Zastosowanie terapii lustrzanej w poprawie funkcji motorycznych po udarze mózgu
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Country: Australia Setting: inpatient Age: adults (mean age: 68 years) Sample size: 40 participants (20 in each group) Sex: 22 women, 18 men Inclusion criteria: acute stroke (< 2 weeks) Exclusion criteria: previous stroke; vision or hearing impairment; acute trauma or impairment of the limbs; inability to sit supported in a high‐backed chair for < 1 hour; MMSE < 22/30; major comorbidities | |
| Interventions | 2 arms
1 and 2: 5 days a week, 20 to 30 minutes for 2 weeks; additional usual rehabilitation programme Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 2 weeks of treatment and 1 month after treatment
| |
| Notes | Unpublished data We used means and SDs of Item 7 of the mAS, and combined the scores on pain intensity of shoulder and hand Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Generated list was used by an independent person for group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Iran Setting: inpatient hospital Age: adults (mean age: 50.9 years) Sample size: 24 participants (12 in each group, no dropouts published) Sex: 9 women, 15 men Inclusion criteria: stroke > 6 months, ability to understand treatment guidelines Exclusion criteria: any structural abnormalities that prevent the execution, any cognitive or perceptual deficit that can affect the implementation of treatment, visual deficits | |
| Interventions | 2 arms
1 and 2: 3 weeks, 5 days a week, 30 minutes a day Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, immediately after treatment and 1 month after treatment
| |
| Notes | Published and unpublished information Funding source: Neuromuscular Rehabilitation Research Centre ‐ Semnan University of Medical Sciences Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with odd‐ and even‐numbered cards |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | Randomised cross‐over trial | |
| Participants | Country: USA Setting: not stated Age: adults (mean age: 58.2 years) Sample size: 9 participants (9 in each group) Sex: 4 women, 5 men Inclusion criteria: at least 6 months post‐stroke Exclusion criteria: not stated | |
| Interventions | 2 arms
Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 2, 4, 6 and 8 weeks
| |
| Notes | Data not included in the analysis Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehablitation centre Age: adults (mean age: 58.8 years) Sample size: 24 participants (9 in experimental group (2 dropped out at follow‐up assessment); 8 in control group 1; 7 in control group 2 (1 dropped out at follow‐up assessment) Sex: 11 women, 13 men Inclusion criteria: ischaemic stroke during the previous 12 months, between 20 and 85 years old, could understand simple verbal instructions (MMSE > 21), BRS between stage 2 and 5 for the hand, mAS < 3 Exclusion criteria: not stated | |
| Interventions | 3 arms 1, 2 and 3: conventional physiotherapy programme
1, 2 and 3: 3 weeks, 5 days a week, 2 hours a day 1 and 2: additional 30 minutes a day Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 3 weeks of treatment and 3 months after treatment
| |
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed as assigned to groups (authors' statement) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: India Setting: inpatient hospital, home after discharge Age: adults (mean age: 45.6 years) Sample size: 33 participants (17 in experimental group, 16 in control group, 1 dropout) Sex: 8 women, 25 men Inclusion criteria: aged < 60 years, single unilateral stroke with hemiparesis, more than 24 weeks post‐stroke, able to understand instructions, Brunnstrom recovery stage of arm (BRS‐A) 2 or above Exclusion criteria: associated neurological complications, severe perceptual and visual deficits (as evaluated by the National Institutes of Health Stroke Subscales and clinical tests: copying and drawing, line‐bisection, cancellation tasks, and functional performance), shoulder subluxation, uncontrolled medical illness | |
| Interventions | 2 arms 1 and 2: usual occupational therapy using principles of Brunnstrom and Bobath approaches
1: 8 weeks, 5 days a week, 45 minutes MT, additional 45 minutes usual occupational therapy 2: 8 weeks, 5 days a week, 90 minutes usual occupational therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of treatment
| |
| Notes | Information partly based on authors' information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement), computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by numbered sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | ITT using 'last measure carried forward' method |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre Age: adults (mean age: 46.4 years) Sample size: 36 participants (19 in experimental group, 17 in control group; 6 dropouts) Sex: 6 women, 30 men Inclusion criteria: post‐stroke hemiparesis due to unilateral stroke; post‐stroke duration > 6 months; paresis of either right or left side; age range between 30 and 60 years; functional ambulation classification (FAC) level 2 and above; ability to walk for a distance of at least 10 metres without any orthosis and walking device Exclusion criteria: any other associated neurological disorder; severe cognitive, perceptual and visual deficits (evaluated by the National Institutes of Health Stroke Subscales and copying, drawing, line bisection, cancellation, and functional tasks); cardiovascular instability; any musculoskeletal disorder affecting locomotion | |
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1: 3 to 4 weeks, 30 sessions, 30 minutes MT and 30 minutes conventional rehabilitation programme 2: 3 to 4 weeks, 30 sessions, 60 minutes conventional rehabilitation programme Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 3 months
| |
| Notes | Information based on abstract and authors' information, full‐text publication received in 2017 Funding source: Pandit Deendayal Upadhayaya National Institute for Persons with Physical Disabilities, 4 VD Marg, New Delhi‐110002, India Declarations of trialists’ interests: no potential conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement), computer‐generated (SPSS software) random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes (authors' statement) |
| Incomplete outcome data (attrition bias) | Low risk | ITT using 'last measure carried forward' method (authors' statement) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors, participants, and therapists were blinded to group allocation (authors' statement) |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 53.9 years) Sample size: 20 (10 in each group; no dropouts published) Sex: 7 women, 13 men Inclusion criteria: onset of stroke within 6 months Exclusion criteria: did not understand treatment method of the study, MMSE < 16, visual impairment, damage on musculoskeletal system or peripheral nerve on paretic side, mAS score > 2, Brunnstrom recovery stage 1, 5 or 6 | |
| Interventions | 2 arms 1 and 2: usual rehabilitation treatment and additional:
1 and 2: 4 weeks, 5 days a week, 30 minutes MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by cards composed of odd and even numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Iran Setting: not stated Age: adults (age not stated) Sample size: 50 participants (25 in each group, no dropouts published) Sex: not stated Inclusion criteria: 1st unilateral stroke (ischaemic or haemorrhagic verified by CT‐scan or MRI), between 1 month and 1 year after stroke, Brunnstrom recovery stages 1 ‐ 3 Exclusion criteria: severe cognitive deficit, severe aphasia, visual deficits, dementia, not able to understand instructions, did not participate in 4 sessions or 2 consecutive sessions | |
| Interventions | 2 arms 1 and 2: physiotherapy and neuromuscular stimulation
1 and 2: 20 sessions, 3 to 5 days a week, 30 minutes 1: 20 sessions, 3 to 5 days a week, additional 30 minutes MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after the 5th, 10th, and 15th session
| |
| Notes | Information based on abstract, partly translated from Persian language Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by coin tossing |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Italy Seting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 58.4 years) Sample size: 48 participants (24 in each group; 6 dropped out post‐treatment, 3 more dropped out after 6 months) Sex: 26 women, 22 men Inclusion criteria: hemiparesis after first‐ever ischaemic or haemorrhagic stroke; during 1st 6 months post‐stroke; diagnosed with CRPS‐type 1 with a VAS pain score > 4 cm Exclusion criteria: intra‐articular injection into the affected shoulder during the previous 6 months or use of systemic corticosteroids during the previous 4 months; presence of another explanation of pain; prior surgery to shoulder or neck; serious uncontrolled medical conditions; global aphasia or cognitive impairments; visual impairments which might interfere with the aims of the study; evidence of recent alcohol or drug abuse; or severe depression | |
| Interventions | 2 arms: 4‐week conventional stroke rehabilitation programme and additional:
1 and 2: 5 days a week, 30 minutes of therapy for the 1st 2 weeks; and 5 days a week, 60 minutes of therapy for the last 2 weeks Date of intervention: October 2000 to December 2006 | |
| Outcomes | Outcomes were recorded at baseline, 1 week after the intervention period and after 6 months
| |
| Notes | Published and unpublished data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Italy Setting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 62 years) Sample size: 24 participants (8 in each group) Sex: 13 women, 11 men Inclusion criteria: 1st ischaemic or haemorrhagic stroke (> 6 months); diagnosis of CRPS‐type 1 (pain VAS > 4 cm) Exclusion criteria: intra‐articular shoulder injection in the previous 6 months or systemic corticosteroid in the previous 4 months; another obvious explanation for pain; prior surgery to shoulder or neck region; serious uncontrolled medical conditions; global aphasia or cognitive impairments interfering with understanding instructions, motor testing and treatment; visual impairments interfering with aims of the study; evidence of recent alcohol or drug abuse; or severe depression | |
| Interventions | 3 arms
1, 2 and 3: 5 days a week; 30 minutes of therapy for 4 weeks Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after the intervention period
| |
| Notes | Published and unpublished data; we only analysed the 1st intervention period (4 weeks); we combined groups 2 and 3 into 1 control group Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation; cards composed with random‐numbers method |
| Allocation concealment (selection bias) | Low risk | A therapist not involved in the treatments opened sealed envelopes and assigned appointments according to treatment group (authors' statement) |
| Incomplete outcome data (attrition bias) | Low risk | Results were analysed on an ITT basis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: not stated Age: adults (mean age: 58.7 years) Sample size: 36 participants (19 in experimental group, 17 in control group, no dropouts published) Sex: 17 women, 19 men Inclusion criteria: stroke onset duration of > 6 months; no neurological deficits in the cerebellum or the brainstem; no hemineglect or visual field deficits; no cognitive problems (> 24 points in the MMSE); independent walking (with or without walking aids) Excludion criteria: not stated | |
| Interventions | 2 arms
1 and 2: 4 weeks, 5 days a week, 40 minutes (20 minutes rTMS and 20 minutes MT or sham therapy) Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after the 4 weeks of therapy:
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by blindly drawing 1 card out of an envelope containing 2 cards that were each marked as experimental group and control group |
| Allocation concealment (selection bias) | Low risk | Concealment by sealed envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated; no dropouts |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: not stated Age: adults (mean age: 59.3 years) Sample size: 27 participants (14 in experimental group, 13 in control group, no dropouts published) Sex: 12 women, 15 men Inclusion criteria: stroke with hemiplegic symptoms, a score of 24 or higher on the MMSE‐K, stroke onset more than 6 months earlier Exclusion criteria: orthopaedic or neurological disease history | |
| Interventions | 2 arms 1 and 2: tDCS 1: MT: participants performed movements of both upper limbs, 10 sets, 20 repetitions of each motion, 2‐minute rest between sets 2: sham therapy: participants performed the same exercises with non‐reflective surface between limbs 1 and 2: 6 weeks, 3 days a week, 20 minutes tDCS + 5 minutes rest + 20 minutes MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after the 6 weeks of therapy
| |
| Notes | Funding source: Wonkwang Health Science University Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Spain Setting: outpatient rehabilitation centre Age: adults (mean age: 53.5 years) Sample size: 34 (17 in experimental group (2 dropped out); 16 in control group (1 dropped out)) Sex: 5 women, 26 men Inclusion criteria: stroke > 6 months, BRS 1 or 2, FM‐UE < 19, sensory impairment assessed by clinical examination, able to maintain sitting position for at least 60 minutes, MMSE > 23 Exclusion criteria: impaired comprehension that hindered understanding of instructions (Mississippi Aphasia screening < 45), upper limb pain that limited participation in rehabilitation protocol, spatial neglect, self‐awareness disorder, emotional circumstances that impeded adequate collaboration | |
| Interventions | 2 arms 1 and 2: usual physical therapy:
1 and 2: 8 weeks, 5 days a week, 60 minutes each, additional 3 days a week, 45 minutes a session MT or passive mobilisation Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of intervention
| |
| Notes | Published information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes and independent investigator |
