L'irrigation saline pour la prise en charge des lésions cutanées causées par des extravasations chez les nouveaux‐nés
Résumé scientifique
Contexte
Les lésions par extravasation sont une complication fréquente dans les unités de soins intensifs néonataux, qui peuvent entrainer des séquelles esthétiques et fonctionnelles. Un large éventail de traitements sont disponibles, dont l'irrigation saline sous‐cutanée (avec ou sans hyaluronidase), la liposuccion, l'utilisation d'antidotes spécifiques, des applications topiques, et les soins de plaie habituels avec des pansements secs ou humides. Tous ces traitements visent à prévenir ou à réduire la gravité des complications.
Objectifs
Objectif principal
Comparer l'efficacité et l'innocuité de l'irrigation saline, avec ou sans injection préalable de hyaluronidase, par rapport à l'absence d'intervention ou par rapport aux soins de plaie habituels pour la cicatrisation des tissus chez les nouveau‐nés ayant des lésions par extravasation.
Objectifs secondaires
Évaluer au moyen d'une analyse en sous‐groupe des essais contrôlés l'influence du type d'extravasation, du timing de l'irrigation suite à l'extravasation, et de l'âge post‐menstruel (APM) du nouveau‐né au moment de la lésion sur les résultats et les effets indésirables.
Plus spécifiquement, nous avions prévu de procéder à une analyse en sous‐groupes pour le critère de jugement principal, en examinant, le cas échéant :
1. Le temps écoulé avant l'irrigation des lésions par extravasation identifiées (< 1 heure ou ≥ 1 heure) ;
2. Le type d'extravasation (liquides de nutrition parentérale ou autres liquides ou médicaments) ;
3. La quantité de solution saline utilisée (< 500 ml ou ≥ 500 ml) ; et
4. L'APM au moment de l'incident (< 37 semaines révolues ou ≥ 37 semaines révolues).
Stratégie de recherche documentaire
Nous avons utilisé la stratégie de recherche standard du groupe de revue Cochrane sur la néonatologie pour consulter le registre Cochrane des essais contrôlés (CENTRAL, 2017, numéro 1), MEDLINE via PubMed (de 1966 au 2 février 2017), Embase (de 1980 au 2 février 2017), et le Cumulative Index to Nursing and Allied Health Literature (CINAHL ; de 1982 au 2 février 2017). Nous avons également effectué des recherches dans des bases de données d'essais cliniques, dans des actes de conférence et dans les références bibliographiques des articles identifiés pour identifier des essais contrôlés randomisés et des essais quasi‐randomisés. Nous avons utilisé l'outil de recherche Google Scholar pour identifier les articles pertinents ayant cité les manuscrits sélectionnés.
Critères de sélection
Les essais contrôlés randomisés (ECR) et les essais contrôlés quasi‐randomisés comparant l'irrigation saline, avec ou sans injection de hyaluronidase par rapport à l'absence d'intervention ou aux soins de plaie habituels pour la prise en charge des lésions par extravasation chez les nouveaux‐nés.
Recueil et analyse des données
Trois auteurs de la revue ont indépendamment passé en revue et identifié des articles pour éventuelle inclusion dans cette revue. Nous avons utilisé l'approche GRADE pour évaluer la qualité des preuves.
Résultats principaux
Nous n'avons trouvé aucune étude éligible. Notre recherche a révélé 10 rapports de cas ou séries de cas décrivant des résultats positifs avec différentes interventions pour cette affection.
Conclusions des auteurs
À ce jour, aucun ECR n'a examiné les effets de l'irrigation saline, avec ou sans injection préalable de hyaluronidase pour la prise en charge des lésions par extravasation chez les nouveaux‐nés. L'irrigation saline est fréquemment rapportée dans la littérature en tant qu'intervention pour la prise en charge des lésions par extravasation chez les nouveaux‐nés. Les recherches devraient tout d'abord évaluer l'efficacité et l'innocuité de cette intervention au moyen d'ECR. Il sera également important pour les chercheurs de déterminer l'ampleur de l'effet en examinant le timing de l'intervention, la nature des liquides perfusés, et la gravité de la lésion au moment de l'intervention.
PICO
Résumé simplifié
L'irrigation saline pour la prise en charge des lésions de la peau causées par des extravasations chez les nouveaux‐nés
Question de la revue : Quel est l'effet de l'irrigation saline, avec ou sans utilisation préalable de hyaluronidase par rapport à l'absence d'intervention ou par rapport aux soins de plaie habituels au niveau de la cicatrisation des tissus chez les nouveau‐nés ayant des lésions causées par des extravasations ?
Contexte : Les nouveaux‐nés prématurés et les enfants nés à terme et malades ayant besoin de liquides intraveineux et de médicaments sont exposés à des risques de lésion de la peau suite à des extravasations, c'est‐à‐dire l'écoulement de liquide dans les tissus biologiques environnants. Ces lésions peuvent entraîner des cicatrices ayant des conséquences au niveau esthétique et, chez certains nouveaux‐nés affecter négativement leur capacité fonctionnelle. Une intervention chirurgicale corrective peut être nécessaire pour certains bébés. L'irrigation saline, avec ou sans injection préalable de hyaluronidase (une protéine qui favorise la dégradation des barrières qui maintiennent ensemble les différentes couches de tissus), est largement utilisée pour la prise en charge des graves lésions par extravasation chez les nouveaux‐nés et vise à prévenir ou à réduire les complications dues aux extravasations. Un traitement conservateur impliquant des soins de plaie habituels et divers pansements appliqués sur la peau, est couramment utilisé face à différents stades de lésions par extravasation et mène à des résultats variables.
