Mirror therapy for improving motor function after stroke
Abstract
Background
Mirror therapy is used to improve motor function after stroke. During mirror therapy, a mirror is placed in the person's midsagittal plane, thus reflecting movements of the non‐paretic side as if it were the affected side.
Objectives
To summarise the effectiveness of mirror therapy compared with no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke. We also aimed to assess the effects of mirror therapy on activities of daily living, pain, and visuospatial neglect.
Search methods
We searched the Cochrane Stroke Group's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, AMED, PsycINFO and PEDro (last searched 16 August 2017). We also handsearched relevant conference proceedings, trials and research registers, checked reference lists, and contacted trialists, researchers and experts in our field of study.
Selection criteria
We included randomised controlled trials (RCTs) and randomised cross‐over trials comparing mirror therapy with any control intervention for people after stroke.
Data collection and analysis
Two review authors independently selected trials based on the inclusion criteria, documented the methodological quality, assessed risks of bias in the included studies, and extracted data. We assessed the quality of the evidence using the GRADE approach. We analysed the results as standardised mean differences (SMDs) or mean differences (MDs) for continuous variables, and as odds ratios (ORs) for dichotomous variables.
Main results
We included 62 studies with a total of 1982 participants that compared mirror therapy with other interventions. Of these, 57 were randomised controlled trials and five randomised cross‐over trials. Participants had a mean age of 59 years (30 to 73 years). Mirror therapy was provided three to seven times a week, between 15 and 60 minutes for each session for two to eight weeks (on average five times a week, 30 minutes a session for four weeks).When compared with all other interventions, we found moderate‐quality evidence that mirror therapy has a significant positive effect on motor function (SMD 0.47, 95% CI 0.27 to 0.67; 1173 participants; 36 studies) and motor impairment (SMD 0.49, 95% CI 0.32 to 0.66; 1292 participants; 39 studies). However, effects on motor function are influenced by the type of control intervention. Additionally, based on moderate‐quality evidence, mirror therapy may improve activities of daily living (SMD 0.48, 95% CI 0.30 to 0.65; 622 participants; 19 studies). We found low‐quality evidence for a significant positive effect on pain (SMD −0.89, 95% CI −1.67 to −0.11; 248 participants; 6 studies) and no clear effect for improving visuospatial neglect (SMD 1.06, 95% CI −0.10 to 2.23; 175 participants; 5 studies). No adverse effects were reported.
Authors' conclusions
The results indicate evidence for the effectiveness of mirror therapy for improving upper extremity motor function, motor impairment, activities of daily living, and pain, at least as an adjunct to conventional rehabilitation for people after stroke. Major limitations are small sample sizes and lack of reporting of methodological details, resulting in uncertain evidence quality.
PICO
Plain language summary
Mirror therapy for improving movement after stroke
Review question
Does mirror therapy improve movement, the performance of daily activities, pain, and lack of attention to and awareness of the affected field of vision (visuospatial neglect) after stroke.
Backround
Paralysis of the arm or leg is common after stroke and frequently causes problems with activities of daily living such as walking, dressing, or eating. Mirror therapy (MT) is a rehabilitation therapy in which a mirror is placed between the arms or legs so that the image of a moving non‐affected limb gives the illusion of normal movement in the affected limb. By this setup, different brain regions for movement, sensation, and pain are stimulated. However, the precise working mechanisms of mirror therapy are still unclear. We conducted a search for literature in various databases and extracted the data of relevant studies.
Search date
This review identified studies up to 16 August 2017.
Study characteristics
We found 62 relevant studies, of which 57 randomly allocated participants to receive either MT or a control therapy (randomised controlled trials) and five provided both therapies to all participants, but in random order (cross‐over trials). The studies involved a total of 1982 participants with a mean age of 59 years (30 to 73 years) after stroke. Mirror therapy was provided three to seven times a week, between 15 and 60 minutes for each session for two to eight weeks (on average five times a week, 30 minutes a session for four weeks).
Key results
At the end of treatment, mirror therapy moderately improved movement of the affected upper and lower limb and the ability to carry out daily activities for people within and also beyond six months after the stroke. Mirror therapy reduced pain after stroke, but mainly in people with a complex regional pain syndrome. We found no clear effect for visuospatial neglect. The beneficial effects on movement were maintained for six months, but not in all study groups. No adverse effects were reported.
Quality of the evidence
The studies provide moderately‐reliable evidence that MT improves movement (motor function, motor impairment) and the performance of daily activities. However, there was only low reliability that MT decreases pain and visuospatial neglect. This may be due to the small number of studies. Further research is needed, with larger methodologically‐sound studies.
Authors' conclusions
Summary of findings
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
| Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 | ⊕⊕⊕⊝ | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 | ⊕⊕⊕⊝ Moderatea | SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 | ⊕⊕⊝⊝ | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 | ⊕⊕⊕⊝ | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 | ⊕⊕⊝⊝ | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) | ⊕⊕⊕⊝ | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 | ⊕⊕⊝⊝ | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded due to several ratings in one or more items with high or unknown risk of bias. | |||||
Background
Description of the condition
Cerebrovascular diseases, taken together with ischaemic heart diseases, are the leading causes of death worldwide. Stroke is one of the leading causes of long‐term disability, particularly in high‐ and middle‐income countries (Murray 2013). Immediately after stroke onset, approximately 80% of survivors have an upper or lower limb motor impairment (Barker 1997; Jorgensen 1995; Nakayama 1994). Full upper limb function is achieved by nearly 80% of people with mild paresis, but only by 20% of people with severe paresis of the upper limb (Nakayama 1994). Of those people with an initial plegic upper limb, only half regain some motor function in the paretic upper limb six months later (Kwakkel 2003). Two‐thirds of people with lower limb impairment are not able to walk independently soon after their stroke, and after rehabilitation only half have independent walking function (Jorgensen 1995). The initial severity of upper and lower extremity paresis is one of the most important predictors of long‐term functional recovery after stroke (Hendricks 2002; Jorgensen 1995; Nakayama 1994), but variability is high, possibly influenced by therapeutic interventions.
Up to 50% of people experience pain of the upper extremity during the first 12 months post‐stroke, especially shoulder pain and complex regional pain syndrome‐type I (CRPS‐type I) (Jönsson 2006; Kocabas 2007; Lundström 2009; Sackley 2008). Pain after stroke may restrict activities of daily living and reduce quality of life (Jönsson 2006; Lindgren 2007).
Additionally, about 40% of people with an acute right hemispheric and 20% of people with a left hemispheric stroke present a unilateral neglect (Ringman 2004), especially visuospatial neglect. After three months a unilateral neglect was present in about 15% of people with a right and 5% of people with a left hemispheric stroke (Ringman 2004). Besides the spatial attention deficits, neglect is a negative factor for functional recovery (Farnè 2004; Katz 1999), and was found to be associated with a reduced health‐related quality of life (Franceschini 2010).
Effective training strategies to promote motor recovery and activities of daily living, to reduce pain or visuospatial neglect or both are therefore needed to reduce the burden of stroke.
Description of the intervention
Evidence suggests that effective therapeutic interventions for regaining motor function should potentially focus on the practice of functional tasks (Van Peppen 2004). However, task‐oriented training strategies, such as constraint‐induced movement therapy (Corbetta 2015; French 2016; Liepert 1998; Miltner 1999; Taub 1993), require some degree of voluntary movement, and are therefore not applicable for people with severe paresis after stroke. Novel training strategies for this patient population use electromechanical training devices (Mehrholz 2015; Mehrholz 2017), electrical muscle stimulation (Hatem 2016; Urton 2007), or repetitive passive or assistive movement stimulation (Feys 2004; Platz 2005).
As an alternative treatment approach, mirror therapy has been proposed as potentially beneficial (Ramachandran 1994). In contrast to other interventions, which employ somatosensory input to assist motor recovery (Feys 2004), mirror therapy is based on visual stimulation. During mirror therapy, a mirror is placed in the person's midsagittal plane, thus reflecting the non‐paretic side as if it were the affected side (Ramachandran 1995). By this setup, movements of the non‐paretic limb create the illusion of normal movements of the paretic limb (Deconinck 2015). One of the advantages of mirror therapy is the relatively easy administration and the possibility of self‐administered home therapy, even for people with severe motor deficits. Clinical studies reported effects of mirror therapy on pain reduction in arm amputees or CRPS‐type I (Ramachandran 1995; Ramachandran 1996; Thieme 2016). Furthermore, mirror therapy was claimed to alleviate hemiparesis after stroke (Ramachandran 1994), which was confirmed in a pilot study (Altschuler 1999).
Recently, some authors have described 'mirror‐like' video or computer‐graphic setups, where a video or computer‐graphic image of the moving limb is presented as if it were the opposite one (Adamovich 2009; Eng 2007; Gaggioli 2004; Hoermann 2017; In 2012; Laver 2017; Morganti 2003).
How the intervention might work
The concept of mirror therapy has been substantiated neurophysiologically. There is long‐standing evidence that observation of movements and performance of the observed actions share similar cortical motor areas (Grèzes 2001). Movement mirroring (i.e. the inversion of the visual feedback) leads to an additional activation of the hemisphere contralateral to the perceived limb laterality (Deconinck 2015; Dohle 2004; Matthys 2009; Shinoura 2008). The mirror illusion may increase cortico‐muscular excitability (Fukumura 2007; Garry 2005; Kang 2011; Kang 2012). However, the precise mechanisms of the effect of mirror therapy in people with stroke remain speculative. As the visual image of the paretic limb is perceived similarly to the person's own moving limb (Dohle 2004), the mirror illusion might prevent or reverse a learned non‐use of the paretic limb (Liepert 1995). Also, by modulation of the cortico‐muscular excitability, mirror therapy might directly stimulate motor recovery. Finally, mirror therapy was regarded as a variant of motor imagery training, which is based on repetitive imagination and mental rehearsal of motor tasks (Miltner 1998; Stevens 2003). Behavioural studies suggest that the experience of agency (the attribution of visual images of body parts as being controlled by oneself) relies on a tight temporal coupling of the visual feedback of active, but not passive, movements (Longo 2009). It is this active performance that seems to distinguish mirror therapy from movement observation therapy (Wang 2013b).
Imaging studies further suggest that mirrored computer‐graphic images are processed similarly to those of real movements (Adamovich 2009; Dohle 2011), as long as the temporal and spatial consistency with real movements does not fall below certain thresholds (Franck 2001). Thus, even technically‐generated images of a human moving limb can be integrated into the body scheme with the same sense of agency as during 'real' mirroring.
Regarding non‐motor symptoms, some studies also found significant effects of mirror therapy on somatosensory impairment after stroke (Acerra 2007; Dohle 2009). Cortical effects might be different from those for rehabilitation of motor function (Fritzsch 2014). Besides, mirror therapy was proposed to reduce unilateral visuospatial neglect after stroke (Dohle 2009). The strong visual stimulus of watching self‐induced movements in the neglected hemifield was postulated to be responsible for this effect. However, this could only be confirmed if the mirror was placed in the affected, rather than the non‐affected, side of the body (Ramachandran 1999).
Finally, mirror therapy was found to be effective in reducing pain in different conditions (Bowering 2013; Thieme 2016). It is hypothesised that mirror therapy may normalise central sensory processing by providing a physiological image of the affected limb (McCabe 2003).
Why it is important to do this review
Since the first publication of our Cochrane Review, a number of new clinical studies about mirror therapy after stroke have been published. An update of the review is therefore required in order to provide a current estimation of the available evidence and to address limitations found in the original review.
Objectives
To summarise the effectiveness of mirror therapy compared with no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke. We also aimed to assess the effects of mirror therapy on activities of daily living, pain, and visuospatial neglect.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cross‐over RCTs comparing mirror therapy (provided by a mirror or a simultaneous video or virtual setup) with any other therapy modality, no therapy, or sham therapy. If we included cross‐over RCTs, we only analysed the first period as a parallel‐group trial.
Types of participants
We included studies examining participants with a paresis of the upper or lower limb, or both, caused by stroke (all types, severity and stages of stroke) aged over 18 years. If we identified studies with mixed populations of people with neurological conditions, we included those studies if separate data for people with stroke were available.