| Incomplete outcome data (attrition bias) | High risk | No ITT analysis was performed |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Switzerland Setting: not stated Age: adults (age not stated) Sample size: 10 participants (no dropouts published) Sex: not stated Inclusion criteria: 3 months after stroke; severe disability (NIHSS 10 ‐ 14), hand paresis Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: TMS: double‐pulse TMS through a figure‐eight focal coil for bilateral intracortical inhibition in primary motor at rest and during movement preparation
1 and 2: 4 weeks, 3 days a week, 15 minutes TMS 1: additional 15 minutes MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after therapy period
| |
| Notes | Information based on authors' information and abstract Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Germany Setting: inpatient rehabiltation centre Age: aduts (mean age: 56.5 years) Sample size: 48 participants (24 in each group, 12 dropped out) Sex: 10 women, 26 men Inclusion criteria: first‐ever ischaemic stroke in the territory of the middle cerebral artery; not more than 8 weeks post‐stroke; between 25 and 80 years old; able to follow therapy instructions; capable of participating in 30‐minute daily therapy sessions Exclusion criteria: experienced previous stroke; major haemorrhagic changes; increased intracranial pressure; hemicraniectomy or orthopaedic, rheumatologic, or other diseases interfering with their ability to sit or to move either upper limb | |
| Interventions | 2 arms
1 and 2: 5 days a week; 30 minutes of therapy for 6 weeks Date of intervention: October 2004 to April 2006 | |
| Outcomes | Outcomes were recorded at baseline and after the intervention
| |
| Notes | Published and unpublished data; we extracted the motor section of the FM‐UE (without reflex activity, 0 to 60) Funding source: rehabilitation research network (refonet) of the German Pension Scheme Rhineland Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed, numbered envelopes were created |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were broken after study inclusion |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors of primary outcome were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: USA Setting: outpatient (at home) Age: adults (34 to 73 years old) Sample size: 6 participants (4 in 2 experimental groups, 2 in control group; dropouts not published) Sex: 3 women, 3 men Inclusion criteria: first‐time unilateral stroke occurring at least 3 months prior with FMA‐UE scores between 10 and 50 Exclusion criteria: not stated | |
| Interventions | 3 arms 1 ‐ 3 : occupational therapy (OT)
1 ‐ 3: 6 weeks, 2 times a week OT in the clinic 1 ‐ 3: 6 weeks, 5 days a week, 30‐minute home programme bimanual MT, unimanual MT or traditional OT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded
| |
| Notes | Information based on abstract Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabiltation centre Age: adults (mean age: 60.9 years) Sample size: 31 (16 in experimental group, 15 in control group, no dropouts published) Sex: 14 women, 17 men Inclusion criteria: unilateral hemiplegia due to first‐ever stroke (verified by CT or MRI); < 6 months; BRS for the upper extremity between I and IV; MMSE 24 and above; lack of excessive spasticity in the joints of the affected upper extremity (stage 2 and below according to the mAS) Exclusion criteria: joint movement limitations in the healthy upper extremity; a visual field defect or neglect syndrome; and those who had previously undergone a rehabilitation programme | |
| Interventions | 2 arms 1 and 2: upper extremity rehabilitation programme
1 and 2: 4 weeks, 5 times a week, 60 to 120 minutes upper extremity rehabilitation programme 1 and 2: 4 weeks, 5 times a week, 20 minutes MT or sham therapy Date of intervention: July 2013 to July 2014 | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated; no dropouts published |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Japan Setting: inpatient hospital Age: adults (mean age: 67.5 years) Sample size: 14 participants (7 in each group, no dropouts published) Sex: 6 women, 8 men Inclusion criteria: 1st episode of stroke with hemiparesis or second episode of stroke with no upper limb motor dysfunction after 1st stroke, > 1 month since stroke, Brunnstrom recovery stage finger 1 ‐ 5, no severe cognitive disorders (MMSE score ≥ 24, and item score of consciousness, gaze, visual fields, language, attention of National Institutes of Health Stroke scale = 0) Exclusion criteria: hypertonia of upper limb, limitation in range of motion of upper limb, other diseases interfering with ability to move upper limbs | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme (physiotherapy, occupational therapy)
1 and 2: 4 weeks, 6 ‐ 7 days a week, daily 2 hours 1: additional 30 minutes MT Date of intervention: October 2010 to March 2011 | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent author who drew sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts and group changes |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 63.9 years) Sample size: 24 participants (14 in experimental group, 10 in control group; 5 dropouts) Sex: 8 women, 11 men Inclusion criteria: onset of stroke at least 6 months prior to study, able to understand and follow simple verbal instructions, MMSE > 21, Brunnstrom stages 1 ‐ 4 Exclusion criteria: apraxia, hemineglect, orthopaedic conditions or digital neuropathy in upper extremities | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 30 minutes additional virtual reality (VR) reflection therapy or additional sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Republic of Korea Seting: inpatient rehabilitation centre Age: adults (mean age: 55.9 years) Sample size: 30 participants (15 in experimental group and 15 in control group; 5 dropouts) Sex: 10 women, 15 men Inclusion criteria: onset of stroke at least 6 months prior to study; were able to understand and follow simple verbal instructions; had a MMSE score over 21; had a Brunnstrom score between stages I and IV Exclusion criteria: had no apraxia or hemineglect; had no orthopaedic and neurologic conditions such as fractures and digital neuropathy on their lower extremities | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 30 minutes conventional stroke rehabilitation programme 1 and 2: 4 weeks, 5 days a week, 30 minutes additional virtual reality (VR) reflection therapy or additional sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks:
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to the participants’ groups |
| Methods | RCT | |
| Participants | Country: Italy Setting: inpatient rehabilitation centre Age: adults (mean age: 66.6 years) Sample size: 26 (13 in each group; 1 dropped out) Sex: 9 women, 17 men Inclusion criteria: hemiplegia after 1st stroke (diagnosed by CT scan) within 4 weeks post‐stroke, absence of severe attentive deficits, presence of movement in shoulder/elbow/hand with Motricity score < 77; Exclusion criteria: haemorrhagic stroke, global aphasia and cognitive impairments that interfere with study or treatment participation (MMSE < 22), concomitant cns‐ or pns‐disorder or myopathia | |
| Interventions | 2 arms: usual rehabilitation programme 1 hour, 5 times a week, additional:
1 and 2: 5 days a week, 30 minutes of MT or sham therapy for 1st 2 weeks, 60 minutes of MT or sham therapy for the last 2 weeks Date of intervention: October 2009 to August 2011 | |
| Outcomes | Outcomes were recorded and reported at baseline and after 4 weeks
| |
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent investigator |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed as allocated (authors' information) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: university hospital Age: adults (mean age: 52.6 years) Sample size: 35 participants (12 in experimental group 1, 11 in experimental group 2, 12 in control group, no dropouts published) Sex: 13 women, 22 men Inclusion criteria: hemiparesis by stroke Exclusion criteria: not stated | |
| Interventions | 3 arms 1, 2 and 3: traditional physiotherapy
1, 2, and 3: 6 weeks, 5 days a week, 30 minutes a session physiotherapy 1, 2, and 3: additional 15 minutes a day MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 6 weeks of therapy
| |
| Notes | Information based on published article Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number blocks |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 64.1 years) Sample size: 81 participants (19 in group 1 (3 dropped out), 25 in group 2 (6 dropped out), 17 in group 3 (2 dropped out),11 in group 4 (2 dropped out), 9 in group 5 (1 dropped out)) Sex: 24 women, 43 men Inclusion criteria: hemiplegia following initial supratentorial stroke, admitted to a convalescent rehabilitation ward Exclusion criteria: time to admission from the onset is within 14 days, difficult communication due to severe cognitive disorder, comorbidity index of 4 or higher, necessity of high‐level consideration and caution for rehabilitation, and scores of hip‐flexion, knee‐extension, and foot‐pat items of the Stroke Impairment Assessment Set (SIAS) lower than 2 | |
| Interventions | 5 arms 1 to 5: standard rehabilitation programme
1 to 5: 4 weeks, 1 hour a day standard rehabilitation programme 1 to 5: 20 minutes within conventional physiotherapy Date of intervention: September 2009 to July 2011 | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Information based on published article Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: university hospital Age: adults (mean age: 55.8 years) Sample size: 27 (14 in experimental group, 13 in control group, 4 dropouts) Sex: 9 women, 14 men Inclusion criteria: onset of stroke within 6 months, MMSE > 21, FMA upper extremity score < 44, Brunnstrom recovery stage 1 ‐ 4, absence of orthopaedic disease in the upper extremity, no visual perception disorder (unilateral neglect, hemianopsia, apraxia), no pacemaker, no anticonvulsant medication, medically stable condition Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: usual rehabilitation treatment
1 and 2: 60 minutes/day, 5 times/week, 4 weeks usual rehabilitation treatment 1 and 2: additional 5 days a week, 30 minutes a day, 4 weeks MT or sham therapy Date of intervention: 1 July to 31 July 2013 | |
| Outcomes | Outcomes were recorded and reported at baseline (t1), after 4 weeks of treatment (t2)
| |
| Notes | Funding source: Sahmyook University Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.7 years) Sample size: 33 participants (20 in 2 experimental groups, 9 in control group; 4 dropouts) Sex: 9 women, 20 men Inclusion criteria: onset of stroke > 6 months, MMSE > 25, absence of cognitive problems, BRS 1 – 4, and the ability to understand the purpose of the study Exclusion criteria: impaired vision, cognitive problems such as a severe decline in cognition or aphasia that would prevent normal progress in the experiment, neurological or musculoskeletal (fracture or balance‐related) disorders not caused by stroke, hemineglect | |
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme
1, 2 and 3: 4 weeks, 5 days a week, 30 minutes a day 1 and 2: additional 4 weeks, 5 days per week, 30 minutes a session Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: outpatient hospital Age: adults (mean age: 49.1 years) Sample size: 25 participants (12 in experimental group, 13 in control group, no dropouts published) Sex: 9 women, 16 men Inclusion criteria: hemiplegia due to stroke, stroke > 6 months. MMSE > 24, understanding the procedure and purpose of the study, volunteer participation in the study Exclusion criteria: not stated | |
| Interventions | 2 arms
1and 2: 4 weeks, 5 days a week, 30 minutes a day MT or control intervention Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by dice with odd and even numbers |
| Allocation concealment (selection bias) | Low risk | Allocation by throwing dice after inclusion in the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information, no drop‐outs |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | Randomised cross‐over trial | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 69.1 years) Sample size: 13 participants (6 in group 1, 7 in group 2, no dropouts) Sex: 3 women, 10 men Inclusion criteria: hemiparesis caused by a single stroke, between 30 and 180 days post‐stroke, MMSE > 20, palpable contraction of paretic wrist and finger extensors, detectable EMG signal (> 5 V) from those muscles Exclusion criteria: cardiac pacemaker; serious contractures or pain in the shoulder, elbow or wrist; shoulder subluxation; severe cognitive impairment or severe aphasia; inability to give informed consent; engagement in any other experimental studies | |
| Interventions | 2 arms 1 and 2: standard physiotherapy and occupational therapy
1 and 2: 8 weeks, 5 days a week, 2 hours a day physiotherapy and occupational therapy 1 and 2: 4 weeks, 5 days a week, two 20‐minute sessions a day; group 1: additional ETMS‐MT for the 1st 4 weeks, group 2: additional ETMS‐MT for the second 4 weeks Date of intervention: November 2009 to May 2012 | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 8 weeks of therapy
| |
| Notes | Based on published and unpublished information Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Assessor not blinded to group allocation |
| Methods | RCT | |
| Participants | Country: India Setting: not stated Age: adults (mean age: 57.3 years) Sample size: 30 (15 in each group, no dropouts) Sex: 8 women, 22 men Inclusion criteria: 1st stroke (ischaemic or haemorrhagic), unilateral stroke with hemiparesis, Brunnstrom recovery stage 2 ‐ 4, age > 25 years, ambulatory before stroke, able to understand simple verbal instructions Exclusion criteria: severe cognitive disorder, previous stroke, orthopaedic or rheumatologic problems restricting lower limbs, other diseases that interfere with ability to sit or moving lower limbs | |
| Interventions | 2 arms 1 and 2: conventional physical therapy and
1 and 2: 40 ‐ 45 minutes/day for 10 days conventional physical therapy 1: twice daily for 15 minutes for 10 days Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 5 and 10 days
| |
