Fluoropirimidinas por vía oral versus intravenosa para el cáncer colorrectal
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial Phase: II Accrual dates: July 1998 to May 2000 | |
| Participants | No. randomised: 77 Stage/treatment line: Metastatic, first‐line Countries/sites: Not specified, study authors from a South Korean centre Setting: Hospital Characteristics (Group A/B): Metastatic colorectal adenocarcinoma; age ≤ 75 years (median 58/57 years); male (50/79%); PS ECOG ≤ 2 (PS ECOG 0: 11/3%) | |
| Interventions | Group A: Oral doxifluridine (5‐dFUR) 333 mg/m2 tds + leucovorin 15 mg bd, D1‐7 and D15‐21 q28d (n randomised = 38) Group B: IV bolus 5‐FU 400 mg/m2/d plus leucovorin 20 mg/m2/d D1‐5, q28d (n randomised = 39) “In the presence of objective response or stable disease, each group of patients was treated for a maximum of 12 cycles. In the case of a complete response, 2 additional cycles were given. The treatment was continued until there was progression, unacceptable toxicity, or a patient’s refusal” (patients and methods, paragraph 2, page 99) | |
| Outcomes | ORR (WHO criteria, 1981) PFS (treated as TTP in this review, based on the definition provided), OS Grade ≥ 3 AEs (WHO toxicity criteria, version not specified) Median follow‐up: 17.0 m (PFS/OS) | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Not specified, blinding unlikely (i) ORR/PFS (treated as TTP): Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: This study was not used for the meta‐analysis for these outcomes |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/PFS (treated as TTP): High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: This study was not used for the meta‐analysis for these outcomes |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/PFS): Low Quote: "All measurable lesions were assessed every three cycles..." (patients and methods, paragraph 3, page 99) Quote: "Each cycle was repeated every four weeks" (patients and methods, paragraph 2, page 99) Therefore, responses were evaluated at the same frequency in both treatment arms (ii) Survival (influences PFS/OS): Unclear Follow‐up duration and assessment frequency for survival events were not specified (iii) Grade ≥ 3 AEs: Low Quote: "Side effects ... were evaluated at the beginning of each cycle" (patients and methods, paragraph 4, page 99) Therefore, grade ≥ 3 AEs were evaluated at the same frequency in both treatment arms |
| Incomplete outcome data (attrition bias) | High risk | (i) ORR/PFS (treated as TTP): High 11/38 (29%) 5‐dFUR/LV and 6/39 (15%) 5‐FU/LV patients were not evaluable. Quote: "... 38 were randomly assigned to group A and 39 to group B" (results, paragraph 1, page 99) Quote: "... the evaluable patients were 27 patients in group A and 33 patients in group B, respectively" (results, paragraph 3, page 99) (ii) OS: Low Although not defined in the Methods, censoring was noted in the KM curves (Fig. 1 and 2, page 100). No evidence suggested bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The effects of the treatments were analysed using both the intent‐to‐treat principle and per‐protocol analysis that included only evaluable patients" (patients and methods, paragraph 5, page 99) Safety analysis: Not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) ECOG PS: Low (Table 1, page 99) (ii) Median age: Low (Table 1, page 99) (iii) Number of metastatic organs: Low (Table 1, page 99) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Design: Factorial (2 × 2), following protocol amendment in October 2005 Accrual dates: July 2004 to August 2010 | |
| Participants | No. randomised: 1608 Stage/treatment type: Stage II or III rectal cancer, neoadjuvant Countries/sites: USA, Canada (multiple sites) Setting: Hospital Characteristics (group 1/2/3/4/5/6): Unresected stage II or III rectal adenocarcinoma; age > 18 years (patients ≤ 59 years: 59.2/52.7/56.1/61.4/57.1/61.2%); male (68.0/67.8/67.0/68.1/67.8/67.6%); PS 0/1 | |
| Interventions | Grp 1 (5‐FU (2 Arm), pre‐amendment): 5‐FU 225 mg/m2/d (n randomised = 147) Grp 2 (CAPE (2 Arm), pre‐amendment): capecitabine 825 mg/m2 oral BD (n randomised = 146) Grp 3 (5‐FU (4 Arm), post‐amendment): 5‐FU 225 mg/m2/d 5 days/wk (n randomised = 330) Grp 4 (5‐FU + OX (4 Arm), post‐amendment): 5‐FU 225 mg/m2/d 5 days/wk, oxaliplatin 50 mg/m2 IV weekly (n randomised = 329) Grp 5 (CAPE (4 Arm), post‐amendment): capecitabine 825 mg/m2 oral BD 5 days/wk (n randomised = 326) Grp 6 (CAPE + OX (4 Arm), post‐amendment): capecitabine 825 mg/m2 oral BD 5 days/wk, oxaliplatin 50 mg/m2 IV weekly (n randomised = 330) All participants received neoadjuvant radiotherapy: 180 cGy per day, 5 doses per week for 25 fractions. Minimal boost: 540 cGy (3 days in 180 cGy fractions) for T3 non‐fixed and distal cancers; 1080 cGy (3 days in 360 cGy fractions) for T4 fixed and/or distal cancers All chemotherapy given for the duration of radiotherapy | |
| Outcomes | Locoregional control at 3 years OS DFS Time to local‐regional recurrence Grade ≥ 3 AEs (NCI CTCAE, version 4.0) No details on median follow‐up | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: US National Cancer Institute at the National Institutes of Health, US Department of Health and Human Services, Public Health Service grants Declarations of interest: None declared | |
| Notes | Following the October 2005 protocol amendment, oxaliplatin was added to form a 2 × 2 factorial design. Daily dose of fluoropyrimidine chemotherapy was unchanged. However, capecitabine and 5‐FU treatment were given 5 days a week (reduced from 7), coinciding with days of planned radiation therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned to treatment groups using the NSABP biased‐coin minimization algorithm" (methods, paragraph 3, page 2) |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) DFS: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Locoregional recurrence (influences DFS): Low Quote: "Following surgery, MRI or CT scans were required every 12 months for two years, and proctoscopy or sigmoidoscopy annually for five years" (methods, paragraph 2, page 2) (ii) Survival (influences DFS/OS): Unclear It was unclear whether any follow‐up (e.g. clinical reviews) other than that described above was performed to detect survival events, and whether they were the same in both oral and intravenous arms (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Low risk | (i) DFS/OS: Low 19/801 participants from the analysis population on the 5‐FU arm had no follow‐up, whereas 9/794 participants on the capecitabine arm had no follow‐up (correspondence with Dr. Carmen Allegra, received 22 July 2016) (ii) Grade ≥ 3 AEs: Low From the analysis population, 21/801 (3%) and 7/794 (1%) participants in the 5‐FU and CAPE arms, respectively, were missing safety outcome data (Table 2, page 6) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low From the analysis population, 5/806 (0.6%) and 8/802 (1%) participants were excluded from the 5‐FU and CAPE arms, respectively, because they were ineligible (Figure 1, page 4) Safety analysis: Unclear ‐ not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ information not specified (ii) Median/mean age: Unclear ‐ information not specified (iii) TNM stage: Unclear ‐ information not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear Quote: "...we do not have complete information concerning the type and use of adjuvant therapy in the study patients" (discussion, paragraph 5, page 7) Risk of bias considerations in a factorial study: Unclear Quote: "... no evidence of oxaliplatin‐treatment‐by‐fluoropyrimidine‐treatment interaction (P = .46)" for 3‐year locoregional recurrence (results, paragraph 2, page 4) No tests for interaction were reported for the other outcomes |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: Not specified | |
| Participants | No. randomised: 60 Stage/treatment line: Inoperable/advanced/recurrent colorectal cancer, no prior 5‐FU or Ftorafur and at least 4 weeks elapsed from previous chemotherapy (only those with no prior chemotherapy were analysed) Countries/sites: Authors from Denmark Setting: Hospital Characteristics (Arm A/B): Inoperable/advanced/recurrent colorectal cancer; age (median 55/59 years); male (43/37%); PS (WHO) ≤ 3 | |
| Interventions | Arm I: Oral Ftorafur 1 gm/m2/d D1‐21, q35d (n randomised = 30) Arm II: IV bolus 5‐FU 500 mg/m2/d D1‐5, q35d (n randomised = 30) Treatment continued until intractable toxicity or PD | |
| Outcomes | ORR (WHO criteria, 1979) OS Median follow‐up: Not specified | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Not specified, blinding unlikely (i) ORR: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Not outcomes for this study |
| Blinding of outcome assessment (detection bias) | High risk | Not specified. (i) ORR: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Not outcomes for this study |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR): Unclear ‐ not specified (ii) Survival (influences OS): This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Not outcomes for this study |
| Incomplete outcome data (attrition bias) | High risk | (i) ORR: High 20% of patients in the 5‐FU arm were not evaluable (Table 1, page 434) (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Not outcomes for this study |
| Incomplete outcome data (ITT analysis) | High risk | Efficacy analysis: High The efficacy analysis population excluded those who refused treatment, had protocol violations, or were lost to follow‐up (10/60) (Table 1, page 434) Safety analysis: The safety analysis population received at least 1 treatment cycle Quote: "They received at least one treatment cycle with 5‐FU or Ftorafur" (material and methods, paragraph 4, page 434) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | i) PS: Unclear ‐ only median and range reported (Table 1, page 434) ii) Median age: Low (Table 1, page 434) iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not provided |
| Methods | Randomised controlled trial Phase: II, non‐comparative Accrual dates: April 1993 to September 1994 | |
| Participants | No. randomised: 130 Stage/treatment line: Metastatic, first‐line (no previous adjuvant chemotherapy) Countries/sites: Italy, 13 institutions Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal adenocarcinoma; age < 80 years (median 61/61 years); male (52/59%); PS ECOG ≤ 2 | |
| Interventions | Arm A: Oral doxifluridine (5‐dFUR) 750 mg/m2 bd plus oral levo‐leucovorin 25 mg/dose D1‐4, q12d (n randomised = 67) Arm B: IV 5‐dFUR 3000 mg/m2 plus l‐leucovorin 25 mg D1‐5, q21d (n randomised = 63) "It was initially planned to deliver five cycles to the patients in arm A and three cycles to those in arm B. In the case of a complete response (CR), partial response (PR), or stable disease (SD), the patients were to receive an additional 4 and 2 cycles, respectively. In the case of a subsequent CR or PR following the documentation of SD or PR at the first evaluation, further 4 cycles for arm A and 2 cycles for arm B were administered" (patients and methods, paragraph 4, page 2088) | |
| Outcomes | ORR (WHO criteria, 1981) Grade ≥ 3 AEs (NCI CTC, 1981) Time to treatment failure (TTF, treated as PFS in this review, based on the definition provided) OS Median follow‐up: 10 m (TTF/OS) | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly allocated to receive oral or i.v. 5‐dFUR, balanced in blocks of varying sizes" (patients and methods, paragraph 2, page 2089) |
| Allocation concealment (selection bias) | Low risk | Quote: "... the patients were registered at a central randomization office ..." (patients and methods, paragraph 2, page 2089) |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/TTF(treated as PFS): Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified. (i) ORR/TTF(treated as PFS): High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTF (treated as PFS)): Unclear Quote: "It was initially planned to deliver five cycles to the patients in arm A and three cycles to those in arm B. In the case of a complete response (CR), partial response (PR), or stable disease (SD), the patients were to receive an additional 4 and 2 cycles, respectively. In the case of a subsequent CR or PR following the documentation of SD or PR at the first evaluation, further 4 cycles for arm A and 2 cycles for arm B were administered" (patients and methods, paragraph 4, page 2089) Quote: "Oral 5‐dFUR ... was administered ... for 4 days repeated every 12 days (arm A); intravenous 5‐dFUR was administered ... for 5 consecutive days every 21 days (arm B)" (patients and methods, paragraph 3, page 2089) Therefore, the schedule of assessment for both treatment arms up until the second evaluation was similar However, no information was provided on the response evaluation schedule following the second evaluation (ii) Survival (influences TTF(treated as PFS)/OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: High Quote: "Side effects were ... evaluated at the beginning of each cycle" (patients and methods, paragraph 8, page 2089) "Oral 5‐dFUR ... was administered ... for 4 days repeated every 12 days (arm A); intravenous 5‐dFUR was administered ... for 5 consecutive days every 21 days (arm B)" (patients and methods, paragraph 3, page 2089) Therefore, safety evaluations occurred more frequently in arm A |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low The sum of participants who achieved CR, PR, SD, and “Treatment failure” appears to be the same as the number randomised (Table 2, page 2091) (ii) TTF (treated as PFS)/OS: Low Although censoring was not defined in the Methods, censoring was noted in the KM curves (Figure 1 and Figure 2, page 2091). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote "The activity of the treatments was analyzed using both the intent‐to‐treat principle (including the entire group of patients) and standard analysis (which excludes inadequately treated cases)" (statistical analysis, paragraph 1, page 2089) Safety analysis: Analyses included those who received treatment Quote: "As three of the patients randomized to arm B were censored because no treatment was administered, the safety analysis was based on the results derived from 127 subjects" (results, paragraph 7, page 2090) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ reported as ECOG PS 0‐1 vs 2 (Table 1, page 2090) (ii) Median age: Low (Table 1, page 2090) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not provided |
| Methods | Randomised controlled trial Phase: III Accrual dates: May 1996 to July 1997 | |
| Participants | No. randomised: 380 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation, 47 sites. Europe (n = 291), Canada (n = 55), Australia (n = 24), New Zealand (n = 5), and Israel (n = 5) Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal adenocarcinoma; age > 18 years (median 61/62 years); male (67/64%); PS ECOG ≤ 2 (ECOG 0: 39/33%) | |
| Interventions | Arm I: Oral UFT 300 mg/m2/d plus leucovorin (LV) 90 mg/d D1‐28, q35d (n randomised = 190) Arm II: IV bolus 5‐FU 425 mg/m2/d plus LV 20 mg/m2/d D1‐5, q35d (n randomised = 190) | |
| Outcomes | TTP OS ORR (WHO criteria ‐ modified) Grade ≥ 3 AEs (NCI CTC, version not specified) Median follow‐up ‐ Not specified | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Taiho Pharmaceutical Company, Bristol‐Myers Squibb Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/TTP: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP: Unclear Quote: "Efficacy was evaluated locally with data subsequently centrally reviewed" (patients and methods, paragraph 5, page 3619) However, the role of the central review in the reported response data was not described (ii) OS: Low Not specified. Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Not specified. Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/TTP): Low Quote: "Tumor reassessment ... was repeated after every two cycles, with an additional computed tomography scan at week 15" (patients and methods, paragraph 5, page 3619) Quote: "On both treatment arms, treatment cycles were to be repeated every 35 days" (patients and methods, paragraph 3, page 3618) Therefore, responses were evaluated at the same frequency in both treatment arms (ii) Survival (influences OS): Unclear The schedule of follow‐up after progression was not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low 1% (2/190) of patients in both arms were not assessed (Table 2, page 3619) (ii) TTP/OS: Low Although not defined in the Methods, censoring was noted from the KM curves for both outcomes (Fig 1 and 2, pages 3621 and 3622). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low The greatest percentage of outcome data missing for an AE in any arm was 11%; there were similar percentages missing from each arm (Tables 4, 5 and 6, page 3623) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "All efficacy analyses have been presented by treatment arm as randomized ..." (patients and methods, paragraph 7, page 3619) Safety analysis: Quote: "All 373 patients who received at least one dose of study medication were evaluated for safety and were analyzed according to the treatment arm as treated" (results, paragraph 18, page 3622) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 1, page 3619) (ii) Median age: Low (Table 1, page 3619) (iii) No. of involved organs: Unclear ‐ not clearly specified. Quote: "The median number of disease sites was two in both treatment arms" (results, paragraph 4, page 3619) |