Types of interventions
Mirror therapy is defined as an intervention that uses a mirror to create a reflection of the non‐paretic upper or lower limb, thus giving the person visual feedback of normal movement of the paretic limb. Using this setup, different variations in the experimental protocol are possible (Bieniok 2011; Dohle 2005). We included studies that used direct mirroring of movement of any regimen and variation (i.e. including video or virtual reality settings). However, we only included those studies where the regimen and delivery of mirror therapy could be identified. Furthermore, for studies with a combination of mirror therapy and other therapies in the experimental condition, we only included studies where a minimum of 50% of the experimental intervention time was applied for mirror therapy.
The control arm of the study could include a no‐treatment group, usual or standard practice, or any other control treatment (i.e. placebo or sham therapy). We excluded studies where the influence of mirror therapy could not be isolated due to the comparison of different mirror therapy regimens or delivery. We contacted trialists if the regimen or delivery (or both) of mirror therapy or the control intervention was unclear.
Types of outcome measures
We evaluated outcome measures post‐intervention and at follow‐up after six months or longer.
Primary outcomes
The primary outcome was motor function. Due to the wide variety of outcome measures, we selected outcome measures to facilitate quantitative pooling. If more than one outcome measure was available we prioritised measures as follows:
-
Upper limb and hand motor function: Action Research Arm Test (Lyle 1981), Wolf Motor Function Test (Wolf 2001), Motor Assessment Scale ‐ upper limb and hand function or both (Carr 1985), Manual Function Test (Miyamoto 2009), Box and Bock Test (Mathiowetz 1985).
-
Lower limb motor function: Motor Assessment Scale ‐ Items 4 or 5 (or both) (Carr 1985), Berg Balance Scale (Berg 1992).
-
Global motor function: Motor Assessment Scale (Carr 1985), Rivermead Motor Assessment Scale (Collen 1991).
However, if these scales were not available, we accepted other measurements that evaluate motor function.
Secondary outcomes
Secondary outcomes included measures of motor impairment (upper limb motor impairment: Fugl‐Meyer Assessment ‐ upper limb or hand function or both (Fugl‐Meyer 1975); Brunnstrom Stages of the Upper Extremity (Brunnstrom 1966); Motricity Index ‐ arm score, muscle or grip strength (Demeurisse 1980)); lower limb motor impairment: Fugl‐Meyer Assessment ‐ lower limb function (Fugl‐Meyer 1975); Brunnstrom Stages of the Lower Extremity (Brunnstrom 1966), activities of daily living (e.g. Functional Independence Measure: Keith 1987), Barthel Index: Mahoney 1965)); pain (Visual Analogue Scale or Numeric Rating Scale), and visuospatial neglect. We also searched for reported adverse effects (e.g. swelling) and dropout rate.
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
We searched the Cochrane Stroke Group's Trials Register (last searched on 16 August 2017); Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library (last searched on 16 August 2017); MEDLINE Ovid (1946 to August 2017); Embase Ovid (1974 to August 2017); Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO; 1982 to August 2017); Allied and Complementary Medicine (AMED Ovid; 1985 to August 2017); PsycINFO Ovid (1806 to August 2017); and the Physiotherapy Evidence Database (PEDro; searched August 2017).
We developed the MEDLINE search strategy for this review with the assistance of the Cochrane Stroke Group's Information Specialist and adapted it to search the other databases (Appendix 1; Appendix 2). We included all languages, and imposed no date limits. As the subject area of this review is quite specific, we did not include a trials filter to maximise the sensitivity of the search.
We also searched ongoing trials and research registers:
-
ISRCTN Registry (www.isrctn.com/, searched December 2016);
-
ClinicalTrials.gov (www.clinicaltrials.gov/, searched December 2016);
-
StrokeTrials Registry (www.strokecenter.org/trials/, searched December 2016);
-
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/, searched December 2016);
Searching other resources
In an effort to identify further published, unpublished and ongoing trials not available in the major databases, we:
-
handsearched the following conference proceedings:
-
Deutsche Gesellschaft für Neurologie (2008 to 2016);
-
Deutsche Gesellschaft für Neurorehabilitation (2000, 2001, 2003, 2005, 2007, 2009, 2010, 2012, 2013, 2014, 2016);
-
Deutsche Gesellschaft für Neurotraumatologie und klinische Neurorehabilitation (2005, 2007, 2009, 2010, 2014, 2016);
-
European Stroke Conference (2001 to 2015);
-
European Congress of Neurorehabilitation (2011, 2013, 2015);
-
World Congress of Neurorehabilitation (1999, 2002, 2006, 2010, 2012, 2014, 2016);
-
World Congress of Physical Therapy (2003, 2007, 2011, 2015);
-
World Stroke Congress (2000, 2004, 2008, 2010, 2012, 2014);
-
-
screened reference lists of all relevant articles and books;
-
contacted trialists, experts, researchers and commercial companies (Reflex Pain Management Ltd) in our field of study to obtain information of unpublished studies and studies not available in the electronic databases;
-
searched System for Information on Grey Literature in Europe (OpenSIGLE ‐ www.opengrey.eu/, searched December 2016); and
-
searched the REHABDATA database (www.naric.com/research/rehab, searched December 2016).
Data collection and analysis
Selection of studies
Two of three review authors (HT, NM and CD) independently screened titles of the references identified from the electronic database searches and ruled out obviously irrelevant references. We obtained abstracts or full texts, or both, of the remaining studies and used our inclusion criteria (types of studies, types of participants, types of interventions and outcome measures) to assess whether they were eligible for inclusion. We resolved disagreements by discussion. If the inclusion of a study was unclear due to missing information, we tried to contact the authors of the studies for further details. Otherwise, we listed the study as 'awaiting classification'.
Data extraction and management
Two of three review authors (HT, NM and CD) independently extracted trial and outcome data of the included trials using a checklist. Because two of the review authors (HT, CD) are principal investigators of included trials, other authors (JB, JM) did the data extraction of those study. The checklists for data extraction contained:
-
methods of randomisation;
-
methods of concealment of allocation;
-
blinding;
-
use of an intention‐to‐treat (ITT) analysis (all participants initially randomised were included in the analysis in their originally‐allocated groups);
-
adverse events;
-
dropouts for all reasons;
-
imbalance of important prognostic factors;
-
participants (country, number of participants, age, gender, type of stroke, time since stroke onset to study entry);
-
inclusion and exclusion criteria;
-
details of interventions in treatment and control groups;
-
outcomes;
-
time points of measurement.
We tried to establish all unclear characteristics of the studies by contacting the trial co‐ordinator or principal investigator. We checked the extracted data for agreement between review authors and entered the data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
We used the 'Risk of bias' assessment tool according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the adequacy of methods for sequence generation (selection bias), concealment of allocation (selection bias), completeness of outcome data or handling of incomplete outcome data (attrition bias), and blinding of assessors (detection bias) (Higgins 2011).
We did not integrate blinding of therapists and participants as an item in the 'Risk of bias' assessment, since this appeared not to be possible for the type of interventions in this review.
We resolved disagreements in methodological assessment by consulting a third review author (MP, JM or JB), and reached consensus through discussion. If an article did not contain information on any methodological criteria, we contacted the study authors for additional information. If no further information was available, we rated the criteria as 'unclear'.
GRADE and 'Summary of findings' table
We assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), for the following main outcomes of analysis: motor function, motor impairment, Fugl‐Meyer Assessment, activities of daily living, pain, pain at the end of intervention phase after excluding studies with CRPS, and visuospatial neglect, each at the end of the intervention phase. We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size, and the overall quality of the evidence, in summary of findings Table for the main comparison.
Measures of treatment effect
The primary and secondary outcome variables of interest were continuous outcomes. We entered data of post‐intervention assessment and follow‐up assessment at six months as means and standard deviations (SDs) and calculated the standardised mean difference (SMD) or mean difference (MD) with 95% confidence intervals (CIs) for each trial. We pooled data through calculation of the overall SMD/MD and 95% CI. For dichotomous data (adverse events, dropouts) we calculated odds ratios (ORs) between groups.
Unit of analysis issues
We considered randomised cross‐over trials prior to cross‐over and analysed only the first intervention phase.
Dealing with missing data
We contacted study authors if appropriate data for analysis were not adequately reported. If study authors did not respond within one month after contact, we tried to contact them at least once more. If data were not sufficient to decide on inclusion or exclusion of studies, we rated the studies as 'awaiting classification'. If data were insufficient for meta‐analysis, we excluded the studies from meta‐analysis. If we were unable to get the missing data for participants who dropped out, we only analysed the participants for which we had data. However, we considered an ITT analysis as part of the 'Risk of bias' assessment and performed a sensitivity analysis in which we excluded studies with no or unreported ITT analysis. We also conducted a sensitivity analysis, excluding studies with missing methodological data (therefore rated as 'unclear' risk of bias).
Assessment of heterogeneity
We evaluated clinical heterogeneity through reported clinical and methodological diversity, variability of participants, interventions, and outcomes in an additional table. We used the I2 statistic to assess heterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed‐effect model approach.
Assessment of reporting biases
We tried to minimise reporting bias through an extensive search of databases, handsearching of references lists and conference abstracts, and by contacting study authors, trialists, and experts in the field for other unpublished or ongoing trials. We also conducted a sensitivity analysis, excluding studies of low methodological quality.
Data synthesis
Where possible, we conducted a pooled analysis of primary outcomes (motor function) and secondary outcomes (motor impairment, activities of daily living, pain, visuospatial neglect, dropout rate) as described above, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis to establish the effectiveness of mirror therapy focused on upper or lower extremity. We also investigated heterogeneity regarding time since stroke. We performed a subgroup analysis separating participants in an acute/subacute stage from those in a chronic stage after stroke; the cut‐off point for separating these subgroups was six months after stroke. We also investigated heterogeneity by the type of control intervention used. We separated subgroups using no (additional) control intervention, another control intervention, and sham intervention with restricted view on the paretic extremity.
Sensitivity analysis
We conducted a sensitivity analysis to test the robustness of the results, removing studies that we assessed to be of lower or ambiguous methodological quality (studies with risk of bias for at least one method of sequence generation, concealment of allocation, ITT analysis, or blinded assessors). We also reanalysed the data by removing cross‐over RCTs.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, Characteristics of ongoing studies and Table 1.
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 | |
| 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r | |
| 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r | |
| 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 | |
| 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 | |
| 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 | |
| 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 | |
| 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 | |
| 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r | |
| 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 | |
| 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 | |
| n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r | |
| 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 | |
| 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 | |
| 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 | |
| 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 | |
| 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 | |
| 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 | |
| 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 | |
| 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 | |
| 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 | |
| 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 | |
| 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 | |
| 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r | |
| 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r | |
| 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r | |
| 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 | |
| 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 | |
| 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 | |
| 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 | |
| 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 | |
| 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 | |
| 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r | |
| 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 | |
| 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 | |
| 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 | |
| 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 | |
| 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 | |
| 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 | |
| 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r | |
| 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 | |
| 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 | |
| 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 | |
| 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r | |
| 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 | |
| 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r | |
| 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 | |
| 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 | |
| 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 | |
| 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 | |
| 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 | |
| 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 | |
| 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
n/r: not reported
Results of the search
We identified 33 new studies from the updated search of the Cochrane Stroke Group's Trials Register. We also identified 8879 references from other electronic databases and 14 references from other sources. After excluding all duplicate references we identified 3588 references from the updated search in all electronic databases (5408 with references in the first version of this review). Two review authors (HT, NM or CD) identified 519 possibly eligible studies (652 with studies in the first version of this review). We discarded 470 studies (599 with studies in the first version of this review). There was insufficient information to determine inclusion eligibility for six trials (Amimoto 2008; ISRCTN40903497; Magni 2014; May 2011; Wang 2013a; Yeldan 2015), but we failed to get in contact with the authors, so the studies are listed as 'awaiting classification' (see Characteristics of studies awaiting classification). We also identified 15 ongoing trials (see Characteristics of ongoing studies). We therefore include 49 new studies (62 with studies in the first version of this review) in this updated version of the review (see Figure 1).

Study flow diagram of updated search and selection process
Included studies
Sixty‐two trials met the inclusion criteria of our review (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Salhab 2016; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014) (see Characteristics of included studies).
We now exclude one study which we had included in the first version of this review, as only 15% of the experimental intervention was spent in mirror therapy (Ietswaart 2011).