| Notes | Unpublished data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Turkey Setting: not stated Age: adults (mean age: 61.4 years) Sample size: 20 participants (10 in experimental group, 10 in control group, no dropouts published) Sex: 10 women, 10 men Inclusion criteria (information based on translation): 1st stroke < 8 weeks; Brunnstrom recovery stages 1 ‐ 4 Exclusion criteria (information based on translation): previously received treatment/rehabilitation; mAS > 3; pain in the paretic side; cognitive impairments; vision impairments/neglect | |
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 5 days a week, daily 1 ‐ 2 hours 1: additional 15 minutes , 4 times daily MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy:
| |
| Notes | Information based on an abstract; partly translated; not possible to contact author Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by envelope method |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated (no dropouts) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.1 years) Sample size: 28 (14 in each group; 2 dropped out) Sex: 11 women, 15 men Inclusion criteria: stroke within last 6 months, able to understand and follow the instructions (MMSE > 21), Brunnstrom recovery stages upper limb 1 ‐ 4 Exclusion criteria: orthopaedic disorders, apraxia, hemineglect, upper‐limb fracture, peripheral nerve injury, participation in other studies or rehabilitation programmes, participation rate < 80% | |
| Interventions | 2 arms 1 and 2: usual rehabilitation programme
1 and 2: 75 minutes, 5 times/week 1: 1st 4 weeks, 5 days/week, 25 minutes twice a day MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded and reported at baseline and after 1 day after therapy period
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not analysed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: rehabilitation hospital Age: adults (mean age: 54.7 years) Sample size: 30 participants (15 in experimental group (1 dropped out), 15 in control group (2 dropped out)) Sex: 13 women, 14 men Inclusion criteria: stroke diagnosed by a neurologist using computed tomography or magnetic resonance imaging, hemiplegia for > 6 months after stroke onset, active ankle dorsiflexion ROM > 10 °, ability to walk > 10 metres independently, MMSE > 21, no visual problems, no adverse effects from NMES, absence of use of any medication that could affect balance or gait Exclusion criteria: uncontrolled blood pressure or angina, history of seizure, pacemaker use, musculoskeletal problems of the lower extremity, any intervention other than conventional therapy | |
| Interventions | 2 arms 1 and 2: conventional physiotherapy
1 and 2: 4 weeks, 5 days a week, 1 hour a day 1: additional 4 weeks, 5 days a week MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and 1 day after therapy period
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | No ITT analysis |
| Blinding of outcome assessment (detection bias) | Low risk | 2 assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehablitation centre Age: adults (mean age: 64.9 years) Sample size: 60 (30 in each group, no dropouts) Sex: 21 women, 39 men Inclusion criteria: hemiplegia due to stroke within 6 months, Korean version of MMSE > 24, BRS upper extremity of 3 to 4 Exclusion criteria: musculoskeletal disease, neglect, mental illness | |
| Interventions | 2 arms
1 and 2: 4 weeks, 5 days a week, 20 minutes/day MT or sham therapy Date of intervention: February to May 2012 | |
| Outcomes | Outcomes were recorded at baseline and after therapy period
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated (no dropouts) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Taiwan Setting: inpatient and outpatient Age: adults (mean age: 55 years) Sample size: 43 participants (14 in experimental group 1, 14 in experimental group 2, 15 in control group, 1 dropout) Sex: 11 women, 32 men Inclusion criteria: ischaemic or haemorrhagic stroke of at least 6 months duration, Brunnstrom stage 3 or above in the arm Exclusion criteria: severe spasticity in any joints of the affected arm (modified AS ≤ 2), serious cognitive deficits (MMSE score > 24), serious vision or visual perception deficits (score of 0 on the best gaze and visual subtest of the National Institutes of Health Stroke Scale), history of other neurologic, neuromuscular, or orthopaedic disease, participation in other studies concurrent with this study | |
| Interventions | 3 arms
1, 2, and 3: 4 weeks, 5 days a week, 1½ hours daily Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after therapy
| |
| Notes | Funding source: National Health Research Institutes, National Science Council, Healthy Ageing Research Center at Chang Gung University, Taiwan Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned , stratified into 4 strata according to the side of lesion and the level of motor impairment |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: USA Setting: home Age: adults (age not stated) Sample size: 10 participants Sex: not stated Inclusion criteria: 6 months or more post‐cerebrovascular accident Exclusion criteria: not stated | |
| Interventions | 2 arms
1 and 2: 4 weeks Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at pretreatment, mid‐treatment, post‐treatment and after 3 months
| |
| Notes | Abstract data only; not included in the analysis due to insufficient data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ability‐matched pairs were created and randomly assigned to groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Australia Setting: inpatient rehabilitation unit Age: adults (mean age: 68.7 years) Sample size: 15 participants (5 in experimental group, 10 in 2 control groups, no dropouts) Sex: 8 women, 7 men Inclusion criteria: first‐ever neurological injury < 8 weeks, affected dorsiflexion strength of < Grade 3, ambulatory prior to admission Exclusion criteria: impaired cognition (MoCA < 21), peripheral neuropathy, impaired ROM of the intact lower limb, medically unfit for rehabilitation | |
| Interventions | 3 arms 1, 2 and 3: individual physiotherapy sessions
1, 2 and 3: 3 weeks, 5 days a week, 45 minutes a day individual physiotherapy 1 and 2: 15 minutes MT or sham therapy during the individual physiotherapy session Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 3 weeks of therapy, and 6 weeks after the intervention
| |
| Notes | Based on unpublished information, only stroke patients included Funding source: National Stroke Foundation, Australia Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by independent person |
| Incomplete outcome data (attrition bias) | Low risk | All data collected, reported and analysed as allocated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Netherlands Setting: home Age: adults (mean age: 57 years) Sample size: 40 participants (20 in each group,; 4 dropped out during intervention period, 4 more dropped out after 6 months) Sex: 20 women, 20 men Inclusion criteria: knowledge of Dutch language, Brunnstrom score upper extremity between 3 and 5; home dwelling status; at least 1 year post‐stroke Exclusion criteria: neglect; comorbidities that influenced upper extremity usage; history of multiple strokes | |
| Interventions | 2 arms
1 and 2: once a week physiotherapeutic supervision for 60 minutes; 5 times a week, 60 minutes of practice at home for 6 weeks Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, post‐treatment and after 6 months
| |
| Notes | Published and unpublished data Funding source: Fonds NutsOhra [SNO‐T‐0602‐23]; Innovatiefonds Zorgverzekeraars [06‐262]; Wetenschappelijk College Fysiotherapie [WU/2007/07] and Hersenstichting Nederland [15F07.54] Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Participants received group allocation after baseline measurement |
| Incomplete outcome data (attrition bias) | Low risk | Results were analysed on an ITT basis (multiple imputation) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Romania Setting: inpatient Age: adults (mean age: 57.5 years) Sample size: 15 participants (7 in experimental group, 8 in control group, no dropouts published) Sex: 8 women, 7 men Inclusion criteria: hemiplegia following a 1st stroke (documented by CT scan), time from stroke between 1 to 3 months, without severe attention deficit Exclusion criteria: global aphasia and cognitive impairments that might interfere with understanding instructions for testing, concomitant progressive central or peripheral nervous system disorders | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme (neuro‐rehabilitation technique, electrical stimulation and occupational therapy)
1 and 2: 6 weeks, 5 times a week, 30 minutes a session conventional stroke rehabilitation programme 1: additional 30 minutes of MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and 1 day after therapy
| |
| Notes | Funding source: not financed Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre Age: adults (mean age: 63 years) Sample size: 22 participants (11 in each group, no dropouts published) Sex: 10 women, 12 men Inclusion criteria: 1st episode of unilateral stroke with hemiparesis (onset ≤ 2 weeks), able to understand and follow simple verbal instructions, Brunnstrom recovery stage 2 and above, no severe cognitive disorders that would interfere with the study’s purpose (MMSE score > 23), stable medical condition to allow participation in the study, ambulatory before stroke Exclusion criteria: neglect, Pusher syndrome, visual deficits, and history of multiple stroke, or comorbidities that influenced lower extremity usage | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation programme: neurodevelopmental facilitation techniques, sensory motor re‐education, active exercises, mobility training, balance, and gait training
1 and 2: 2 weeks, 6 days a week, 60 minutes a day and additional 30 minutes of MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
| |
| Notes | Funding source: not financed (according to authors) Declarations of trialists’ interests: there are no conflicts of interest (according to authors) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) by block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed as intended |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | Randomised cross‐over trial | |
| Participants | Country: France Setting: not stated Age: adults (mean age: 53.5 years) Sample size: 8 participants (4 in each group, 2 dropouts) Sex: 4 women, 4 men Inclusion criteria: neglect (according to Negligence Evaluation Battery) secondary to a unilateral stroke of the right hemisphere Exclusion criteria: other concomitant cerebral injuries, Illetrism or cognitive dysfunction altering comprehension | |
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes a day; 1: MT for 5 consecutive days, 1 session a day, after 10 days sham therapy for 5 consecutive days; 2: same protocol as group 1, but participants received sham therapy before MT Date of intervention: not stated | |
| Outcomes | Outcomes were recorded before and after each session
| |
| Notes | Based on unpublished information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by randomised‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by drawing lots |
| Incomplete outcome data (attrition bias) | High risk | No ITT analysis was performed |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: India Seting: hospital Age: adults (mean age: 44.9 years) Sample size: 60 participants (40 in 2 experimental groups, 20 in control group, 1 dropout) Sex: 20 women, 40 men Inclusion criteria: unilateral hemiplegic stroke, between 6 weeks and 6 months post‐stroke, ischaemic stroke, age 18 to 60 years, both men and women, BRS 2 ‐ 5, modified AS ≥ 1, voluntary extension of wrist and fingers of at least 10 ° from the resting position Exclusion criteria: > 60 years of age, BRS 1 or 6, wrist and/or finger contracture, cardiac pacemaker or other metal implants, significant visual, auditory and cognitive impairment | |
| Interventions | 3 arms 1, 2 and 3: conventional therapy
1, 2 and 3: 2 weeks, 6 days a week, 30 minutes daily MT, MT + FES, or FES Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
| |
| Notes | Based on published information Funding source: not stated Declarations of trialists’ interests: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by cards composed of odd and even numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | All data were collected and analysed as allocated |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded to group allocation |
| Methods | RCT | |
| Participants | Country: India Setting: inpatient rehabilitation centre and home training after discharge Age: adults (mean age: 63.4 years) Sample size: 48 participants (27 in experimental group, 21 in control group, 2 dropouts) Sex: 20 women, 28 men Inclusion criteria: stroke patients with thalamic and parietal lobe lesions within 48 hours of stroke onset who had upper limb weakness, provided informed consent Exclusion criteria: Glasgow Coma Scale score < 7, unco‐operative patients | |
| Interventions | 2 arms 1 and 2: home programme
1: 4 weeks, 5 days a week, 1 hour a day MT and 1 hour limb activation 2: 4 weeks, 5 days a week, 1 hour a day sham therapy and 1 hour limb activation Date of intervention: January 2011 to August 2013 | |
| Outcomes | Outcomes were recorded at baseline and at 1, 3 and 6 months
| |
| Notes | Published and unpublished information Funding source: Christian Medical College, Department of Neurology, India, Intramural research fund Declarations of trialists’ interests: there are no conflicts of interest to the manuscript; full disclosures at http://n.neurology.org/content/83/11/1012/tab‐article‐info | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed numbered envelopes |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis performed ('last observation carried forward' method) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient Age: adults (mean age: 56.3 years) Sample size: 30 participants (15 in each group) Sex: 13 women, 17 men Inclusion criteria: diagnosis of hemiplegia due to stroke of at least a 6‐month duration, scores of ≥ 24 points on the MMSE‐Korean (MMSE‐K; no difficulty with cognitive functions), Brunnstrom’s upper extremity stage IV, no difficulties with perceptual abilities including hemineglect based on the MVPT, voluntary consent to participate in the study Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: conventional occupational therapy