| Other bias | Low risk | Subsequent therapies: Low Similar percentages of participants and types of chemotherapy were used in the second‐line setting after progression, in each arm Quote: "Secondary chemotherapy was administered to 41% (78 of 190) of patients receiving UFT/LV, and 39% (75 of 190) of patients receiving 5‐FU/LV" (results, paragraph 16, page 3622) Quote: "The most frequently administered secondary chemotherapy was fluoropyrimidines only, in 49% of the 78 UFT/LV patients and in 47% of the 75 5‐FU/LV patients who took secondary chemotherapy, followed by irinotecan only (28% in each arm). In patients receiving secondary chemotherapy, oxaliplatin alone or in combination with irinotecan was given to 13% (10 of 78) of UFT/LV treated patients and 16% (12 of 75) of 5‐FU/LV treated patients" (results, paragraph 16, page 3622) |
| Methods | Randomised controlled trial Phase: III Design: Part One ‐ 2‐arm, 1:1 randomisation to XELOX vs FOLFOX4 Part Two ‐ 2 × 2 factorial (protocol amendment to include randomisation to bevacizumab (BEV) or placebo); 1:1:1:1 randomisation to XELOX + placebo, XELOX + BEV, FOLFOX‐4 + placebo, FOLFOX‐4 + BEV Accrual dates: Part One ‐ July 2003 to May 2004; Part Two ‐ February 2004 to February 2005 | |
| Participants | No. randomised: 2035 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation; Europe (n = 1048), Canada (n = 343), Oceania (n = 188), US (n = 178), Central/Eastern Asia (n = 163), South America (n = 65), and South Africa (n = 49) Setting: Hospital Characteristics Part One (Arm I/II): Metastatic colorectal adenocarcinoma; age ≥ 18 years (median 61/62 years); male (61/64%); PS ECOG ≤ 1 (PS ECOG 0: 50/51%) Characteristics Part Two (Arm I/II/III/IV): Metastatic colorectal adenocarcinoma; age ≥ 18 years (median 61/61/60/60 years); male (59/61/53/59%); PS ECOG ≤ 1 (PS ECOG 0: 59/59/60/57%) | |
| Interventions | Part One: Arm I (XELOX): oxaliplatin 130 mg/m2 D1 plus capecitabine 1000 mg/m2 bd for 14 d, q21d (n randomised = 317) Arm II (FOLFOX4): oxaliplatin 85 mg/m2 D1 plus leucovorin 200 mg/m2/d, IV bolus 5‐FU 400 mg/m2/d and 5‐FU 600 mg/m2/d 22‐hour infusion D1‐2, q14d (n randomised = 317) Part Two: Arm I: XELOX + placebo (n randomised = 350) Arm II: XELOX + BEV 7.5 mg/kg D1, q21d (n randomised = 350) Arm III: FOLFOX‐4 + placebo (n randomised = 351) Arm IV: FOLFOX‐4 + BEV 5 mg/kg D1, q14d (n randomised = 350) Treatment continued until PD or for 48 weeks, whichever came first (study treatment phase). Participants who completed the 48‐week treatment phase without PD were eligible to continue treatment until PD in a post‐study treatment phase | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) TTF Grade ≥ 3 AEs (NCI CTC, version 3) Median follow‐up: PFS 17.7 m (cut‐off date 31 January 2006), OS cut‐off date 31 July 2008 | |
| Study Details | Journal articles | |
| Funding sources and declarations of interest | Declarations of interest: Roche, Sanofi‐Aventis Funding sources: Roche | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were assigned to treatment using an interactive voice response system" (patients and methods, paragraph 4, page 2007) |
| Blinding of participants and personnel (performance bias) | High risk | Quote "... open‐label" (patients and methods, paragraph 1, page 2007) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low Quote: "Tumor responses were assessed by investigators and also by an independent response review committee" (patients and methods, paragraph 9, page 2007) Similar ORs were obtained for the response rates, which were "Investigator assessed" and "IRC assessed" (Table 2, page 2010) (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/PFS): Low Quote: "Tumor assessments (computed tomography scan, magnetic resonance imaging) were ... repeated after every two XELOX cycles and every three FOLFOX‐4 cycles (i.e., every sixth week in both arms and at the end of treatment)" (patients and methods, paragraph 9, page 2007) (ii) Survival (influences PFS/OS): Low Quote: "After completion of study treatment, patients were followed every 3 months until PD and/or death" (patients and methods, paragraph 9, page 2007) (iii) Grade ≥ 3 AEs: Unclear ‐ no schedule was provided |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Unclear ‐ not specified (ii) PFS/OS: Low Quote: "Patients who were not reported as having died at the time of the analysis were censored using the date they were last known to be alive" (Cassidy et al, British Journal of Cancer, 2011: patients and methods, paragraph 9, page 59). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The intent‐to‐treat (ITT) patient population included all patients who underwent randomization and signed the informed consent form" (patients and methods, paragraph 11, page 2007) Safety analysis: Quote: "All patients receiving at least one dose of study drug were included in the safety analysis" (patients and methods, paragraph 11, page 2007) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, 2009) (ii) Median age: Low (Table 1, 2009) (iii) No. of involved organs: Low (Table 1, 2009) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "There were no major imbalances between the treatment groups with respect to the use of second‐line therapy ..." (results, paragraph 9, page 2009) Risk of bias considerations in a factorial study: Low For efficacy analysis ‐ Quote: "Both a clinically relevant and statistically significant (P = .7025) treatment interaction was ruled out" (results, paragraph 4, page 2008) For safety analysis ‐ Quote: "The addition of bevacizumab did not alter the similarities and differences in safety profile between XELOX and FOLFOX‐4" (results, paragraph 14, page 2010) |
| Methods | Randomised controlled trial Phase: III Accrual dates: May 2004 to April 2007 | |
| Participants | No. randomised: 322 Stage/treatment line: Metastatic, first‐line Countries/sites: Italy, 23 Southern Italy Cooperative Oncology group (SICOG) centres Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal adenocarcinoma; age ≥ 18 years (median 65/64 years); male (54/66%); PS ECOG ≤ 2 (PS ECOG 0: 60/61%) | |
| Interventions | Arm I (OXAFAFU): IV oxaliplatin 85 mg/m2, 6S‐leucovorin 250 mg/m2 D1, IV bolus fluorouracil 850 mg/m2 D2, q14d (n randomised = 164) Arm II (OXXEL): IV oxaliplatin 100 mg/m2 D1 plus capecitabine 1000 mg/m2 bd D1‐D11, q14d (n randomised = 158) Treatment continued until PD, unacceptable toxicity, or participant refusal, or a maximum of 12 cycles | |
| Outcomes | ORR (WHO criteria, 1981) PFS OS Grade ≥ 3 AEs (WHO criteria, 1981) Median follow‐up: 24 months (PFS/OS) | |
| Study Details | Journal article and abstracts | |
| Funding sources and declarations of interest | Funding sources: SICOG Declarations of interest: No conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/PFS: High Outcome assessment at risk of bias if there was lack of blinding (ii)OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/PFS): Low Quote: "In both arms, cycles were repeated every 2 weeks" (methods, paragraph 5, page 219) Quote: "CT or MRI scan was repeated after every 4 cycles" (methods, paragraph 4, page 219) Therefore, responses were evaluated at the same frequency in both treatment arms Quote: "Response was ... reassessed 8 weeks after the date of their first documentation; only confirmed responses were computed in the activity analysis" (methods, paragraph 4, page 219) (ii) Survival (influences PFS/OS): Low Quote: "After discontinuation of first‐line treatment, patients were followed every 2 months to assess the disease status (iii) Grade ≥ 3 AEs: Low Quote: "During treatment, WBC count with differential was performed weekly. Biochemistry, symptoms, body weight, and nonhematological toxicity were checked before each cycle" (methods, paragraph 2, page 218‐9) |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low 11% of patients in the OXXEL arm and 10% of patients in the OXAFAFU arm were not assessed (Comella et al, ASCO Gastrointestinal Cancers Symposium, 2008 ‐ slides associated with abstract 344) (ii) PFS/OS: Low It is likely that censoring has occurred (Figures 1 and 2, pages 222 and 223). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "... 322 eligible patients ... were randomized to the OXXEL (158 patients) or OXAFAFU (164 patients) arm" This was the same as the number of participants at risk at t = 0 in Figure 1 (PFS curve) and Figure 2 (OS curve) (pages 222 and 223), as well as the total number of participants described in the ITT response data (Comella et al, ASCO Gastrointestinal Cancers Symposium, 2008 ‐ slides associated with abstract 344) Safety analysis: Not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 220) (ii) Median age: Low (Table 1, page 220) (iii) No. of involved organs: Low (Table 1, page 220) |
| Other bias | Low risk | Subsequent therapies: Low Post‐study treatment was similar in both arms (Comella et al, ASCO Gastrointestinal Cancers Symposium, 2008 ‐ slides associated with abstract 344) |
| Methods | Randomised controlled trial Phase: III Accrual dates: December 2004 to June 2007 | |
| Participants | No. randomised: 3451 Stage/treatment type: High‐risk stage II or stage III colon cancer, adjuvant Countries/sites: 330 centres in 34 countries (including USA, Europe, Asia, and Australia) Setting: Hospital Characteristics (Arm A/B/C) for ITT population: Adjuvant colon cancer; age ≥ 18 years (median 58/58/58 years); male (57/51/55%); PS ECOG 0‐1 (PS ECOG 0: 86/85/85%) | |
| Interventions | Arm A (FOLFOX4): D1 oxaliplatin 85 mg/m2, LV 200 mg/m2, IV bolus fluorouracil 400 mg/m2, followed by IV fluorouracil 600mg/m2 22‐hour continuous infusion on D1 and 2, q14d from W1‐24; W25‐48 observation only (n randomised = 1151) Arm B (Bevacizumab (Bev)‐FOLFOX4): FOLFOX4 + Bev 5 mg/kg D1 q14d from W1‐24, then Bev alone 7.5 mg/kg D1 q21d from W25‐48 (n randomised = 1155) Arm C (Bev‐XELOX): capecitabine 1000 mg/m2 oral bd D1‐14, oxaliplatin 130 mg/m2 D1 + Bev 7.5 mg/kg D1 q21d from W1‐24, then Bev alone 7.5 mg/kg D1 q21d from W25‐48 (n randomised = 1145) Total of 12 courses | |
| Outcomes | DFS OS Grade ≥ 3 AEs (NCI CTCAE, version 3.0) Median follow‐up: For patients with stage III disease who did not have DFS events at the data cut‐off date ‐ Arm A 48.5 m, Arm B 48.3 m, Arm C 48.3 m Data cut‐off dates: DFS ‐ 30 June 2010, OS ‐ 30 June 2012 | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Genentech, Roche, Chugai Declarations of interest: Honoraria from Roche‐Genentech, Merck‐Serono, Sanofi‐Aventis, Amgen | |
| Notes | All efficacy results were only reported for stage III participants in all arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A block design randomisation procedure (block size of six) was used" (methods, paragraph 3, page 1226) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done after surgery using a centralised interactive computerised system" (methods, paragraph 3, page 1226) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study had an open‐label design" (methods, paragraph 3, page 1226) (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Disease recurrence (influences DFS): Low Quote: "Recurrences or new occurrences were based on investigator tumour assessments, and pre‐scheduled every 6 months after randomisation until year 4, then annually thereafter" (methods, paragraph 7, page 1227) (ii) Survival (influences DFS/OS): Low Quote: "Survival status was assessed every 6 months in the first 4 years after randomisation, then annually thereafter" (methods, paragraph 8, page 1227) (iii) Grade ≥ 3 AEs: Unclear Quote: "Adverse events were monitored until at least 28 days after the last dose of study treatment or end of observation However, the frequency/schedule of monitoring in both arms was unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) DFS/OS: Low Quote: "Event‐free patients at the clinical cutoff date were censored at the last date at which they were known to be disease‐free" (methods, paragraph 7, page 1227) Quote: "Patients who were still alive at the clinical cutoff date were censored at the date at which they were last confirmed to be alive" (methods, paragraph 8, page 1227) No evidence of bias related to censoring (ii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "Patients who were event‐free at a given time point were censored at that time point" (methods, paragraph 11, page 1228) ITT population included all participants randomised to their allocated treatments (Figure 1, page 1227) Safety analysis: Quote: "The safety population comprised all patients who received at least one dose of study treatment" (methods, paragraph 12, page 1228) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 1227) (ii) Median age: Low (Table 1, page 1227) (iii) TNM stage: Low (stage II vs stage III, and stage III N1 vs N2) (Table 1, page 1227) |
| Other bias | Low risk | Subsequent therapies: Low Participants in the Bev‐FOLFOX4 and Bev‐XELOX groups received similar subsequent drug therapy after a recurrence or a new occurrence of colorectal cancer in the stage III ITT population (supplementary appendix, Table 1, page 6) |
| Methods | Randomised controlled trial Phase: III Accrual dates: January 1999 to September 2004 | |
| Participants | No. randomised: 155 Stage/treatment type: T3 or T4 rectal adenocarcinoma, with or without nodal metastasis, or any T stage tumours with nodal metastasis; neoadjuvant Countries/sites: Spain, 3 sites Setting: Hospital Characteristics (Arm I/II): Locally advanced rectal adenocarcinoma; age ≤ 80 years (median 65/63 years); male (74/66%); PS WHO 0‐2 (PS ECOG 0: 63/64%) | |
| Interventions | Arm I (FU+LV): LV 20 mg/m2 followed by IV bolus FU 350 mg/m2 for 5 consecutive days during first and fifth weeks of radiotherapy (n randomised = 77) Arm II (UFT + LV): Single course of oral LV 12.5 mg bd and oral UFT 300 mg/m2/d on D 8‐36 of the radiotherapy course (n randomised = 78) Radiotherapy consisted of a total dose of 45 Gy given in 25 fractions of 1.8 Gy, 5 fractions per week | |
| Outcomes | DFS OS Grade ≥ 3 AEs (ECOG CTC) Median follow‐up: 22 m, insufficient for DFS outcome | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: No conflicts of interest | |
| Notes | The scientific committee decided to stop the study because of slow accrual after 155 participants (63% of planned accrual) from the 3 participating hospitals had been randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in blocks of 10 ... " (methods and materials, paragraph 3, page 103) |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were centrally randomized ..." (methods and materials, paragraph 3, page 103) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...open‐label clinical trial ..." (methods and materials, paragraph 1, page 103) (i) DFS: This study was not used for the meta‐analysis for this outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: This study was not used for the meta‐analysis for this outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Disease recurrence (influences DFS) and (ii) Survival (influences DFS/OS) Note: DFS and OS were not included in the meta‐analysis owing to < 3 years of median follow‐up Quote: "Patients were evaluated every 2 months for the first 6 months, every 3 months for the next 6 months, at 6‐month intervals for the next 4 years, and then yearly" (methods and materials, paragraph 10, page 103) (iii) Grade ≥ 3 AEs: Low Quote: "During therapy, patients were monitored weekly for acute toxicity" (methods and materials, paragraph 9, page 103) Quote: "Follow‐up was planned every 3 months following completion of therapy....The same schedule was applied for both arms." (correspondence with Dr. de la Torre, received 13 August 2012) |
| Incomplete outcome data (attrition bias) | Low risk | (i) Survival (DFS and OS): Low Censoring was noted in the KM curves (Fig. 1, page 106). No evidence of bias related to censoring This study was not used for the meta‐analysis of DFS and OS outcomes (ii) Grade ≥ 3 AEs: Low No missing data from participants in the safety analysis population (correspondence with Dr. de la Torre, received 13 August 2012) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low "One patient randomized to the FU+LV arm and 2 patients randomized to the UFT+LV arm were excluded from all Safety analysis: Analyses were also performed in the same population as described above |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 104) (ii) Median age: Low (Table 1, page 104) (iii) TNM stage: Low (Table 1, page 104) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Accrual dates: April 2002 to August 2004 | |