Caractéristiques des études : Nous n'avons pas trouvé d'études randomisées ou quasi‐randomisées de haute qualité permettant actuellement de répondre à cette question.
Principaux résultats : A ce jour, aucun essai contrôlé randomisé n'a examiné les effets de l'irrigation saline, avec ou sans injection préalable de hyaluronidase, pour la prise en charge des lésions par extravasation chez les nouveaux‐nés. De nombreux rapports dans la littérature indiquent que l'irrigation saline est utilisée pour la prise en charge des lésions par extravasation chez les nouveaux‐nés. Les recherches devraient tout d'abord évaluer l'efficacité et l'innocuité de cette intervention au moyen d'essais contrôlés randomisés. Il serait également important de déterminer l'ampleur de l'effet en examinant le timing de l'intervention, la nature du soluté perfusé, et la gravité de la lésion au moment de l'intervention.
Qualité des preuves : Nous avons obtenu des preuves de très faible qualité issues de séries de cas ou de rapports.
Authors' conclusions
Summary of findings
| Saline irrigation with or without prior infiltration of hyaluronidase vs standard wound care for management of extravasation injury in neonates | ||||||
| Patient or population: neonates requiring management of extravasation injury | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard wound care | Risk with saline irrigation with or without prior infiltration of hyaluronidase | |||||
| Complete wound healing after saline irrigation with or without prior hyaluronidase infiltration (wound healing): clinical assessment | Study population | Not estimable | 237 | ⊕⊝⊝⊝ | Pooled data for 237 neonates from 10 case report studies who underwent saline irrigation. Three neonates received further surgical intervention. For 234 neonates, complete wound healing was reported | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| All 10 studies analysed in GRADE pro GDT are case reports, and the intervention methods mentioned are highly variable. In view of this, "relative effect (95% CI)" and "absolute effect (95% CI)" were not derived. | ||||||
Background
Description of the condition
Many preterm and sick term infants in neonatal intensive care units (NICUs) require intravenous nutrition and medication as part of their care and are particularly vulnerable to extravasation (leakage of fluid into surrounding tissue) injury. Extravasation or infiltration of intravenous fluid into surrounding tissue is known to occur at some point in up to 70% of neonates undergoing intensive care (Irving 1999). A survey of regional NICUs in the United Kingdom (UK) reported the prevalence of extravasation injury resulting in skin necrosis (local tissue death) as 38/1000 neonates, with 70% of these injuries occurring in infants at 26 weeks' gestation or less (Wilkins 2004). About 4% of infants leave the NICU with cosmetically or functionally significant scars caused by extravasation injury (Wilkins 2004). Extravasation of intravenous infusion into the interstitial space may result from displacement of the intravascular catheter or increased vascular permeability. Some medications and infusions are more toxic to the veins than others. The mechanism of extravasation necrosis is not completely understood, but the degree of damage appears to be related to osmolality, pH, and dissociability of ions within the infusate (intravenous fluid) (Davies 1994; Goutos 2014).
Depending on the nature and volume of the infusate, extravasation may go unnoticed or may cause severe inflammation. In the short term, this may lead to partial or complete skin loss, infection, nerve or tendon damage, and, infrequently, compartment syndrome leading to amputation of the affected limb (Gault 1993). Scarring leading to contractures and disfigurement, functional loss of the affected part, complex regional pain syndrome (Hadaway 2007), and major limb deformities (Fullilove 1997) are well known long‐term sequelae (Cartlidge 1990). It has been reported that 70% of scars sustained by minor extravasation injury disappeared by eight years of age, and that more serious but less prominent scars were still seen at eight years of age (Fox 1998).
Researchers have classified extravasation injury into four stages (Millam 1988; Flemmer 1993; McCullen 2006).
-
Stage 1: painful intravenous site, no erythema and swelling, flushes with difficulty.
-
Stage 2: painful intravenous site, slight swelling, redness, no blanching, brisk capillary refill below infiltration site, good pulse volume below infiltration site.
-
Stage 3: painful intravenous site, marked swelling, blanching, cool to touch, brisk capillary refill below infiltration site, good pulse volume below infiltration site.
-
Stage 4: painful intravenous site, very marked swelling, blanching, cool to touch, capillary refill longer than four seconds, decreased or absent pulse, skin breakdown or necrosis.