1 and 2: 4 weeks, 5 days a week, 30 minutes MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after therapy
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random card selection |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: South Korea Setting: rehabilitation unit Age: adults (mean age: 60 years) Sample size: 30 participants (15 in experimental group, 15 in control group, no dropouts published) Sex: 15 women, 15 men Inclusion criteria: stroke > 3 months identifiable by CT or MRI, no cognitive dysfunction that would interfere with the study purpose as indicated by a MMSE‐K > 24, no perceptual disorder or unilateral neglect that would have interfered with the study purpose as indicated by the MVPT, Brunnstrom score between stages I – IV for the UE Exclusion criteria: aphasia, vision or hearing disorders, or had had MT previously | |
| Interventions | 2 arms
1 and 2: 6 weeks, 5 days a week task‐oriented MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and immediately after treatment and 1 month after treatment
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Thailand Setting: inpatient rehabilitation centre Age: adults (mean age: 56 years) Sample Size: 47 participants (20 in each group; 7 dropped out) Sex: 19 women, 21 men Inclusion criteria: 1st stroke hemiparesis onset more than 3 months, age > 18 years, able to follow 2‐step command, upper extremity Brunnstrom stage between 1 and 4, able to sit with or without support more than 30 minutes, cognitive function evaluated by MMSE ≥ 24, no previous disease of the hemiparetic side | |
| Interventions | 2 arms
1 and 2: 30 minutes/session, 10 sessions Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and at the end of 2 weeks, 4 weeks and 12 weeks
| |
| Notes | Published and unpublished data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by blocked randomisation, computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Central randomisation by a third party |
| Incomplete outcome data (attrition bias) | High risk | Dropouts not analysed |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Malaysia Setting: nursing homes Age: adults (mean age: 58 years) Sample size: 30 participants (15 in each group: 1 dropped out from the experimental group) Sex: 9 women, 21 men Inclusion criteria: men and women, age 50 to 70 years, 1st episode of unilateral stroke with hemiparesis, 2 to 12 months post‐stroke, diagnosis of stroke with involvement of middle cerebral artery on MRI or CT scan by neurologist Exclusion criteria: MMSE < 24, uncontrolled systemic hypertension, perceptual or apraxic deficits, visual deficit such as homonymous hemianopia, reflex sympathetic dystrophy, severe shoulder subluxation, contracture in the affected upper limb and botox injection within past 6 months to the affected upper limb | |
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 5 days a week, 1 hour a day conventional rehabilitation programme 1 and 2: additional 30 minutes a day MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Information based on unpublished data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in the analysis |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: India Setting: outpatient Age: adults (mean age: 54.8/57.9 years) Sample size: 20 participants (6 in experimental group, 6 in control group, 8 dropped out) Sex: not stated Inclusion criteria: age 45 to 65 years, 1st episode of ischaemic and haemorrhagic stroke, stroke between 1 to 6 months, men and women, MMSE > 23, BRS 4 and 5 Exclusion criteria: any musculoskeletal disorders, neurological disorder other than stroke, visual impairment, systemic disease, non‐cooperative patients, psychological problems | |
| Interventions | 2 arms 1 and 2: conventional therapy
1 and 2: 4 weeks, 6 days a week, 30 minutes a day conventional therapy 1 and 2: additional 30 minutes a day MT or MRP Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | Information based on unpublished data Funding source: not stated Declarations of trialists’ interests: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent investigator |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: Brazil Setting: home Age: adults (mean age: 57.5 years) Sample size: 16 participants (8 in each group: no dropouts published) Sex: 6 women, 10 men Inclusion criteria: stroke > 6 months, spasticity < 3 modified AS for horizontal shoulder adductors, elbow flexors, and wrist and finger flexors; FM‐UE score 30 ‐ 49 points Exclusion criteria: other neurological diseases, orthopaedic upper limb problems which interfered with their activity level, uncontrolled shoulder pain, significant uncorrectable visual impairment, aphasia or difficulty understanding simple tasks, visual hemineglect, those who were receiving other upper‐limb interventions | |
| Interventions | 2 arms
1 and 2: 4 weeks, 3 days a week, 1 hour a day MT or sham therapy Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 4 weeks of therapy, and 2 weeks after training
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation by independent person and stapled, sealed envelopes |
| Incomplete outcome data (attrition bias) | Low risk | ITT was performed (authors' information) |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome measures were videotaped and rated by a trained physiotherapist blinded to the group allocation |
| Methods | RCT; 2 baseline subgroups | |
| Participants | Country: Netherlands Setting: inpatient and outpatient rehabilitation centre Age: adults (mean age: 73.4 years) Sample size: 16 participants (6 in the outpatient centre group (Rothgangel 2004a), 10 in the inpatient rehabilitation group (Rothgangel 2004b) Sex: 10 women, 6 men Inclusion criteria: 1st stroke in the territory of the middle cerebral artery; minimum 3 months post‐stroke; minimum score of 1 in the ARAT Exclusion criteria: bilateral stroke; severe neglect; severe visual impairments | |
| Interventions | 2 arms
1 and 2: day hospital group (6 participants): 17 treatments during 5 weeks for 30 minutes each; inpatient rehabilitation group (10 participants): 37 treatments during 5 weeks for 30 minutes each Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, in the middle of the treatment, after 5 weeks of treatment and 10 weeks after baseline
| |
| Notes | Due to sufficient differences in treatment intensity, we analysed both experimental and both control groups separately Significant differences in baseline characteristics (age, ARAT, participant‐specific problem scale) Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Participants received group allocation after baseline measurement |
| Incomplete outcome data (attrition bias) | Low risk | All participants were analysed as allocated to groups. No dropouts |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT; subgroup: outpatient centre | |
| Participants | see Rothgangel 2004 | |
| Interventions | see Rothgangel 2004 | |
| Outcomes | see Rothgangel 2004 | |
| Notes | see Rothgangel 2004 | |
| Methods | RCT; subgroup: inpatient rehabilitation | |
| Participants | see Rothgangel 2004 | |
| Interventions | see Rothgangel 2004 | |
| Outcomes | see Rothgangel 2004 | |
| Notes | see Rothgangel 2004 | |
| Methods | Randomised cross‐over trial | |
| Participants | Country: Lebanon/USA Setting: not stated Age: adults (age not stated) Sample size: 18 participants (9 in experimental group, 9 in control group, no dropouts published) Sex: not stated Inclusion criteria: stroke (subacute stage) Exclusion criteria: not stated | |
| Interventions | 2 arms
1 and 2: 2 weeks, 4 times a week, 50 minutes MT + ES or conventional therapy; followed by 2 weeks vice versa Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 1st 2 weeks, and immediately after the last 2 weeks, and 4 weeks after end of training
| |
| Notes | Information based on abstract Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: India Seting: inpatient rehabilitation centre Age: adults (mean age: 51.2 years) Sample size: 20 participants (10 in each group, no dropouts published) Sex: 4 women, 16 men Inclusion criteria: aged between 18 and 60 years, first‐time ischaemic or haemorrhagic stroke of the middle cerebral artery tertiary, occurring < 6 months before the start of the study, Brunnstrom recovery stages I to IV for the arm and hand, MMSE > 24 Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: conventional stroke rehabilitation 1: additional MT: participants performed unilateral movements while watching in the mirror 2: additional sham therapy: participants performed the same exercises as in MT group using the non‐reflecting surface of the mirror 1 and 2: 3 weeks, 5 days a week, 6 hours conventional stroke rehabilitation 1 and 2: 3 weeks, 5 days a week, 2 x 30 minutes additional MT or sham therapy a day Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after therapy
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Austria/Germany Setting: 3 inpatient rehabilitation centres Age: adults (mean age: 63 years) Sample size: 32 participants (15 in experimental group, 17 in control group, 2 dropouts) Sex: 13 women, 19 men Inclusion criteria: had suffered their 1st ischaemic or haemorrhagic stroke within 6 months prior to entering the study, had severe (FM‐UE ≥ 18 ≤ 33 points) or very severe arm paresis (FM‐UE ≤ 17 points) as assessed with the Fugl‐Meyer Assessment, had arm/hand function that could be electrically stimulated and EMG‐triggered pulses that could be elicited, reported to have been independent in their activities of daily living before stroke, reported to have had full functionality of their upper extremities before the stroke, and were able to understand study tasks and test instructions Exclusion criteria: were pregnant, had an implanted cardiac pacemaker, defibrillator, brain stimulation, drug pump, or metal implant, had wounds, thrombosis, or phlebitis in the stimulation area; severe forms of Dupuytren’s contracture, dementia and concomitant severe neurological diseases; or profound neurocognitive deficits | |
| Interventions | 2 arms 1 and 2: conventional therapy
1 and 2: 3 weeks, 5 days a week conventional therapy 1 and 2: 3 weeks, 5 days a week, 30 minutes a day EMG‐MES + MT or EMG‐MES Date of intervention: September 2013 to August 2014 | |
| Outcomes | Outcomes were recorded at baseline and after therapy
| |
| Notes | Abstract published in 2015, full‐text publication received in 2017 Funding source: not stated Declarations of trialists' interests: the first author was employed by MED‐EL after the end of study (STILLWELL, one of the distributors and developers of the stimulation device which was used in the study) and gives seminars for EMG‐triggered multichannel electrostimulation; the senior author delivers seminars and has authored two manuals on MT; the other authors declared no potential conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence and block randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by sealed envelopes and independent investigator |
| Incomplete outcome data (attrition bias) | Low risk | All data included as intended |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: South Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 51.4 years) Sample size: 40 participants (19 in mirror therapy group, 21 in control group) Sex: 22 women, 18 men Inclusion criteria: stroke within 6 months Exclusion criteria: not able to understand treatment instructions; communication difficulties due to aphasia; MMSE < 15 points; musculoskeletal or neurological damage of the unaffected upper extremity; modified AS of 3 or more points; Brunnstrom stage of recovery (arm) of 1 or more than 5 points | |
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: September 2008 to February 2009 | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of treatment
| |
| Notes | Published data only, extracted in part on the basis of an unauthorised, automatic translation of the original publication in Korean; Significant difference in MFT between groups at baseline measurement Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabilitation centre Age: adults (mean age: 63.4 years) Sample size: 40 participants (20 in each group; 7 dropped out at 6 months follow‐up) Sex: 17 women, 23 men Inclusion criteria: 1st unilateral stroke during previous 12 months; a score of 1 or 2 in the Brunnstrom stages of lower extremity; ambulatory before stroke Exclusion criteria: severe cognitive disorders | |
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 4 weeks and after 6 months
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated allocation of blocks |
| Allocation concealment (selection bias) | Low risk | The physicians who assessed potential participants to determine eligibility did not know to which group the participants would be allocated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | Randomised cross‐over trial | |
| Participants | Country: Japan Setting: inpatient rehabilitation centre Age: adults (mean age: 63.7 years) Sample size: 15 participants (9 in mirror therapy group; 6 dropped out, 4 during the 1st interval) Sex: 9 women, 6 men Inclusion criteria: people admitted or planned to be admitted to rehabilitation ward on the hospital due to post‐stroke hemiparesis; within 1 month post‐stroke; informed consent was obtained from the participant and their family Exclusion criteria: higher brain dysfunction | |
| Interventions | 2 arms
1 and 2: 10 to 15 minutes a day for 4 weeks, followed by 4 weeks vice versa Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline and after 4 weeks of therapy
| |
| Notes | We only analysed the 1st intervention period of 4 weeks Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation to groups |
| Allocation concealment (selection bias) | High risk | Stated by authors (unpublished information) |