| Participants | No. randomised: 348 Stage/treatment line: Metastatic, first‐line Countries/sites: Spain, 29 sites Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age ≥ 18 years (median 64/65 years); male (63/58%); KPS ≥ 70% (KPS > 70%: 89/90%) | |
| Interventions | Arm I (XELOX): capecitabine 1000 mg/m2 bd D1‐14 plus oxaliplatin 130 mg/m2 D1, q21d for 12C (n randomised = 174) Arm II (FUOX): Infusional FU 2250 mg/m2 D1, 8, 15, 22, 29, 36 plus oxaliplatin 85 mg/m2 D1, 15 and 29 q42d for 6C (n randomised = 174) or until PD, intolerable AEs, or participant refusal | |
| Outcomes | TTP Grade ≥ 3 AEs (NCI CTC, version 2.0); HFS assessed using 3‐grade scale previously described (Blum 1999) ORR (RECIST, version 1.0) OS Median follow‐up: 17.5 m (TTP/OS; cut‐off date 15 June 2006) | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Treatment of Digestive Tumors (TTD), Madrid, Spain, Roche, Sanofi‐Aventis Declarations of interest: Sanofi‐Aventis, Roche | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned ... using a centrally generated computer randomization code" (patients and methods, paragraph 4, page 4225) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...open‐label, phase III trial..." (patients and methods, paragraph 1, page 4225) (i) ORR/TTP: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP: High Quote: "The response was assessed only by the investigators" (patients and methods, paragraph 8, page 4225) Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTP): Low Quote: " Imaging studies ... were repeated every 12 weeks during treatment" (patients and methods, paragraph 7, page 4225) (ii) Survival (influences OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: High Quote: "Patients were evaluated for adverse events before oxaliplatin administration" (patients and methods, paragraph 5, page 4225) Quote: "XELOX consisted of oral capecitabine ... ... plus oxaliplatin ... on day 1 every 3 weeks. FUOX consisted of FU ... ... plus oxaliplatin ... on days 1, 15, and 29 every 6 weeks" (patients and methods, paragraph 4, page 4225) Therefore, participants were evaluated for AEs more frequently in the FUOX arm than in the XELOX arm |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low 13% of patients in the XELOX arm and 9% of patients in the FUOX arm were not assessable (Table 2, page 4227) (ii) TTP/OS: Low Although not defined in Methods, censoring was noted in the KM curves (Fig 1 & 2, page 4227). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Quote: “Safety was evaluated in all patients who received treatment..." (results, paragraph 5, page 4227) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "...348 patients (intent‐to‐treat population) were ... randomly assigned to treatment: 174 to XELOX and 174 to FUOX. Six patients (three in each treatment arm) did not initiate study treatment, leaving 342 patients who constituted the per‐protocol population" (results, paragraph 1, page 4225) Quote: "The primary statistical analysis of efficacy was ... between groups in the per‐protocol population" (patients and methods, paragraph 9, page 4225) Safety analysis: Quote: “Safety was evaluated in all patients who received treatment (XELOX, n = 171; FUOX, n = 171) ..." (results, paragraph 5, page 4227) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ presented as KPS ≤ 70% vs > 70% (Table 1, page 4226) (ii) Median age: Low (Table 1, page 4226) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Low risk | Subsequent therapies: Low Quote: "A total of 199 patients (58.2%) received second‐line chemotherapy: 99 patients (57.9%) in the XELOX arm and 100 patients (58.5%) in the FUOX group" (results, paragraph 4, page 4226) Other Quote: "... significantly more patients in the XELOX arm (26%) than in the FUOX arm (16%) had received previous adjuvant chemotherapy (P = .032), which consisted of fluoropyrimidine therapy with or without LV" (results, paragraph 1, page 4225) |
| Methods | Randomised controlled trial Phase: III Accrual dates: June 1995 to August 1997 | |
| Participants | No. randomised: 816 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation, 85 sites ‐ USA and Puerto Rico (n = 466), Canada (n = 100), Europe (n = 250) Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age > 18 years (median 64/64 years); male (61/60%); PS ECOG 0‐2 (PS ECOG 0: 45/43%) | |
| Interventions | Arm I (UFT/LV): UFT 300 mg/m2/d and LV 75 mg/d (US) or 90 mg/d (non‐USA countries) D1‐28, q35d (n randomised = 409) Arm II (5‐FU/LV): 5‐FU 425 mg/m2/d plus LV 20 mg/m2/d D1‐5, q28d (n randomised = 407) | |
| Outcomes | OS TTP ORR (WHO criteria, modified) Grade ≥ 3 AEs (CTC, version not specified) Median follow‐up: Not specified. | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Taiho Pharmaceutical Company, Bristol‐Myers Squibb Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were centrally randomized ..." (patients and methods, paragraph 2, page 3607) |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/TTP: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/TTP: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTP): High Quote: "Tumor reassessment, including tumor measurements and a computed tomography scan of the Quote: "In the UFT/LV treatment arm ...cycles repeated every 35 days...In the 5‐FU/LV treatment arm ... cycles repeated every 28 days" (patients and methods, paragraph 3, page 3607) Therefore, responses were evaluated more frequently in the 5‐FU/LV arm (ii) Survival (influences OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low 1 patient in the 5‐FU/LV arm (< 1%) and no patients in the UFT/LV arm were not assessable (Table 2, page 3608) (ii) TTP/OS: Low Although not defined in the Methods, censoring was noted in the KM curves (Figure 1, page 3610). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Other than data for LFTs, which were not of interest for the review, relevant AEs have < 7% of safety outcome data missing (Table 4, 5, and 6, pages 3611 and 3612) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "All efficacy analyses are presented by treatment arm as randomized" (patients and methods, paragraph 8, page 3608) Safety analysis: Quote: "All 802 patients who received at least one dose of study medication were evaluated for safety and were analyzed based on the treatment arm as treated" (results, paragraph 18, page 3610) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 1, page 3608) (ii) Median age: Low (Table 1, page 3608) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Low risk | Subsequent therapies: Low Quote: "Secondary chemotherapy was administered to 52% of patients assigned to UFT/LV and 50% of patients assigned to 5‐FU/LV. Information about the type of drugs included in subsequent chemotherapy was not collected" (results, paragraph 17, page 3610) |
| Methods | Randomised controlled trial Phase: II, non‐comparative Accrual dates: February 2007 to June 2008 | |
| Participants | No. randomised: 302 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation; Argentina, Australia, Austria, Belgium, Brazil, France, Germany, Greece, Hong Kong, Israel, Italy, Mexico, Poland, Thailand Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age ≥18 years (median 60.0/61.5 years); male (63/63%); KPS ≥ 60 (PS ECOG 0: 79/79%) | |
| Interventions | Arm I (UFOX + cetuximab): D1 cetuximab (loading dose 400 mg/m2 then 250 mg/m2 weekly, thereafter) followed by oxaliplatin 85 mg/m2 D1 and 15, UFT (tegafur 250 mg/m2/d, uracil 560 mg/m2/d) and folinic acid 90 mg/d D1‐21, q28d (n randomised = 152) Arm II (FOLFOX4 + cetuximab): D1 cetuximab (as per Arm I) followed by oxaliplatin 85 mg/m2 D1 and 15, folinic acid 200 mg/m2, IV bolus 5‐FU 400 mg/m2 and 5‐FU 600 mg/m2 22‐hour infusion D1, 2, 15, and 16, q28d (n randomised = 150) Treatment continued until disease progression, withdrawal of consent, or unacceptable toxicity | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (NCI CTC, version 3.0) Median follow‐up: Clinical cut‐off for PFS ‐ 30 June 2009; clinical cut‐off for OS ‐ 31 August 2011 | |
| Study Details | Journal article and abstract/poster | |
| Funding sources and declarations of interest | Funding sources: Merck KGaA Declarations of interest: Merck‐Serono, Roche, Amgen | |
| Notes | Of note, this study was designed before demonstration of KRAS mutation status was a predictive biomarker for cetuximab response. Enrollment was therefore independent of KRAS mutation status. Additionally, recruitment was curtailed after demonstration of KRAS mutation status as a predictive biomarker for cetuximab response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomly assigned ... using a centralized stratified permuted block randomization procedure" (patients and methods, paragraph 4, page 15) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...open‐label phase II study" (patients and methods, paragraph 4, page 15) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High The study was not blinded, and independent review was not performed (correspondence with Dr. Peter Eggleton, received 22 July 2012) Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/PFS): Low Quote: "Investigators assessed response to treatment every 8 weeks based on radiological imaging (CT or MRI scans)..." (patients and methods, paragraph 6, page 16) Quote: "After permanent treatment cessation, patients were followed every 3 months to collect data on progression..." (patients and methods, paragraph 6, page 16) Quote: "This particular regimen of UFT and oxaliplatin was chosen to allow a 4‐week dosing cycle, which ensured that all assessments were carried out at the same time in each treatment arm" (patients and methods, paragraph 5, page 15) Survival assessment was performed 6 weeks after final tumour assessment (correspondence with Dr. Peter Eggleton, received 2 August 2012) Subsequently, quote: "After permanent treatment cessation, patients were followed every 3 months to collect data on ... survival ..." (patients and methods, paragraph 6, page 16) (iii) Grade ≥ 3 AEs: Low Assessments for adverse events followed identical schedules (correspondence with Dr. Peter Eggleton, received 2 August 2012) |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low Similar proportions of participants were not evaluable in both arms (Table 3, page 20) (ii) PFS/OS: Low Although not defined in the Methods, censoring was noted in the KM curves for PFS/OS (Figure 2, page 21). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Data related to grade ≥ 3 adverse events were collected until the clinical cut‐off date (30 June 2009). No missing outcome data before that time (correspondence with Dr. Peter Eggleton, received 24 July 2016) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The primary efficacy analysis of PFS ... was carried out on the intention‐to‐treat (ITT) population, comprising all randomized patients" (patients and methods, paragraph 9, page 16) Safety analysis: Quote: "Safety analyses were performed on the safety population, which comprised all randomized patients who received any dose of any study treatment" (patients and methods, paragraph 10, page 16) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | High risk | (i) PS: Low (Table 1, page 18) (ii) Median age: Low (Table 1, page 18) (iii) No. organs involved: Low (Table 1, page 18) (iv) KRAS mut: High Information on KRAS mut status was available for 93/150 (62%) of the ITT population in the FOLFOX4 + cetuximab arm and 87/152 (57%) of the ITT population in the UFOX + cetuximab arm (Figure 1, page 17) A greater proportion of participants with known KRAS mutant status in the UFOX + cetuximab arm 47/87 (54%) were KRAS mutant than in the FOLFOX4 + cetuximab arm 37/93 (40%) – 14% difference (Table 1, page 18) |
| Other bias | Low risk | Subsequent therapies: Low A similar proportion of participants received different types of subsequent second‐line therapy after disease progression, in each arm (supplementary table 1) |
| Methods | Randomised controlled trial Phase: III Accrual dates: May 2003 to August 2004 | |
| Participants | No. randomised: 306 Stage/treatment line: Metastatic, first‐line Countries/sites: France, 33 sites Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age ≥ 18 years (median 66/64 years); male (64/60%); PS ECOG ≤ 2 (PS ECOG 0‐1 92/93%) | |
| Interventions | Arm I (XELOX): oxaliplatin 130 mg/m2 D1 plus oral capecitabine 1000 mg/m2 bd D1‐14, q21d (n randomised = 156) Arm II (FOLFOX): 6 D1 oxaliplatin 100 mg/m2, leucovorin 400 mg/m2, IV bolus 5‐FU 400 mg/m2, and 5‐FU 2400‐3000 mg/m2 46‐hour infusion, q14d (n randomised = 150) Treatment was continued for 24 weeks or until disease progression, whichever came first | |
| Outcomes | ORR (RECIST, version 1.0) PFS OS Grade ≥ 3 AEs (NCI‐CTC, version 3) Median follow‐up: 18.8 months for ITT population, all outcomes | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Roche Declarations of interest: Roche | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned in a 1:1 ratio to a treatment group by centralised, adaptive randomisation (minimisation method) ..." (materials and methods, paragraph 3, page 683) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...open‐label study" (materials and methods, paragraph 1, page 683) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low Quote: "Tumour responses were validated in a centralised, blinded review of CT scans by an independent response committee (IRC)" (materials and methods, paragraph 7, page 684) (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): High Quote: "Tumour assessments (CT scan and MRI) were ... repeated at cycles 3 and 6 in the XELOX group and cycles 4 Quote: "XELOX ...every 3 weeks. FOLFOX‐6 ...every 2 weeks" (materials and methods, paragraph 4, page 683) Therefore, the first and second response assessments were performed later in the XELOX arm (weeks 9 and 18) compared with the FOLFOX‐6 arm (weeks 8 and 16) (ii) Survival (influences PFS/OS): Low Quote: "After completion of study treatment, patients were reassessed every 3 months until 18 months after the end of treatment for the last randomised patient" (materials and methods, paragraph 7, page 684) (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low Quote: "...a small imbalance between the two treatment groups with respect to the percentage of patients not assessable for response (12.2% in XELOX vs. 8.7% in FOLFOX‐6)..." (discussion, paragraph 2, pages 687 and 688) (ii) PFS/OS: Low Although not defined in the Methods, censoring was noted in the KM curves (Figures 2 and 3, pages 687 and 688). No evidence of bias related to censoring. (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The intent‐to‐treat (ITT) population included all patients who underwent randomisation" (materials and methods, paragraph 10, page 684) Safety analysis: Quote: "All patients receiving at least one dose of study treatment were included in the safety population" (materials and methods, paragraph 10, page 684) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear Reported as ECOG 0‐1 vs 2 (Table 2, page 686) (ii) Median age: Low (Table 2, page 686) (iii) No. of involved organs: Low (Table 2, page 686) |
| Other bias | Unclear risk | Subsequent therapies: Unclear Quote: "Although the protocol did not include monitoring of second‐line therapies following study drug discontinuation, the possibility that second‐line therapies may have influenced the OS results cannot be ruled out" (discussion, paragraph 1, page 687) |
| Methods | Randomised controlled trial Phase: II, non‐comparative Accrual dates: March 2006 to January 2008 | |
| Participants | No. randomised: 145 Stage/treatment line: Metastatic, first‐line Countries/sites: 15 centres in France, La Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) study Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age 18 to 75 years (median 61/61 years); male (64/48%); PS ECOG 0‐2 (PS ECOG 0: 54/60%) | |
| Interventions | Arm I (XELIRI + bevacizumab (BEV)): irinotecan 200 mg/m2 D1 and capecitabine 1000 mg/m2 (800 mg/m2 if ≥ 65 years) bd D1‐14 plus BEV 7.5 mg/kg D1, q21d for a maximum of 8 cycles (n randomised = 72) Arm II (FOLFIRI + BEV): Irinotecan 180 mg/m2, leucovorin 400 mg/m2, and IV bolus fluorouracil 400 mg/m2 followed by 2400 mg/m2 46‐hour infusion plus 5 mg/kg BEV D1, q14d for a maximum of 12 cycles (n randomised = 73) For participants whose disease was controlled after 6 months of BEV and chemotherapy, BEV (7.5 mg/kg every 3 weeks) was continued as a single‐agent maintenance therapy in both arms until progressive disease | |
| Outcomes | PFS ‐ 6 months, PFS Grade ≥ 3 AEs (NCI CTCAE, version 3.0) ORR (RECIST, version 1.0) OS Median follow‐up: 36 months for both Arm I/II; final analysis 15 March 2010 | |