Because the two groups in Rothgangel 2004 received significantly different treatment sessions, we decided to split the data and analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
Design
Fifty‐seven studies were RCTs with a parallel‐group design (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Nagapattinam 2015; Pandian 2014; Park 2015a; Park 2015b; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011; Zacharis 2014), and five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006).
Sample Size
The 62 studies included a total of 1982 participants. Individual sample sizes of identified trials ranged from six (Geller 2016) to 94 (Tyson 2015). A detailed description of individual sample sizes can be found in Characteristics of included studies.
Participants
Not all studies provided data on characteristics of participants. Detailed descriptions of participant characteristics are given in Table 1.
The mean age of participants in the included studies was 59 years, with a range from 30 years (Moustapha 2012) to 78 years (Tezuka 2006). There were more participants with a hemiparesis of the left side (53%). There were more men (60%) than women (40%). Twenty‐four studies included participants after their first‐ever stroke. Mean time post‐stroke ranged between five days (Acerra 2007), and five years (Altschuler 1999). Twenty‐nine studies included participants in the acute or subacute phase after stroke (within six months post‐stroke) and 21 trials included participants in the chronic phase (more than six months). Among those participants with known aetiology, 67% had an ischaemic and 33% a haemorrhagic stroke.
Fifty‐two studies provided information on the study setting: 39 inpatient rehabilitation settings or hospitals; three inpatient and outpatient rehabilitation settings; four home settings; two inpatient and home setting; and four outpatient settings (Table 2). The included studies were conducted in 21 different countries.
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion | 20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r | |
| Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge | |
| Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient and outpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r | |
| Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre | |
| Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r | |
| Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting | |
| Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital | |
| Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; | 15 | 5 | 6 | 450 | University hospital | |
| Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital | |
| Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital | |
| Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r | |
| Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r | |
| Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward | |
| Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital | |
| Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward | |
| Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting | |
| Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home | |
| Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit | |
| Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home | |
| Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation | |
| Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r | |
| Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital | |
| Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit | |
| Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes | |
| Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home | |
| Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre | |
| See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre | |
| Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres | |
| Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services | |
| Upper extremity | n/r | 1: no additional therapy; | n/r | n/r | n/r | n/r | n/r | n/r | |
| Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals | |
| Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm | Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r |
EMG: electromyography
ETMS: electromyography‐triggered neuromuscular stimulation
FES: functional electrical stimulation
Hz: hertz
MT: mirror therapy
NMES: neuromuscular electrical stimulation
n/r: not reported
rTMS: repetitive transcranial magnetic stimulation
tDCS: transcranial direct current stimulation
TMS: transcranial magnetic stimulation
μs: microsiemens
Inclusion and exclusion criteria of studies are listed in Characteristics of included studies.
Interventions
Characteristics of interventions are summarised in Table 2. All except two included studies (In 2012; In 2016), provided mirror therapy using a mirror or a mirror box in the midsagittal plane between the upper or lower limbs. Thus the mirror reflected movements of the non‐affected side as if these movements were executed with the affected side. In 2012 and In 2016 used a virtual reflection setting where the affected extremity was placed under a screen while the non‐affected extremity was placed under a camera. The screen displayed the mirrored picture of the unaffected limb.
Ten studies examined the effects of mirror therapy for the lower extremity (Arya 2017; Cha 2015; In 2016; Kawakami 2015; Kumar 2013; Lee 2016; Marquez 2012; Mohan 2013; Salhab 2016; Sütbeyaz 2007); all other studies examined the effects of mirror therapy for the upper extremity.
Eleven studies used a combination of mirror therapy and other interventions. Kim 2014, Kim 2015a, Lee 2016, and Yun 2011 integrated a combination of mirror therapy with functional or neuromuscular electrical stimulation, Kojima 2014 and Schick 2017 with electromyographic‐triggered electrical muscle stimulation, and Lin 2014a combined mirror therapy with electrical sensory stimulation using a mesh‐glove. Mirror therapy was further combined with transcranial direct current stimulation (Cho 2015), or transcranial magnetic stimulation (Cha 2015; Dalla Libera 2015; Ji 2014a). If studies used two experimental groups, we combined both intervention groups for analysis.
Mirror therapy was provided for between three and seven days a week, and for between two and eight weeks. Each session lasted between 15 and 60 minutes. The total time for experimental intervention was between 225 and 2160 minutes.
Rothgangel 2004 included 16 participants and randomised them to mirror therapy or bilateral arm training. However, six of the participants were treated in an outpatient rehabilitation centre, and 10 in an inpatient care facility, which led to a significant difference in treatment time: the outpatient group received 17 treatment sessions of 30 minutes each; the inpatient group received 37 treatment sessions of 30 minutes each. Because these two groups are considerably different in total treatment time, we decided to analyse them separately (outpatient group: Rothgangel 2004a, and inpatient group: Rothgangel 2004b).
In 29 studies participants performed bilateral movements, moving the affected limb behind the mirror as best they could. In 22 studies participants only moved the unaffected side while looking in the mirror. In two studies participants performed both uni‐ and bilateral movements (In 2016; Kawakami 2015). In Rothgangel 2004 participants with muscle hypotonia had to move the affected arm as best they could; participants with muscle hypertonia should only move the unaffected arm while looking into the mirror. In two studies, a therapist passively moved the affected arm behind the mirror according to the movements of the unaffected one (Pandian 2014; Tezuka 2006).
In 11 studies the control group received no additional intervention other than standard rehabilitation. Twenty‐two studies used a form of sham therapy where the reflecting side of the mirror was covered, or the non‐reflecting side of the mirror was placed in the direction of the unaffected arm while practising. Eleven studies provided interventions with an unrestricted view of the affected side using the same training as in the experimental groups but without a mirror or with a plexiglas between limbs. Eighteen studies used other interventions in the control groups: electromyographic‐triggered muscle stimulation (Amasyali 2016; Kawakami 2015; Schick 2017; Wang 2015); (functional) electrical muscle stimulation (Kawakami 2015; Nagapattinam 2015; Yun 2011); conventional therapy (Arya 2015; Arya 2017; Geller 2016; Kim 2016; Salhab 2016; Wu 2013); motor imagery (Cacchio 2009b); passive mobilisation of the affected limb (Colomer 2016; Kawakami 2015); transcranial magnetic stimulation (Dalla Libera 2015); task‐oriented training (Lin 2014a); motor relearning programme (Rehani 2015); lower limb activities (Tyson 2015); or constraint‐induced movement therapy (Yoon 2014). In one study a therapist passively moved the affected arm according to the movements of the unaffected one, but without a mirror between limbs (Tezuka 2006). If studies integrated two control groups we combined both groups for analysis (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9). However, for testing the influence of different control treatments, we analysed single control groups in a subgroup analysis. Based on the difference of using a covered mirror, another intervention without mirror (also transparent plexiglas), or no additional therapy, we performed a subgroup analysis differentiating the effects of types of control intervention (covered mirror versus another intervention with unrestricted view versus no additional therapy) (Analysis 3.1).
Outcome
The included studies used a number of different outcomes. A description of the outcome measures used can be found in Characteristics of included studies.
Primary outcome: motor function
For analysis of our primary outcome of motor function we used the Motor Assessment Scale Item 7 (Acerra 2007; Marquez 2012; Piravej 2012), the Box and Block Test (Alibakhshi 2016; Amasyali 2016; Cho 2015; Kim 2015a; Lin 2014a; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a), the Action Research Arm Test (Dohle 2009; Geller 2016; Invernizzi 2013; Kim 2016; Michielsen 2011; Nagapattinam 2015; Thieme 2013; Tyson 2015), the Wolf Motor Function Test (functional ability) (Cacchio 2009a; Cacchio 2009b; Colomer 2016; Hiragami 2012; Kojima 2014; Yoon 2014), the Manual Function Test (Bae 2012; In 2012; Kim 2014; Lee 2012; Park 2015b; Seok 2010), the Berg Balance Scale (Cha 2015; In 2016; Lee 2016), the Brunnel Balance Assessment (Mohan 2013), the CAHAI (Rehani 2015), and the TEMPA (Rodrigues 2016).
Secondary outcomes: motor impairment, activities of daily living, pain and visuospatial neglect
For analysing motor impairment we used the Fugl‐Meyer score (Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009;Geller 2016; Gurbuz 2016; Hiragami 2012; In 2012; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lim 2016; Lin 2014a; Michielsen 2011; Mirela 2015; Mohan 2013; Park 2015a; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yoon 2014; Yun 2011), the Brunnstrom stages of motor recovery (Piravej 2012; Sütbeyaz 2007; Yavuzer 2008), muscle or grip strength (Acerra 2007; Lee 2016; Marquez 2012), the Motricity Index (Invernizzi 2013; Tyson 2015), and the Manual Muscle Test (Seok 2010).
In our pooled analysis of the secondary outcome activities of daily living we used the Functional Independence Measure (Dohle 2009; Geller 2016; Hiragami 2012; Invernizzi 2013; Kim 2015a; Kim 2016; Pandian 2014; Park 2015a; Park 2015b; Sütbeyaz 2007; Yavuzer 2008), the Barthel Index (Kuzgun 2012; Lim 2016; Piravej 2012; Schick 2017; Thieme 2013 ; Yoon 2014), and the Motor Activity Log (amount of use) (Kojima 2014; Lin 2014a; Wu 2013).
For the analysis of the secondary outcome of pain we included the measurement of pain at rest (Acerra 2007; Cacchio 2009b; Michielsen 2011), and during movement (Cacchio 2009a; Dohle 2009). The investigators used Numerical Rating Scales between 0 and 10 (Acerra 2007), Visual Analogue Scales between 0 and 10 (Cacchio 2009a), or between 0 mm and 100 mm (Cacchio 2009b; Michielsen 2011), or the pain section of the Fugl‐Meyer Assessment, normalised on the average score for each item (0 to 2; 2 indicating no pain) (Dohle 2009, Thieme 2013).
Visuospatial neglect as an outcome was analysed using the Star Cancellation Test (Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015), and a self‐developed score (Dohle 2009).
Follow‐up assessment
For analysis of sustained treatment effects for our primary outcome of motor function, we used only the data of follow‐up assessments after six months (Cacchio 2009a; Michielsen 2011), as well as for motor impairment (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008).
Adverse effects
Twenty‐one studies explicitly reported the assessment of adverse effects (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Colomer 2016; Hiragami 2012; Invernizzi 2013; Kojima 2014; Kuzgun 2012; Lin 2014a; Marquez 2012; Mohan 2013; Rodrigues 2016; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Tyson 2015; Wu 2013; Yavuzer 2008; Zacharis 2014). No adverse events were reported.
Excluded studies
We discarded 470 studies following consideration of abstracts, full texts or both (see: Characteristics of excluded studies). In the Excluded studies section, we mention only those studies that might in a superficial view appear to meet the eligibility criteria and those studies that we classified as well‐known and likely to be considered relevant by some readers (Characteristics of excluded studies).
Risk of bias in included studies
All details about the methodological quality of the included studies using the 'Risk of bias' assessment tool (Higgins 2011) are provided in Characteristics of included studies and Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We emailed all trialists of the included studies to clarify some methodological or design issues, or both. Most trialists provided at least some of the requested information. Two review authors (from HT, NM, CD, JB or JM) independently evaluated the methodological quality of the studies. The assessing authors discussed all disagreements and resolved them by contacting another author or by obtaining additional information through contact with the principal investigator of the study.
Allocation
Fifty‐two studies used adequate randomisation procedures, and were therefore at low risk of bias (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Bae 2012; Bahrami 2013; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dalla Libera 2015; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Nagapattinam 2015; Pandian 2014; Park 2015a; Piravej 2012; Rajappan 2016; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yoon 2014; Yun 2011). We were not able to rate risk of bias for 10 trials due to missing information about the sequence generation process (Altschuler 1999; Cacchio 2009a; Geller 2016; Kumar 2013; Manton 2002; Mirela 2015; Park 2015b; Salhab 2016; Wang 2015; Zacharis 2014). Five studies used a cross‐over design with random allocation to the order of treatment (Altschuler 1999; Kojima 2014; Moustapha 2012; Salhab 2016; Tezuka 2006). We only analysed the first treatment period as a parallel‐group design in these five studies. Eight studies used block randomisation methods (Cacchio 2009b; Hiragami 2012; Kojima 2014; Lin 2014a; Mohan 2013; Piravej 2012; Sütbeyaz 2007; Yavuzer 2008). One study randomly allocated ability‐matched pairs to treatment groups (Manton 2002).