| Incomplete outcome data (attrition bias) | High risk | Stated by authors (unpublished information) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Germany Setting: inpatient rehabilitation centre Age: adults (mean age: 67.2 years) Sample size: 60 participants (21 in the mirror therapy group intervention, 18 in the mirror therapy single therapy, 21 in the sham group; 11 dropped out in the intervention period) Sex: 25 women, 35 men Inclusion criteria: 1st supratentorial stroke within the previous 3 months; aged between 18 and 80 years; clinically diagnosed severe hemiparesis or hemiplegia of the distal upper limb with MRC grading of 0 or 1 of wrist and finger extensors Exclusion criteria: visual impairments that may limit participation in mirror therapy; severe cognitive and/or language deficits which preclude participants from following instructions in the group training protocol; other neurological or musculoskeletal impairments of the upper extremity not due to stroke; severe neglect (head is not turned to the affected side due to instruction) | |
| Interventions | 3 arms 1, 2 and 3: standard rehabilitation programme, additional:
1, 2 and 3: 5 weeks, additional 20 sessions, 30 minutes MT, MT group or sham therapy Date of intervention: April 2009 to July 2011 | |
| Outcomes | Outcomes assessed before and after treatment, and 7 months after treatment
| |
| Notes | Published and unpublished data Funding source: Klinik Bavaria Kreischa, Germany Declarations of trialists’ interests: the first author received and will receive honorarium for presentations and seminars on MT; the other authors declared no potential conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation by an independent person |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed ('last observation carried forward' method) |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors of primary outcome (motor function) were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: UK Setting: 12 inpatient stroke services Age: adults (mean age: 64 years) Sample size: 94 participants from 12 sites: 63 in experimental group (6 dropped out), 31 in control group (3 dropped out) Sex: 34 women, 60 men Inclusion criteria: stroke at least 1 week previously and inpatient in a stroke rehabilitation unit, no premorbid conditions limiting upper or lower limb function, sufficient cognitive and communication to give consent, medically stable and able to participate in rehabilitation, upper or lower limb weakness which limits activity Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: conventional rehabilitation programme
1 and 2: 4 weeks, 7 days a week, 30 minutes a day MT or lower‐limb exercises Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, after 4 weeks of therapy, and 8 weeks after baseline
| |
| Notes | Published and unpublished data, full‐text publication received in 2016 Funding source: National Institute for Health Research under its Research for Patient Benefit (RfPB) Programme Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation by an independent web‐based randomisation service |
| Incomplete outcome data (attrition bias) | High risk | No ITT analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation |
| Methods | RCT | |
| Participants | Country: China Setting: not stated Age: adults (mean age: 64.9 years) Sample size: 90 participants (30 in experimental group, 60 in 2 control groups, no dropouts) Sex: 40 women, 50 men Inclusion criteria: 1st ischaemic or haemorrhagic stroke (CT or MRI); neurological deficit; aged 30 to 75 years; unilateral paralysis of upper limb; stable vital signs; mental health; normal intelligence; no significant cognitive dysfunction; MMSE > 24; middle school education and above; no visual impairment; no aphasia and dementia; can execute instructions Exclusion criteria: unstable condition; severe disease or infection of heart; liver or kidney; other complicated diseases which could affect motor function | |
| Interventions | 3 arms 1, 2 and 3: routine rehabilitation and task‐oriented training
1, 2 and 3: 8 weeks, 6 days a week, 60 minute routine rehabilitation 1: 8 weeks, 6 days a week, 30 minutes additional mirror therapy 2: 8 weeks, 6 days a week, 20 minutes additional EMGBF Date of intervention: March 2012 to June 2014 | |
| Outcomes | Outcomes were recorded at baseline and after therapy
| |
| Notes | Information based on abstract; extracted in part on the basis of an unauthorised, automatic translation of the original publication in Chinese Funding source: Changsha Economics Office Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | Multicentre RCT; stratified by lesion side and motor impairment level | |
| Participants | Country: Taiwan Setting: 4 hospitals Age: adults (mean age: 54.2 years) Sample size: 33 (16 in the MT group, 17 in the control group; 12 lost to 6 months follow‐up) Sex: 10 women, 23 men Inclusion criteria: 1st unilateral ischaemic or haemorrhagic cerebrovascular accident before > 6 months, mild to moderate motor impairment (FM‐UE 26‐56), mild spasticity (mAS < 3), able to understand and follow the instructions (MMSE > 24); Exclusion criteria: participation in another study or experimental rehabilitation project < 6 months, serious visual or visual perception impairment (e.g. neglect and poor visual fields) assessed by NIHSS, severe neuropsychologic, neuromuscular or orthopaedic disease | |
| Interventions | 2 arms: usual rehabilitation programme, additional:
1: 4 weeks, 5 days a week, 60 minutes a day of MT, followed by 30 minutes task‐oriented training 2: 4 weeks, 5 days a week, 90 minutes a day Date of intervention: not stated | |
| Outcomes | Outcomes were recorded at baseline, and after 4 weeks and 6 months after treatment
| |
| Notes | Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by randomly‐selected numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed numbered envelopes |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Turkey Setting: inpatient rehabilitation centre Age: adults (mean age: 63.3 years) Sample size: 40 participants (20 in each group; 4 dropped out at 6 months follow‐up) Sex: 17 women, 19 men Inclusion criteria: 1st unilateral stroke during previous 12 months; a Brunnstrom recovery stage between 1 and 4 of the upper extremity; able to understand and follow simple instructions Exclusion criteria: severe cognitive disorders (MMSE < 24) | |
| Interventions | 2 arms
1 and 2: 5 days a week, 30 minutes of therapy for 4 weeks Date of intervention: February 2006 to April 2006 | |
| Outcomes | Outcomes were recorded at baseline, after 4 weeks and after 6 months
| |
| Notes | We combined the Brunnstrom stages of upper extremity and hand into 1 item using raw data Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated allocation of blocks |
| Allocation concealment (selection bias) | Low risk | The physicians who assessed potential participants to determine eligibility did not know to which group the participants would be allocated |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in the analysis |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to group allocation |
| Methods | RCT | |
| Participants | Country: Republic of Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 57.8 years) Sample size: 26 participants (8 in experimental group, 9 each in 2 control groups, no dropouts published) Sex: 10 women, 16 men Inclusion criteria: hemiplegia due to stroke < 6 weeks after onset, no past history of stroke, able to perform an active extension of the affected wrist and more than 2 fingers at an angle of > 10 ° and an active abduction of the affected thumb at an angle of > 10 °, capable of simple communication, can receive care by guardians or caregivers, able to maintain a sitting position for > 30 minutes Exclusion criteria: people with depression who were unable to co‐operate in the treatment, not able to perform active task training due to musculoskeletal problems, spasticity of mAS II or higher, complex regional pain syndrome or secondary adhesive capsulitis | |
| Interventions | 3 arms 1, 2 and 3: conventional therapy
1, 2 and 3: 2 weeks, 5 days a weeks, 40 minutes a day of conventional therapy
Date of intervention: October 2012 to May 2013 | |
| Outcomes | Outcomes were recorded at baseline and after 2 weeks of therapy
| |
| Notes | Funding source: 2‐year research grant of Pusan National University, Republik of Korea Declarations of trialists’ interests: there are no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by random cards with numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Methods | RCT | |
| Participants | Country: South Korea Setting: inpatient rehabilitation centre Age: adults (mean age: 63.3 years) Sample size: 60 participants (40 in 2 experimental groups, 20 in control group, no dropouts published) Sex: 21 women, 39 men Inclusion criteria: 1st unilateral stroke; Brunnstrom recovery stage I ‐ IV; MMSE > 21 Exclusion criteria: unco‐operative due to cognitive impairment; medically unstable; neurologic deficit; neglect | |
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme, additional
1, 2 and 3: 3 weeks, 5 days a week, 30 minutes MT, MT + NMES, or sham therapy Date of intervention: March 2009 to March 2010 | |
| Outcomes | Outcomes were recorded at baseline and after 3 weeks of treatment
| |
| Notes | Published and unpublished information Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling number table as stated by authors (unpublished information) |
| Allocation concealment (selection bias) | High risk | Stated by authors (unpublished information) |
| Incomplete outcome data (attrition bias) | High risk | Stated by authors (unpublished information) |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blinded; stated by authors (unpublished information) |
| Methods | RCT | |
| Participants | Country: Greece Setting: not stated Age: adults (age not stated) Sample size: 30 participants (15 in experimental group, 15 in control group, no dropouts published) Sex: not stated Inclusion criteria: > 4 weeks after stroke, upper limb plegia (Motricity Index ≤ 77) Exclusion criteria: not stated | |
| Interventions | 2 arms 1 and 2: routine rehabilitation treatment
1 and 2: 8 weeks (20 ‐ 24 sessions) 1: additional 30 minutes MT a day Date of intervention: March 2013 to November 2013 | |
| Outcomes | Outcomes were recorded at baseline and after 8 weeks of therapy
| |
| Notes | Information based on an abstract Funding source: not stated Declarations of trialists’ interests: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (authors' statement) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
ABILHAND: a measure of manual ability for adults with upper limb impairment
ADL: activities of daily living
ARAT: Action Research Arm Test
AS: Ashworth Scale
BBT: Box and Block Test
BF‐FES: biofeedback functional electrical stimulation
BI: Barthel Index
BRS: Brunnstrom Recovery Stages
CAHAI: Chedoke Arm and Hand Activity Inventory
CIMT: Constraint‐Induced Movement Therapy
CRPS‐type 1: complex regional pain syndrome ‐ type I
CT: computerised tomography
EMG: electromyography
EMGBF: electromyographic biofeedback
FAC: Functional Ambulatory Categories
FES: functional electrical stimulation
FIM: Functional Independence Measure
FM/FMA: Fugl‐Meyer Assessment
FM‐UE: Fugl‐Meyer Assessment upper extremity
FM‐LE: Fugl‐Meyer Assessment lower extremity
FRT: functional reach test
GAS: goal attainment scaling
iEMG: integrated electro‐myogram
ITT: intention‐to‐treat
JTHFT: Jebson Taylor Hand Function Test
LBT: Line Bisection Test
MAL: Motor Activity Log
MAS: Motor Assessment Scale
mAS: modified Ashworth Scale
MBI: modified Barthel index
MCSI: modified composite spasticity index
MFT: Manual Function Test
MI‐UL: Motricity Index ‐ upper limb
MMSE: Mini Mental State Examination
MMT: Manual Muscle Test
MoCA: Montreal Cognitive Assessment
MRC: Medical Research Council
MRI: magnetic resonance imaging
MT: mirror therapy
MVPT: Motor‐free Visual Perception Test
NIHSS: National Institutes of Health Stroke Scales
NMES: neuromuscular electrical stimulation
NRS: Numeric Rating Scale
NSA: Nottingham Sensory Assessment
QOM: quality of movement
QST: quantitative sensory testing
RASP: Rivermead Assessment of Somatosensory Performance
RCT: randomised controlled trial
ROM: range of motion
rTMS: repetitive transcranial magnetic stimulation
SCT: Star Cancellation Test
SD: standard deviation
SIS: Stroke Impact Scale
SSQOL: Stroke‐specific quality of life scale
tDCS: transcranial direct current stimulation
TEMPA: Upper Extremity Performance Evaluation Test for the Elderly (English title)
TS: Tardieu Scale
TUG: timed‐up and go test
UEFI: Upper Extremity Functional Index
UEFT: Upper Extremity Function Test
Stroke‐ULAM: Stroke Upper‐Limb Activity Monitor
VAS: Visual analogue scale
WMFT: Wolf Motor Function Test
WMFT/FA: Wolf Motor Function Test ‐ functional ability
WMFT/PT: Wolf Motor Function Test ‐ performance time
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study of healthy people | |
| Study did not use relevant outcomes | |
| Only 10% of the experimental intervention was used for mirror therapy | |
| Mirror therapy is also part of the control intervention | |
| No relevant outcomes included | |
| The paper was withdrawn permanently, because of plagiarism (Wu 2013) | |
| Intervention was not mirror therapy as defined in this review | |
| Control intervention included mirror therapy | |
| Study did not include people after stroke | |
| Randomisation procedure not adequate | |
| Study is not a RCT | |
| Time spent in mirror therapy did not reach inclusion criteria | |
| Study is not an RCT | |
| Randomisation procedure not adequate |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised cross‐over trial |
| Participants | Country: Japan Sample size: 14 participants Inclusion criteria: 4 months and longer after stroke |
| Interventions | 2 arms
|
| Outcomes |
|
| Notes | We were not able to include this trial because of unclear outcome of motor function |
| Methods | RCT |
| Participants | Country: Canada Inclusion criteria: adults, people with stroke, with normal vision, admitted to the rehabilitation programme at Toronto Rehabilitation Institute |
| Interventions | 2 arms
|
| Outcomes | Outcomes: not stated |
| Notes | Information based on www.isrctn.com/ISRCTN40903497 |
| Methods | RCT |
| Participants | Country: not published Sample size: 10 (5 in experimental group, 5 in control group) Inclusion criteria: chronic stroke, paresis of the upper limb, aged 40 ‐ 75 Exclusion criteria: not published |
| Interventions | 2 arms
1 and 2: 6 weeks, 24 minutes twice a day |
| Outcomes | Outcomes were recorded:
|
| Notes | Information based on abstract |
| Methods | Cohort study (randomisation not published) |