| Study Details | Journal article, abstract, oral poster presentation, and journal article for translational sub‐study | |
| Funding sources and declarations of interest | Funding sources: Roche, Pfizer, and Chugai Declarations of interest: Roche, Chugai, Pfizer, Amgen, Boeringer, Merck Serono | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using the minimisation method (using a 10% random factor) (correspondence with Dr. Jean‐Pierre Pignon, received 1 August 2012) |
| Allocation concealment (selection bias) | Low risk | Central randomisation by fax was used (correspondence with Dr. Jean‐Pierre Pignon, received 1 August 2012) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "... open‐label, non‐comparative phase II study" (patients and methods, paragraph 1, page 1237) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): Low Quote: "Tumour assessments using abdominal and/or thoracic computed tomography (CT) or magnetic resonance imaging (MRI) were performed at baseline and every 8 weeks until progression" (patients and methods, paragraph 5, page 1238) (ii) Survival (influences PFS/OS): Low Quote: "After disease progression, patients were followed up at least every 2 months until death" (patients and methods, paragraph 4, page 1238) (iii) Grade ≥ 3 AEs: High Quote: "During treatment, physical examination, ECOG performance status, BP, and blood and biochemistry analyses were repeated every cycle" (patients and methods, paragraph 4, page 1237) Quote: "treatment with either ... XELIRI ... every 3 weeks ... or FOLFIRI ... every 2 weeks" (patients and methods, paragraph 3, page 1237) Quote: "During bevacizumab maintenance, clinical examination, BP, blood/urine analysis and ECOG performance status were performed every 3 weeks" (patients and methods, paragraph 4, page 1237 and 1238) Therefore, safety evaluation was performed more frequently in the FOLFIRI + BEV group during combination treatment Safety assessment was 3‐weekly during treatment with BEV alone for both arms |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low Outcome data were available for all randomised participants, including those who had 'early stopping' (correspondence with Dr. Jean‐Pierre Pignon, received 1 August 2012) (ii) PFS/OS: Low As above (iii) Grade ≥ 3 AEs: Low Quote: "All 145 patients were evaluable for safety..." (results, paragraph 5, page 1239) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Analysis was according to treatment as randomised (Fig. 1, page 1239) Safety analysis: Analysis was according to treatment as randomised (Fig. 1, page 1239) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 1239) (ii) Median age: Low (Table 1, page 1239) (iii) No. of involved organs: Low (Table 1, page 1239) |
| Other bias | Low risk | Similar proportions of participants in the XELIRI + BEV and FOLFIRI + BEV groups received different types of subsequent drug therapy following progression on first‐line treatment (supplementary material, supplementary Tables 1 and 2, pages 4‐7) |
| Methods | Randomised controlled trial Phase: III Accrual dates: April 1999 to September 2000 (closed) | |
| Participants | No. randomised: 125 of planned 950 Stage/treatment line: Metastatic, first‐line Countries/sites: USA, 24 study sites and one Expanded Participation Project (EPP) site Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal cancer; age ≥ 18 years (median 64/65 years); male (64/62%); PS ECOG 0‐2 (PS ECOG 0: 45/43%) | |
| Interventions | Arm A: Continuous 5‐FU infusion 300 mg/m2/d D1‐28, q35d (n randomised = 64) Arm B: Oral eniluracil 11.5 mg/m2 and oral 5‐FU 1.15 mg/m2 bd D1‐28, q35d (n randomised = 61) | |
| Outcomes | ORR (ECOG Solid Tumour Response Criteria) PFS OS Grade ≥ 3 AEs (CTC, version 2.0) Median follow‐up: 1.3 years, all outcomes | |
| Study Details | Technical report and study protocol | |
| Funding sources and declarations of interest | Funding sources: National Cancer Institute, DHHS Declarations of interest: None declared | |
| Notes | This study was closed early, after accrual of 125 of a planned 950 participants, following results of the Van Cutsem 2001a and Schilsky 2002a studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with permuted blocks within strata algorithm, with override protection for treatment imbalances that could occur within the main institutions of the cooperative group due to the stratified algorithm (correspondence with Dr. Paul Catalano, received 10 July 2012) |
| Allocation concealment (selection bias) | Low risk | Permuted blocks of undisclosed size were generated within strata. Additionally, accruals occurred over time across many treating centres, making it very unlikely that one could decode the randomisation algorithm and predict the next assignment in the sequence (correspondence with Dr. Paul Catalano, received 10 July 2012) |
| Blinding of participants and personnel (performance bias) | High risk | No blinding occurred in the trial (correspondence with Dr. Paul Catalano, received 10 July 2012) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding (i) ORR/PFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/PFS): Low Response assessments were performed every 10 weeks. For those with measurable disease, if a CR/PR was achieved, this was confirmed after 4 weeks (E5296 study protocol, page 14) (ii) Survival (influences OS): Low Post‐treatment follow‐up was the same for both arms: every 3 months if < 2 years from study entry, every 6 months if 2 to 5 years from study entry, and every 12 months if > 5 years from study entry (E5296 study protocol, page 20) (iii) Grade ≥ 3 AEs: Low Complete blood count (CBC) was examined weekly and other AE assessments were performed before each treatment cycle (E5296 protocol, page 14). One cycle was every 35 days for both arms (E5296 study protocol, page 6) |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low The sum of those with unevaluable and unknown responses was 5/62 (8%) in the IV 5‐FU arm and 3/61 (5%) in the oral 5‐FU + eniluracil arm (Table 11, page 22) (ii) PFS/OS: Low Survival outcomes were known for all participants who were eligible, were ineligible, and had withdrawn (correspondence with Dr. Paul Catalano, received 10 July 2012) Quote: "Toxicity data was submitted for 63 patients randomized to arm A and 59 patients randomized to arm B" (results, paragraph 6, page 9) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Presented results were analysed according to allocated treatment (section 7.1, page 8 and Table 1, page 12). This excluded patients who were found to be ineligible (after randomisation) and who withdrew from the study before treatment (2/125, 1.6%) (results, paragraph 1, page 8 and Table 1, page 12) Safety analysis: Analysis was performed for those who had toxicity data submitted (results, paragraph 6, page 9, and Table 8, pages 20 and 21) |
| Selective reporting (reporting bias) | Low risk | The same outcomes were reported in the study protocol and in the technical report |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 3, page 13) (ii) Median age: Low (Table 3, page 13) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Design: For Period 1 (the only period of interest for this review), 3 × 2 factorial design with randomisation to FOLFIRI vs mIFL vs CapeIRI (open‐label), and randomisation to celecoxib vs placebo (double‐blind) Accrual dates: Period 1 February 2003 to March 2004 | |
| Participants | No. randomised: 430 Stage/treatment line: Metastatic, first‐line Countries/sites: For Periods 1 and 2, participants were enrolled in USA, Canada, Australia, and New Zealand, at 99 sites Setting: Hospital Characteristics (Arm A/B/C): Metastatic colorectal cancer; age ≥ 18 years (median 61/62/62 years); male (63.9/58.9/54.5%); PS ECOG 0‐1 (PS ECOG 0: 52.1/49.6/48.3%) | |
| Interventions | Arm A (FOLFIRI + *celecoxib/placebo): irinotecan 180 mg/m2, LV 400 mg/m2, IV bolus FU 400 mg/m2, followed by 5FU 2400 mg/m2 46‐hour infusion, q14d (n randomised = 144) Arm B (mIFL + celecoxib/placebo): irinotecan 125 mg/m2, LV 20 mg/m2, IV bolus FU 500 mg/m2 D1 and 8, q21d (n randomised = 141) Arm C (CapeIRI + celecoxib/placebo): irinotecan 250 mg/m2 D1 and oral capecitabine 1000 mg/m2 bd D1‐14, q21d (n randomised = 145) *Oral celecoxib 400 mg bd or placebo tablets; permanently discontinued on 19 January 2005. Treatment continued until PD, unacceptable toxicity from chemotherapy, or withdrawal of consent | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (NCI CTC, version 2.0) Median follow‐up: 34 months (cut‐off date Nov 17 2006) | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Pfizer Declarations of interest: Pfizer, Sanofi‐Aventis, Genentech, AstraZeneca, Bristol‐Myers Squibb, Amgen, Imclone Systems Inc, Roche, Boerhinger Ingelheim | |
| Notes | Study was terminated after enrolment of 547 of a planned 900 participants. Accrual to this trial had slowed after report of cardiovascular concerns with celecoxib, despite celecoxib/placebo administration for all participants was permanently discontinued in January 2005 16 participants (11.1%) in Arm I chose to have bevacizumab after the study amendment (bevacizumab 5 mg/kg IV on D1, repeated every 2 weeks), and 7 participants (5.0%) in Arm II chose to have bevacizumab after the study amendment (bevacizumab 7.5 mg/kg IV on D1, repeated every 3 weeks) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "...randomly assigning patients to one of three open‐label chemotherapy arms ..." (patients and methods, paragraph 2, page 4780) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): Low Quote: "During chemotherapy, a follow‐up CT/MRI of the abdomen/pelvis and chest x‐ray or chest CT/MRI were to be performed every 6 weeks. Assessments were performed every 6 weeks until PD or on chemotherapy discontinuation" (patients and methods, paragraph 10, page 4781) (ii) Survival (influences PFS/OS): Low Quote: "After PD, the patient was observed every 3 months for survival" (patients and methods, paragraph 10, page 4781) (iii) Grade ≥ 3 AEs: High Participants were reviewed for safety assessments every week during the first cycle, and then every cycle (communication with Dr. Justin Binko, received 26 July 2012) Quote: "...FOLFIRI ... repeated every 2 weeks ... mIFL ... repeated every 3 weeks. CapeIRI ... repeated every 3 weeks" (patients and methods, paragraph 5, page 4780) Therefore, following cycle 1, participants in the FOLFIRI arm underwent more frequent safety evaluations than those in the mIFL and CapeIRI arms |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Unclear ‐ not specified (ii) PFS/OS: Low PFS‐ Quote: "For patients without documented PD, data were censored on the date of the last tumor assessment with nonprogression status or, for patients who started a second‐line therapy, at the date of the start of new therapy" (patients and methods, paragraph 11, page 4781). No evidence of bias related to censoring OS‐ Quote: "...in the absence of confirmation of death, data were censored at the last date the patient was known to be alive" (patients and methods, paragraph 12, page 4781). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "Efficacy analyses included all patients randomly assigned on an intent‐to‐treat basis" (patients and methods, paragraph 14, page 4781) Safety analysis: Quote: "Safety analyses included all treated patients ..." (patients and methods, paragraph 14, page 4781) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 2, page 4783) (ii) Median age: Low (Table 2, page 4783) (iii) No. of involved organs: Unclear ‐ not specified A similar median number of measurable target metastatic lesions for all 3 treatment arms was reported in a published author reply, quote: "The median number of measurable target metastatic lesions was 3.2 for FOLFIRI, 3.1 for mIFL, and 3.1 for CapeIRI" (Fuchs et al, Journal of Clinical Oncology 2008, paragraph 2) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "The rates of utilization of poststudy salvage chemotherapy did not differ significantly between the three first‐line chemotherapy arms in period 1 (77% for FOLFIRI, 75% for mIFL, and 77% for CapeIRI)" (results, paragraph 4, page 4783) Other bias: Some participants in the FOLFIRI and mIFL arms from period 1 received BEV, although this was only a small percentage in each arm Quote: "After activation of this study amendment, patients randomly assigned to FOLFIRI or mIFL during period 1 had the option of adding bevacizumab to their current regimen. Among patients enrolled during period 1, 16 patients on the FOLFIRI arm added bevacizumab to their regimen, and seven patients on the mIFL arm added bevacizumab to their regimen" (patients and methods, paragraph 6, page 4780) Risk of bias considerations in a factorial study: Unclear ‐ tests for interaction not specified |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: December 2002 to November 2003 | |
| Participants | No. randomised: 150 Stage/treatment line: Metastatic, first‐line Countries/sites: USA, 33 sites Setting: Hospital Characteristics (Arm I/II/III): Metastatic colorectal cancer; age ≥ 18 years (median 62/62/62.5 years); male (57/62/65%); PS ECOG 0‐1 (PS ECOG 0: 61/58/52%) | |
| Interventions | Arm I (mFOLFOX6): oxaliplatin 85 mg/m2, LV 350 mg, IV bolus FU 400 mg/m2 and 2400 mg/m2 46‐hour infusion, q14d (n randomised = 50) Arm II (bFOL): oxaliplatin 85 mg/m2 D1 and 15, LV 20 mg/m2, IV bolus FU 500 mg/m2 D1, 8, and 15, q28d (n randomised = 50) Arm III (CapeOx): oxaliplatin 130 mg/m2 D1 and capecitabine 1000 mg/m2 bd D1‐15, q21d (n randomised = 50) Treatment continued until PD, unacceptable toxicity, extended toxicity‐related dose delay or withdrawal of consent | |
| Outcomes | ORR (RECIST, version 1.0) TTP (treated as PFS in this review, based on the definition provided) Grade ≥ 3 AEs (NCI CTC, version 2.0) OS Median follow‐up: All outcomes ‐ 16.9, 15.1, 15.0 months, for Arms 1 through 3, respectively | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Sanofi‐Aventis Declarations of interest: Sanofi‐Aventis, Genentech BioOncology, Bristol Myers‐Squibb, Taiho, Samyang Confirma Biotech, Amgen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "TREE‐1 and TREE‐2 were two sequentially conducted, randomized, open‐label cohorts in this study" (patients and methods, paragraph 1, page 3524) (i) ORR/TTP (treated as PFS): Low Outcome assessment unlikely to be influenced by lack of blinding This study was not used for the meta‐analysis of the TTP (treated as PFS) outcome (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP (treated as PFS): High Outcome assessment at risk of bias from lack of blinding This study was not used for the meta‐analysis of the TTP (treated as PFS) outcome (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTP (treated as PFS)): Low Quote: "Tumor assessments were repeated every 12 weeks in TREE‐1 ..." (patients and methods, paragraph 5, page 3524) This study was not used for the meta‐analysis of the TTP (treated as PFS) outcome (ii) Survival (influences TTP (treated as PFS)/OS): High Quote: "After treatment discontinuation, patients in TREE‐2 were followed for survival at 3‐month intervals for at least 2 years and every 6 months thereafter until lost to follow‐up or consent withdrawal; these data were collected for patients in TREE‐1 who consented retrospectively" (patients and methods, paragraph 5, page 3524) No information was provided regarding the relative numbers of participants in each arm of TREE‐1 who consented retrospectively This study was not used for meta‐analysis of the TTP (treated as PFS) outcome (iii) Grade ≥ 3 AEs: High Quote: "Clinical assessments and toxicities were recorded on day 1 of each cycle and at the end of treatment" (patients and methods, paragraph 5, page 3524) Quote: "In TREE‐1, patients received mFOLFOX6 ... every 2 weeks ..., bFOL ... every 4 weeks ... , or CapeOx ... every 3 weeks ..." (patients and methods, paragraph 1, page 3524) Therefore, participants underwent safety evaluations more frequently in the mFOLFOX arm |
| Incomplete outcome data (attrition bias) | High risk | (i) ORR: High 23% of participants were missing reported confirmed response data in the CapeOx arm (Table 4, page 3527) (ii) TTP (treated as PFS): Low Quote: "TTP was censored at the last date the patient was known to be progression free for patients who did not have objective tumor progression and who were either still on study at the time of the analysis or who were removed from follow‐up before documentation of objective tumor progression. For patients who received second‐line treatment prior to progression or death, TTP was censored at the time of starting the new therapy" (Table 4, page 3527) No evidence of bias related to censoring This study was not used for the meta‐analysis of TTP (treated as PFS) outcome OS: Unclear ‐ Not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "All analyses are for the as‐treated population, which includes all randomly assigned patients receiving at least one treatment" (patients and methods, paragraph 6, page 3524) Quote: "In TREE‐1, 147 of 150 patients were treated (one was ineligible for prior chemotherapy and two did not start treatment)" (results, paragraph 2, page 3524) Therefore, 2% of randomised participants were excluded from the efficacy analysis population Safety analysis: Same as for efficacy |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 1, page 3525) (ii) Median age: Low (Table 1, page 3525) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified for the different arms within TREE‐1 |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: November 2003 to April 2004 | |