Twenty‐five studies used an adequate concealment of allocation, and we therefore considered them to be at low risk of bias (Acerra 2007; Arya 2015; Arya 2017; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Hiragami 2012; Invernizzi 2013; Kim 2016; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rehani 2015; Rodrigues 2016; Rothgangel 2004; Nagapattinam 2015; Schick 2017; Sütbeyaz 2007; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). There was no description of the allocation concealment process, so we rated 35 trials at unclear risk of bias (Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Bae 2012; Bahrami 2013; Cacchio 2009a; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; In 2012; In 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Yoon 2014; Zacharis 2014). Two studies were at high risk of bias because the authors of the trials confirmed that no concealment of allocation process had occurred (Tezuka 2006; Yun 2011). The methods used for concealment of allocation are presented in Characteristics of included studies.
Blinding
We rated 37 studies at low risk of bias, since at least the primary outcome measures were assessed by people blinded to group allocation (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Cha 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; Hiragami 2012; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Kim 2016; Kuzgun 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Moustapha 2012; Pandian 2014; Piravej 2012; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008). In 22 studies the process of blinding was not described (Bae 2012; Bahrami 2013; Cho 2015; Dalla Libera 2015; Geller 2016; In 2012; Kawakami 2015; Kim 2015a; Kumar 2013; Lee 2012; Lim 2016; Manton 2002; Mirela 2015; Mohan 2013; Park 2015a; Park 2015b; Rajappan 2016; Rehani 2015; Salhab 2016; Wang 2015; Yoon 2014; Zacharis 2014). In three trials the study authors stated that the assessors of the primary outcome measure were not blinded, so we considered them to be at high risk of bias (Kojima 2014; Nagapattinam 2015; Yun 2011)
Incomplete outcome data
Seventeen studies conducted an ITT analysis that included incomplete outcome data (Acerra 2007; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Hiragami 2012; Invernizzi 2013; Marquez 2012; Michielsen 2011; Mohan 2013; Nagapattinam 2015; Pandian 2014; Rodrigues 2016; Rothgangel 2004; Schick 2017; Thieme 2013). No description of handling incomplete outcome data was available in 28 studies, and we considered them to be at unclear risk of bias for this domain (Alibakhshi 2016; Altschuler 1999; Bae 2012; Bahrami 2013; Cha 2015; Cho 2015; Dalla Libera 2015; Geller 2016; Gurbuz 2016; Ji 2014a; Kawakami 2015; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lim 2016; Manton 2002; Mirela 2015; Park 2015a; Park 2015b; Salhab 2016; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015; Wu 2013; Yoon 2014; Zacharis 2014). Seventeen studies reported that no ITT analysis was performed, and we rated them at high risk of bias (Colomer 2016; Dohle 2009; In 2012; In 2016; Kim 2015a; Lee 2012; Lee 2016; Lin 2014a; Moustapha 2012; Piravej 2012; Rajappan 2016; Rehani 2015; Sütbeyaz 2007; Tezuka 2006; Tyson 2015; Yavuzer 2008; Yun 2011)
Selective reporting
We did not evaluate studies for selective reporting.
Other potential sources of bias
Twenty studies did not report whether or not participants dropped out during the intervention. In the remaining 42 studies, 109 participants dropped out, which is a rate of 5.5%. Seventeen studies reported no dropouts during the intervention period, 17 trialists reported dropout rates of 15% or less, and in eight studies the dropout rate was above 15%. Fifty‐nine participants dropped out of the experimental groups and 51 participants dropped out of the control groups, giving balanced dropout rates between groups. A detailed description of study characteristics can be found in Characteristics of included studies.
Effects of interventions
Comparison 1: Mirror therapy versus all other interventions
Outcome 1.1: Motor function at the end of the intervention phase
We included 36 studies in a pooled analysis of motor function after study end, with a total of 615 participants in the intervention and 558 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016;Kojima 2014; Lee 2012; Lee 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Nagapattinam 2015; Seok 2010; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). Mirror therapy had a statistically significant effect on motor function in participants after stroke compared with all other types of interventions (SMD 0.47, 95% CI 0.27 to 0.67; 1173 participants; 36 studies; I2 = 62%; Analysis 1.1).
Based on our sensitivity analysis for the influence of trial methodology, we found robust effects on motor function except for concealment of allocation. By analysing only those studies with adequate methods of concealment, the effect on motor function was not significant (Analysis 5.1). We therefore downgraded the quality of evidence to moderate, due to several ratings of unclear risk of bias.
Outcome 1.2: Motor impairment at the end of intervention phase
We included 39 studies in a pooled analysis of motor impairment after study end, with a total of 672 participants in the intervention and 620 in the control groups in the post‐assessment data analysis (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Arya 2015; Arya 2017; Cho 2015; Colomer 2016; Dohle 2009;Gurbuz 2016; In 2012; Invernizzi 2013; Kim 2014; Kim 2016; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lee 2016; Lin 2014a; Lim 2016; Marquez 2012; Michielsen 2011; Mirela 2015; Mohan 2013; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wang 2015; Wu 2013; Yavuzer 2008; Yun 2011; Yoon 2014). Mirror therapy has a statistically significant effect on motor impairment in participants after stroke compared with all other types of interventions (SMD 0.49, 95% CI 0.32 to 0.66; 1292 participants; 39 studies; I2 = 53%; Analysis 1.2). The quality of evidence for motor impairment was moderate.
The effect was robust even after excluding studies with no or inadequate methods of allocation concealment (Analysis 5.2)
Outcome 1.3: Fugl‐Meyer Assessment for the upper extremity at the end of intervention phase
Since 29 studies used the Fugl‐Meyer Asssessment for analysing treatment effects on motor impairment, we analysed the effect on motor impairment for this outcome measure, using mean differences. We included 28 studies in a pooled analysis on Fugl‐Meyer Assessment for the upper extremity after study end, with a total of 463 participants in the intervention and 435 in the control groups in the post‐assessment data analysis (Alibakhshi 2016; Amasyali 2016; Arya 2015; Cho 2015; Colomer 2016; Dohle 2009; Gurbuz 2016; In 2012; Kim 2014; Kim 2015a; Kojima 2014; Kumar 2013; Kuzgun 2012; Lee 2012; Lin 2014a; Lim 2016; Michielsen 2011; Mirela 2015; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Tezuka 2006; Thieme 2013; Wang 2015; Wu 2013; Yun 2011; Yoon 2014). Mirror therapy had a statistically significant effect on Fugl‐Meyer‐Assessment in participants after stroke compared with all other types of interventions (MD 4.32, 95% CI 2.46 to 6.19; 898 participants; 28 studies; I2 = 77%; Analysis 1.3). We rated the evidence for this outcome as of low quality.
Outcome 1.4: Activities of daily living at the end of the intervention phase
We included 19 studies in the analysis of the outcome of activities of daily living (Dohle 2009; Gurbuz 2016; Hiragami 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lim 2016; Lin 2014a; Pandian 2014; Park 2015a; Piravej 2012; Schick 2017; Sütbeyaz 2007; Thieme 2013; Wu 2013; Yavuzer 2008; Yoon 2014). These studies included 333 participants in the intervention and 289 in the control groups. Mirror therapy had a statistically significant effect on activities of daily living for participants with stroke, compared with all other interventions (SMD 0.48, 95% CI 0.30 to 0.65; 622 participants; 19 studies; I2 = 15%; Analysis 1.4). We rated the evidence for this secondary outcome as of moderate quality.
Outcome 1.5: Pain at the end of the intervention phase
For analysing the effects of mirror therapy on pain at the end of the intervention, we included six studies presenting data on pain at rest or during movement (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Dohle 2009; Michielsen 2011; Thieme 2013). These studies included 129 participants in the intervention and 119 in the control groups. Mirror therapy had a statistically significant effect on pain reduction for participants after stroke, compared with all other interventions (SMD −0.89, 95% CI −1.67 to −0.11; 248 participants; 6 studies; I2 = 87%; Analysis 1.5). We rated the quality of the evidence for this secondary outcome pain as low.
However, two studies only included participants after stroke with a diagnosis of CRPS‐type I, which might have influenced the effects of the intervention (Cacchio 2009a; Cacchio 2009b). We therefore performed a post hoc sensitivity analysis and removed the studies that only included participants with CRPS after stroke. After removing those two studies, we were left with four studies with 97 participants in the intervention and 79 in the control groups (Acerra 2007; Dohle 2009; Michielsen 2011; Thieme 2013). We found no statistically significant effect on pain for mirror therapy compared with all other interventions in this subgroup (SMD −0.23, 95% CI −0.53 to 0.08; 176 participants; 4 studies; I2 = 0%; Analysis 6.1).
Outcome 1.6: Visuospatial neglect at the end of the intervention
Five studies reported outcome on visuospatial neglect (Dohle 2009; Moustapha 2012; Pandian 2014; Thieme 2013; Tyson 2015). These studies included 109 participants in the intervention and 66 in the control groups. Based on these data, we found a statistically non‐significant effect of mirror therapy versus all other interventions on visuospatial neglect after stroke (SMD 1.06, 95% CI −0.10 to 2.23; 175 participants; 5 studies; I2 = 89%; Analysis 1.6). We rated the quality of evidence for this secondary outcome as low.
Outcome 1.7: Motor function at follow‐up after six months
Two studies provided data on motor function at a follow‐up period of six months (Cacchio 2009a; Michielsen 2011). These studies included 44 participants each in the experimental and control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically non‐significant effect on motor impairment in people after stroke, compared with all other interventions (SMD 1.20, 95% CI −0.78 to 3.18; 88 participants; 2 studies; I2 = 94%; Analysis 1.7).
Outcome 1.8: Motor impairment at follow‐up after six months
Three studies provided data on motor impairment at a follow‐up period of six months (Michielsen 2011; Sütbeyaz 2007; Yavuzer 2008). These studies included 54 participants in the experimental and 55 in the control groups. At follow‐up after six months from the end of intervention, mirror therapy had a statistically significant effect on motor function in people after stroke, compared with all other interventions (SMD 0.69, 95% CI 0.26 to 1.12; 109 participants; 3 studies; I2 = 17%; Analysis 1.8).
Outcome 1.9: Dropouts at the end of intervention phase
We included 42 studies that provided data for the dropout rate at the end of the intervention phase in this analysis (Acerra 2007; Alibakhshi 2016; Altschuler 1999; Amasyali 2016; Arya 2015; Arya 2017; Cacchio 2009a; Cacchio 2009b; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; In 2016; Invernizzi 2013; Kawakami 2015; Kim 2014; Kim 2015a; Kojima 2014; Kuzgun 2012; Lee 2012; Lee 2016; Lim 2016; Lin 2014a; Marquez 2012; Michielsen 2011; Mohan 2013; Moustapha 2012; Pandian 2014; Piravej 2012; Rajappan 2016; Rodrigues 2016; Rothgangel 2004; Samuelkamaleshkumar 2014; Schick 2017; Nagapattinam 2015; Sütbeyaz 2007; Tezuka 2006; Thieme 2013; Tyson 2015; Wu 2013; Yavuzer 2008; Yun 2011). We found a statistically non‐significant effect for dropping out in the mirror‐therapy groups compared with the control groups (OR 1.14, 95% CI 0.74 to 1.76; 1438 participants; 42 studies; I2 = 0%; Analysis 1.9).
Comparison 2: Subgroup analysis ‐ upper versus lower extremity
Outcome 2.1: Motor function at the end of the intervention phase
We performed a subgroup analysis for those studies examining mirror therapy for the upper extremity (subgroup 2.1.1) and lower extremity (subgroup 2.1.2) (Analysis 2.1). We included 31 studies in the analysis of motor function after mirror therapy for the upper extremity. Studies included 553 participants in the experimental and 495 in the control groups (Acerra 2007; Alibakhshi 2016; Amasyali 2016; Bae 2012; Cacchio 2009a; Cacchio 2009b; Cho 2015; Colomer 2016; Dohle 2009; Hiragami 2012; In 2012; Invernizzi 2013; Kim 2014; Kim 2015a; Kim 2016; Kojima 2014; Lee 2012; Lin 2014a; Michielsen 2011; Park 2015b; Piravej 2012; Rodrigues 2016; Samuelkamaleshkumar 2014; Schick 2017; Ji 2014a; Seok 2010; Nagapattinam 2015; Thieme 2013; Tyson 2015; Wang 2015; Yoon 2014). We found a statistically significant effect of mirror therapy on motor function of the upper extremity for people after stroke compared to all other interventions (SMD 0.46, 95% CI 0.23 to 0.69; 1048 participants; 31 studies; I2 = 66%; Analysis 2.1.1).
