalcin levels.

Comparison 1 Comparison of 1 mg/day vs 5 mg/day oral vitamin K, Outcome 2 Serum vitamin K levels.
| Core elements | Issues to consider | Status of research for this review |
| Evidence | What is the current state of evidence? | A systematic review found only limited high quality evidence in relation to the effectiveness or otherwise of vitamin K supplementation for people with CF. |
| Population | Diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Any age group with a diagnosis of CF (defined by sweat test or genetic testing or both). Pancreatic insufficient. |
| Intervention | Type, prognostic | All preparations of vitamin K used as a supplement at any dose and for any duration. |
| Comparison | Type, prognostic factor | Placebo with a dose, frequency, duration comparable to the intervention, or no supplementation. |
| Outcome | Which clinical or patient related outcomes will the researcher need to measure, improve, influence or accomplish? | Clinical outcomes related to:
Biochemical analysis:
Quality of life:
Adverse events Data type: continuous and dichotomous |
| Time stamp | Date of literature search or recommendation | 15 April 2010. |
| Study type | What is the most appropriate study design to address the proposed question? | RCT (adequately powered/large sample size, sufficient duration) |
| BMI: body mass index | ||
| Dose | n | UcOC % | UcOC % |
| 1 mg/day | 7 | 46 (14.4) | 28 (8.26) |
| 5 mg/day | 6 | 47.6 (9.45) | 30.2 (13.09) |
| SD: standard deviation | |||
| Dose | n | Serum vitamin K levels (nmol/L) | Serum vitamin K levels (nmol/L) |
| 1 mg/day | 7 | 0.28 (0.25) | 2.52 (2.61) |
| 5 mg/day | 6 | 0.15 (0.19) | 6.98 (9.95) |
| SD: standard deviation | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum undercarboxylated osteocalcin levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Serum vitamin K levels Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