| Participants | No. randomised: 223 Stage/treatment line: Metastatic, first‐line Countries/sites: USA, 57 sites Setting: Hospital Characteristics (Arm I/II/III): Metastatic colorectal cancer; age ≥ 18 years (median 64/57/62 years); male (61/49/58%); PS ECOG 0‐1 (PS ECOG 0: 61/54/65%) | |
| Interventions | Arm I (mFOLFOX6 + bevacizumab (BEV)): oxaliplatin 85 mg/m2, LV 350 mg, IV bolus 5FU 400 mg/m2, and 5‐FU 2400 mg/m2 46‐hour infusion, q14d + BEV 5 mg/kg D1, q14d (n randomised = 75) Arm II (bFOL + BEV): oxaliplatin 85 mg/m2 D1 and 15, LV 20 mg/m2, IV bolus FU 500 mg/m2 D1, 8, and 15, q28d + BEV 5 mg/kg D1, q14d (n randomised = 74) Arm III (CapeOx + BEV): oxaliplatin 130 mg/m2 D1 and capecitabine 850 mg/m2 bd D1‐15, q21 plus BEV 7.5 mg/kg D1, q21d (n randomised = 74) Treatment continued until PD, unacceptable toxicity, extended toxicity‐related dose delay, or withdrawal of consent | |
| Outcomes | ORR (RECIST, version 1.0) TTP (treated as PFS in this review, based on the definition provided) Grade ≥ 3 AEs (NCI CTC, version 2.0) OS Median follow‐up: All outcomes, 17.9, 17.6 and 18.5 months in Arm I‐III, respectively. | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Sanofi‐Aventis Declarations of interest: Sanofi‐Aventis, Genentech BioOncology, Bristol Myers‐Squibb, Taiho, Samyang Confirma Biotech, Amgen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "TREE‐1 and TREE‐2 were two sequentially conducted, randomized, open‐label cohorts in this study" (patients and methods, paragraph 1, page 3524) (i) ORR/TTP (treated as PFS): Low Outcome assessment unlikely to be influenced by lack of blinding This study was not used for the meta‐analysis of the TTP (treated as PFS) outcome (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP (treated as PFS): High Outcome assessment at risk of bias from lack of blinding This study was not used for meta‐analysis of the TTP (treated as PFS) outcome (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTP (treated as PFS)): Low Quote: "Tumor assessments were repeated ... every 6 weeks in TREE‐2" (patients and methods, paragraph 5, page 3524) This study was not used for meta‐analysis of the TTP (treated as PFS) outcome (ii) Survival (influences TTP (treated as PFS)/OS): Low Quote: "After treatment discontinuation, patients in TREE‐2 were followed for survival at 3‐month intervals for at least 2 years and every 6 months thereafter until lost to follow‐up or consent withdrawal ..." (patients and methods, paragraph 5, page 3524) This study was not used for the meta‐analysis of the TTP (treated as PFS) outcome. (iii) Grade ≥ 3 AEs: High Quote: "Clinical assessments and toxicities were recorded on day 1 of each cycle and at the end of treatment" (patients and methods, paragraph 5, page 3524) Quote: "In TREE‐1, patients received mFOLFOX6 ... every 2 weeks ..., bFOL ... every 4 weeks ... , or CapeOx ... every 3 weeks ... In TREE‐2, patients received one of the same three chemotherapy regimens as in TREE‐1 but with the addition of bevacizumab ..." (patients and methods, paragraph 1, page 3524) Therefore, participants underwent safety evaluations more frequently in the mFOLFOX arm |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low Confirmed tumour response data were reported for ≥ 85% of participants in all arms (Table 4, page 3527) (ii) TTP (treated as PFS): Low Quote: "TTP was censored at the last date the patient was known to be progression free for patients who did not have objective tumor progression and who were either still on study at the time of the analysis or who were removed from follow‐up before documentation of objective tumor progression. For patients who received second‐line treatment prior to progression or death, TTP was censored at the time of starting the new therapy" (Table 4, page 3527) There was no evidence of bias related to censoring This study was not used for meta‐analysis of the TTP (treated as PFS) outcome OS: Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | High risk | Efficacy analysis: High Quote: "All analyses are for the as‐treated population, which includes all randomly assigned patients receiving at least one treatment" (patients and methods, paragraph 6, page 3524). 4/75 (5.3%) , 4/74 (5.4%) and 2/74 (3%) patients were excluded from the mFOLFOX6 + BEV, bFOL + BEV and CapeOx + BEV arms, respectively (from results, paragraph 1, page 3524 and Table 2, page 3525) Safety analysis: Same as for efficacy |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. |
| Similarity of arms at baseline | High risk | (i) PS: Low (Table 1, page 3525) (ii) Median age: High 5‐year difference between bFOL + BEV and CapeOx + BEV arms (Table 1, page 3525) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified for the different arms within TREE‐2 |
| Methods | Randomised controlled trial Phase: III Accrual dates: September 1996 to February 1998 | |
| Participants | No. randomised: 605 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation, 61 sites ‐ USA (n = 48), Canada (n = 9), Brazil (n = 2), and Mexico (n = 2) Setting: Hospital Characteristics (Arm I/II/): Metastatic colorectal cancer; age ≥ 18 years (median 64/63 years); male (59.9/65.0%); KPS ≥ 70% (median 90/90%) | |
| Interventions | Arm I: capecitabine 1250 mg/m2 bd D1‐14, q21d (n randomised = 302) Arm II: IV bolus 5‐FU 425 mg/m2 plus LV 20 mg/m2 D1‐5, q28d (n randomised = 303) Treatment continued until PD, unacceptable toxicity, or scheduled assessment at 30 weeks. Participants with a tumour response or SD were allowed to enter a continuation phase up to a total of 48 weeks. Treatment continuation beyond 48 weeks (post‐continuation phase) for participants without PD was provided at the discretion of the investigator | |
| Outcomes | ORR (WHO criteria, 1979) TTP (treated as PFS in this review, based on the definition provided) OS Grade ≥ 3 AEs (NCI CTC, revised December 1994) No details on median follow‐up | |
| Study Details | Journal articles | |
| Funding sources and declarations of interest | Funding sources: F. Hoffman‐La Roche Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomly assigned to treatment with capecitabine or 5‐FU/LV according to a computer‐generated randomization code" (patients and methods, paragraph 3, page 2283) |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomized centrally by country in blocks of four patients" (patients and methods, paragraph 3, page 2283) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "... open‐label, randomized, parallel‐group study ..." (patients and methods, paragraph 3, page 2283) (i) ORR/TTP (treated as PFS): Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP (treated as PFS): Low Quote: "Investigator assessments of tumor response were reviewed, solely on the basis of imaging, by an independent review committee (IRC) composed of radiologists who were blinded to the treatment received, the clinical condition of the patient, and the investigator’s evaluation" (patients and methods, paragraph 8, page 2284) The absolute ORR was higher for capecitabine than for 5‐FU/LV for both of these assessments (Table 2, page 2285) (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/TTP (treated as PFS)): Low Quote: "Assessments of tumor dimensions and involved sites were performed before the start of treatment and were scheduled after weeks 6, 12, 18, 24, and 30 of therapy. Further assessments were performed after weeks 39 and 48 for patients who received prolonged therapy (up to 48 weeks). Follow‐up assessments for disease progression and survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 8, page 2284) (ii) Survival (influences TTP (treated as PFS)/OS): Low Quote: "Follow‐up assessments for ... survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 8, page 2284) (iii) Grade ≥ 3 AEs: Low Quote: "Safety evaluations were conducted at least monthly until 4 weeks after the end of therapy..." (patients and methods, paragraph 9, page 2284) |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low For Investigator assessed responses, missing post‐baseline data for 7.3% of participants in the capecitabine arm and for 12.5% in the 5‐FU/LV arm (Table 2, page 2285) (ii) TTP (treated as PFS)/OS: Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The analyses of efficacy were based on all randomized patients" (patients and methods, paragraph 12, page 2284) Safety analysis: Quote: "The analyses of toxicity were based on the safety population, which included all patients who received at least one dose of study treatment" (patients and methods, paragraph 13, page 2284) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear KPS was reported as a mean/median (Table 1, page 2285) (ii) Median age: Low (Table 1, page 2285) (iii) No. of organs: Unclear‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Accrual dates: March 2002 to December 2007 | |
| Participants | No. randomised: 401 Stage/treatment type: Rectal adenocarcinoma; adjuvant‐ R0 resection, neoadjuvant‐ cT3‐4 N0 or cTany N+ Countries/sites: Germany, 35 sites Setting: Hospital Characteristics (Arm I/II): Rectal adenocarcinoma; age ≥ 18 years (median 65/64 years); male (65/67%); PS WHO 0‐1 (PS WHO 0: 61/49%) | |
| Interventions | Adjuvant cohort: Arm I: Two cycles of capecitabine 2500 mg/m2 D1‐14, q21d, followed by chemoradiotherapy 50.4 Gy plus capecitabine 1650 mg/m2 D1‐38, then 3 cycles of capecitabine (n randomised and with post‐randomisation data = 116) Arm II: Two cycles IV bolus fluorouracil 500 mg/m2 D1‐5, repeated D29‐33, followed by chemoradiotherapy 50.4 Gy plus infusional fluorouracil 225 mg/m2 daily, then 2 cycles of bolus fluorouracil (n randomised and with post‐randomisation data = 115) Neoadjuvant cohort: Arm I: Chemoradiotherapy (50.4 Gy plus capecitabine 1650 mg/m2 daily), followed by radical surgery and 5 cycles of capecitabine 2500 mg/m2 per day for 14 days (n randomised and with post‐randomisation data = 81) Arm II: Chemoradiotherapy (50.4 Gy plus infusional fluorouracil 1000 mg/m2 D1‐5 and 29‐33), followed by radical surgery and four cycles of bolus fluorouracil 500 mg/m2 for 5 days (n randomised and with post‐randomisation data = 80) Surgery: TME for tumours of the lower two‐thirds of the rectum and PME for the upper third, assuming a 5 cm distal margin without coning, were mandatory for the adjuvant cohort and recommended for the neoadjuvant cohort. For low‐lying tumours, the decision between low anterior resection and abdominoperineal excision was left to the surgeon’s discretion | |
| Outcomes | OS (5 years) DFS Grade ≥ 3 AEs (NCI‐CTC, version 2.0) Median follow‐up: 52 months for all outcomes | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Roche Pharma AG Declarations of interest: Roche Pharma AG, Chugai Pharma, Amgen, Merck KGaA, Ariad, Bristol‐Myers Squibb, Novartis, Merck Sharp & Dohme | |
| Notes | Study protocol was amended in March 2005, to include patients with locally advanced rectal cancer receiving preoperative chemoradiotherapy (neoadjuvant cohort). Recruitment to the adjuvant cohort was continued | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated ... using permuted blocks with stratification by centre and clinical or pathological tumour stage (T3–4 N0 vs T1–2 Npositive vs T3–4 Npositive)" (methods, paragraph 5, page 580) |
| Allocation concealment (selection bias) | Low risk | Quote: "Local investigators were masked to next assignment in the sequence" (methods, paragraph 5, pages 580 and 581) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "... open‐label, non‐inferiority, phase 3 trial ..." (methods, paragraph 1, page 580) (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: High Quote: "The study was open‐label; patients, treating physicians, and data managers and analysts were not masked to group assignment" (methods, paragraph 5, page 581) Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Disease recurrence (influences DFS): Low Quote: "Follow‐up, done for 5 years after the start of therapy..." (methods, paragraph 12, page 582) (ii) Survival (influences DFS/OS): Low Quote: "Follow‐up, done for 5 years after the start of therapy..." (methods, paragraph 12, page 582) (iii) Grade ≥ 3 AEs: High Quote: "Vital signs, haematology, and biochemistry were monitored weekly during chemoradiotherapy and before each chemotherapy cycle" (methods, paragraph 10, page 581) Quote: "Capecitabine was given twice daily ... on days 1–14, and repeated on day 22" (methods, paragraph 8, page 581) Quote: "Fluorouracil bolus was administered on five consecutive days (days 1–5) and repeated on day 29" (methods, paragraph 9, page 581) Therefore, these safety evaluations occurred more frequently in the capecitabine arm |
| Incomplete outcome data (attrition bias) | Low risk | (i) DFS: Low Quote: "DFS was analysed using censored failure times ..." (methods, paragraph 15, page 583). No evidence of bias related to censoring (ii) OS: Low OS was also analysed with censoring using the last date of contact or death (correspondence with Dr. Ralf‐Dieter Hofheinz, received 1 August 2012). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Data were available for all participants in the analysis population (correspondence with Dr. Ralf‐Dieter Hofheinz, received 1 August 2012) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Participants were analysed according to the treatment arm allocated (Figure 3, page 582) However, quote: "All analyses were based on all patients with post‐randomisation data" (methods, paragraph 13, page 582). Therefore, 9/401 (2.2%) participants were excluded from the analyses (Figure 3, page 582) Safety analysis: Same as for efficacy |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 583) (ii) Median age: Low (Table 1, page 583) (iii) TNM stage: Low (Table 1, page 583) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ similarity of therapy following recurrence or new occurrence of disease not specified |
| Methods | Randomised controlled trial Phase: II Accrual dates: November 2007 to February 2010 | |
| Participants | No. randomised: 60 Stage/treatment line: Unresectable primary or metastases (all enrolled patients had metastases); first‐ or second‐line (if second‐line, first‐line therapy with FOLFOX was mandated), patients who had previous adjuvant chemotherapy had > 6 months elapsed since treatment Countries/sites: Japan, from 12 institutes of theTohoku Clinical Oncology Research and Education Society (T‐CORE) Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age 20 to 75 years (median 62.0/62.5 years); male (56.7/60.0%); PS ECOG 0‐1 | |
| Interventions | Arm I (Sequential IRIS‐bevacizumab (BEV)): D1 irinotecan 150 mg/m2 plus BEV 7.5 mg/kg, followed by S‐1 40‐60 mg* oral bd D3‐16, q21d (n randomised = 30) *S‐1 doses: 80 mg/d if BSA < 1.25 m2; 100 mg/d if BSA 1.25 to 1.5 m2; 120 mg/d if BSA > 1.5 m2 Arm II (mFOLFIRI‐BEV): D1 irinotecan 150 mg/m2, LV 200 mg/m2, IV bolus 5‐FU 400 mg/m2, followed by 5‐FU 2400 mg/m2 46‐hour continuous infusion, plus BEV 5 mg/kg D1, q14d (n randomised = 30) | |
| Outcomes | PFS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (AE assessments occurred up to 12 weeks) (CTCAE, version 3.0) Median follow‐up: 324 days (range, 41 to 843 days) | |
| Study Details | Journal article and abstract/poster | |
| Funding sources and declarations of interest | Funding sources: Tohoku Clinical Oncology Research and Education Declarations of interest: Chugai Pharmaceutical Co., Ltd., and Novartis Pharma, Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dynamic allocation was performed (UMIN‐CTR UMIN000000770) |