Five studies with 62 participants in the experimental and 63 in the control groups were included in the analysis for the lower extremity (Cha 2015; In 2016; Lee 2016; Marquez 2012; Mohan 2013). The positive effect of mirror therapy on motor function of the lower extremity for people after stroke compared with all other interventions was statistically significant (SMD 0.56, 95% CI 0.19 to 0.92; 125 participants; 5 studies; I2 = 0%; Analysis 2.1.2). There was a statistically non‐significant difference between subgroups.
Comparison 3: Subgroup analysis ‐ sham intervention (covered mirror) versus other intervention (unrestricted view) versus no intervention
We found two different groups of control interventions. In all studies, participants in the control group performed the same movements as participants in the experimental groups. However, in one type of control intervention the view of the affected side was obscured with a covered mirror, or with the non‐reflective side of the mirror (sham intervention). In the other type of control intervention participants had an unrestricted view of both; the unaffected and the affected limb (other intervention). Because we believed that this may have influenced the effect of therapy, we performed a subgroup analysis, differentiating between these two types of studies.
Outcome 3.1: Motor function at the end of the intervention phase
Sixteen studies with the outcome of motor function used a covered mirror in the control group (Acerra 2007; Cacchio 2009a; Cacchio 2009b; Cha 2015; Cho 2015; In 2016; Invernizzi 2013; Ji 2014a; Kim 2014; Marquez 2012; Mohan 2013; Nagapattinam 2015; Park 2015b; Piravej 2012; Rodrigues 2016; Thieme 2013). These studies included 281 participants in the intervention and 225 in the control groups. For this subgroup we found a statistically significant effect of mirror therapy on motor function after stroke (SMD 0.67, 95% CI 0.36 to 0.99; 506 participants; 16 studies; I2 = 63%).
Fourteen studies with an intervention using an unrestricted view in the control groups, thus providing a view of both limbs, were analysed in this subgroup (Alibakhshi 2016; Amasyali 2016; Bae 2012; Colomer 2016; Dohle 2009; In 2012; Kim 2016; Lee 2016; Lin 2014a; Michielsen 2011; Schick 2017; Tyson 2015; Wang 2015; Yoon 2014). These studies included 259 participants in the experimental and 215 in the control groups. The effect of mirror therapy on motor function after stroke in these studies was not statistically significant (SMD 0.27, 95% CI −0.05 to 0.59; 474 participants; 14 studies; I2 = 62%).
We included eight studies with no additional control therapy in this analysis (Amasyali 2016; Hiragami 2012; Kim 2015a; Kojima 2014; Lee 2012; Samuelkamaleshkumar 2014; Seok 2010; Wang 2015). Studies included 114 participants in the experimental and 105 in the control groups. This subgroup showed no statistically significant effect in favour of mirror therapy (SMD 0.57, 95% CI −0.02 to 1.15; 219 participants; 8 studies; I2 = 75%; Analysis 3.1).
However, subgroup differences did not demonstrate statistical significance.
Comparison 4: Subgroup analysis: subacute versus chronic stage after stroke
In this subgroup analysis we differentiated between studies that included participants within six months (subacute stage) and those at more than six months after stroke (chronic stage). Eighteen studies with participants in the subacute stage after stroke were included in this analysis. We found a statistically significant effect of mirror therapy compared to all other interventions for this subgroup (SMD 0.45, 95% CI 0.18 to 0.73; 596 participants; 18 studies; I2 = 59%). Fourteen studies in this analysis included participants in the chronic phase after stroke. The effect on motor function was also significant for this subgroup (SMD 0.43, 95% CI 0.06 to 0.81; 398 participants; 14 studies; I2 = 68%; Analysis 4.1). Subgroup difference did not demonstrate statistical significance.
Comparison 5: Sensitivity analysis by trial methodology
We tested the robustness of the results by analysing only RCTs and excluding randomised cross‐over trials, and by using specific methodological variables that could influence the observed treatment effects (randomisation procedure, concealment of allocation, blinding of assessors and ITT analysis; Analysis 5.1).
Outcome 5.1: Motor function at the end of the intervention phase
All studies without randomised cross‐over trials
We included 35 studies in a subgroup analysis of all studies without randomised cross‐over trials. The studies included 609 participants in the experimental and 551 in the control groups. Based on this analysis, mirror therapy had a statistically significant effect on motor function in people after stroke, compared to all other treatments (SMD 0.47, 95% CI 0.27 to 0.68; 1160 participants; 35 studies; I2 = 63%; Analysis 5.1.1).
All studies with adequate sequence generation
We analysed 33 studies with 546 participants in the intervention and 459 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.36, 95% CI 0.19 to 0.54; participants = 1005; studies = 33; I2 = 45%; Analysis 5.1.2)
All studies with adequate concealed allocation
We analysed 16 studies as having used an adequate method of allocation concealment. These studies included 313 participants in the experimental and 259 in the control groups. Based on this analysis, we found a non‐significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.21, 95% CI −0.04 to 0.47; 572 participants; 16 studies; I2 = 51%; Analysis 5.1.3).
All studies with adequate intention‐to‐treat (ITT) analysis
We included 12 studies in our analysis of studies with an adequate ITT analysis. Based on our analysis of 204 participants in the experimental and 184 in the control groups with post‐intervention data, mirror therapy had a significant effect on motor function compared with all other interventions (SMD 0.55, 95% CI 0.14 to 0.95; 388 participants; 12 studies; I2 = 70%; Analysis 5.1.4).
All studies with blinded assessors
In this analysis we included 25 studies with 437 participants in the experimental and 383 in the control groups. Mirror therapy had a statistically significant positive effect on motor function compared with all other interventions (SMD 0.44, 95% CI 0.17 to 0.70; 820 participants; 25 studies; I2 = 69%; Analysis 5.1.5).
Outcome 5.2: Motor impairment at the end of the intervention phase
All studies with adequate sequence generation
We analysed 36 studies with 620 participants in the intervention and 537 in the control groups in this subgroup analysis of studies that we rated as having adequate sequence generation. We found a statistically significant effect of mirror therapy compared with all other therapies for people after stroke (SMD 0.46, 95% CI 0.29 to 0.63; 1157 participants; 36 studies; I2 = 47%; Analysis 5.2).
Discussion
Summary of main results
The main purpose of this review was to evaluate the effect of mirror therapy for improving motor function, motor impairment, activities of daily living, and reducing pain and visuospatial neglect for people after stroke. We included 62 studies (57 RCTs and five randomised cross‐over studies), with a total of 1982 included participants that compared mirror therapy with other interventions. We found moderate‐quality evidence that mirror therapy improves motor function and motor impairment and activities of daily living. Furthermore, with low‐quality evidence we found reduced pain after stroke and improved motor impairment six months after the end of the intervention. However, after excluding studies that included participants with a complex regional pain syndrome only, we found no statistically significant effect on pain, based on moderate‐quality evidence. Results for motor function after six months and for visuospatial neglect were not statistically significant and were of low‐quality evidence. Acceptability of the intervention was high, without significantly more dropouts from the intervention groups compared with control groups, and with no reported adverse events during or after mirror therapy.
Fifty‐two of the included studies evaluated the effect of mirror therapy on motor function of the upper extremity, and 10 studies evaluated the effect of mirror therapy on the lower extremity. Mirror therapy was effective in improving both upper and lower limb motor function.
Based on a subgroup analysis, we found statistically significant effects on motor function in those studies that compared mirror therapy with a sham intervention using a covered mirror (thus avoiding any view of the affected limb), but not in studies that used unrestricted view (no mirror or a transparent plexiglas) or no additional intervention in the control groups. However, there were no statistically significant differences between subgroups with different control interventions.
In a further subgroup analysis, we compared studies that included participants in the acute/subacute phase after stroke (within six months after stroke) and participants in the chronic phase (more than six months after stroke). Mirror therapy was effective for both subgroups of participants.
Overall completeness and applicability of evidence
Based on the available and included evidence, we were able to answer the research question, especially for the outcomes of motor function and motor impairment for the upper and lower extremity, as well as activities of daily living and pain. However, for visuospatial neglect, the number of studies and participants was low, so we could draw no final conclusion. Furthermore, we found some indications for a selective effect of mirror therapy on pain in participants with CRPS. However, this is based on only two studies, so we could draw no final conclusion. The positive results for motor impairment were consistent with follow‐up assessment after six months, but not for motor function. The results are limited because our subgroup analysis indicates evidence of a greater effect of mirror therapy on motor function when compared with a sham intervention (using a covered mirror) than when compared with other (using unrestricted view) or no interventions. The positive effects in this review therefore at least indicate that mirror therapy as an adjunct to routine therapy can improve motor function for people after stroke. Furthermore, the effect on motor function was statistically significant both in acute/subacute and in chronic participants.
One of the potential advantages of mirror therapy compared with other interventions may be due to the possibility of training by moving the unaffected arm, or both arms, while looking in the mirror. Even people with severe paresis could therefore practise on their own without a therapist. Furthermore, mirror therapy could be applied at home, at least after inpatient training, as evaluated in five studies (Arya 2015; Manton 2002; Michielsen 2011; Pandian 2014; Rodrigues 2016).
Quality of the evidence
We used several methodological domains (adequate sequence generation, adequate concealment of allocation, adequate handling of missing outcome data and blinding of assessors) to assess the risks of bias in the included studies. We assessed nine studies as having unclear sequence generation. We found 33 studies with no or unclear use of concealed allocation of participants to study groups, 40 studies with no or unclear use of an adequate handling of missing outcome data, and 24 studies with no or unclear blinding of assessors. Results must therefore be interpreted with caution due to risks of bias. On this basis, we downgraded the quality of the evidence.
Some of the analyses showed significant heterogeneity. However, in the case of motor function and motor impairment this was no longer present when we excluded from the analysis those studies with unclear methods of sequence generation.
In order to test for potential biases through methodological issues, we performed a sensitivity analysis excluding randomised cross‐over studies, studies with unclear adequacy of sequence generation, studies with inadequate concealment of allocation, studies not providing adequate handling of missing outcome data, and studies that did not use assessors blinded to the intervention. Based on these sensitivity analyses, the effects of mirror therapy on motor function for people after stroke were robust, except for studies with adequate methods of allocation concealment. For those studies, the effects on motor function, but not motor impairment, did not demonstrate statistical significance.
Additionally, overall limitations of the included studies were the small sample sizes of most studies and differences in study participants (e.g. severity of motor impairment) and therapy delivery between the studies (i.e. amount and frequency of the treatment period).
Potential biases in the review process
Through an extensive searching process, we are confident that we have identified all relevant studies in the field. However, there remains a risk of publication bias towards a selection of positive results. Furthermore, there is a small possibility of additional (published or unpublished) studies that we did not identify. As stated above, there was heterogeneity between studies in terms of trial design (i.e. parallel‐group and cross‐over trials, duration of follow‐up and selection criteria for participants), characteristics of participants (i.e. severity of motor impairment and time since stroke onset) and characteristics of interventions (i.e. total amount of time of therapy, percentage of the intervention dedicated to mirror therapy only, and therapy for upper or lower extremity). We also identified methodological limitations of studies. However, as stated above, a sensitivity analysis of methodological limitations and participant characteristics revealed the robustness of the results across the stated potential confounding factors, except for concealment of allocation. Blinding of therapists and participants would be an additional item in the 'Risk of bias' assessment, but we decided not to integrate this item, since blinding of therapists or participants appears not to be not practicable for the type of intervention in this review.
Agreements and disagreements with other studies or reviews
The results of this review are in line with the results of other reviews (Ezendam 2009; Rothgangel 2011). These reviews were systematic in terms of their methods. However, they had more limited search strategies, only included studies that were published before 2009, and did not use a pooled analysis of identified studies. A narrative review also describes positive effects of mirror therapy after stroke (Ramachandran 2009).