The best management approach is preventative and requires hypervigilant monitoring of the intravenous site (Patnaik 2004). However, once extravasation has occurred, the best practice for reducing tissue damage and its consequent complications is a topic of controversy. Available treatments include irrigation with saline (with or without prior infiltration of hyaluronidase), liposuction, use of specific antidotes, various topical applications, and normal wound management with dry or wet dressings (Ramasethu 2004). Successful outcomes without sequelae have been reported with multiple needle puncture of the extravasation site, followed by gentle squeezing (Chandanvasu 1986). Various topical applications such as silver sulphadiazine cream (Friedman 1998); silver sulphadiazine ointment, topical povidone‐iodine ointment, and saline wash (Brown 1979); topical application of silver sulphadiazine and paraffin tulle (Kumar 2001); hydrocolloid gel (Thomas 1997; Sawatzky‐Dickson 2006); fibrinolysin and deoxyribonuclease ointment (Falcone 1989); and a combination of antibacterial ointment, sesame oil, and an herbal mixture (Cho 2007) have all been reported to be effective. In a tense extravasation injury, gentle massaging of the affected limb to allow good capillary flush has been found to be beneficial (Davidson 1985). Infiltration of 'recombinant human hyaluronidase' (rHuPH20) into the extravasation injury site has been reported (Kuensting 2010). Topical application of maternal platelet‐rich plasma (PRP) has been shown to produce encouraging results for a necrotic extravasation wound (Lee 2013). Use of topical hyalomatrix PA over the eschar wound that developed after extravasation injury and was treated with saline irrigation resulted in restoration of re‐epithelialisation and dermal neoformation (Onesti 2012). It has been suggested that the effectiveness of the intervention may depend on the injury‐intervention interval (Casanova 2001), or the necrosis interval (Goutos 2014). Limb elevation has proved effective when extravasation resulted in erythema and swelling (Reynolds 2007). The stage of injury at the time of intervention may be important, and it has been suggested that saline irrigation for stage 3 or 4 extravasation may prevent further damage (Sawatzky‐Dickson 2006; Kostogloudis 2015). Non‐pharmacological intervention measures have been described in a recently published paper (Reynolds 2014).
Description of the intervention
Subcutaneous irrigation for extravasation injury is carried out by the 'flush‐out technique’, which was first proposed by David Gault in 1993 (Gault 1993). Dilute hyaluronidase is infiltrated into the affected area following local anaesthetic infiltration under aseptic conditions. Four small stab incisions are made around the periphery of the extravasated area. Saline is then infiltrated into the subcutaneous tissue in small volumes of 20 to 50 mL (Gault 1993) with a blunt‐ended needle with a side hole, so it dilutes the extravasate and comes out freely through exit stab incisions. A total of 500 mL of saline is recommended for the ‘flush‐out’ (Gault 1993; Davies 1994; Harris 2001). Residual fluid is then manipulated down to the exit holes and is expressed. After flush‐out, a wound dressing is applied, and the limb is elevated for 24 hours.
Although this is the standard procedure described by Gault, investigators have noted wide variation in the flush‐out process, the dosage of hyaluronidase infiltrated (Bruera 1999), and the total volume of saline used for irrigation. The published literature does not provide specific guidance on the time interval allowed between injection of hyaluronidase and subsequent saline flush‐out. However, most reports mention that flush‐out should be undertaken soon after hyaluronidase infiltration.
How the intervention might work
Immediate saline irrigation with or without hyaluronidase is used increasingly in neonatal units across the world. The idea is that saline irrigation will dilute the extravasate and flush it out from the local area as quickly as possible. This technique is theoretically possible in neonates, who have very little subcutaneous fat.
Hyaluronidase is an enzyme that hydrolyses the mucopolysaccharides present in subcutaneous connective tissue. Hyaluronidase reversibly hydrolyses hyaluronic acid polymers within the extracellular space (Jaworski 1950). This is thought to enhance permeability within the subcutaneous tissue compartment while allowing diffusion of the extravasate through larger areas in the subcutaneous space, which then can be flushed out more easily by saline irrigation (PPAG 2005).
Why it is important to do this review
Extravasation injury is associated with serious morbidity in neonates. In the short term, this injury may cause pain, tissue necrosis, ulceration, and infection. Extravasation may prevent infusion of needed medications, which in itself can cause dire consequences. In the medium to long term, this may lead to scar formation with disfigurement and functional impairment. If effective, saline irrigation with or without hyaluronidase infiltration may reduce this morbidity. However, the intervention is highly invasive and involves a great deal of manual handling of the sick and vulnerable neonate. It can lead to pain, hypothermia, infection, and cardiorespiratory destabilisation. The multiple stab incisions integral to the procedure may result in scar formation. It is important to establish if, for the short and long term, this form of intervention is superior to conservative wound management.
Objectives
Primary objective
To compare the efficacy and safety of saline irrigation or saline irrigation with prior hyaluronidase infiltration versus no intervention or normal wound care for tissue healing in neonates with extravasation injury.
Secondary objective
To evaluate by subgroup analysis of controlled trials the influence of type of extravasate, timing of irrigation following extravasation, and postmenstrual age (PMA) of the neonate at the time of injury on outcomes and adverse effects.
Specifically, we planned to perform subgroup analysis for the primary outcome, if appropriate, by examining:
-
time to irrigation from identified extravasation injury (< 1 hour or ≥ 1 hour);
-
type of extravasate (parenteral nutrition fluid or other fluids or medications);
-
amount of saline used (< 500 mL or ≥ 500 mL); and
-
PMA at injury (< 37 completed weeks or ≥ 37 completed weeks).
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised controlled trials comparing saline irrigation, with or without hyaluronidase infiltration, versus no intervention or normal wound care only. We considered as eligible relevant studies published only as abstracts in conference proceedings, and we contacted principal trial authors for further details. We applied no language restrictions.
Types of participants
Neonates of any gestational age at birth (defined as up to 28 days of postnatal age at enrolment) with extravasation of intravenous infusate from peripheral or central venous catheters, when extravasation has led to any one of the following.
-
Moderate swelling and erythema of the overlying area.