| Allocation concealment (selection bias) | Low risk | Central allocation was used (UMIN‐CTR UMIN000000770) |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded (UMIN‐CTR UMIN000000770) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low Quote: "Effectiveness was judged comprehensively using blinded tests on the treatment methods by 3 or more physicians not including primary physicians" (patients and methods, paragraph 7, page 103) (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR): Low Imaging was performed at the same time‐points in both arms. Physician assessments for PFS were performed using the same schedule in both arms (correspondence with Dr. Shunsuke Kato, received 15 October 2013) (ii) Survival (influences PFS): Low Physician assessments for PFS were performed using the same schedule in both arms (correspondence with Dr. Shunsuke Kato, received 15 October 2013) (iii) Grade ≥ 3 AEs: High Quote: "With regard to safety data, the patients’ health status was observed and blood samples were tested during weekly medical examinations by the attending physician until 4 weeks after commencing treatment and repeated after the fifth week at the start of each new course of treatment" (patients and methods, paragraph 7, page 103) Treatment cycles were every 3 weeks for the sequential IRIS‐BEV group and every 2 weeks for the mFOLFIRI‐BEV group (patients and methods, paragraph 3, page 103) Therefore, safety evaluations were performed more frequently in the mFOLFIRI‐BEV arm |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low The reason for 'NE' (non‐evaluable) disease (Table 3, page 105) in 16.7% of the sequential IRIS‐BEV group and 13.3% of the mFOLFIRI‐BEV group was non‐measurable disease in all cases. No missing outcome data (correspondence with Dr. Shunsuke Kato, received 15 October 2013) (ii) PFS: Low Participants lost to follow‐up without progression were censored on the last day if it was confirmed that no progression or death occurred (correspondence with Dr. Shunsuke Kato, received 15 October 2013). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low The maximum percentage of participants missing data for the grade ≥ 3 AEs of interest was 16.7% (Table 2, page 105). Similar number of participants were missing data in both arms (correspondence with Dr. Shunsuke Kato, received 15 October 2013) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Analysis for efficacy outcomes kept participants in the intervention groups to which they were randomised, regardless of the intervention received (correspondence with Dr. Shunsuke Kato, received 15 October 2013) Safety analysis: Safety analysis population comprised those with available grade ≥ 3 AE data (correspondence with Dr. Shunsuke Kato, received 15 October 2013) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 1, page 104) (ii) Median age: Low (Table 1, page 104) (iii) No. of involved organs: Unclear ‐ not specified (Similar number of patients with "Number of metastases ‐ 1/2/3" in both arms) (Table 1, page 104) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: October 1997 to February 1999 | |
| Participants | No. randomised: 166 Stage/treatment type: Stage II/III resected rectal adenocarcinoma, adjuvant Countries/sites: South Korea, single site (Yonsei University College of Medicine) Setting: Hospital Characteristics (Arm I/II): Resected rectal adenocarcinoma; age < 70 years (median 52.3/59.5 years); male (61/64%); PS ECOG ≤ 2 | |
| Interventions | Arm I: IV bolus 5‐FU 450 mg/m2/daily and leucovorin 20 mg/m2/d D1‐5, q28d for 12 cycles with *radiotherapy (n randomised = 74) Arm II: Oral doxifluridine 700 mg/m2/d with oral leucovorin 20 mg/m2/d D1‐21, q28d for 12 cycles with radiotherapy (n randomised = 92) *Radiotherapy commenced with C3 at a dose of 5400 cGy at 180 Gy/d, 5 days per week for 6 consecutive weeks | |
| Outcomes | Grade ≥ 3 AEs (WHO criteria, version not specified) Median follow‐up: more than 15 months, range, 6 to 26 months. Less than 3 year follow‐up | |
| Study Details | Journal article (in Korean) | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization lists were stratified by a medical statistician, using randomly permuted blocks of varying sizes" (materials and methods, paragraph 1, page 675) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | (i) DFS: This study was not used for the meta‐analysis for this outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Not specified, blinding unlikely. Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: This study was not used for the meta‐analysis for this outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Not specified. Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Disease recurrence (influences DFS): Unclear ‐ not specified (ii) Survival (influences DFS/OS): This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) DFS: This study was not used for the meta‐analysis for this outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Unclear risk | Efficacy analysis: Unclear Not specified. Note that the number of participants reported in the IV vs oral arm differed substantially‐ 74 vs 92 participants, respectively (Table 1, page 676) Safety analysis: Not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | High risk | (i) PS: Unclear ‐ not specified (ii) Mean age: High 7.2 year difference in mean age between arms (Table 1, page 676) (iii) TNM stage: Low (stage II vs III) Quote: "There was no difference of TNM stage distribution between two groups of patients (P = .454); stage II was 25 in the IV arm and 41 in the oral arm; and stage III was 49 in the IV arm and 51 in the oral arm (results, paragraph 1, page 675) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ similarity of therapy following recurrence or new occurrence of disease not specified |
| Methods | Randomised controlled trial Phase: III Design: Factorial, 2 × 2 Accrual dates: May 2003 to April 2004 (closed 12 January 2005) | |
| Participants | No. randomised: 85 Stage/treatment line: Metastatic, first‐line Countries/sites: European Organisation for Research and Treatment of Cancer (EORTC) study Setting: Hospital Characteristics (Arm I/II/III/IV): Metastatic colorectal cancer; age ≥ 18 years (median 66.0/60.5/63.0/65.0 yrs); male (79/64/52/57%); PS WHO 0‐2 (PS WHO 0: 53/64/61/57%) | |
| Interventions | Arm I (FOLFIRI + celecoxib): irinotecan 180 mg/m2 D1, 15, 22, FA 200 mg/m2 D1, 2, 15, 16, 29, 30, IV bolus 5‐FU 400 mg/m2 followed by 22‐hour continuous infusion 600 mg/m2 D1, 2, 15, 16, 29, 30 + celecoxib 800 mg daily (n randomised = 19) Arm II: FOLFIRI plus placebo (n randomised = 22) Arm III (CAPIRI plus celecoxib): irinotecan 250 mg/m2 D1 and 22 and capecitabine 1000 mg/m2 bd D1‐15 and 22‐36 + celecoxib 800 mg daily (n randomised = 23) Arm IV: CAPIRI plus placebo (n randomised = 21) Treatment continued up to a planned total of 6 cycles or until PD, unacceptable toxicity, or withdrawal of consent. Participants with a response or SD were allowed to continue treatment beyond 6 cycles at the discretion of the investigator | |
| Outcomes | PFS Grade ≥ 3 AEs (NCI CTC, version 2) ORR (RECIST, version 1.0) OS Median follow‐up: 14.6 months for all outcomes | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Roche, Pharmacia (currently Pfizer), Aventis (currently Sanofi‐Aventis) Declarations of interest: None declared | |
| Notes | "...after the enrolment of only 85 patients, recruitment was suspended as a consequence of seven deaths not due to disease progression. One more patient subsequently died following the suspension of recruitment. Six deaths occurred in patients receiving CAPIRI and two in those receiving FOLFIRI ... Five deaths in the CAPIRI arm and both of those in the FOLFIRI arm were deemed to be treatment related. Underlying risk factors could not be identified as a likely explanation for these fatal toxic effects. On the basis of the outcome of this review, the trial was officially closed on 12 January 2005" (results, paragraph 1, page 922) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to receive centrally using a minimization technique" (patients and methods, paragraph 4, page 922) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified. (i) ORR/PFS: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/PFS): Low Quote: "Evaluation of disease status was carried out every 6 weeks during treatment and every 8 weeks subsequently until the documentation of disease progression" (patients and methods, paragraph 3, page 921) (ii) Survival (influences PFS/OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low Response was not assessable or early death occurred in 8/44(18%) participants in the CAPIRI arm and in 4/41(10%) participants in the FOLFIRI arm (Table 3, page 924) (ii) PFS/OS: Low Quote: "Patients with no evidence of PD at the time of their last visit were censored at that time" (patients and methods, paragraph 3, page 922) (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "... assessed in the intention‐to‐treat (ITT) population" (patients and methods, paragraph 3, page 922) Quote: "...enrollment of ... 85 patients ..." (results, paragraph 1, page 922). This is the same number of participants presented in all of the efficacy analyses. (Table 3 and 4, page 924, and Figure 1, page 925) Safety analysis: Analyses included those who received study drug, quote: "Three patients (4%) did not receive study drugs and are therefore not included in the safety analysis" (results, paragraph 3, page 922) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 2, 923) (ii) Median age: Low (Table 2, 923) (iii) No. of involved organs: Low (Table 2, 923) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified Risk of bias considerations in a factorial study: Unclear Quote: "...in view of the fact that response rates were lower in both celecoxib arms compared with the corresponding placebo arms for both regimens, it is possible that celecoxib may actually reduce the response to chemotherapy..." (discussion, paragraph 5, page 925) Quote: "Celecoxib did not appear to modulate the toxicity of the chemotherapy; thus it is very unlikely that the toxicity observed with CAPIRI was due to celecoxib" (discussion, paragraph 5, page 925) No tests for interaction were reported |
| Methods | Randomised controlled trial Phase: III Accrual dates: February 1997 to March 1999 | |
| Participants | No. randomised: 1608 Stage/treatment type: Stage II/III adenocarcinoma of the colon, adjuvant Countries/sites: National Surgical Adjuvant Breast and Bowel Project (NSABP) study Setting: Hospital Characteristics (Arm I/II): resected Stage II/III colon cancer (age < 60 years: 41.7/41.2%); male (51.6/53.5%); PS ECOG ≤2 | |
| Interventions | Arm I (FU/LV): LV 500 mg/m2 and IV bolus FU 500 mg/m2 weekly W1 to 6, q56d for 3 cycles (n randomised = 803) Arm II (UFT/LV): UFT 300 mg/m2/d and LV 90 mg/d D1‐28, q35d for 5 cycles (n randomised = 805) | |
| Outcomes | OS DFS Grade ≥ 3 AEs (NCI toxicity criteria, 1958) Median follow‐up: 62.3 months among surviving patients | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: NSABP Foundation Declarations of interest: Taiho | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely. (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) DFS: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Disease recurrence (influences DFS): Low Quote: "Starting 6 months after the completion of protocol chemotherapy and continuing through 5 years after random assignment, patients were to be re‐evaluated semiannually. Five years after random assignment, a status of disease report was required on a yearly basis" (patients and methods, paragraph 4, page 2060) (ii) Survival (influences DFS/OS): Low Quote as above Quote: "Before the administration of each cycle of chemotherapy, patients had a physical examination, CBCs, and chemistry profiles including hepatic and renal function studies. During active chemotherapy, patients in both groups underwent weekly CBCs" (patients and methods, paragraph 3, page 2060) Quote: "Patients randomly assigned to the FU+LV group received three 8‐week cycles of intravenous chemotherapy" (patients and methods, paragraph 2, page 2060) Quote: "Patients randomly assigned to the UFT+LV group received five 5‐week cycles ..." (patients and methods, paragraph 2, page 2060) Therefore, participants in the UFT+LV arm had more frequent safety evaluations than those in the FU+LV arm |
| Incomplete outcome data (attrition bias) | Unclear risk | (i)(ii) DFS/OS: Unclear Quote: "Our primary analyses include all patients who were eligible and had follow‐up information (intent to treat)" (patients and methods, paragraph 8, page 2060) However, no indication whether this follow‐up information was complete for all outcomes (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low ‐ Analyses were performed on what was described as an ’intent to treat’ population but included only those who were eligible and had follow‐up information. Quote: "Fifty patients (3.1%) were deemed ineligible Safety analysis: Analyses performed on those who were eligible and had follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ not specified (ii) Median age: Unclear ‐ not specified Baseline age was dichotomised into < 60 years vs ≥ 60 years (Table 1, page 2061) (iii) TNM stage: Low (stage II vs stage III and stage III N1 vs N2) N1 vs N2: Low (Table 1, page 2061) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ similarity of therapy following recurrence or new occurrence of disease not specified |
| Methods | Randomised controlled trial Phase: II Accrual dates: December 2001 to March 2005 | |
| Participants | No. randomised: 118 Stage/treatment line: Metastatic, first‐line Countries/sites: Gruppo Oncologico Aree Metropolitane (GOAM) study Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal adenocarcinoma; age > 18 years (median 64/67 years); male (50/53.2%); KPS ≥ 70% (Median 90/90%) | |
| Interventions | Arm A (pviFOX): oxaliplatin 130 mg/m2 D1 and protracted venous infusion 5‐FU 250 mg/m2/d D1‐21, q21d (n randomised = 56) Arm B (XELOX): oxaliplatin 130 mg/m2 D1 and oral capecitabine 1000 mg/m2 bd D1‐14, q21d (n randomised = 62) Treatment continued for 6 cycles or until PD, at the investigators' discretion | |
| Outcomes | ORR (RECIST, version 1.0) TTP Grade ≥ 3 AEs (CTC criteria, version 2.0) No details on median follow‐up | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: No conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/TTP: Low Outcome unlikely to be influenced by lack of blinding (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/TTP: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/TTP): Low Quote: "Every three cycles, re‐evaluation was scheduled with the recording of the symptoms, weight, KPS, physical examination and chest‐abdominal‐pelvic CT...." (patients and methods, paragraph 5, page 3163) Quote: "In both arms, the treatment was repeated every 21 days ..." (patients and methods, paragraph 3, page 3162) (ii) Survival (influences OS): Not applicable This was not an outcome of interest for this study (iii) Grade ≥ 3 AEs: Low Quote: " ... the recording of the symptoms, side‐effects and physical examination was carried out prior to each cycle, blood count and blood‐chemistry tests for liver and kidney function before and 10 days after each cycle. ..." (patients and methods, paragraph 5, page 3163) Quote: "In both arms, the treatment was repeated every 21 days ..." (patients and methods, paragraph 3, page 3162) |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR/TTP: Low Response was not evaluable in 3/56 (5.4%) participants in the pviFOX arm and in 5/62 (8.1%) participants in the XELOX arm (Table 5, page 3166) (ii) OS: N/A Not applicable, as this was not an outcome of interest for this study (iii) Grade ≥ 3 AEs: Low Analyses were performed in those with evaluable data ‐ 54/56 in the pviFOX arm and 61/62 in the XELOX arm (Table 4, page 3165) Although the reasons for participants not having evaluable data were unclear, only a low percentage had non‐evaluable data. |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "Efficacy analyses were based on an intent‐to‐treat analysis" (patients and methods, paragraph 7, page 3164) Quote: "One hundred and twenty‐two patients were enrolled between December 2001 and March 2005. Four patients resulted [sic] ineligible and were excluded from the randomisation" (results, paragraph 1, page 3164) These 118 randomised participants were included in the efficacy analyses (Table 5 and Fig. 1, page 3166) Safety analysis: Analyses were performed in those with evaluable data ‐ 54/56 in the pviFOX arm and 61/62 in the XELOX arm (Table 4, page 3165) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | High risk | (i) PS: Unclear Reported as median KPS only (Table 1, page 3163) (ii) Median age: Low (Table 1, page 3163) (iii) No. of involved organs: High A 16.5% difference between arms with respect to the number of participants with 1 vs more than 1 metastatic site (30/56 (53.6%) in the pviFOX arm and 23/62 (37.1%) in the XELOX arm) (Table 1, page 3163) |
| Other bias | Unclear risk | Subsequent therapies: Quote: "...60 have received a second‐line chemotherapy, 25 and 35 in arms A and B, respectively" (results, paragraph 7, page 3165) Similar proportions in each arm received second‐line therapy, but OS was not an outcome in this study |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: June 2010 to September 2012 | |