Study flow diagram of updated search and selection process

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
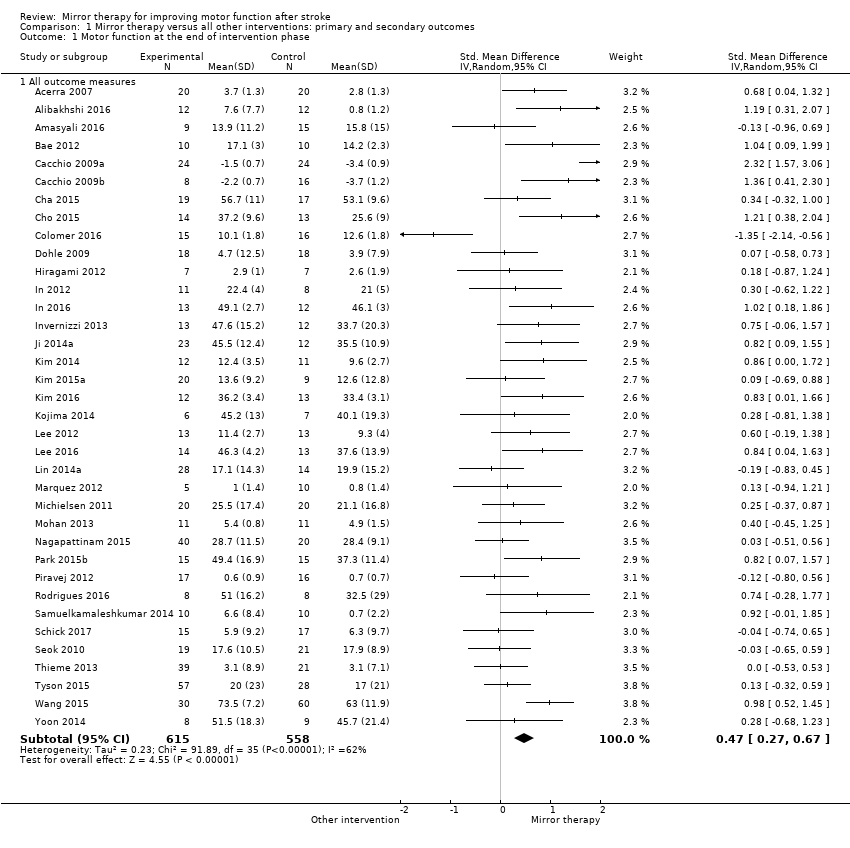
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 1 Motor function at the end of intervention phase.
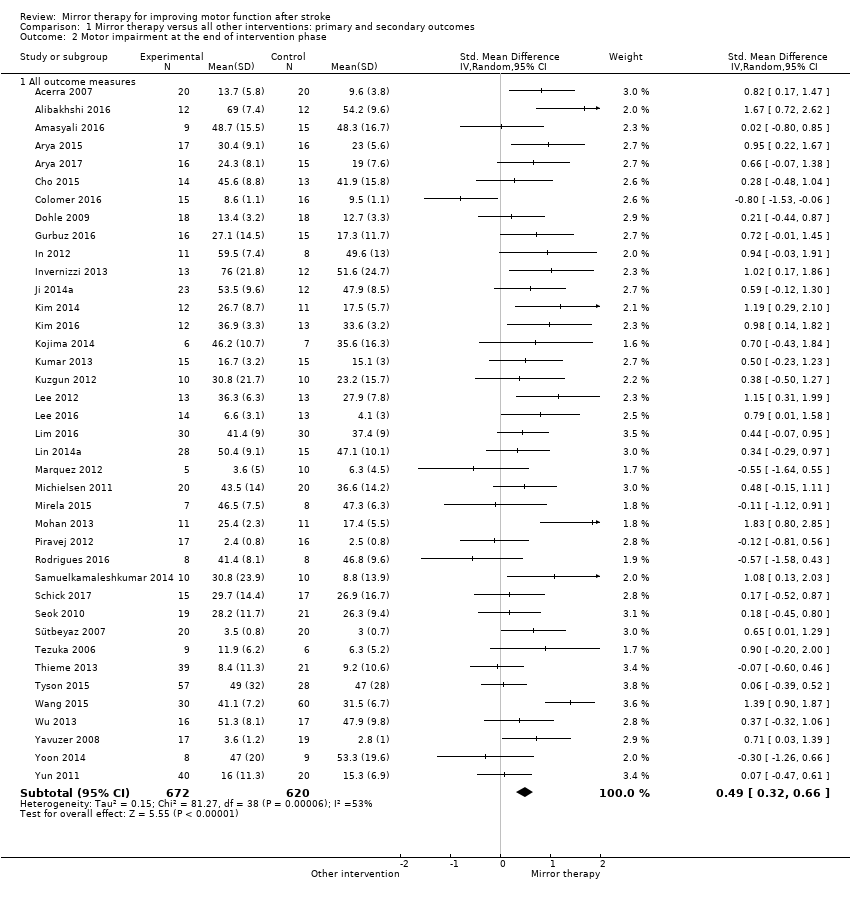
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 2 Motor impairment at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 4 Activities of daily living at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 5 Pain at the end of intervention phase.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 6 Visuospatial neglect at the end of intervention.
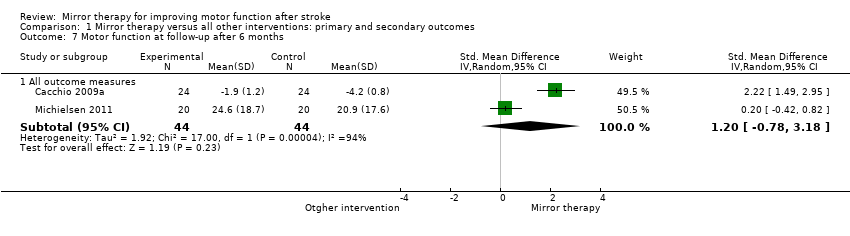
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 7 Motor function at follow‐up after 6 months.
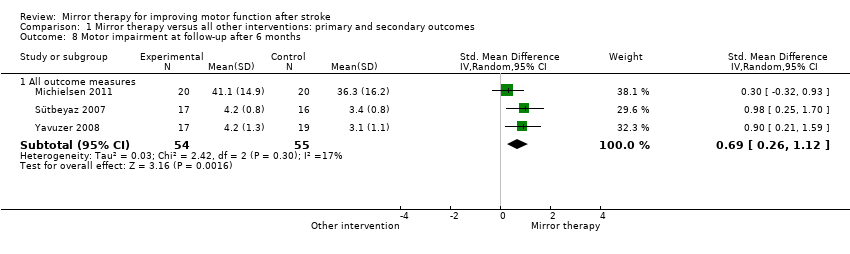
Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 8 Motor impairment at follow‐up after 6 months.

Comparison 1 Mirror therapy versus all other interventions: primary and secondary outcomes, Outcome 9 Dropouts at the end of intervention phase.

Comparison 2 Subgroup analysis: upper versus lower extremity, Outcome 1 Motor function at the end of intervention.

Comparison 3 Subgroup analysis: sham intervention (covered mirror) versus other intervention (unrestricted view), Outcome 1 Motor function at the end of intervention phase.

Comparison 4 Subgroup analysis: subacute versus chronic stage after stroke, Outcome 1 Motor function at the end of intervention phase.
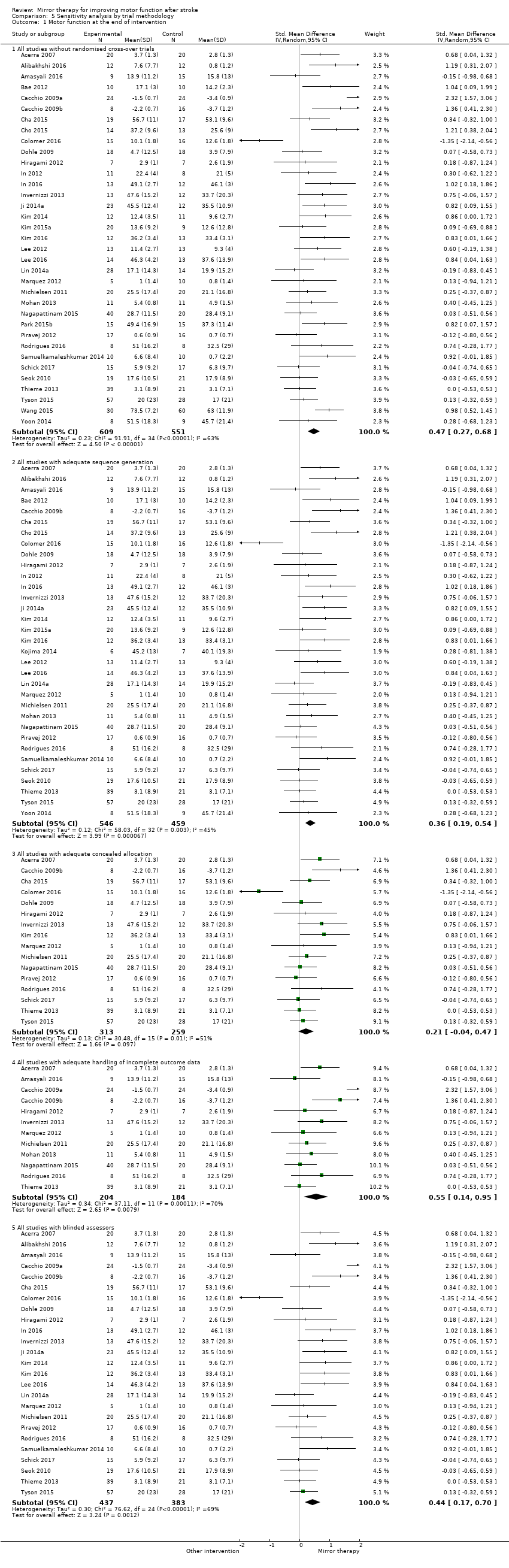
Comparison 5 Sensitivity analysis by trial methodology, Outcome 1 Motor function at the end of intervention.

Comparison 5 Sensitivity analysis by trial methodology, Outcome 2 Motor impairment at the end of intervention.

Comparison 6 Post hoc sensitivity analysis removing studies that only included participants with CRPS after stroke. Subgroup analysis: pain without complex regional pain syndrome (CRPS), Outcome 1 Pain at the end of intervention.