| Participants | Country: not published Sample size: 42 participants (21 in experimental group, 21 in control group) Inclusion criteria: stroke, paresis of the lower limb Exclusion criteria: not published |
| Interventions | 2 arms 1 and 2: conventional therapy programme
1 and 2: 4 weeks, 5 days a week, 60 to 120 minutes conventional therapy programme 1: 4 weeks, 5 days a week, 30 minutes MT |
| Outcomes | Outcomes were recorded at baseline, after therapy period, and 12 weeks
|
| Notes | Information based on abstract |
| Methods | Cohort study (randomisation not published) |
| Participants | Country: not published Inclusion criteria: stroke, hemiplegia Exclusion criteria: not published |
| Interventions | 2 arms 1 and 2: conventional therapy programme
1 and 2: 4 weeks |
| Outcomes | Outcomes were recorded at baseline, and 2 weeks and 4 weeks after therapy period
|
| Notes | Information based on abstract |
| Methods | Cohort study (randomisation not published) |
| Participants | Country: Turkey Sample size: 8 participants (4 in experimental group, 4 in control group) Inclusion criteria: diagnosis of a stroke (within 1 month), partial anterior circulation infarction (PACI), upper extremity motor functional level according to Brunnstrom stages between 1 and 4, and no musculoskeletal injury history in the affected upper extremity Exclusion criteria: a residual upper extremity deficit from a previous stroke, intolerance to upright position, visual problem, cognitive deficit preventing them from following instructions, and unilateral neglect preventing them from being able to view the mirror |
| Interventions | 2 arms 1 and 2: neurodevelopmental treatment
|
| Outcomes | Outcomes were recorded at baseline and after therapy period
|
| Notes |
ARAT: Action Research Arm Test
BBS: Berg balance scale
BI: Barthel Index
FIM: functional independence measure
FM/FMA: Fugl‐Meyer Assessment
FM‐UE: Fugl‐Meyer Assessment upper extremity
MI: Motricity Index
MT: Mirror therapy
RCT: Randomised controlled trial
STEF: Simple Test for Evaluating Hand Function
WMFT: Wolf Motor Function Test
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Developing new ways to minimise disability after stroke, a randomized controlled trial of Functional Electrical Stimulation (FES) of the arm and mirror therapy |
| Methods | RCT |
| Participants | Country: New Zealand Inclusion criteria: over 18 years, admitted to Waikato Hospital with a confirmed diagnosis of stroke, living in the community within the Hamilton area on admission to hospital, a score of greater than 16/30 on MoCA, ARAT score of < 30/57 Exclusion criteria: severe cognitive impairment (< 16/30 on the MoCA), severe or unstable cardiovascular disease (i.e. unstable angina, pacemaker fitted, dysrhythmia other than controlled atrial fibrillation), near‐terminal disease (including advanced lung, heart, kidney, liver failure resistant to medical management), acute musculoskeletal disorder |
| Interventions | 3 arms 1, 2 and 3: task‐specific training, additional
1, 2 and 3: 4 ‐ 6 weeks, 2 times a day 30 minutes of task‐specific training 1, 2 and 3: 4 ‐ 6 weeks, 2 times a day 30 minutes of MT, MT + FES or FES |
| Outcomes | Outcomes will be recorded at baseline and after therapy period:
|
| Starting date | Not stated |
| Contact information | Principal Investigator: Dr John Parsons, The University of Auckland, Auckland, New Zealand, Email: [email protected] |
| Notes | Estimated sample size: 100 participants (in 2 experimental groups and 1 control group) www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000121763 |
| Trial name or title | Graded motor imagery based on mirror neuron on rehabilitative training for stroke patients: a BOLD‐fMRI study |
| Methods | RCT |
| Participants | Country: China Inclusion criteria: participants signed informed consent, unconscious obstacles, condition is relatively stable, no obvious lack of eyesight, aged 40 ‐ 75 years, no history of cerebrovascular disease, with cerebral infarction diagnosis standards, the course in 2 weeks to 3 months, right‐handed, left hemiplegia, no metal implants in the body, no MRI testing taboo, NIHSS score > 4 minutes, paresis test positive, muscle strength level 1 ‐ 3 Exclusion criteria: people who are not diagnosed with cerebral infarction by imaging, the acute stage of cerebrovascular diseases, unstable vital signs, persons with serious mental illness, with understanding disabilities who cannot meet the test, persons with serious heart, liver and kidney dysfunction, those who have contraindications to MRI examination |
| Interventions | 2 arms 1 and 2: routine training
|
| Outcomes | Outcomes:
|
| Starting date | Not stated |
| Contact information | Principal Investigator: Tu Wenzhan, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, China Email: [email protected] |
| Notes | Estimated sample size: 30 participants (in experimental group and in control group) |
| Trial name or title | Zentrale Gesichtslähmung nach Schlaganfall: eine randomisierte, kontrollierte Studie [German] |
| Methods | RCT |
| Participants | Country: Germany Inclusion criteria: 1st episode of stroke with facial paresis (within 7 days to 6 months), 18 years of age and over Exclusion criteria: Speech disorder, which prevents understanding of the questionnaires, pre‐existing brain lesions, degenerative diseases of the brain |
| Interventions | 4 arms 1 to 4: training of oral motor skills (MMT)
1 to 4: 3 weeks, 4 days a week, 30 minutes a day MMT 1 and 2: 3 weeks, 4 days a week, 30 minutes a day additional MMT or additional MT |
| Outcomes | Outcomes will be recorded at baseline and after treatment
|
| Starting date | October 2015 |
| Contact information | Moritz Klinik Bad Klosterlausnitz, Hermann‐Sachse Strasse 46, 07639 Bad Klosterlausnitz, Deutschland |
| Notes | Estimated sample size: 80 participants www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009288 |
| Trial name or title | The effect of mirror therapy on motor ability of patients after stroke |
| Methods | RCT |
| Participants | Country: Iran Inclusion criteria: people with age between 30 to 65 years, with the 1st‐ever stroke that are diagnosed by neurologists and confirmed by computed tomography or magnetic resonance imaging, 1 month has passed since their stroke, 1 to 4 point from Brunnstrom scale, not to have severe cognitive disorders and be able follow simple verbal instructions (MMSE score > 24). Exclusion criteria: participants excluded if they do not participate in 4 sessions intermittently or 2 sessions constantly in intervention |
| Interventions | 3 arms 1, 2 and 3: conventional rehabilitation programme
|
| Outcomes | Outcomes will be recorded before intervention and the end of 5, 10, 15, and 20 sessions
|
| Starting date | 23 July 2015 |
| Contact information | Principal Investigator: Tahereh Khaleghdoost Mohammadi, Physiotherapy centre for elderly and handicap city of Rasht, Rasht, Iran Email: [email protected] |
| Notes | Estimated sample size: 93 participants (in 1 experimental group and 2 control groups) |
| Trial name or title | Use of tendon vibration and mirror for the improvement of upper limb function and pain reduction |
| Methods | RCT |
| Participants | Country: Israel Inclusion criteria: stroke; 18 to 85 years of age; stroke onset between 1 month and 1 year ago; NIHSS 3 to 15 on study admission; affected upper‐limb function 10% to 90% on Fugl‐Meyer Scale; ability to understand instructions and to move the unaffected limb freely Exclusion criteria: severe cognitive impairment; severe aphasia; severe neglect that impairs ability to understand instructions or to execute tasks |
| Interventions | 3 arms
1, 2 and 3: 10 sessions, 30 minutes each |
| Outcomes | Outcomes will be assessed after treatment and 3 months after treatment
|
| Starting date | September 2009 |
| Contact information | Principal Investigator: Elior Moreh, MD, Hadassah University Hospital, Jerusalem, Israel Email: [email protected] |
| Notes | Estimated enrolment: 30 |
| Trial name or title | Robot‐ versus mirror‐assisted motor interventions in rehabilitating upper‐limb motor and muscle performance and daily functions post‐stroke: a comparative effectiveness study |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: willing to provide written informed consent, > 6 months onset of unilateral stroke, an initial 25 ‐ 56 or 18 ‐ 50 scores of the FM‐UE, sufficient cognitive ability (MMSE ≧ 24 points), without upper limb fracture within 3 months, 40 years to 75 years of age Exclusion criteria: recurrence of stroke or seizure episode during the intervention, occurrence of serious or continuous pain on affected upper extremity, history of other neurological disease or severe orthopaedic condition |
| Interventions | 5 arms
1 to 5: 4 weeks, 5 days a week, 1 hour MT, RR, RR with FES, CR, or RR‐PI and ½ hour functional training a day |
| Outcomes | Outcomes:
|
| Starting date | August 2011 |
| Contact information | Principal Investigator: Keh‐chung Lin, ScD, School of Occupational Therapy, College of Medicine, National Taiwan University |
| Notes | Estimated sample size: 100 participants (in 3 experimental groups and 2 control groups) |
| Trial name or title | Hybrid approach to mirror therapy and transcranial direct current stimulation for stroke recovery: a follow‐up study on brain reorganisation, motor performance of upper extremity, daily function, and activity participation |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: 1st episode of stroke in cortical regions, time since stroke > 6 months, initial motor part of UE of FMA score ranging from 24 to 52, indicating moderate to mild movement impairment, no severe spasticity in any joints of the affected arm (mAS ≤ 2), no serious cognitive impairment (i.e. MMSE score ≧ 24), willing to sign the informed consent form Exclusion criteria: visual/attention impairments that might interfere with seeing mirror illusion, including hemineglect/hemianopsia, major health problems or poor physical conditions that might limit participation, currently participating in any other research, previous brain neurosurgery, metallic implants within the brain |
| Interventions | 4 arms
1: 2 weeks, 20 minutes tDCS, 40 minutes MT, 30 minutes functional training; 2 weeks 60 minutes MT and 30 minutes functional training 2: 2 weeks, 20 minutes sham tDCS, 40 minutes MT, 30 minutes functional training; 2 weeks 60 minutes MT and 30 minutes functional training 3: 4 weeks, 60 minutes MT and 30 minutes functional training |
| Outcomes | Outcomes will be recorded at baseline, after 2 weeks, after 4 weeks, 16 weeks, and 28 weeks
|
| Starting date | August 2014 |
| Contact information | Principal Investigator: Ching‐Yi Wu, ScD, Chang Gung Memorial Hospital, Taipei City,Taiwan Email: [email protected] |
| Notes | Estimated sample size: 80 participants (in 3 experimental groups and 1 control group) |
| Trial name or title | A pilot randomised controlled trial (RCT) of mirror box therapy in upper limb rehabilitation with sub‐acute stroke patients |
| Methods | RCT |
| Participants | Country: UK Inclusion criteria: 18 years and over; newly‐admitted inpatient of the rehabilitation ward; diagnosis of CVA in the last 3 months resulting in upper‐limb motor loss; able to follow 2‐part spoken or written commands in the English language; upper‐limb therapy designated as a main portion of goal directed treatment programme; consent to take part in the study Exclusion criteria: not stated |
| Interventions | 2 arms 1 and 2: standard occupational therapy for upper limb rehabilitation
1 and 2: 6 weeks, 3 ‐ 5 sessions a week of approximately 45 minutes OT |
| Outcomes | Outcomes will be recorded at baseline and after treatment and 3 and 6 months after treatment
|
| Starting date | April 2015 |
| Contact information | Principal Investigator: Alison Porter‐Armstrong, DPhil, University of Ulster, Belfast, Northern Ireland Email: [email protected] |
| Notes | Estimated sample size: 50 participants (25 in experimental group and 25 in control group) |
| Trial name or title | Effects of robot‐assisted combined therapy in upper limb rehabilitation in stroke patients |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: 1st‐ever unilateral stroke with more than 3 months onset, an initial UE subsection of the Fugl‐Meyer Assessment score of 18 to 56 indicating moderate to severe and moderate upper‐limb movement impairment, no excessive spasticity in any of the joints of the affected upper limb (shoulder, elbow, wrist, fingers), be able to follow study instructions and perform study tasks, and willing to provide written informed consent Exclusion criteria: with neural or psychological medical problem that may influence the study, with severe joint pain, with upper limb fracture within 3 months, participation in any experimental rehabilitation or drug studies during the study period; and refusing to provide written informed consent |
| Interventions | 6 arms 1 to 6: OT, additional
1 to 6: 4 weeks, 5 days a week, 90 minutes OT |
| Outcomes | Outcomes will be recorded within 3 days and immediately after therapy period
|
| Starting date | August 2014 |
| Contact information | Principal Investigator: Chia‐Yi Lee, MD, Cathay General Hospital, Taipei City, Taiwan |
| Notes | Estimated sample size: 120 participants (in 3 experimental groups and 3 control groups) |
| Trial name or title | Effects of home‐based mirror therapy combined with task‐oriented training for patients with stroke: a randomised controlled trial |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: diagnosed as having a unilateral stroke, at least 3 months after stroke onset, from 20 to 80 years of age, having completed acute rehabilitation care or discharged home, a baseline score of the Fugl‐Meyer Assessment (FMA) of 20 to 60, able to follow the therapy instructions (cognition status will be measured by the Montreal Cognitive Assessment), capable of participating in therapy and assessment sessions Exclusion criteria: neglect, global or receptive aphasia, major medical problems, comorbidities that influenced UE usage or caused severe pain |
| Interventions | 3 arms
1 and 2: 4 weeks (12 sessions), 3 days a week, 30 minutes MT followed by 30 minutes TOT |