| Participants | No. randomised: 70 Stage/treatment line: Locally advanced or metastatic; first‐line chemotherapy for locally advanced or metastatic CRC (if recurrence occurred after neoadjuvant therapy or adjuvant chemotherapy an interval of at least 6 months from completing therapy was mandated) Countries/sites: Single‐centre study in China (The No. 3 People's Hospital of Zhengzhou, Zhengzhou Tumour Hospital) Setting: Hospital Characteristics (Arm I/II): Locally advanced or metastatic; age 18 to 75 years; PS ECOG 0‐1 (unclear) | |
| Interventions | Arm I (SOX): L‐OHP 130 mg/m2 D1 IV infusion, S‐1 oral for 14 days. S‐1 dose calculated according to BSA: 80 mg/d for BSA < 1.25 m2; 100 mg/d for BSA ≥ 1.25 m2 but < 1.5 m2; 120 mg/d for BSA ≥ 1.5 m2 but < 1.8 m2; 140 mg/d for BSA > 1.8 m2. Schedule repeated every 3 weeks (n randomised = 35) Arm II (FOLFOX4): L‐OHP 85 mg/m2 IV D1, leucovorin (CF) 200 mg/m2 IV D1‐2, 5‐FU 400 mg/m2 IV bolus D1‐2, infusional 5‐FU 1200 mg/m2 IV continuous over 44 hours (n randomised = 35) | |
| Outcomes | Grade ≥ 3 AEs (NCI‐CTC, version 3.0) ORR (reported only after 2 cycles of chemotherapy) (RECIST, version not specified) No details on median follow‐up | |
| Study Details | Journal article (in Chinese) | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ not specified |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ not specified |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR: This study was not used for the meta‐analysis for this outcome (ii) OS: Not an outcome for this study (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified. (i) ORR: This study was not used for the meta‐analysis for this outcome (ii) OS: Not an outcome for this study (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR): This study was not used for the meta‐analysis for this outcome (ii) Survival: No survival outcomes in this study (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: This study was not used for the meta‐analysis for this outcome (ii) OS: Not an outcome for this study (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Unclear risk | Efficacy analysis: Unclear, but not an outcome for this study Safety analysis: Quote (translated from Chinese to English): "70 cases are randomly allocated into trial group and control group, each 35 cases" (information and methods, paragraph 1, page 821) Denominator appears to be the randomised population in Table 1, page 821 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of arms at baseline | Unclear risk | All Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: Not specified (journal), abstracts stated phase IV Accrual dates: September 1997 to December 2000 | |
| Participants | No. randomised: 237 Stage/treatment line: Metastatic/unresectable, first‐line Countries/sites: Spain, 16 sites Setting: Hospital Characteristics (Arm I/II): Metastatic/unresectable colorectal cancer; age ≥ 18 years (median 67/68 years); male (62/68%); KPS ≥ 60% (KPS 100%: 27/29%) | |
| Interventions | Arm I (FT/LV): Tegafur 750 mg/m2/d D1‐21, q28d with LV 15 mg tds (n randomised = 114) Arm II (5‐FU/LV): IV bolus 5‐FU 425 mg/m2 D1‐5 with LV 20 mg/m2, q28d for 2 cycles, then q35d thereafter (n randomised = 123) Treatment continued until PD, unacceptable toxicity, or withdrawal of consent | |
| Outcomes | ORR (WHO criteria, 1981) TTP OS Grade ≥ 3 AEs (NCI CTC, version 2.0) No details on median follow‐up | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Prasfarma Almirall‐Prodesfarma, Spain Declarations of interest: No conflicts of interest | |
| Notes | Owing to slow enrolment, recruitment was suspended after 85% of the expected sample size (246 participants) was randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned in blocks of 4 and stratified by center ..." (patients and methods, paragraph 4, page 2242) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients ... were centrally randomised to treatment with either oral FT/LV or i.v. 5‐FU/LV" (patients and methods, paragraph 4, page 2242) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was a randomised, multicenter, open‐label clinical trial ..." (patients and methods, paragraph 1, page 2242) (i) ORR/TTP: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/TTP): High Quote: "Responses were evaluated every 3 cycles" (Salud et al, Journal of Clinical Oncology 2004‐ slides associated with abstract 3547) In the FT/LV arm, treatment was given in 28‐day cycles. In the 5‐FU/LV arm, treatment was given in 28‐day cycles for 2 cycles, and in 35‐day cycles thereafter (patients and methods, paragraph 5, page 2242) Therefore, after cycle 2, response assessments were performed more frequently in the FT/LV treatment arm (ii) Survival (influences OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: High Quote: "Before each cycle, adverse events were documented and a physical examination, differential blood count and blood biochemistry test were performed" (patients and methods, paragraph 7, page 2243) In the FT/LV arm, treatment was given in 28‐day cycles. In the 5‐FU/LV arm, treatment was given in 28‐day cycles for 2 cycles, and in 35‐day cycles thereafter (patients and methods, paragraph 5, page 2242) Therefore, after cycle 2, safety assessments were performed more frequently in the FT/LV treatment arm |
| Incomplete outcome data (attrition bias) | High risk | (i) ORR/TTP: High 27/114 (24%) participants in the FT/LV arm and 24/123 (20%) in the 5‐FU/LV arm were not evaluable for response owing to “deviations from protocol in the response evaluation methodology” (Fig. 1, page 2244) (ii) OS: Low No participants in the FT/LV arm and 2/123 (1.6%) in the 5‐FU/LV arm were not evaluable for survival (Fig. 1, page 2244) Censoring was noted in the KM curves for OS (Fig. 2, page 2246). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | High risk | Efficacy analysis: High Quote: "All efficacy analyses were conducted on the intent‐to‐treat (ITT) population" (patients and methods, paragraph 10, page 2243) However, 0/144 (0%) and 2/123 (2%) participants randomised to the FT/LV and 5‐FU/LV arms, respectively, were excluded from the survival analysis owing to being unevaluable (Fig. 1, page 2244). The 27/144 (24%) and 24/123 (20%) participants randomised to the FT/LV and 5‐FU/LV arms, respectively, who were not evaluable for response were excluded from the analysis (Fig. 1, page 2244) Safety analysis: Quote: "Toxicity analyses were performed on patients who received at least one dose of study treatment (safety population)" (patients and methods, paragraph 10, page 2243) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 2245) (ii) Median age: Low (Table 1, page 2245) (iii) No. of involved organs: Low (Table 1, page 2245) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "Overall, 94 of 237 treated patients continued receiving treatment after the end of the study with second‐line agents. In the FT/LV treatment arm, 48 patients were mainly treated with irinotecan and combination of oxaliplatin + irinotecan in 27% and 20% of cases, respectively. Likewise, in the 5‐FU/LV treatment arm, 46 patients involved in second‐line therapy received irinotecan and combination of oxaliplatin + irinotecan in 30.5% and 28.3% of cases, respectively" (results, paragraph 3, pages 2243 and 2244) |
| Methods | Randomised controlled trial Phase: III Accrual dates: January 2006 to January 2008 | |
| Participants | No. randomised: 302 Stage/treatment line: Metastatic, first‐line Countries/sites: Greece, multiple sites Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal cancer; age ≥ 18 years (median 66/66 years); male (55/65%); PS ECOG 0‐2 (PS ECOG 0: 64/66%) | |
| Interventions | Arm A (XELIRI + bevacizumab (BEV)): irinotecan 240 mg/m2 D1, capecitabine 1000 mg/m2 D1‐14 and BEV 7.5 mg/kg D1, q21d, up to 6 cycles (n randomised and eligible = 143) Arm B (FOLFIRI + BEV): irinotecan 180 mg/m2 D1, leucovorin 200 mg/m2 D1, IV bolus fluorouracil 400 mg/m2 followed by fluorouracil 2400 mg/m2 46‐hour infusion, and BEV 5 mg/kg D1, q14d, up to 12 cycles (n randomised and eligible = 142) Single agent BEV was administered as maintenance until unacceptable toxicity or PD | |
| Outcomes | PFS ORR (RECIST, version 1.0) OS Grade ≥ 3 AEs (NCI CTC, version 2.0) Median follow‐up: 42 months | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Hellenic Oncology Research Group (HeCOG) Declarations of interest: Pfizer, Roche Hellas SA, Genesis Pharma SA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used stratified blocked randomisation balanced by centre (correspondence with Anastasia Eleftheraki, received 6 July 2012) |
| Allocation concealment (selection bias) | Low risk | Quote: " ... randomization, done centrally at the HeCOG Data Office ..." (methods, paragraph 3, page 2) |
| Blinding of participants and personnel (performance bias) | High risk | (i) ORR/PFS: Low This was an open‐label study (correspondence with Anastasia Eleftheraki, received 6 July 2012) Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High As above, this was an open‐label study (correspondence with Anastasia Eleftheraki, received 6 July 2012) Quote: "No central review of the imaging material was done" (methods, paragraph 5, page 2) Outcome assessment at risk of bias from lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): High Quote: "Disease evaluation was carried out after 3 cycles of treatment in group A and after 6 cycles in group B, at the end of treatment, and every 3 months thereafter by chest X‐rays and CT scans of the abdomen and pelvis" (methods, paragraph 5, page 2) Treatments were quote: " ...repeated every 21 days for 6 cycles (group A, XELIRI) or ... repeated every 14 days for 12 cycles (group B, FOLFIRI)" (methods, paragraph 3, page 2) Therefore, disease assessment was performed more frequently in the XELIRI + BEV arm than in the FOLFIRI + BEV arm during treatment (every 9 weeks vs every 12 weeks) (ii) Survival (influences PFS/OS): Low The follow‐up schedule for survival assessment was the same in both arms ‐ approximately every 6 months (correspondence with Anastasia Eleftheraki, received 6 July 2012) (iii) Grade ≥ 3 AEs: High Participants were examined for adverse events every cycle of treatment and 1 month after last treatment administration (both groups) (correspondence with Anastasia Eleftheraki, received 6 July 2012) Treatments were quote: "...repeated every 21 days for 6 cycles (group A, XELIRI) or ... repeated every 14 days for 12 cycles (group B, FOLFIRI)" (methods, paragraph 3, page 2) Therefore, disease assessment was performed more frequently in the FOLFIRI + BEV arm than in the XELIRI + BEV arm during treatment (every 2 weeks vs every 3 weeks) |
| Incomplete outcome data (attrition bias) | High risk | (i) ORR/PFS: High Quote: "In group A, 43 patients (30.1%) were not evaluated for response because of treatment discontinuation (24 patients, 16.8%), early death (5, 3.5%), missing data (3, 2.1%), or non‐evaluable disease (11, 7.7%). In group B, 28 patients (19.7%) were not evaluated for response because of treatment discontinuation (5 patients, 3.5%), early death (2, 1.4%), or non‐evaluable disease (21, 14.8%) (results, paragraph 3, page 5) (ii) OS: Low Although not defined in the Methods, censoring was noted in the KM curves for OS (Figure 2, page 5). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Outcome data were missing for 5/138 participants in the XELIRI + BEV arm and for 2/132 in the FOLFIRI + BEV arm (results, paragraph 2, page 5 and Table 2, page 6) |
| Incomplete outcome data (ITT analysis) | High risk | Efficacy analysis: High Quote: "PFS, OS and ORR were analyzed on an intent‐to‐treat basis..." (methods, paragraph 10, page 4) However, whilst 302 participants were randomised, 17 of these participants were excluded owing to ineligibility. This left 285 participants who were eligible and included in the analysis for all efficacy outcomes (Figure 1, page 3) Safety analysis: Quote: " ...in the safety analysis ... only the treated population was included" (methods, paragraph 10, page 4) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 4) (ii) Median age: Low (Table 1, page 4) (iii) No. of involved organs: Low (Table 1, page 4) |
| Other bias | Unclear risk | Subsequent therapies: Unclear Only information on maintenance BEV was reported Quote: "In total, 64 patients in group A and 46 in group B received Bev as maintenance for a median of 4 cycles (range, 1–35) and 6 cycles (range, 2–48), respectively" (results, paragraph 2, page 5) |
| Methods | Randomised controlled trial Phase: III Accrual dates: November 2005 to January 2008 | |
| Participants | No. randomised: 441 Stage/treatment type: High‐risk AJCC stage II or AJCC stage III CRC, adjuvant Countries/sites: Greece, multiple sites Setting: Hospital Characteristics: Characteristics (Arm A/B): High‐risk AJCC stage II CRC (high histological grade, lymphovascular/perineural invasion, mucinous component, T4 stage, extramural vein invasion, symptomatic bowel obstruction or perforation at diagnosis, < 12 lymph nodes removed); or AJCC stage III; age 18 to 75 years (median 62.4/63.7 years); male (56.4/55.5%); PS ECOG 0‐1 (PS ECOG 0 91.7/93.7%) | |
| Interventions | Arm I (XELOX): capecitabine 1000 mg/m2 bd D1‐14 and oxaliplatin 130 mg/m2 D1, q21d for 8 cycles (n randomised and eligible = 211) Arm II (mFOLFOX6): leucovorin 200 mg/m2, IV bolus 5‐FU 400 mg/m2 and 5‐FU 600 mg/m2 22‐hour infusion D1 and D2 plus oxaliplatin 85 mg/m2 D1, q14d for 12 cycles (n randomised and eligible = 197) | |
| Outcomes | 3‐year DFS OS Grade ≥ 3 AEs (NCI CTC, version 2.0) Median follow‐up: 74.7 months (range, 0 to 155.5 months) | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Hellenic Oncology Research Groups Declarations of interest: No conflicts of interest | |
| Notes | The accrual target was 824, but the study was closed prematurely after enrolment of 441 participants owing to slow accrual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomization, done centrally at the Hellenic Cooperative Oncology Group (HeCOG) data office" (methods, paragraph 2, page 2) |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely. (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) DFS: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | Unclear risk | (i) Disease recurrence (influences DFS): Low Quote: "Follow‐up evaluation for disease recurrence was carried out after the completion of treatment in all patients, every 3 months for the first year, every 4 months for the second and third year and every 6 months for the fourth and fifth year ..." (methods, paragraph 4, page 3) (ii) Survival (influences DFS/OS): Unclear ‐ not specified (iii) Safety: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) Recurrence: Unclear ‐ not specified (ii) DFS/OS Censoring was noted in the KM curves (Fig. 2, page 8). There was no evidence of bias related to censoring (ii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | High risk | Efficacy analysis: High Quote: "Analyses of survival parameters and objective response rates were performed in all randomized patients (intention to treat, ITT population) ..." (methods, paragraph 14, page 4) However, this was not an ITT analysis as defined in our review 33 (7.5%) randomised participants were excluded from the analysis owing to ineligibility (Table 1, page 5). Furthermore, whilst the efficacy analysis population was described in the text as all randomised participants, the number of participants at risk at t(0) for PFS and OS in Figure 2 is not consistent with this. 4/197 (2%) and 2/211 (1%) of participants randomised to the mFOLFOX6 and CAPOX arms, respectively, were excluded from the DFS analysis. 4/197 (2%) and 3/211 (1%) of participants randomised to the mFOLFOX6 and CAPOX arms, respectively, were excluded from the OS analysis (Fig. 2, page 8) Safety analysis: Quote: "...analyses of toxicity ... were performed only in patients who did receive treatment (treated patient population)" (methods, paragraph 14, page 4) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of arms at baseline | Low risk | (i) PS: Low 2% difference in ECOG 1 (Table 1, page 6) (ii) Median age: Low (Table 1, page 6) 1.3 year difference (iii) TNM: Low (stage II vs stage III, stage II T3 vs T4, stage III N1 vs N2) (Table 1, page 6) |
| Other bias | Unclear risk | Subsequent therapies: Unclear regarding subsequent drug therapy after a recurrence or a new occurrence of colorectal cancer |
| Methods | Randomised controlled trial Phase: III Accrual dates: August 2002 to August 2004 | |
| Participants | No. randomised: 476 Stage/treatment line: Metastatic, first‐line Countries/sites: Germany, 68 sites; Austria, 1 site Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal cancer; age > 18 years (median 64/66 years); male (63/62%); PS ECOG 0‐2 (PS ECOG 0‐1: 93/91%) | |
| Interventions | Arm A (FUFOX): oxaliplatin 50 mg/m2, leucovorin 500 mg/m2, and 22‐hour infusional FU 2000 mg/m2 D1, 8, 15, and 22, q35d. After cycle 4, oxaliplatin on D1 and 15 of cycle only (n randomised = 234) Arm B (CAPOX): capecitabine 1000 mg/m2 bd D1‐14 and oxaliplatin 70 mg/m2 D1 and 8, q21d. After cycle 6, oxaliplatin on D1 only (n randomised = 242) Treatment continued until PD or severe toxicity | |