| Mirror therapy compared to all other interventions: primary and secondary outcomes for improving motor function after stroke | |||||
| Participants: people with paresis of the upper or lower limb, or both, caused by stroke Setting: inpatient and outpatient Intervention: mirror therapy Control: no treatment, placebo or sham therapy, or other treatments for improving motor function and motor impairment after stroke | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | № of participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | ||||
| Control | Mirror therapy versus all other interventions | ||||
| Motor function at the end of intervention phase: all outcome measures | The mean motor function at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor function at the end of intervention phase ‐ all studies in the intervention groups was 0.47 SDs higher (0.27 to 0.67 higher) | 1173 | ⊕⊕⊕⊝ | SMD 0.47, 95% CI 0.27 to 0.67; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Motor impairment at the end of intervention phase: all outcome measures | The mean motor impairment at the end of intervention phase ‐ all studies in the control groups was NA | The mean motor impairment at the end of intervention phase ‐ all studies in the intervention groups was 0.49 SDs higher (0.32 to 0.66 higher) | 1292 | ⊕⊕⊕⊝ Moderatea | SMD 0.49, 95% CI 0.32 to 0.66; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Fugl‐Meyer Assessment upper extremity at the end of intervention phase | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the control groups was NA | The mean Fugl‐Meyer Assessment score at the end of intervention phase ‐ all studies in the intervention groups was 4.32 pointshigher (2.46 to 6.19 higher) | 898 | ⊕⊕⊝⊝ | MD 4.32, 95% CI 2.46 to 6.19; the minimum important difference is approximately 5.25 |
| Activities of daily living at the end of intervention phase: all studies | The mean activities of daily living at the end of intervention phase ‐ all studies in the control groups was NA | The mean activities of daily living at the end of intervention phase ‐ all studies in the intervention groups was 0.48 SDs higher (0.29 to 0.67 higher) | 622 | ⊕⊕⊕⊝ | SMD 0.48, 95% CI 0.30 to 0.65; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase: all studies | The mean pain at the end of intervention phase ‐ all studies in the control groups was NA | The mean pain at the end of intervention phase ‐ all studies in the intervention groups was 0.89 SDs lower (1.67 to 0.11 lower) | 248 | ⊕⊕⊝⊝ | SMD −0.89, 95% CI −1.67 to −0.11; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Pain at the end of intervention phase after excluding studies with CRPS | The mean pain at the end of intervention phase ‐ studies without CRPS in the control groups was NA | The mean pain at the end of intervention phase ‐ studies without CRPS in the intervention groups was 0.23 SDs lower (0.53 lower to 0.08 higher) | 176 (4 RCTs) | ⊕⊕⊕⊝ | SMD −0.23, 95% CI −0.53 to 0.08; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| Visuospatial neglect at the end of intervention: all studies | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the control groups was NA | The mean visuospatial neglect at the end of intervention phase ‐ all studies in the intervention groups was 1.06SDs higher (0.10 lower to 2.23 higher) | 175 | ⊕⊕⊝⊝ | SMD 1.06, 95% CI −0.10 to 2.23; as a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate, and 0.8 a large difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded due to several ratings in one or more items with high or unknown risk of bias. | |||||
| Study ID | Mean age | Sex | Side of paresis | Time since stroke | Type of stroke | |||
| Years | Women | Men | Left | Right | Mean time | Ischaemic | Haemorrhagic | |
| 68 | 22 | 18 | 16 | 24 | 5.3 days | 40 | 0 | |
| 50.9 | 9 | 15 | 15 | 9 | n/r | n/r | n/r | |
| 58.2 | 4 | 5 | 8 | 1 | 4.8 years | n/r | n/r | |
| 58.8 | 11 | 13 | 8 | 16 | 5.3 months | 24 | 0 | |
| 45.6 | 8 | 25 | 7 | 26 | 12.9 months/12.3 months. | 17 | 16 | |
| 46.4 | 6 | 30 | 16 | 20 | 15.9 months | 17 | 9 | |
| 53.9 | 7 | 13 | 13 | 7 | 4.6 months | 9 | 11 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 58.4 | 26 | 22 | 34 | 14 | 5 months | 35 | 13 | |
| 62 | 13 | 11 | 15 | 9 | 15.7 months | 19 | 5 | |
| 58.7 | 17 | 19 | n/r | n/r | 1.8 months | n/r | n/r | |
| 59.3 | 12 | 15 | 14 | 13 | 13.2 months/15.5 months | 17 | 10 | |
| 53.5 | 5 | 26 | 24 | 7 | 551 days | 23 | 8 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 56.5 | 10 | 26 | 25 | 11 | 27 days | 48 | 0 | |
| n/r | 3 | 3 | n/r | n/r | n/r | n/r | n/r | |
| 60.9 | 14 | 17 | 14 | 17 | 44.3 days | 25 | 6 | |
| 67.5 | 6 | 8 | 6 | 8 | 47 days | 9 | 5 | |
| 63.9 | 8 | 11 | 9 | 10 | 14.1 months | 10 | 9 | |
| 55.9 | 10 | 15 | 13 | 12 | 13.1 days | 16 | 9 | |
| 66.6 | 9 | 17 | 13 | 13 | 23 days | 26 | 0 | |
| 52.6 | 13 | 22 | 14 | 21 | 8.9 months | 19 | 16 | |
| 64.1 | 24 | 43 | 35 | 32 | 32.3 days | 28 | 39 | |
| 55.8 | 9 | 14 | 13 | 10 | 34.5 days | 14 | 9 | |
| 57.7 | 9 | 20 | 20 | 9 | 404.4 days | 14 | 15 | |
| 49.1 | 9 | 16 | 16 | 9 | n/r | 8 | 17 | |
| 69.1 | 3 | 10 | 5 | 8 | 78.8 days | 10 | 3 | |
| 57.3 | 8 | 22 | n/r | n/r | n/r | n/r | n/r | |
| 61.4 | 10 | 10 | 10 | 10 | n/r | n/r | n/r | |
| 57.1 | 11 | 15 | 11 | 15 | 3.6 months | n/r | n/r | |
| 54.7 | 13 | 14 | 8 | 19 | 39.6 months | 8 | 20 | |
| 64.9 | 21 | 39 | 31 | 29 | 52 days | 19 | 41 | |
| 55 | 11 | 32 | 22 | 21 | 19.6 months | 20 | 28 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 68.7 | 8 | 7 | 9 | 6 | 24.3 days | 10 | 5 | |
| 57 | 20 | 20 | 28 | 12 | 4.6 years | 28 | 12 | |
| 57.5 | 8 | 7 | 5 | 10 | 53.2 days | 15 | 0 | |
| 63 | 10 | 12 | 6 | 16 | 6.4 days | 14 | 8 | |
| 53.5 | 4 | 4 | 4 | 4 | 4.5 months | n/r | n/r | |
| 44.9 | 20 | 40 | n/r | n/r | 4.2 months | 60 | 0 | |
| 63.4 | 20 | 28 | 37 | 11 | 2 days | 26 | 22 | |
| 56.3 | 13 | 17 | 14 | 16 | 20.9 months | 16 | 14 | |
| 60 | 15 | 15 | 17 | 13 | 8.2 months | 17 | 13 | |
| 56 | 19 | 21 | 25 | 15 | 7.2 months | 27 | 13 | |
| 58 | 9 | 21 | 3 | 27 | 5 months | 20 | 10 | |
| 56.3 | n/r | n/r | n/r | n/r | 83.9 days | n/r | n/r | |
| 57.5 | 6 | 10 | 11 | 5 | 34.8 months | 16 | 0 | |
| 73.4 | 10 | 6 | 8 | 8 | 9.5 months | 16 | 0 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| 51.2 | 4 | 16 | 9 | 11 | 4.1weeks | 14 | 6 | |
| 63 | 13 | 19 | 15 | 17 | 50 days | 27 | 5 | |
| 51.4 | 22 | 18 | n/r | n/r | 4.0 months | n/r | n/r | |
| 63.4 | 17 | 23 | 27 | 13 | 3.7 months | 33 | 7 | |
| 63.7 | 9 | 6 | 6 | 9 | 32.7 days | n/r | n/r | |
| 67.2 | 25 | 35 | 37 | 23 | 45 days | 45 | 15 | |
| 64 | 34 | 60 | 56 | 38 | 29 days | 76 | 18 | |
| 64.9 | 40 | 50 | 39 | 51 | 63.7 days | 57 | 33 | |
| 54.2 | 10 | 23 | 18 | 15 | 20.6 months | 20 | 13 | |
| 63.3 | 17 | 19 | 21 | 19 | 5.5 months | 29 | 7 | |
| 57.8 | 10 | 16 | 15 | 11 | 22.7 days | 16 | 10 | |
| 63.3 | 21 | 39 | 31 | 29 | 25.8 days | 46 | 14 | |
| n/r | n/r | n/r | n/r | n/r | n/r | n/r | n/r | |
| n/r: not reported | ||||||||
| Study ID | Extremity | Mirror therapy variation | Control intervention | Type of movements | Minutes per session | Sessions per week | Total duration (weeks) | Total amount of therapy (minutes) | Setting |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Functional motor tasks (i.e. with objects); motor co‐ordination tasks; sensory discrimination tasks; grip strength; active range of motion | 20 to 30 | 7 | 2 | 280 ‐ 420 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities without mirror | n/r | 30 | 5 | 3 | 450 | Inpatient hospital | |
| Upper extremity | Bilateral activities | Bilateral activities; transparent plastic between limbs | Proximal and distal movements | 15 (2 times a day) | 12 | 4 (1st period) | 720 | n/r | |
| Upper extremity | Activities of the unaffected limb | 1. EMG‐triggered electrostimulation; | Wrist, hand flexion, extension and forearm circumduction, and supination–pronation | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy based on Brunnstrom and Bobath principles | Task‐based mirror therapy: finger dexterity, mass grasp/finger flexion, release/finger extension, wrist dorsiflexion, | 45 | 5 | 8 | 1800 | Inpatient hospital, home after discharge | |
| Lower extremity | Activities of the unaffected limb | Conventional motor therapy based on neurophysiological approaches | Activity‐based MT: ball‐rolling, rocker‐board and pedaling | 60 | n/r | 3 ‐ 4 (30 session) | 1800 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Activities of the non‐paretic arm, without mirror | Flexion/extension of the shoulder, radial/ulnar deviation and pro‐/supination of the forearm, flexion/extension of the fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper and lower extremity | Activities of the unaffected limbs | Routine programme (physiotherapy and neuromuscular stimulation) | Range of motion of the healthy limbs | 30 | 5 | 4 | 600 | n/r | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient and outpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror (control group 1); imagination of movements of the affected limb (control group 2) | Flexion/extension of shoulder, elbow and wrist; prone/supination forearm | 30 | Daily | 4 | 840 | Inpatient and outpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb + rTMS | Activities of the unaffected limb; covered mirror + rTMS | Flexing and extending the hip, knee, and ankle at a self‐selected speed under supervision but without additional verbal feedback | 20 | 5 | 4 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb + tDCS /anode attached over primary motor cortex | Activities of the unaffected limb; covered mirror + tDCS | Pronation, supination, flexion, and extension of both wrists, flexion and extension of the fingers, and flexion and extension of the elbows (10 sets, 20 repetitions per motion and set, 2 min rest between sets) | 20 | 3 | 6 | 360 | n/r | |
| Upper extremity | Activities of the unaffected limb | Passive mobilisation of the affected limb | Flexion and extension of shoulder, pronation and supination of forearm, gross and fine motor movements of wrist, hand and fingers (also with objects) | 45 | 3 | 8 | 1080 | Outpatient rehabilitation centre | |
| Upper extremity | 10 Hz TMS applied by 8‐coil on the ipsilesional somatosensory cortex, followed by MT | TMS only | n/r | 30 | 3 | 4 | 360 | n/r | |
| Upper extremity | Bilateral activities | Bilateral activities; without mirror | Execution of arm, hand and finger postures | 30 | 5 | 6 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral and unilateral activities | Traditional occupational therapy | n/r | 30 | 5 | 6 | 900 | Home setting | |
| Upper extremity | Activities of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion and extension of wrist and finger | 20 | 5 | 4 | 400 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | No additional therapy | Supination and eversion of the forearm, flexion and extension of the wrist and finger, grasp a block | 30 | 6 or 7 | 4 | 720 ‐ 840 | Inpatient Hospital | |
| Upper extremity | Bilateral activities; virtual mirror on a screen; arm projected by a camera | Bilateral activities; without mirror (screen was off) | 1st week: wrist flexion/ extension, forearm pro‐/supination, clenching and opening the hand, 2nd week gross motor tasks, 3rd and 4th week fine motor tasks; 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Lower extremity | Uni‐ and bilateral activities; virtual mirror on the screen, leg projected by a camera | Uni‐ and bilateral activities; without mirror (screen was off) | 1st week: dorsiflexion and plantarflexion (lifting of the heel) of the unaffected ankle; adduction and abduction of forefoot and rear foot; and adduction and abduction of the hip (moving the knees inward and outward), 2nd week mimicked the movements (1st week) of the unaffected lower limb on the monitor with the affected lower limb, 3rd dorsiflexion, adduction and abduction of the unaffected ankle; plantar flexion, adduction and abduction of the ankle; and adduction and abduction of the hip; 4th week: complex movements and different tasks (remote control with up and down buttons); 3 sets of 10 repetitions, comfortable speed of movement, supervision of caregivers, using checklist | 30 | 5 | 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Movements of the unaffected limb | Movements of the unaffected limb; covered mirror | Flexion/extension of shoulder, elbow and wrist, pro‐ /supination of the forearm, self selected speed, no additional verbal feedback | 30 1st 2 weeks; 60 last 2 weeks | 5 | 4 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: MT: Movements of the unaffected limb + rTMS; Experimental 2: MT: Movements of the unaffected limb | Activities of the unaffected limb, covered mirror | Experimental 1: finger flexion and extension + 10Hz rTMS on lesioned hemisphere; | 15 | 5 | 6 | 450 | University hospital | |
| Lower extremity | Bilateral activities and activities of the unaffected limb | 4 control groups: (1) EMG triggered electrical muscle stimulation; (2) electrical muscle stimulation; (3) repetitive facilitation exercises; (4) passive and active‐assistive range of motion exercises | Dorsiflexion of the ankle joint, stepping over, and abduction/adduction of the hip joint) | 20 | 7 | 4 | 560 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities + FES | Bilateral activities + FES; covered mirror | Extension of wrist and fingers to lift of the hand from an FES switch, at the same time attempt to extend affected hand supported by electrical stimulation (20 Hz), pulse rate 300 μs, individual intensity for muscle contraction and complete extension | 30 | 5 | 4 | 600 | University hospital | |