| Outcomes | Outcomes will be recorded at baseline, immediately after treatment, and 3 months follow‐up
|
| Starting date | March 2016 |
| Contact information | Principal investigator: not stated, Chang Gung Memorial Hospital, Taipei City, Taiwan Email: not stated |
| Notes | Estimated sample size: 90 participants (in 2 experimental groups and 1 control group) |
| Trial name or title | Effect of mirror therapy versus bilateral arm training for rehabilitation after chronic stroke: a pilot randomised controlled trial |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: > 6 months after onset of an ischaemic or haemorrhage stroke, no excessive spasticity on any joints of the affected arm, aged 21 years and older Exclusion criteria: history of stroke or other neurologic, neuromuscular, or orthopaedic disease; participation in other experimental rehabilitation or drug studies concurrent with this study |
| Interventions | 2 arms 1 and 2: home programme
1 and 2: 4 weeks, 3 days a week, 1½ hours/day MT or bilateral arm training 1 and 2: 4 weeks, 5 days a week, ½ hour home programme |
| Outcomes | Outcomes will be assessed within 4 weeks (± 3 days) after intervention
|
| Starting date | September 2015 |
| Contact information | Principal Investigator: Keh‐chung Lin, ScD, School of Occupational Therapy, College of Medicine, National Taiwan University |
| Notes | Estimated sample size: 60 participants (in experimental group and in control group) |
| Trial name or title | Effects of mirror box therapy on neuroplasticity and functional outcome in hemiparetic upper limb post‐stroke |
| Methods | Randomised cross‐over trial |
| Participants | Country: UK Inclusion criteria: 18 years to 105 years, hemiparetic upper limb post‐stroke, capable of providing informed consent, intact vision: if diagnosis of peripheral field defect, participant should be able to compensate for it Exclusion criteria: any contraindication to MRI scanning, clinically‐significant psychiatric disorder (e.g. depression), pre‐existing neurological or psychiatric disease that could confound the study results |
| Interventions | 2 arms
|
| Outcomes | Outcomes will be recorded at baseline, after 3 weeks, and 6 weeks
|
| Starting date | April 2016 |
| Contact information | Principal Investigator: Iris Grunwald, Anglia Ruskin University, Cambridge, England |
| Notes | Estimated sample size: 40 participants (in experimental group and in control group) |
| Trial name or title | Self‐directed box (mirror) therapy after stroke: a dosing study |
| Methods | RCT |
| Participants | Country: USA Inclusion criteria: adults status post‐stroke (ischaemic or haemorrhagic) between the ages 18 and 85 years, receiving inpatient rehabilitation, using the impaired arm, ability to lift and release a wash cloth off a table with any means of prehension in either the sitting or standing positions, a score > 21/30 on the MMSE, ability to consent Exclusion criteria: serious visual or visual‐perceptual deficits, neuropsychological impairments, or orthopaedic conditions that would prevent participation in the BT protocol as determined by the treatment team, involvement in another study protocol related to motor function after stroke, anticipated length of stay > 2 weeks, < 6 months post‐stroke |
| Interventions | 3 arms 1 to 3: treatment as usual
1: Additional 30 minutes MT a day (twice for 15 minutes) 2: Additional 60 minutes MT a day (4 times for 15 minutes) |
| Outcomes | Outcomes will be recorded at pre‐ and post‐intervention up to 12 months
|
| Starting date | January 2017 |
| Contact information | Principal Investigator: Glenn Gillen, EdD, OTR, Columbia University, New York City, USA E‐mail: [email protected] (Dawn M. Nilsen EdD, OTR) |
| Notes | Estimated sample size: 45 participants (in 2 experimental groups and 1 control group) |
| Trial name or title | Efficacy and time dependent effects of transcranial direct current stimulation (tDCS) combined with mirror therapy for rehabilitation after subacute and chronic stroke |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: experienced a 1st‐ever unilateral stroke with stroke onset ≥ 1 week, UE‐FMA score between 18 and 56, able to follow instructions to perform the tasks (MMSE ≥ 24) Exclusion criteria: participants are currently involved in other rehabilitation or drug research trial(s), have neurological or psychological disorders other than stroke, have joint contracture or excessive spasticity of the paretic upper limb that prohibits them performing the tasks, received Botulinum toxin injections 3 months prior to enrolment, have unstable cardiovascular status such as uncontrolled hypertension or New York Heart Association (NYHA) Class III/IV heart failure, have contradictions to tDCS including a history of epilepsy, migraine headache, uncontrolled medical status, being pregnant, having a pacemaker, or metal implanted in their head or body, have a history of drug or alcohol abuse, skin lesions on the electrode sites, brain tumour, brain injury, arteriovenous malformation (AVM), had brain surgery, other brain diseases (such as intracranial hypertension or cerebral oedema), or being not suitable for using tDCS by the physician's assessment. |
| Interventions | 3 arms
1: 4 weeks, 5 days a week, 90 minutes: 20 minutes tDCS, 20 minutes MT + sham tDCS, 20 minutes MT without tDCS, 30 minutes functional task practice 2: 4 weeks, 5 days a week, 90 minutes: 20 minutes sham tDCS, 20 minutes MT + tDCS, 20 minutes MT without tDCS, 30 minutes functional task practice |
| Outcomes | Primary outcomes will be recorded at baseline, and after 4 weeks therapy period
|
| Starting date | August 2016 |
| Contact information | Contact: Ching‐Yi Wu, ScD, Chang Gung Memorial Hospital, Taipei City, Taiwan E.mail: cywu@mail,cgu.edu.tw |
| Notes | Estimated sample size: 99 participants (in 2 experimental groups and 1 control group) |
| Trial name or title | Comparative efficacy study of action observation therapy and mirror therapy after stroke: rehabilitation outcomes and neural mechanisms by MEG |
| Methods | RCT |
| Participants | Country: Taiwan Inclusion criteria: diagnosed as having a unilateral stroke, 1 to 6 months after stroke onset, from 20 to 80 years of age, a baseline score of the Fugl‐Meyer Assessment (FMA) of 20 to 60, able to follow the study instructions (measured by the MoCA), capable of participating in therapy and assessment sessions Exclusion criteria: people with global or receptive aphasia, severe neglect, major medical problems, or comorbidities that influenced UE usage or cause severe pain |
| Interventions | 3 arms
1: 3 weeks, 15 sessions 2: 3 weeks, 15 sessions, 60 minutes MT |
| Outcomes | Outcomes will be recorded at baseline and after treatment and 3 months after treatment
|
| Starting date | August 2016 |
| Contact information | Contact: Yu‐Wei Hsieh, PhD, Chang Gung Memorial Hospital, Taipei City, Taiwan E‐mail: [email protected] |
| Notes | Estimated sample size: 90 participants (60 in 2 experimental groups and 30 in control group) |
ABILHAND: a measure of manual ability for adults with upper limb impairment
ARAT: Action Resarch Arm Test
AROM: active range of motion
BBT: Box and Block test
CAHAI: Chedoke arm and hand activity inventory
EQ‐5D‐5L: health questionnaire for adults
FES: Functional electrical stimulation
FAM: Functional Assessment Measure
FIM: Functional Independence Measure
FM‐UE: Fugl‐Meyer Assessment‐ upper extremity
gWMFT: Graded Wolf Motor Function Test
Hz: Hertz
JTHFT: Jebson Taylor Hand Function Test
MAL: Motor Activity Log
MAS: Motor Assessment Scale
mAS: Modified Ashworth Scale
mBI: Modified Barthel Index
MMSE: Mini Mental State Examination
MoCA: Montreal cognitive assessment
MRC: Medical research council Scale for Muscle Strength
MRI: magnetic resonance imaging
mRS: Modified Rankin scale
MT: Mirror therapy
NEADL: Nottingham Extended Activities of Daily Living Scale
NIHSS: National Institutes of Health Stroke Scales
NMES: Neuromuscular Electrical Stimulation
OT: occupational therapy
QMI: Questionnaire upon Mental Imagery
RCT: randomised controlled trial
rNSA: Revised Nottingham sensory assessment
RR: robotic rehabilitation
tDCS: Transcranial direct current stimulation
UE: upper extremity
(WHOQOL)‐BREF: World Health Organization Quality of Life
WMFT: Wolf Motor Function Test
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 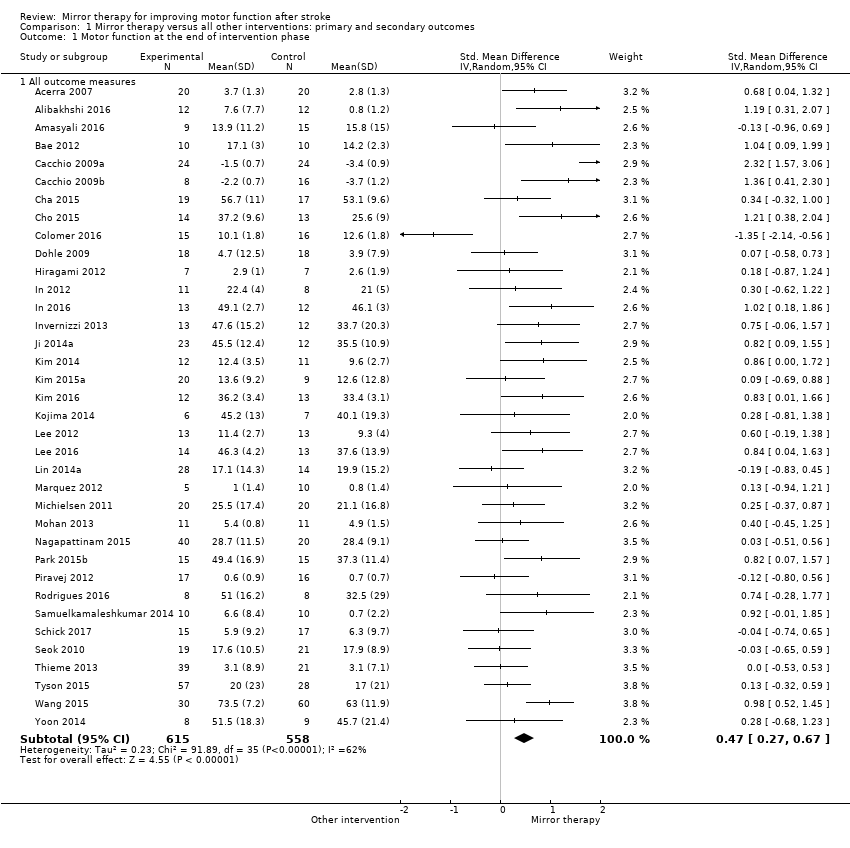 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 1 Motor function at the end of intervention phase. | ||||
| 1.1 All outcome measures | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 2 Motor impairment at the end of intervention phase Show forest plot | 39 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 2 Motor impairment at the end of intervention phase. | ||||
| 2.1 All outcome measures | 39 | 1292 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.32, 0.66] |
| 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase Show forest plot | 28 | 898 | Mean Difference (IV, Random, 95% CI) | 4.32 [2.46, 6.19] |
| Analysis 1.3  Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase. | ||||
| 4 Activities of daily living at the end of intervention phase Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 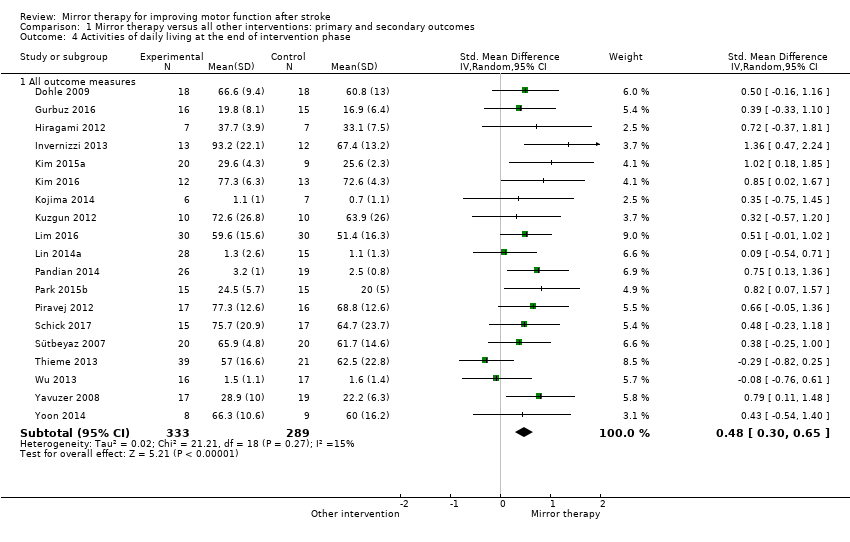 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 4 Activities of daily living at the end of intervention phase. | ||||
| 4.1 All outcome measures | 19 | 622 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.30, 0.65] |
| 5 Pain at the end of intervention phase Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5 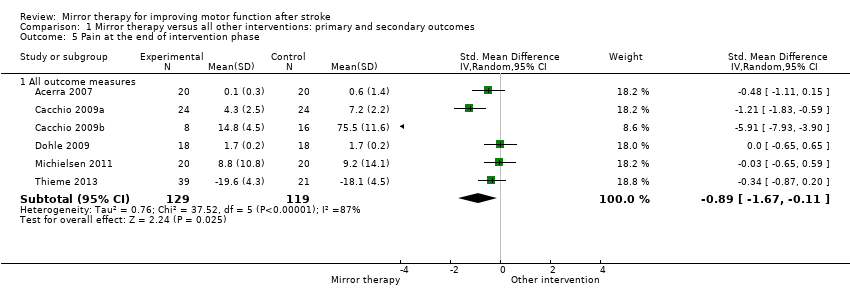 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 5 Pain at the end of intervention phase. | ||||
| 5.1 All outcome measures | 6 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.67, ‐0.11] |
| 6 Visuospatial neglect at the end of intervention Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6 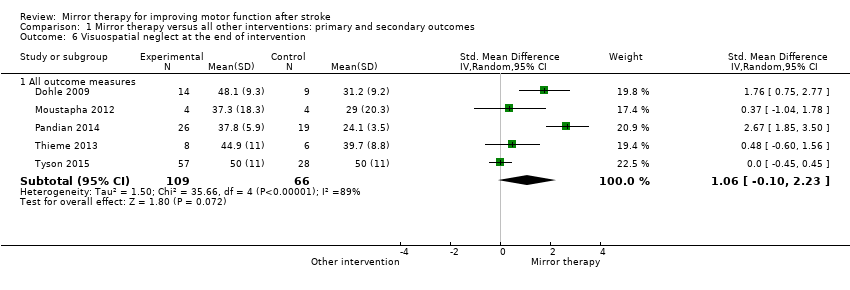 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 6 Visuospatial neglect at the end of intervention. | ||||
| 6.1 All outcome measures | 5 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.10, 2.23] |
| 7 Motor function at follow‐up after 6 months Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 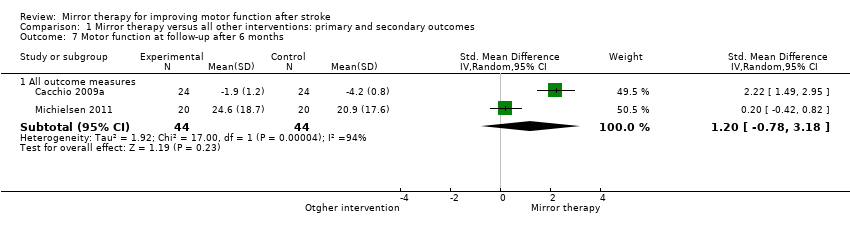 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 7 Motor function at follow‐up after 6 months. | ||||
| 7.1 All outcome measures | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.78, 3.18] |
| 8 Motor impairment at follow‐up after 6 months Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 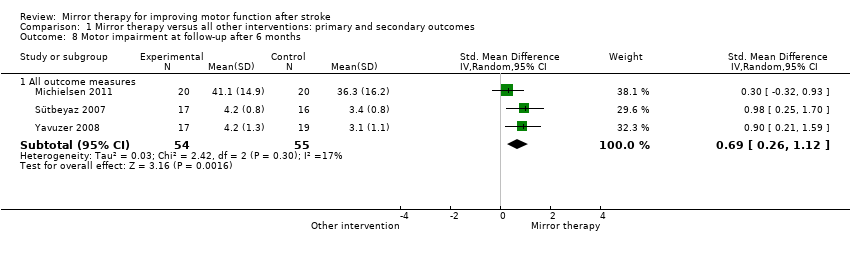 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 8 Motor impairment at follow‐up after 6 months. | ||||
| 8.1 All outcome measures | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.26, 1.12] |
| 9 Dropouts at the end of intervention phase Show forest plot | 42 | 1438 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.76] |
| Analysis 1.9 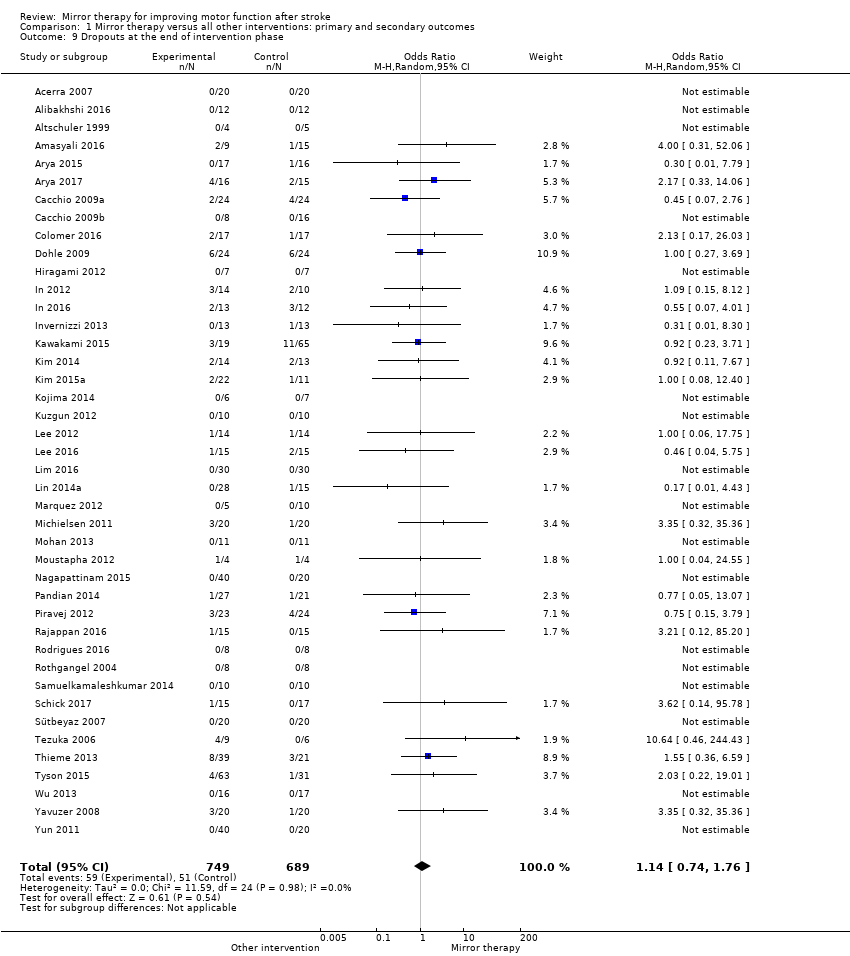 Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 9 Dropouts at the end of intervention phase. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| Analysis 2.1 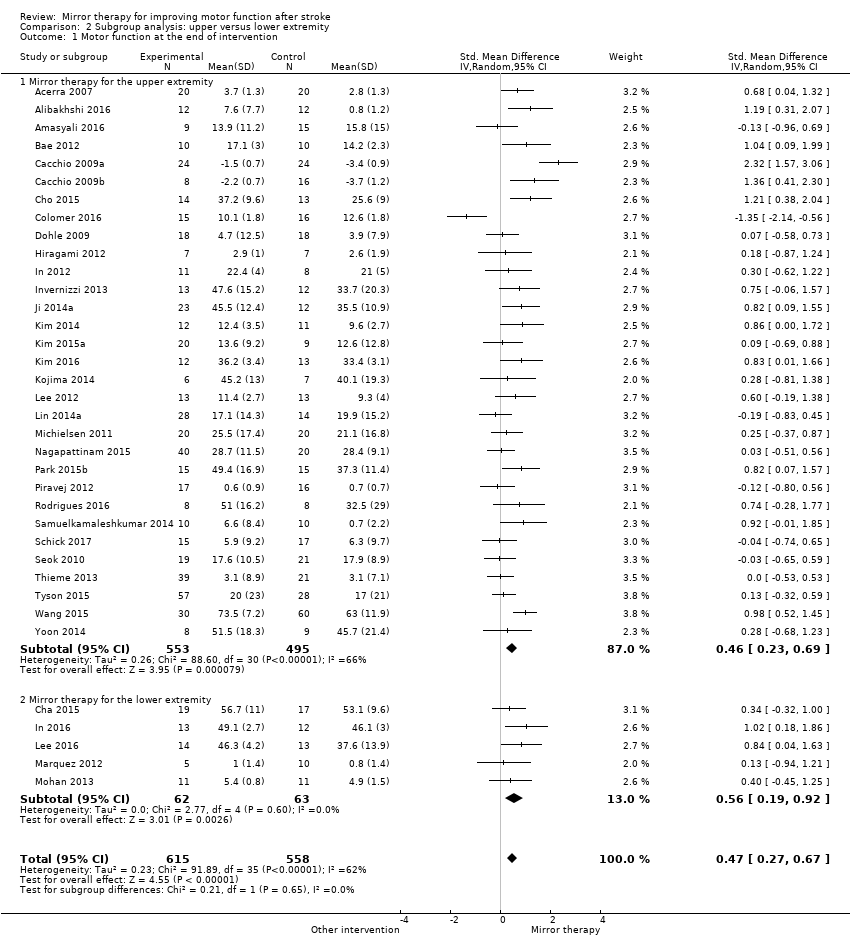 Comparison 2 Subgroup analysis: upper versus lower extremity, Outcome 1 Motor function at the end of intervention. | ||||
| 1.1 Mirror therapy for the upper extremity | 31 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Mirror therapy for the lower extremity | 5 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.29, 0.72] |
| Analysis 3.1 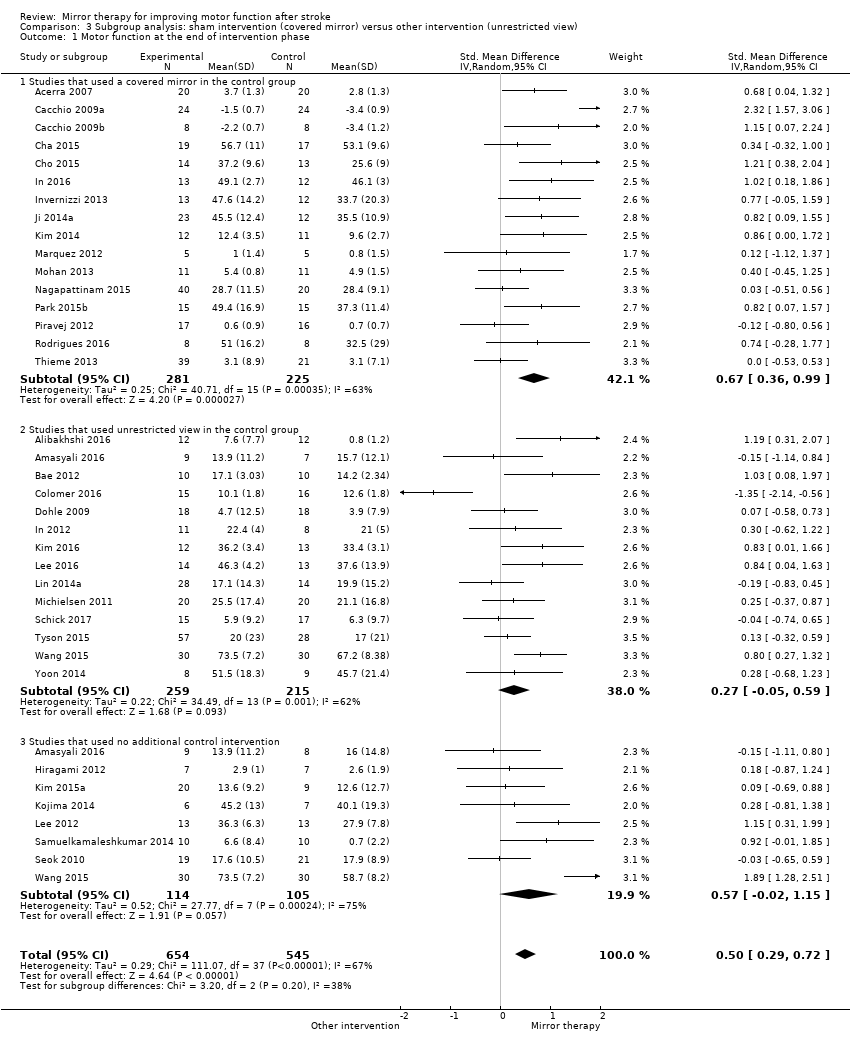 Comparison 3 Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view), Outcome 1 Motor function at the end of intervention phase. | ||||
| 1.1 Studies that used a covered mirror in the control group | 16 | 506 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.36, 0.99] |
| 1.2 Studies that used unrestricted view in the control group | 14 | 474 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.59] |
| 1.3 Studies that used no additional control intervention | 8 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 32 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| Analysis 4.1  Comparison 4 Subgroup analysis: subacute versus chronic stage after stroke, Outcome 1 Motor function at the end of intervention phase. | ||||
| 1.1 All studies including participants within 6 months after stroke | 18 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.18, 0.73] |
| 1.2 All studies including participants with more than 6 months after stroke | 14 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Sensitivity analysis by trial methodology, Outcome 1 Motor function at the end of intervention. | ||||
| 1.1 All studies without randomised cross‐over trials | 35 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.68] |
| 1.2 All studies with adequate sequence generation | 33 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.19, 0.54] |
| 1.3 All studies with adequate concealed allocation | 16 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.4 All studies with adequate handling of incomplete outcome data | 12 | 388 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.14, 0.95] |
| 1.5 All studies with blinded assessors | 25 | 820 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.17, 0.70] |
| 2 Motor impairment at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Sensitivity analysis by trial methodology, Outcome 2 Motor impairment at the end of intervention. | ||||
| 2.1 All studies with adequate sequence generation | 36 | 1157 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.29, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at the end of intervention Show forest plot | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.08] |
| Analysis 6.1  Comparison 6 Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS), Outcome 1 Pain at the end of intervention. | ||||
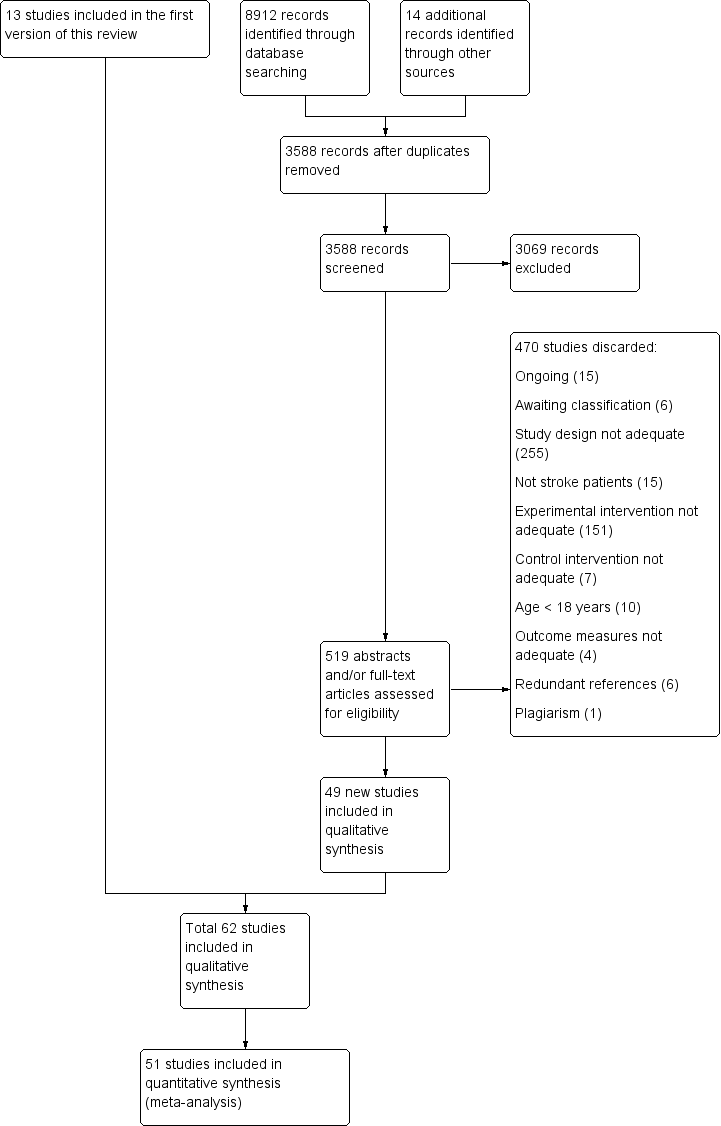
Study flow diagram of updated search and selection process

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
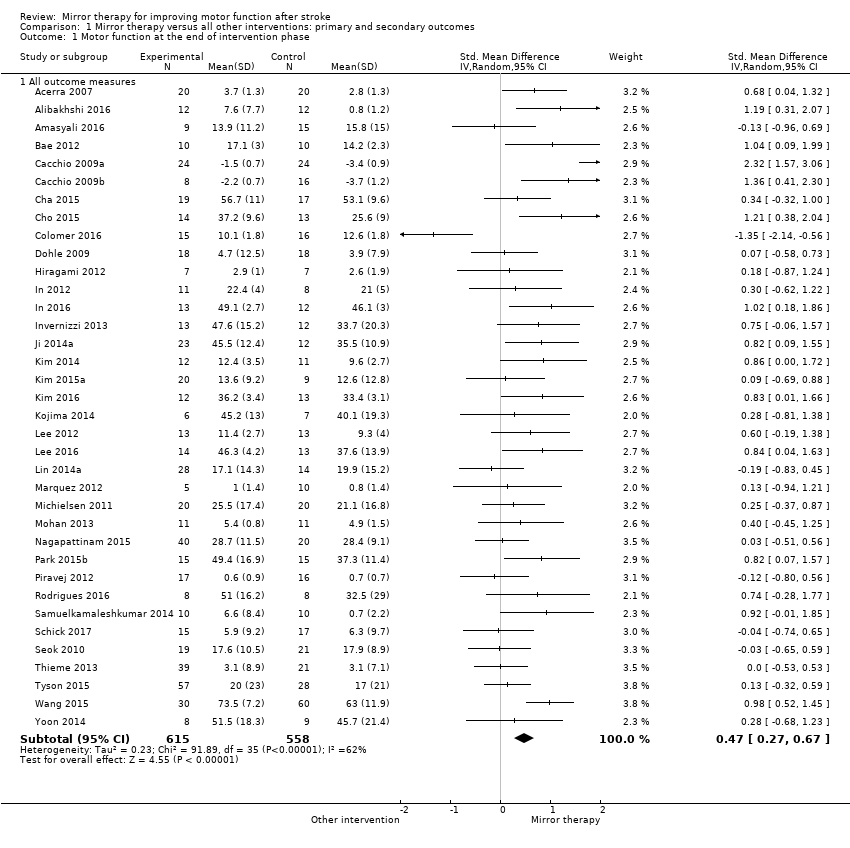
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 1 Motor function at the end of intervention phase.