-
Blanching or discolouration of the overlying skin.
-
Pressure symptoms (capillary refill longer than four seconds and reduced or absent pulse below the site of extravasation).
-
Ulceration of the overlying skin.
Types of interventions
Irrigation therapy is defined as any intervention by which saline is instilled into the site of extravasation, with the aim of diluting the extravasate, followed by provision for it to drain through the skin incisions. Any prior infiltration with hyaluronidase and its dosage are also recorded.
Normal wound care is defined as care involving no irrigation or other invasive interventions but including wound care with dry or wet dressings and wound debridement if clinically indicated.
Types of outcome measures
Primary outcomes
Complete tissue healing (defined as no residual breach of skin or inflammatory signs) without scar formation at discharge or latest assessment, preferably at least three months from the time of injury, as complete wound healing is expected by this time.
Secondary outcomes
Short‐term outcomes
-
Time to complete tissue healing.
-
Infection, defined as at least one of the following.
-
Purulent discharge from the site.
-
Organisms isolated from an aseptically obtained culture of fluid or tissue derived from the site.
-
Clinical diagnosis made by the physician requiring topical or systemic antibiotics.
-
-
Pain during the intervention (measured on validated neonatal pain scales).
-
Hypothermia during or at the end of the intervention (defined as temperature < 36°C or a drop of 2°C from baseline measurement).
-
Anaphylactic reactions.
-
Fluid and electrolyte status.
-
Any other adverse effects reported by trial investigators.
Long‐term outcomes
These include the following outcomes as measured in the latest assessment, preferably at least three months after injury.
-
Severity of the scar, as measured by a quantifiable validated scar scale. Scar measurement scales could include, but are not limited to, the Vancouver Scar Scale, a visual analogue scale, the Patient and Observer Scar Assessment Scale, and the Manchester Scale.
-
Contracture – permanent tightening of non‐bony tissues such as muscles, tendons, ligaments, or skin, with or without restriction of joint motion (dichotomous outcome).
-
Functional impairment – restriction of function of the injured body part, as reported by caregivers or by the physician (dichotomous outcome).
-
Disfigurement ‐ substantial scarring that is cosmetically offensive, as measured by caregivers or by the reporting physician (dichotomous outcome).
-
Need for additional surgical procedures.
Search methods for identification of studies
We used the criteria and standard methods of the Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal search strategy for the specialised register).
Electronic searches
We conducted a comprehensive search of the following databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library; MEDLINE via PubMed (1966 to 2 February 2017); Embase (1980 to 2 February 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 2 February 2017). We used the following search terms: (extravasation OR ((inflammation OR swelling OR edema OR erythema) AND catheter* OR intravenous fluid*)) AND (saline OR hyaluronidase OR hyaluronoglucosaminidase OR irrigation), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategy for each database). We removed language restrictions during the search update for this review.
We searched clinical trial registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Clinical Trials Registry and Platform ‐ www.whoint/ictrp/search/en/; and the ISRCTN Registry ‐ www.isrctn.com).
Ms Jennifer Spano from the Cochrane Neonatal Review Group performed a parallel search; Figure 1 presents the search flow diagram.

Study flow diagram: review update.
We performed a 'reverse citation' search of relevant articles using Google Scholar.
See Appendix 2 for searches run for the previously published review.
Searching other resources
We reviewed reference lists from the above articles and from review articles on the topic. When relevant, we attempted to communicate with primary authors of the studies above to identify unpublished data.
We searched the following.
-
Proceedings of the Nutrition Society: Nestle Foundation (2004).
-
Proceedings of summer meetings of the British Association of Plastic Surgeons (2001 to 2010).
-
Proceedings of annual conferences of the European Society for Paediatric Research (2001 to 2010).
-
Proceedings of the Pediatric Academic Societies (2001 to 2010).
We retrieved one abstract from the abstract archives of the Pediatric Academic Societies meetings and found that it was not relevant to this review (Valente 2001).
We could not retrieve in full one abstract from the Proceedings of the British Association of Plastic Surgeons Summer Meeting held in July 2001. Therefore, we contacted the primary author of the presentation (Moss 2001) by email. The primary author replied to inform us that currently he has no access to the abstract.
Data collection and analysis
We used standardised methods of the Cochrane Collaboration and the Cochrane Neonatal Review Group.
Selection of studies
Three review authors independently reviewed all identified articles for possible inclusion in this review. In the event of disagreement on the suitability of a trial for inclusion, we reached consensus by discussion.
Data extraction and management
Each review author extracted data separately using pre‐designed data extraction forms; we then compared results. However, we identified no suitable randomised controlled trial (RCT) or quasi‐RCT for further analysis.
Assessment of risk of bias in included studies
Two review authors (NG, SB) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We planned to use standardised statistical methods of the Cochrane Collaboration. For categorical data, we aimed to calculate risk ratio (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), and number needed to treat for an additional harmful outcome (NNTH). For continuous data, we aimed to calculate weighted mean difference (WMD). We planned to report the 95% confidence interval (CI) for all estimates.
Unit of analysis issues
We planned to analyse results reported for individual infants.
Dealing with missing data
We planned to contact principal authors when data were missing; as the search for this review yielded only case reports or series, we did not need to do so.
Assessment of heterogeneity
We planned to undertake an assessment of heterogeneity, if applicable, by using the I2 statistic, and to consider 25% as low and 75% as high heterogeneity.