| Upper extremity | Bilateral activities + FES | No additional therapy | 2 experimental groups: (1) EMG‐triggered FES (due to unaffected limb) of affected wrist extension + physiological and object‐related movements; (2) FES of affected wrist extension + physiological and object‐related movements | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | Conventional therapy | Arm bicycling, peg board exercise, skateboard‐supported exercises on a tabletop, donut on base putty kneading, double curved arch, bimanual placing cone, block stacking, graded pinch exercise, plastic cone stacking, shoulder curved arch | 30 | 5 | 4 | 300 | Outpatient hospital | |
| Upper extremity | Bilateral activities + EMTS | No additional therapy | Extension of wrist and fingers to reach EMG threshold on 50 ‐ 70% of maximum wrist extension, neuromuscular stimulation 10 seconds symmetrical biphasic pulses at 50 Hz, pulse width 200 μs, followed by 20 seconds of rest to assist full range of motion; bimanual wrist and finger extension during 'on' and 'off' period, difficulty of exercises dependent upon participants’ levels of functioning with regard to wrist and finger flexion and extension or thumb opposition | 20 (2 times a day) | 5 | 4 | 800 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | No additional therapy | Flexion/ extension of the knee and ankle; self‐selected speed; under supervision | 2 times daily for 15 minutes | 5 | 2 | 300 | n/r | |
| Upper extremity | n/r | No additional therapy | Wrist extension | 4 times daily for 15 minutes | 5 | 4 | 1200 | n/r | |
| Upper extremity | Bilateral activities | No additional therapy | Lifting both arms, flexion/ extension of the elbow, pronation of the forearm, wrist extension, internal/ external rotation of the wrist, clenching and opening the fist, tapping on the table; self‐performed; supervision of a guardian | 2 times daily for 25 minutes | 5 | 4 | 1000 | Inpatient rehabilitation ward | |
| Lower extremity | Bilateral activities + NMES | Conventional therapy | Dorsiflexion movements of the ankle | n/r | 5 | 4 | n/r | Rehabilitation hospital | |
| Upper extremity | Bilateral activities | Bilateral activities, covered mirror | Task‐oriented MT: forearm pronation‐supination and wrist flexion/extension, finger flexion‐extension, counting numbers, tapping, and opposing; simple manipulating tasks (such as picking up coins and beans, flipping over cards); complicated tasks (plugging and unplugging pegboards, drawing simple figures, and colouring) | 20 | 5 | 4 | 400 | Inpatient rehabilitation ward | |
| Upper extremity | Experimental 1: MT: Bilateral activities; Experimental 2: MT and sensory electrical stimulation by a mesh‐glove | Task‐oriented training | Transitive movements (e.g. gross motor tasks, such as reaching out to put a cup on a shelf, or fine motor tasks, such as picking up marbles); intransitive movements (e.g. gross motor movements, such as pronation and supination, or fine motor movements, such as finger opposition) | 60 | 5 | 4 | 1200 | In‐ and outpatient setting | |
| Upper extremity | n/r | n/r; transparent plastic between limbs | n/r | n/r | n/r | 4 | n/r | Home | |
| Lower extremity | Bilateral activities | 1: Bilateral activities, covered mirror; | Alternate dorsiflexion and plantarflexion in both ankles as best as possible, self‐paced speed | 15 | 5 | 3 | 225 | Inpatient rehabilitation unit | |
| Upper extremity | Bilateral activities | Bilateral activities | Exercises based on the Brunnstrom phases of motor recovery; functional tasks (i.e. with objects) | 60 | 1 (under supervision) + 5 (at home) | 6 | 2160 | Home | |
| Upper extremity | Bilateral activities | No additional therapy | Flexion and extension of shoulder, elbow, wrist and finger, prone‐supination of the forearm | 30 | 5 | 6 | 900 | Inpatient | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Lying position: hip‐knee‐ankle flexion, with the hip and knee placed in flexion, moving the knee inward and outward, hip abduction with external rotation followed by hip adduction with internal rotation; sitting position: Hip‐knee‐ankle flexion, knee extension with ankle dorsiflexion, knee flexion beyond 90 °; each exercise 2 sets of 10 repetitions | 60 | 6 | 2 | 720 | Inpatient rehabilitation | |
| Upper extremity | Bilateral activities | Landscape images were shown to participants, they should try to describe the images, without movements | Finger and hand movements | 30 | 5 | 1 | 150 | n/r | |
| Upper extremity | Bilateral activities | functional electrical stimulation, covered mirror | Experimental 1: wrist and finger extension, grasping and releasing a bottle; Experimental 2: combined MT and functional electrical stimulation | 30 | 6 | 2 | 360 | Hospital | |
| Upper extremity | Bilateral activities, therapist supported if patients were not able to move paretic limb | Bilateral activities, covered mirror | Flexion and extension movements of wrist and fingers | 60 | 5 | 4 | 1200 | inpatient rehabilitation and home training after discharge | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Pronation and supination of the forearm and the flexion and extension movements of the wrist and fingers; 5 sets each motion, 30 repetitions per set | 30 | 5 | 4 | 600 | Inpatient | |
| Upper extremity | Activities of the unaffected limb | Activities of the unaffected limb, non‐reflecting surface | Task‐oriented activities consisted with reaching, grasping, lifting and releasing objects | n/r | 5 | 6 | n/r | Rehabilitation unit | |
| Upper extremity | Not stated | Same tasks; covered mirror | Task‐oriented activities consisting of grasping and releasing objects | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | bilateral activities | Same tasks; covered mirror | Finger and wrist movements, grasping different objects | 30 | 5 | 4 | 600 | Nursing homes | |
| Upper extremity | Bilateral activities | Motor relearning programme | Hand‐opening, wrist flexion/ extension, forearm pronation/ supination, hand sliding on surface | n/r | 6 | 4 | n/r | Outpatient | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | Task‐orientend activities consisted with manipulating objects | 60 | 3 | 4 | 720 | Home | |
| Upper extremity | Bilateral activities (hypotone muscles); unilateral activities (hypertone muscles) | Bilateral activities; without mirror | Gross motor arm and hand movements; functional activities (i.e. with objects); fine motor activities (i.e. with objects) | 30 | Total number of sessions: 17 | 5 | 510 | Outpatient centre | |
| See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | See Rothgangel 2004a | 30 | Total number of sessions: 37 | 5 | 1110 | Inpatient rehabilitation centre | |
| Lower extremity | MT + Electrical stimulation | Conventional therapy | n/r | 50 | 4 | 2 | 400 | n/r | |
| Upper extremity | Activities of the unaffected limb | No additional therapy | Wrist flexion, extension, radial and ulnar deviation, circumduction, fisting, releasing, abduction, and adduction of all fingers; activities such as squeezing a ball, stacking rings, flipping cards, placing pegs on a board | 2 times for 30 | 5 | 3 | 900 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Electromyographic‐triggered muscular electrical stimulation | Grasping movements in combination with electromyographic‐triggered muscular electrical stimulation | 30 | 5 | 3 | 450 | 3 inpatient rehabilitation centres | |
| Upper extremity | Activities of the unaffected limb | No therapy | 5 movements of wrist and fingers, each 6 minutes | 30 | 5 | 4 | 500 | Inpatient rehabilitation centre | |
| Lower extremity | Activities of the unaffected limb | Activities of the unaffected limb; covered mirror | Dorsiflexion movements of the ankle | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb; affected limb passively moved by therapist | Activities of the unaffected limb; affected limb passively moved by therapist; without mirror | 13 kinds of movements, i.e. flexion/extension of wrist, pinching fingers, gripping ball | 10 to 15 | Daily | 4 (1st period) | 280 to 420 | Inpatient rehabilitation centre | |
| Upper extremity | Bilateral activities | Bilateral activities; covered mirror | 1st week: isolated movements of fingers, wrist, lower arm, elbow and shoulder in all degrees of freedom, up to 50 repetitions per series, up to 4 series; | 30 | 3 ‐ 5 | 4 ‐ 5 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Not stated; self‐performed, daily checking by therapist | Lower limb activities; without a mirror | n/r | 30 | 5 | 4 | 600 | 12 inpatient stroke services | |
| Upper extremity | n/r | 1: no additional therapy; | n/r | n/r | n/r | n/r | n/r | n/r | |
| Upper extremity | Bilateral activities | Usual occupational therapy | Transitive movements: fine motor tasks of squeezing sponges, placing pegs in holes, flipping a card, gross motor tasks (reaching out for touch); intransitive movements (repetitive wrist flexion/extension, finger opposition, forearm pro‐/supination) | 60 | 5 | 4 | 1200 | 4 hospitals | |
| Upper extremity | Bilateral activities | Bilateral activities; nonreflecting side of the mirror | Flexion/extension of wrist and fingers | 30 | 5 | 4 | 600 | Inpatient rehabilitation centre | |
| Upper extremity | Activities of the unaffected limb | 1: constraint induced movement therapy (6 hours/day) + palliative rehabilitation programme + self‐exercise; | Flexion/extension of the shoulder, elbow, wrist, finger, and pronation/supination of the forearm | 30 | 5 | 2 | 300 | Inpatient rehabilitation centre | |
| Upper extremity | Experimental 1: activities of the unaffected limb Experimental 2: activities of the unaffected limb and additionally neuromuscular electrical stimulation of the affected arm | Neuromuscular electrical stimulation of finger and wrist extensors of the affected arm | Flexion/extension of wrist and fingers | 30 | 5 | 3 | 450 | Inpatient rehabilitation centre | |
| n/r | n/r | n/r | n/r | 30 | Total: 20 ‐ 24 | 8 | 600 ‐ 720 | n/r | |
| EMG: electromyography | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All outcome measures | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 2 Motor impairment at the end of intervention phase Show forest plot | 39 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All outcome measures | 39 | 1292 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.32, 0.66] |
| 3 Fugl‐Meyer Assessment upper extremity at the end of intervention phase Show forest plot | 28 | 898 | Mean Difference (IV, Random, 95% CI) | 4.32 [2.46, 6.19] |
| 4 Activities of daily living at the end of intervention phase Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All outcome measures | 19 | 622 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.30, 0.65] |
| 5 Pain at the end of intervention phase Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 All outcome measures | 6 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.67, ‐0.11] |
| 6 Visuospatial neglect at the end of intervention Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 All outcome measures | 5 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.10, 2.23] |
| 7 Motor function at follow‐up after 6 months Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All outcome measures | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.78, 3.18] |
| 8 Motor impairment at follow‐up after 6 months Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 All outcome measures | 3 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.26, 1.12] |
| 9 Dropouts at the end of intervention phase Show forest plot | 42 | 1438 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.74, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | 1173 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 1.1 Mirror therapy for the upper extremity | 31 | 1048 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.23, 0.69] |
| 1.2 Mirror therapy for the lower extremity | 5 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 36 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.29, 0.72] |
| 1.1 Studies that used a covered mirror in the control group | 16 | 506 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.36, 0.99] |
| 1.2 Studies that used unrestricted view in the control group | 14 | 474 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.59] |
| 1.3 Studies that used no additional control intervention | 8 | 219 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.02, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention phase Show forest plot | 32 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.22, 0.66] |
| 1.1 All studies including participants within 6 months after stroke | 18 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.18, 0.73] |
| 1.2 All studies including participants with more than 6 months after stroke | 14 | 398 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.06, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Motor function at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies without randomised cross‐over trials | 35 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.68] |
| 1.2 All studies with adequate sequence generation | 33 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.19, 0.54] |
| 1.3 All studies with adequate concealed allocation | 16 | 572 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.47] |
| 1.4 All studies with adequate handling of incomplete outcome data | 12 | 388 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.14, 0.95] |
| 1.5 All studies with blinded assessors | 25 | 820 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.17, 0.70] |
| 2 Motor impairment at the end of intervention Show forest plot | 36 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies with adequate sequence generation | 36 | 1157 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.29, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at the end of intervention Show forest plot | 4 | 176 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.08] |