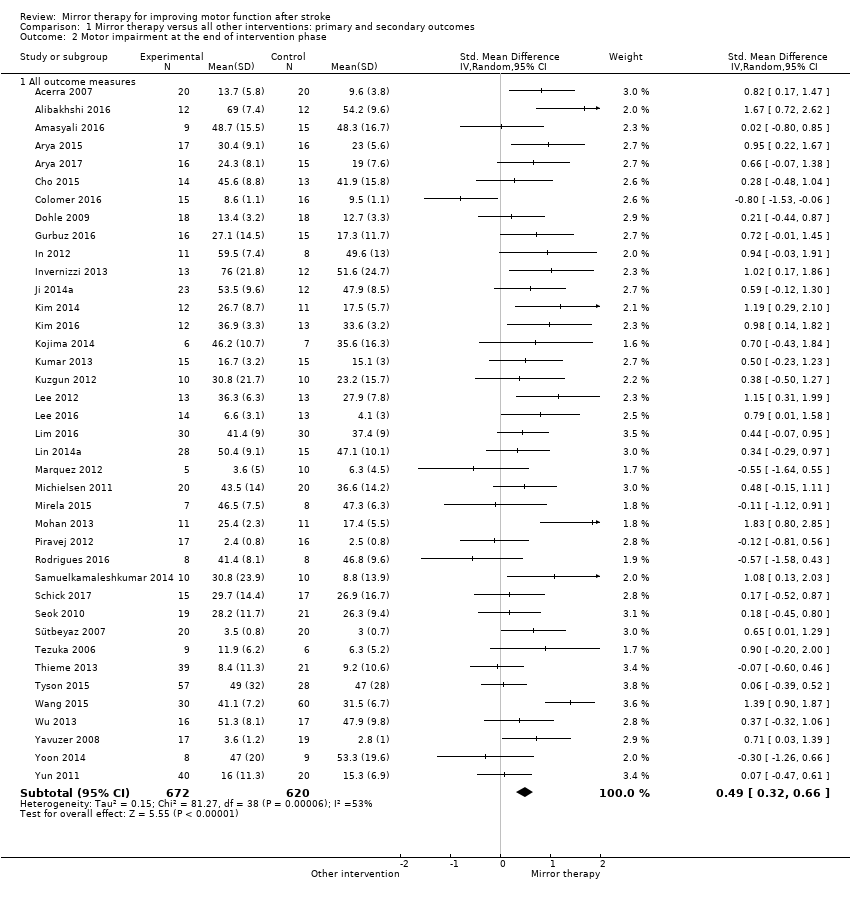
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 2 Motor impairment at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 4 Activities of daily living at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 5 Pain at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 6 Visuospatial neglect at the end of intervention.
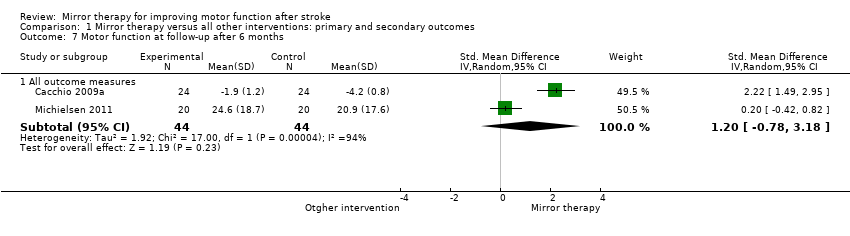
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 7 Motor function at follow‐up after 6 months.
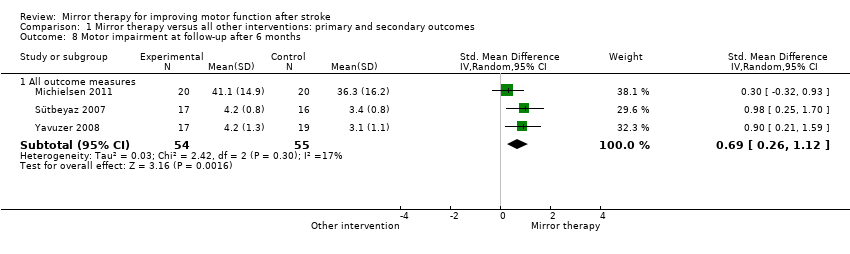
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 8 Motor impairment at follow‐up after 6 months.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 9 Dropouts at the end of intervention phase.

Comparison 2 Subgroup analysis: upper versus lower extremity, Outcome 1 Motor function at the end of intervention.

Comparison 3 Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view), Outcome 1 Motor function at the end of intervention phase.

Comparison 4 Subgroup analysis: subacute versus chronic stage after stroke, Outcome 1 Motor function at the end of intervention phase.
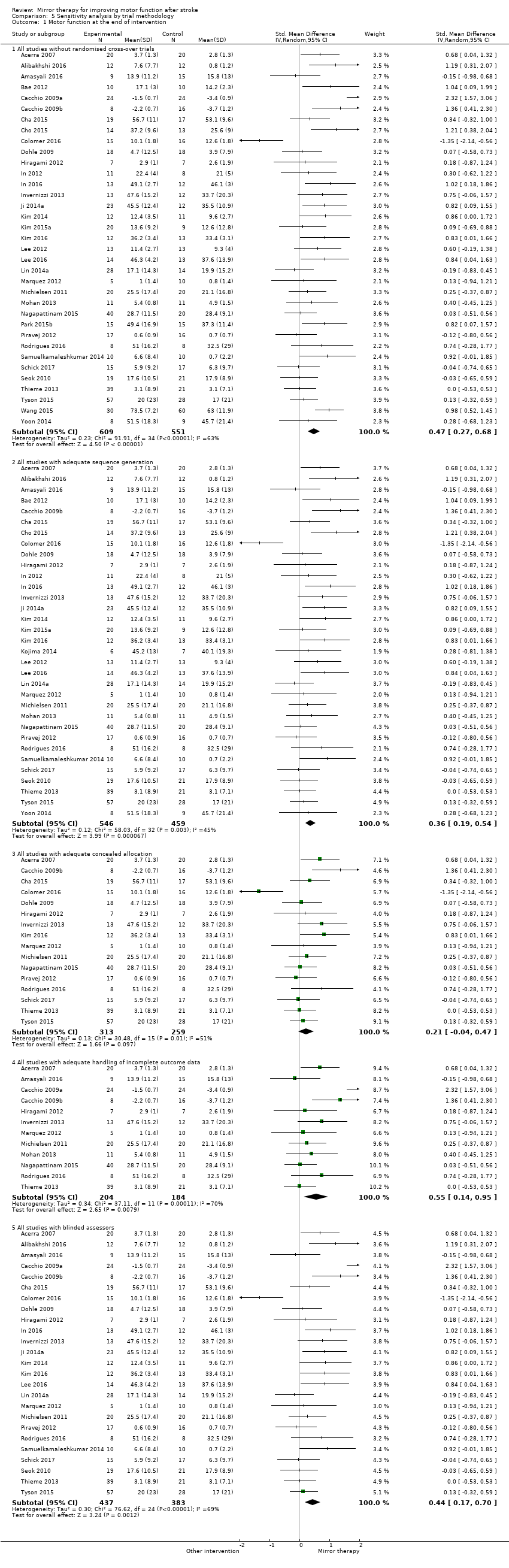
Comparison 5 Sensitivity analysis by trial methodology, Outcome 1 Motor function at the end of intervention.

Comparison 5 Sensitivity analysis by trial methodology, Outcome 2 Motor impairment at the end of intervention.

Comparison 6 Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS), Outcome 1 Pain at the end of intervention.
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
| Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 | ⊕⊕⊕⊝ | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 | ⊕⊕⊕⊝ Moderatea | SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 | ⊕⊕⊝⊝ | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 | ⊕⊕⊕⊝ | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 | ⊕⊕⊝⊝ | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) | ⊕⊕⊕⊝ | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 | ⊕⊕⊝⊝ | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded due to several ratings in one or more items with high or unknown risk of bias. | |||||
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 | |
| 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r | |
| 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r | |
| 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 | |
| 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 | |
| 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 | |
| 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 | |
| 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 | |
| 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r | |
| 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 | |
| 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 | |
| n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r | |
| 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 | |
| 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 | |
| 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 | |
| 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 | |
| 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 | |
| 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 | |
| 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 | |
| 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 | |
| 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 | |
| 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 | |
| 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 | |
| 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r | |
| 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r | |
| 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r | |
| 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 | |
| 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 | |
| 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 | |
| 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 | |
| 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 | |
| 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 | |
| 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r | |
| 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 | |
| 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 | |
| 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 | |
| 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 | |
| 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 | |
| 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 | |
| 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r | |
| 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 | |
| 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 | |
| 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 | |
| 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r | |
| 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 | |
| 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r | |
| 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 | |
| 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 | |
| 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 | |
| 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 | |
| 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 | |
| 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 | |
| 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| n/r: not reported | ||||||||
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion | 20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r | |
| Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge | |
| Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient and outpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r | |
| Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre | |
| Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r | |
| Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting | |
| Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital | |
| Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; | 15 | 5 | 6 | 450 | University hospital | |
| Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital | |
| Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital | |
| Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r | |
| Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r | |
| Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward | |
| Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital | |
| Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward | |
| Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting | |
| Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home | |
| Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit | |
| Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home | |
| Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation | |
| Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r | |
| Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital | |
| Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit | |
| Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes | |
| Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home | |
| Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre | |
| See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre | |
| Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres | |
| Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services | |
| Upper extremity | n/r | 1: no additional therapy; | n/r | n/r | n/r | n/r | n/r | n/r | |
| Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals | |
| Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm | Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r | |
| EMG: electromyography | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All outcome measures | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 2 Motor impairment at the end of intervention phase Show forest plot | 39 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All outcome measures | 39 | 1292 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.32, 0.66] |
| 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase Show forest plot | 28 | 898 | Mean Difference (IV, Random, 95% CI) | 4.32 [2.46, 6.19] |
| 4 Activities of daily living at the end of intervention phase Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All outcome measures | 19 | 622 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.30, 0.65] |
| 5 Pain at the end of intervention phase Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 All outcome measures | 6 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.67, ‐0.11] |
| 6 Visuospatial neglect at the end of intervention Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All outcome measures | 5 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.10, 2.23] |
| 7 Motor function at follow‐up after 6 months Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All outcome measures | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.78, 3.18] |
| 8 Motor impairment at follow‐up after 6 months Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 All outcome measures | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.26, 1.12] |
| 9 Dropouts at the end of intervention phase Show forest plot | 42 | 1438 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 1.1 Mirror therapy for the upper extremity | 31 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Mirror therapy for the lower extremity | 5 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.29, 0.72] |
| 1.1 Studies that used a covered mirror in the control group | 16 | 506 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.36, 0.99] |
| 1.2 Studies that used unrestricted view in the control group | 14 | 474 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.59] |
| 1.3 Studies that used no additional control intervention | 8 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 32 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| 1.1 All studies including participants within 6 months after stroke | 18 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.18, 0.73] |
| 1.2 All studies including participants with more than 6 months after stroke | 14 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies without randomised cross‐over trials | 35 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.68] |
| 1.2 All studies with adequate sequence generation | 33 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.19, 0.54] |
| 1.3 All studies with adequate concealed allocation | 16 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.4 All studies with adequate handling of incomplete outcome data | 12 | 388 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.14, 0.95] |
| 1.5 All studies with blinded assessors | 25 | 820 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.17, 0.70] |
| 2 Motor impairment at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies with adequate sequence generation | 36 | 1157 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.29, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at the end of intervention Show forest plot | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.08] |