Assessment of reporting biases
This assessment was not applicable.
Data synthesis
Quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: complete wound healing with no functional and cosmetic complications.
Two review authors (PG, NG) independently assessed the quality of evidence for each of the outcomes above. We planned to consider evidence from RCTs as high quality but to downgrade the evidence by one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
-
High. We are very confident that the true effect lies close to the estimate of effect.
-
Moderate. We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
-
Low. Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
-
Very low. We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis, if appropriate, for the primary outcome by examining:
-
time to irrigation from identified extravasation injury (< 1 hour or ≥ 1 hour);
-
type of extravasate (parenteral nutrition fluid or other fluids OR medications);
-
amount of saline used (< 500 mL or ≥ 500 mL); and
-
corrected gestation at injury (< 37 completed weeks or ≥ 37 completed weeks).
However, we did not undertake this analysis, as we identified no suitable data for analysis.
Sensitivity analysis
This was not applicable.
Results
Description of studies
By applying the search strategy, we identified no RCTs or quasi‐RCTs for this review, but we found several case reports, case series, and cohort studies. As we retrieved no RCTs or quasi‐RCTs for the intended study age group, we repeated the search with no age restrictions and still did not identify any relevant studies that matched our inclusion criteria.
Results of the search
We identified one relevant case report published since the original review was presented in 2012 (Kostogloudis 2015).
Included studies
We found no eligible RCTs or quasi‐RCTs and included no studies in this review.
Excluded studies
Ten studies identified by the search were not eligible for inclusion but have been used for discussion and are mentioned below under additional tables (Gault 1993; Davies 1994; Martin 1994; Chen Y Y 1996; Casanova 2001; Harris 2001; Chowdhury 2004; Siu 2007; Kuensting 2010; Kostogloudis 2015 (Table 1; Table 2; Characteristics of excluded studies).
| Study | Type of study | Number of neonates | Extravasation | Intervention | Outcome |
| Case series | 14 neonates | Dopamine ‐ 9 Caffeine ‐ 2 Calcium ‐ 2 Beta blocker ‐ 1 | Hyaluronidase infiltration and aspiration ‐ 12 Saline irrigation and aspiration ‐ 1 Hyaluronidase infiltration alone ‐ 1 | 3 cases developed skin necrosis but healed spontaneously | |
| Case report | 1 neonate | Total parenteral nutrition (TPN) | Infiltration with hyaluronidase with saline irrigation | Healing in 3 days | |
| Retrospective review of 31 cases | 31 neonates had 36 extravasations | TPN ‐ 13 Medications ‐ 10 Blood products ‐ 6 Crystalloids ‐ 5 Contrast ‐ 2 | Hyaluronidase infiltration and saline irrigation used for 24 extravasation injuries | No surgical graft required | |
| Case report | 2 preterm neonate less than 29 weeks gestation | TPN | Hyaluronidase infiltration and saline irrigation | Minimal or no scarring, no functional sequelae | |
| Retrospective review of 96 cases | Total 96 cases referred to a tertiary plastic surgical unit (age range ‐ preterm neonate to 72 years ‐ exact distribution not mentioned) 44 early referrals, i.e. within 24 hours 52 late referral, i.e. after 24 hours | TPN Inotrope Ca KCl Bicarbonate Dextrose: 10%‐20% Chemotherapeutics Contrast Antibiotics (exact numbers not mentioned) | Early referral group: 44 (saline irrigation alone ‐ 37, liposuction and saline irrigation ‐ 6, liposuction alone ‐ 1) Late referral group: wound and surgical management | Early referral group: 39 (88.5%) no soft tissue damage, 5 (11.5%) minor skin blistering Late referral group: 8 (15%) healed without tissue necrosis, 17 (33%) minor skin sloughing, 27 (52%) extensive damage to tissues ‐ 3 amputations | |
| Retrospective review | 56 neonates | TPN Inotrope Calcium Potassium chloride Sodium bicarbonate High concentration of dextrose (exact numbers not mentioned) | Saline irrigation alone | No skin loss. None required reconstructive surgery | |
| Case report | 34 neonates | TPN ‐ 28 10% dextrose ‐ 4 Second‐generation cephalosporin ‐ 2 | Saline irrigation followed by topical application of paraffin‐impregnated gauze and povidone‐iodine‐soaked gauze dressing | In 21 infants, no signs of soft tissue damage by 24 hours of treatment Minor findings still present for a few days in 7 patents after treatment Ischaemic signs present in 6 patients subsided within 25 days | |
| Case report | 1 neonate | Antibiotics | Infiltration with recombinant human hyaluronidase (rHuPH20) | Wound healed completely by 8 days | |
| Case report | 1 neonate | Dobutamine, adrenaline, 8.4% sodium bicarbonate, 10% calcium gluconate | Infiltration of hyaluronidase followed by liposuction and saline irrigation | No signs of soft tissue damage at 2 weeks | |
| Case report | 1 neonate | TPN | Hyaluronidase infiltration and saline irrigation | Healed fully by 5 days |
| Study | Stage of extravasation | Intervention | Outcome | Reason for exclusion |
| Skin necrosis (stage 3): n = 3 Swelling, oedema, discolouration (stage 1‐2): n = 11 | Hyaluronidase and aspiration ‐ 12 cases Hyaluronidase infiltration alone ‐ 1 Saline irrigation and aspiration ‐ 1 Treatment allocation not specified by stage of injury | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Swelling, erythema, and induration (stage 3): n = 1 | Hyaluronidase and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Erythema and oedema (stage 1‐2): n = 12 Skin necrosis (stage 3): n = 24 | Stage 1‐2 ‐ standard wound management Stage 3 ‐ hyaluronidase and saline irrigation | No sequelae No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): n = 2 | Hyaluronidase and saline irrigation | Minimal scarring, no sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): Early referral: n = 44 Delayed referral (stage 3‐4): n = 52 | Stage 3: Saline irrigation alone: n = 37 Liposuction and saline irrigation: n = 6 Liposuction alone: n = 1 Stage 3‐4: Wound debridement and surgical flap | No sequelae 8 (15%) healed without tissue necrosis, 17 (33%) minor skin sloughing, 27 (52%) extensive damage to tissues ‐ 3 amputations | Neither a randomised nor a quasi‐randomised study | |
| Exact stage of extravasation injury not specified: n = 56 | Saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Stage 3 and stage 4 extravasation | Saline irrigation within 30 minutes of extravasation injury | None of the wounds needed secondary skin coverage procedures | Neither a randomised nor a quasi‐randomised study | |
| Discolouration, oedema, cool, pulses not palpable (stage 4): n = 1 | Infiltration with recombinant human hyaluronidase (rHuPH20) | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Oedema, pallor, cold, and no capillary filling | Infiltration with hyaluronidase, liposuction, and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): n = 1 | Hyaluronidase and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study |
Risk of bias in included studies
This was not applicable, as we found no eligible studies.
Allocation
This was not applicable, as no randomised controlled trials are available.
Blinding
This was not applicable.
Incomplete outcome data
This was not applicable, as the search for this review identified only case reports.
Selective reporting
This was not applicable.
Other potential sources of bias
This was not applicable.
Effects of interventions
No results are forthcoming, as we identified no eligible studies.
Discussion
Although our search identified no relevant trials for this review, we present a brief summary of case reports and case series on this topic that highlight important issues related to current practice and practice variations when saline irrigation with or without hyaluronidase infiltration is the main intervention. Most reports describe good outcomes, but this may reflect the publication bias that is inherent in case reports.
In a retrospective review of all venous line‐related extravasation injuries among neonates in the neonatal intensive care unit (NICU) and in the surgical ward over an 18‐month period, healthcare providers treated 24 stage 3 and 4 injuries out of 36 extravasation injuries with hyaluronidase infiltration and saline irrigation. None of the 36 cases required surgical debridement (Chowdhury 2004), suggesting good outcomes of the intervention, even at advanced stages of extravasation injury.
A case series highlighting drug‐related extravasation injuries and intervention variations with saline and hyaluronidase also reported good outcomes. Physicians identified extravasation injury secondary to leakage of dopamine (n = 9), caffeine (n = 2), calcium (n = 2), and beta blocker (n = 1) in 14 neonates, 12 of whom were treated with hyaluronidase infiltration and aspiration, one with saline irrigation followed by aspiration, and another with hyaluronidase infiltration alone. Three patients developed skin necrosis, but all cases healed spontaneously (Casanova 2001). Clinicians have reported complete wound healing following extensive subcutaneous extravasation caused by use of corrosive drugs at cardiac catheterisation. Injury was managed with infiltration of hyaluronidase followed by liposuction and saline irrigation (Martin 1994).
Clinicians reported good outcomes in two preterm neonates at less than 29 weeks' gestation with total parenteral nutrition (TPN) fluid extravasation and treated with hyaluronidase infiltration and saline irrigation. Both lesions healed with minimal or no scarring (Davies 1994).
Two separate case reports described rapid tissue healing within three to five days in neonates with TPN fluid extravasation. Both individuals were treated with saline irrigation with prior hyaluronidase infiltration, but injury‐irrigation intervals were different (90 minutes vs 4 hours) (Chen Y Y 1996; Siu 2007).
A retrospective review over three years at a regional neonatal unit described 56 confirmed cases of extravasation injury secondary to leakage of infusate of TPN, inotropic agents, calcium, potassium, sodium bicarbonate, and high‐concentration dextrose. All injuries were irrigated with 500 mL of normal saline. Healthcare providers reported no episode of skin loss, and none of these neonates required reconstructive surgery (Harris 2001).
In a retrospective review of outcomes of extravasation injury following referral to a tertiary plastic surgical unit, review authors divided patients into two groups ‐ 'early', that is, outcomes within the first 24 hours of extravasation; and 'late', that is, outcomes 24 hours after injury. Of 96 patients referred (ranging from premature infants to 70‐year‐olds), 44 were in the 'early' group and 52 in the 'late' group. A range of interventions were offered to the 'early' group, including saline flush‐out (n = 37), saline flush‐out and liposuction (n = 6), and liposuction alone (n = 1). The 'late' group was offered conservative wound management only. Of 52 patients referred late, 8 (15%) healed without tissue necrosis, 17 (33%) showed only minor sloughing of the skin, and 27 (52%) had extensive damage to the soft tissue. Practitioners noted several complications including three amputations among babies in this 'late' group. The 'early' group, for whom saline irrigation was used, showed no signs of soft tissue damage in 39 (88.5%) cases and minor skin blistering with delayed healing in only five cases (11.5%). The range of agents causing extravasation was similar in the two groups (Gault 1993). This report describes only serious extravasation injuries referred to a tertiary plastic surgery centre, which may not be representative of all such injuries.
A recent case report describes infiltration with 'recombinant human hyaluronidase' (rHuPH20) without saline irrigation for the management of antibiotic extravasation injury for a term neonate (Kuensting 2010). The appearance of the swollen and tense foot returned to normal within 24 hours.
A report of 34 neonates with stage 3 and 4 extravasation injuries meticulously monitored and treated within 30 minutes of injury with saline irrigation and occlusive paraffin dressing indicated that neonates responded well to treatment without the need for secondary surgical intervention or secondary skin coverage procedures (Kostogloudis 2015).
A survey of current practice in the management of extravasation injury among neonates in Australia and New Zealand revealed that 16 of 24 centres followed written policy/standard practice in using saline wash‐out via small incisions (Restieaux 2013).
We found no reports that advocated complete non‐intervention (masterly inactivity) for the management of severe skin extravasation injury in neonates.
Extravasation injury is an inevitable complication of neonatal intensive care. Increasingly, babies born at the borderline of viability are being successfully treated in intensive care. Such babies require prolonged periods of intravenous access, including the need for vasoactive medications, TPN, and other infusions. Adequate training of medical and nursing staff in the care of central and peripheral lines, including frequent monitoring of the infusion site, has been advocated extensively in the literature as the most effective way of preventing extravasation injury (Brown 1979; Irving 2001; McCullen 2006; Tong 2007). Our review shows that many different methods are used in the management of extravasation injury for the neonate, including saline irrigation with or without prior hyaluronidase infiltration. All methods are reported to be successful without significant adverse events. Evidene suggests that mild extravasation injuries (stage 1 and stage 2) heal well with conservative management, but invasive methods are frequently required for more severe injuries (stage 3 and stage 4).
Given the limitations of the evidence at present, it seems prudent to use the saline irrigation technique for advanced stages of extravasation injury, especially stage 3 and 4 injury.
Summary of main results
We identified no randomised controlled trials evaluating whether saline irrigation or saline irrigation with prior hyaluronidase improves tissue healing in neonates with extravasation injury when compared with no intervention or normal wound care. We found 10 case reports or case series relevant to the question. Pooled data from these 10 case reports show that 234 out of 237 neonates who underwent saline irrigation with or without hyaluronidase infiltration exhibited complete wound healing without functional or cosmetic complications.
Overall completeness and applicability of evidence
Evidence provided in this review was obtained neither from randomised controlled trials (RCTs) nor from quasi‐RCTs. No ongoing trials are registered at clinicaltrials.gov; the World Health Organization International Clinical Trials Registry Platform (www.whoint/ictrp/search/en/); or the ISRCTN Registry (www.isrctn.com). Saline irrigation for management of extravasation injuries in neonates cannot be advocated as standard treatment.
Quality of the evidence
We found no evidence from RCTs to support or refute the efficacy of saline irrigation or saline irrigation with prior hyaluronidase for improving tissue healing among neonates with extravasation injury when compared with no intervention or normal wound care. The quality of evidence derived for this review only from case series and case reports is 'very low', and true effects could be substantially different from those reported.
Potential biases in the review process
This was not applicable.
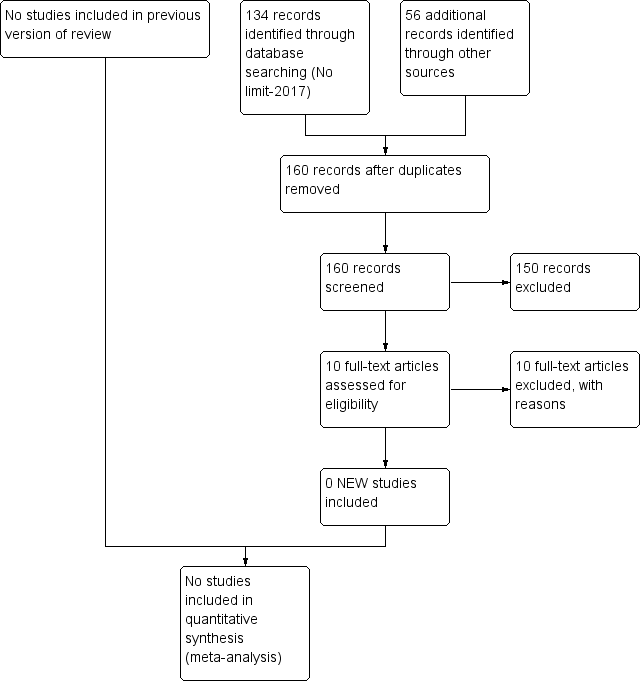
Study flow diagram: review update.
| Saline irrigation with or without prior infiltration of hyaluronidase vs standard wound care for management of extravasation injury in neonates | ||||||
| Patient or population: neonates requiring management of extravasation injury | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard wound care | Risk with saline irrigation with or without prior infiltration of hyaluronidase | |||||
| Complete wound healing after saline irrigation with or without prior hyaluronidase infiltration (wound healing): clinical assessment | Study population | Not estimable | 237 | ⊕⊝⊝⊝ | Pooled data for 237 neonates from 10 case report studies who underwent saline irrigation. Three neonates received further surgical intervention. For 234 neonates, complete wound healing was reported | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| All 10 studies analysed in GRADE pro GDT are case reports, and the intervention methods mentioned are highly variable. In view of this, "relative effect (95% CI)" and "absolute effect (95% CI)" were not derived. | ||||||
| Study | Type of study | Number of neonates | Extravasation | Intervention | Outcome |
| Case series | 14 neonates | Dopamine ‐ 9 Caffeine ‐ 2 Calcium ‐ 2 Beta blocker ‐ 1 | Hyaluronidase infiltration and aspiration ‐ 12 Saline irrigation and aspiration ‐ 1 Hyaluronidase infiltration alone ‐ 1 | 3 cases developed skin necrosis but healed spontaneously | |
| Case report | 1 neonate | Total parenteral nutrition (TPN) | Infiltration with hyaluronidase with saline irrigation | Healing in 3 days | |
| Retrospective review of 31 cases | 31 neonates had 36 extravasations | TPN ‐ 13 Medications ‐ 10 Blood products ‐ 6 Crystalloids ‐ 5 Contrast ‐ 2 | Hyaluronidase infiltration and saline irrigation used for 24 extravasation injuries | No surgical graft required | |
| Case report | 2 preterm neonate less than 29 weeks gestation | TPN | Hyaluronidase infiltration and saline irrigation | Minimal or no scarring, no functional sequelae | |
| Retrospective review of 96 cases | Total 96 cases referred to a tertiary plastic surgical unit (age range ‐ preterm neonate to 72 years ‐ exact distribution not mentioned) 44 early referrals, i.e. within 24 hours 52 late referral, i.e. after 24 hours | TPN Inotrope Ca KCl Bicarbonate Dextrose: 10%‐20% Chemotherapeutics Contrast Antibiotics (exact numbers not mentioned) | Early referral group: 44 (saline irrigation alone ‐ 37, liposuction and saline irrigation ‐ 6, liposuction alone ‐ 1) Late referral group: wound and surgical management | Early referral group: 39 (88.5%) no soft tissue damage, 5 (11.5%) minor skin blistering Late referral group: 8 (15%) healed without tissue necrosis, 17 (33%) minor skin sloughing, 27 (52%) extensive damage to tissues ‐ 3 amputations | |
| Retrospective review | 56 neonates | TPN Inotrope Calcium Potassium chloride Sodium bicarbonate High concentration of dextrose (exact numbers not mentioned) | Saline irrigation alone | No skin loss. None required reconstructive surgery | |
| Case report | 34 neonates | TPN ‐ 28 10% dextrose ‐ 4 Second‐generation cephalosporin ‐ 2 | Saline irrigation followed by topical application of paraffin‐impregnated gauze and povidone‐iodine‐soaked gauze dressing | In 21 infants, no signs of soft tissue damage by 24 hours of treatment Minor findings still present for a few days in 7 patents after treatment Ischaemic signs present in 6 patients subsided within 25 days | |
| Case report | 1 neonate | Antibiotics | Infiltration with recombinant human hyaluronidase (rHuPH20) | Wound healed completely by 8 days | |
| Case report | 1 neonate | Dobutamine, adrenaline, 8.4% sodium bicarbonate, 10% calcium gluconate | Infiltration of hyaluronidase followed by liposuction and saline irrigation | No signs of soft tissue damage at 2 weeks | |
| Case report | 1 neonate | TPN | Hyaluronidase infiltration and saline irrigation | Healed fully by 5 days |
| Study | Stage of extravasation | Intervention | Outcome | Reason for exclusion |
| Skin necrosis (stage 3): n = 3 Swelling, oedema, discolouration (stage 1‐2): n = 11 | Hyaluronidase and aspiration ‐ 12 cases Hyaluronidase infiltration alone ‐ 1 Saline irrigation and aspiration ‐ 1 Treatment allocation not specified by stage of injury | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Swelling, erythema, and induration (stage 3): n = 1 | Hyaluronidase and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Erythema and oedema (stage 1‐2): n = 12 Skin necrosis (stage 3): n = 24 | Stage 1‐2 ‐ standard wound management Stage 3 ‐ hyaluronidase and saline irrigation | No sequelae No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): n = 2 | Hyaluronidase and saline irrigation | Minimal scarring, no sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): Early referral: n = 44 Delayed referral (stage 3‐4): n = 52 | Stage 3: Saline irrigation alone: n = 37 Liposuction and saline irrigation: n = 6 Liposuction alone: n = 1 Stage 3‐4: Wound debridement and surgical flap | No sequelae 8 (15%) healed without tissue necrosis, 17 (33%) minor skin sloughing, 27 (52%) extensive damage to tissues ‐ 3 amputations | Neither a randomised nor a quasi‐randomised study | |
| Exact stage of extravasation injury not specified: n = 56 | Saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Stage 3 and stage 4 extravasation | Saline irrigation within 30 minutes of extravasation injury | None of the wounds needed secondary skin coverage procedures | Neither a randomised nor a quasi‐randomised study | |
| Discolouration, oedema, cool, pulses not palpable (stage 4): n = 1 | Infiltration with recombinant human hyaluronidase (rHuPH20) | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Oedema, pallor, cold, and no capillary filling | Infiltration with hyaluronidase, liposuction, and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study | |
| Skin necrosis (stage 3): n = 1 | Hyaluronidase and saline irrigation | No sequelae | Neither a randomised nor a quasi‐randomised study |