| Outcomes | ORR (RECIST, version 1.0) PFS OS Grade ≥ 3 AEs (NCI CTC, version 2) Median follow‐up: 17.3 months | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Hoffman La‐Roche, Sanofi‐Aventis Declarations of interest: Roche, Sanofi‐Aventis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐based randomization was performed centrally by fax" (patients and methods, paragraph 4, page 4218) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at high risk of bias if there was lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/PFS: High Outcome assessment at risk of bias if there was lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias if there was lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): High Quote: "During therapy, tumor assessments were repeated after three cycles of CAPOX and two cycles of FUFOX ...Follow‐up for disease progression and survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 5, page 4218) Arm A (FUFOX) was given in 5‐week cycles, and Arm B (CAPOX) was given in 3‐week cycles (Table 1, page 4218) Therefore, tumour assessments were performed more frequently in the CAPOX arm than in the FUFOX arm during treatment (every 9 weeks vs every 10 weeks) (ii) Survival (influences PFS/OS): Low Quote: "Follow‐up for disease progression and survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 5, page 4218) (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Unclear ‐ not specified (ii) PFS/OS: Low Although not defined in the Methods, censoring was noted in the KM curve for PFS (Fig. 3, page 4220). No evidence of bias related to censoring. (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The efficacy analysis was based on the intent‐to‐treat population" (patients and methods, paragraph 8, page 4218) 476 participants were randomly assigned, and subsequently, 2 participants were excluded from the allocation population because they did not meet inclusion criteria. Analysis was performed on a population that excluded a further 4 participants owing to withdrawal of consent, and loss to follow‐up after random assignment (excluded 6/476, 1.3%) (Fig. 1, page 4219). Analysis does include participants who were allocated to a treatment arm but did not receive that treatment (total of 239 participants in the CAPOX arm, and 231 in the FUFOX arm) (Fig. 1, page 4219) Safety analysis: Not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear Reported as PS ECOG 0‐1 vs 2 (Table 2, page 4220) (ii) Median age: Low (Table 2, page 4220) (iii) No. of involved organs: Low (Table 2, page 4220) |
| Other bias | Low risk | Subsequent therapies: Low A similar proportion of participants in each arm received the same types of post‐progression treatment Quote: "In both the CAPOX and FUFOX arms, 66% of patients received second‐line treatment. Most of the patients received irinotecan‐based chemotherapy in the second‐line treatment (81% in both CAPOX and FUFOX arms). Additional treatments included reintroduction with oxaliplatin (CAPOX, 13%; FUFOX, 21%), cetuximab (CAPOX, 22%; FUFOX, 21%), or mitomycin (CAPOX, 9%; FUFOX, 9%). On subsequent treatment lines, patients in the CAPOX arm changed to FU (43%) and 29% continued with capecitabine. In the FUFOX arm, 56% continued with FU and 30% received capecitabine. In total, 56% patients of the entire study population received all three drugs: fluoropyrimidine, oxaliplatin, and irinotecan (CAPOX, 57%; FUFOX, 55%)" (results, paragraph 12, pages 4219 and 4220) |
| Methods | Randomised controlled trial Phase: III Accrual dates: July 2003 to May 2005 | |
| Participants | No. randomised: 627 Stage/treatment line: Metastatic, second‐line Countries/sites: 19 countries, 87 centres ‐ Oceania, Central and Eastern Asia, South Africa, Canada, USA, Israel, Mexico, South America, Europe Characteristics (Arm I/II): Metastatic colorectal cancer; age ≥ 18 years (median 60.7/59.7 years); male (62/61%); PS ECOG ≤ 2 (PS ECOG 0: 48/46%) | |
| Interventions | Arm I (XELOX): oxaliplatin 130 mg/m2 D1 plus oral capecitabine 1000 mg/m2 bd D1‐15, q21d, up to 24 weeks of treatment. Could receive treatment beyond week 24 in a post‐study treatment phase until PD (n randomised = 313) Arm II (FOLFOX‐4): oxaliplatin 85 mg/m2 D1 and LV 200 mg/m2/d, IV bolus 5FU 400 mg/m2/d and 22‐hr infusion 600 mg/m2/d D1‐2, q14d. Post study treatment as per Arm I (n randomised = 314) Treatment continued until PD, intolerable AEs, or participant refusal | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (NCI‐CTCAE, version 3) Median follow‐up: 25.7 months | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Roche Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Dynamic randomization was used to assign patients to treatment" (patients and methods, paragraph 5, page 1721) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study was open label because of the different routes of administration of the fluoropyrimidine components of these regimens" (patients and methods, paragraph 1, page 1721) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Quote: "The study was open label because of the different routes of administration of the fluoropyrimidine components of these regimens" (patients and methods, paragraph 1, page 1721) Quote: "Assessments of tumor response were made by investigators and also by an independent response review committee (IRC) that was blinded to treatment assignment" (patients and methods, paragraph 9, page 1721) ‐ Low for ORR Quote: "PFS was the primary end point of the study and was defined as the time from the date of randomization to the first documentation of disease progression by the investigators or death from any cause" (patients and methods, paragraph 12, page 1721) It is unclear if PFS was assessed by a local investigator. If so, this outcome assessment would be at risk of bias from lack of blinding ‐ Unclear for PFS (ii) OS: Low |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/PFS): Low Quote: "Assessments were then repeated using the same imaging technique approximately every 6 weeks and again within 2 weeks of study completion, withdrawal or treatment discontinuation....Confirmation of response was required after a minimum of 4 weeks" (patients and methods, paragraph 9, page 1721) (ii) Survival (influences PFS/OS): Low Quote: "After completion of study treatment, patients were followed up every 3 months until disease progression or death" (patients and methods, paragraph 9, page 1721) (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low 13.1% of participants in the XELOX arm and 13.7% in the FOLFOX‐4 arm were missing response data (Rothenberg et al, Journal of Clinical Oncology 2007‐slides associated with abstract 4031) (ii) PFS/OS: Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The intention‐to‐treat (ITT) patient population included all patients who underwent randomization" (patients and methods, paragraph 11, page 1721) The ITT population was used for all efficacy analyses, included PFS (results, paragraph 6, page 1722; Figure 2A, page 1724; Table 2, page 1723), OS (results, paragraph 7, page 1722; Figure 2B, page 1724; Table 2, page 1723) and ORR (results, paragraph 8, page 1723 and Table 2, page 1723) Safety analysis: Quote: "The safety population was defined as all patients receiving at least one dose of study drug" (patients and methods, paragraph 11, page 1721) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 1723) (ii) Median age: Low (Table 1, page 1723) (iii) No. of involved organs: Low (Table 1, page 1723) |
| Other bias | Low risk | Subsequent therapies: Low A similar proportion of participants in both arms received further therapy after progression Quote: "A similar proportion of patients in the two treatment groups received further anticancer therapy after discontinuing study treatment (60% with XELOX and 62% with FOLFOX‐4), including drug therapy, surgery and radiotherapy. The most commonly used treatments were 5‐FU (25% in the XELOX group versus 25% in the FOLFOX‐4 group), capecitabine (10% versus 26%), irinotecan (16% versus 21%), cetuximab (15% versus 19%), oxaliplatin (17% versus 14%), radiotherapy (18% versus 14%) and bevacizumab (6% versus 7%)" (results, paragraph 9, page 1723) |
| Methods | Randomised controlled trial Phase: III Accrual dates: October 1997 to May 1999 | |
| Participants | No. randomised: 981 Stage/treatment line: Metastatic, first‐line Countries/sites: USA and Canada, 136 centres Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age ≥ 18 years (median 64/64 years); KPS ≥ 70% (KPS 100%: 30/32%) | |
| Interventions | Arm I (EU/5‐FU): eniluracil 11.5 mg/m2 and 1.15 mg/m2 5‐FU oral bd D1‐28, q35d (n randomised and treated = 485) Arm II (5‐FU/LV): 20 mg/m2 LV and IV 5‐FU 425 mg/m2 D1‐5, q28d (n randomised and treated = 479) Continue treatment until PD, unacceptable toxicity, or withdrawal of consent | |
| Outcomes | OS ORR (SWOG criteria, 1992 ‐ adapted) PFS Grade ≥ 3 AEs (SWOG criteria, 1992 ‐ adapted) Median follow‐up: N/A | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was a randomized, multicenter, open‐label, phase III trial..." (patients and methods, paragraph 1, page 1520) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs:High Outcome assessment at risk of bias from lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low An independent review panel, blinded to treatment allocation, reviewed all responses (correspondence with Dr. Jeremey Levin, received 23 July, 2012) (ii) OS: Low Outcome assessment unlikely to be influenced by lack of blinding (iii) Grade ≥ 3 AEs: High Outcome assessment at risk of bias from lack of blinding |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): High Quote: "Efficacy assessments were performed at baseline and repeated at the beginning of course 3 and every other course thereafter until discontinuation of treatment ... confirmed objective response required two consecutive assessments performed at least 4 weeks apart." (patients and methods, paragraph 6, page 1520) Quote: "Patients randomized to the EU/5‐FU treatment arm received 5‐week courses ... Patients randomized to the 5‐FU/LV treatment arm ... 28‐day cycle" (patients and methods, paragraph 3, page 1520) Therefore, participants in the 5FU/LV arm had more frequent disease assessments (ii) Survival (influences PFS/OS): Low Quote: "At the end of study treatment, all patients were followed quarterly for survival" (patients and methods, paragraph 7, page 1520) (iii) Grade ≥ 3 AEs: High While on treatment, participants were evaluated for safety at the beginning of each cycle, and a final evaluation was performed approximately 28 days after the last dose of study drug in both arms (correspondence with Dr. Jeremey Levin, received 23 July, 2012) Quote: "Patients randomized to the EU/5‐FU treatment arm received 5‐week courses ... Patients randomized to the 5‐FU/LV treatment arm ... 28‐day cycle" (patients and methods, paragraph 3, page 1520) Therefore, participants in the 5FU/LV arm had more frequent safety assessments |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low Unknown/unable to determine responses in 8% of participants in the EU/5‐FU arm and in 10% in the 5‐FU/LV arm because of loss to follow‐up, withdrawal of consent, or incomplete measurements (Table 4, page 1522) (ii) PFS/OS: Low Quote: "If a patient had not died, duration of survival was censored on the date of last contact" (patients and methods, paragraph 9, page 1520). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Analysis for each outcome kept participants in the intervention groups to which they were randomised, regardless of the intervention received (correspondence with Dr. Jeremey Levin, received 23 July, 2012) However, quote: "Efficacy and safety were summarized for all patients who received at least one dose of study drug" (patients and methods, paragraph 9, page 1520). Of 981 participants randomised, 964 were treated and included in the analyses (1.7% excluded) (results, paragraphs 1 and 6, pages 1521 and 1522) Safety analysis: Analyses as per Efficacy, in all participants who received at least 1 dose of study drug |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 1521) (ii) Median age: Low (Table 1, page 1521) (iii) No. of involved organs: Low (Table 1, page 1521) |
| Other bias | Low risk | Subsequent therapies: Low A similar proportion of participants in both arms received further therapy after progression (Table 3, page 1521) |
| Methods | Randomised controlled trial Phase: Not specified Design: Factorial, 2 × 2 Accrual dates: January 2004 to July 2006 | |
| Participants | No. randomised: 459 Stage/treatment line: Metastatic, first‐line Countries/sites: UK, 61 centres Setting: Hospital Characteristics (Arm A/B/C/D): Metastatic colorectal cancer; “elderly and frail” population considered by the treating oncologist to be unsuitable for upfront full‐dose chemotherapy"; no upper or lower age limit (median 75/75/73/75 years); male (63/60/59/60%); PS WHO ≤ 2 (PS WHO 0: 22/20/20/24%) | |
| Interventions | Arm A (FU): IV levofolinate 175 mg, IV bolus fluorouracil 320 mg/m2 and fluorouracil 2240 mg/m2 46‐hour infusion, q14d (n randomised = 115) Arm B (OxFU): IV levofolinate 175 mg/m2, oxaliplatin 68 mg/m2, bolus fluorouracil 320 mg/m2, and fluorouracil 1920 mg/m2 46‐hour infusion, q14d (n randomised = 115) Arm C (Cap): capecitabine 1000 mg/m2 bd D1‐15, q21d (n randomised = 115) Arm D (OxCap): oxaliplatin 104 mg/m2 D1 and capecitabine 800 mg/m2 bd D1‐15, q21d (n randomised = 114) Treatment continued until PD or clinical deterioration In Arms A and B, second‐line treatment was considered with OxFU or OxCap, respectively, upon progression | |
| Outcomes | ORR (reported for after 12‐14 weeks) (RECIST, version 1.0) PFS OS Grade ≥ 3 AEs (CTCAE, version 3.0) No details on median follow‐up | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Cancer Research UK, National Institute of Health Research. Drugs and infusors supplied by Wyeth and Baxter Declarations of interest: Roche, Sanofi‐Aventis, UK MRC, British Geriatrics Society | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done by use of the method of minimisation" (methods, paragraph 5, page 1750) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1:1:1 ratio by telephone with a computerised algorithm developed and maintained centrally at the MRC CTU" (methods, paragraph 5, page 1750) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Treatment allocation was not masked" (methods, paragraph 5, page 1750) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding This study was not used for the meta‐analysis of the ORR outcome (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Outcome assessment at risk of bias from lack of blinding This study was not used for the meta‐analysis of the ORR outcome (ii) OS: Low |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): Low Quote: "After week 12, radiological response was assessed ..." (methods, paragraph 8, page 1751) This study was not used for the meta‐analysis of the ORR outcome (ii) Survival (influences PFS/OS): Low Quote: "Thereafter, patients without radiological or clinical evidence of deterioration could continue the same regimen, immediately or after a planned break, with reassessment every 12 weeks" (methods, paragraph 9, page 1751) (iii) Grade ≥ 3 AEs: High Quote: "Before each cycle, toxicity was scored ..." (methods, paragraph 7, page 1750) Quote: "The cycle was repeated every 14 days (FU regimen)" (methods, paragraph 6, page 1750) Quote: "The cycle was repeated every 14 days (OxFU regimen)" (methods, paragraph 6, page 1750) Quote: "The cycle was repeated every 21 days (OxCap regimen)" (methods, paragraph 6, page 1750) Quote: "The cycle was repeated every 21 days (Cap regimen)" (methods, paragraph 6, page 1750) Therefore, safety assessments were performed more frequently in the FU and OxFU groups than in the OxCap and Cap arms |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Unclear Quote: "RR and toxic effects are reported as percentage of assessable patients..." (methods, paragraph 13, page 1752). However, the latter was not specified. This study was not used for the meta‐analysis of the ORR outcome (ii) PFS/OS: Low Quote: "For time‐to‐event endpoints, Kaplan‐Meier curves were produced with patients alive and event‐free being censored at the time last seen" (methods, paragraph 13, page 1751). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low Quote: "440 (96%) patients had complete data for toxic effects" (results, paragraph 7, page 1754) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "PFS was defined as time from randomisation to first progression or death from any cause, assessed by intention to treat" (methods, paragraph 11, page 1751). Furthermore, the same number randomised (Figure 1, page 1751) are included in the analysis population for PFS and OS (Figure 2, page 1753) Safety analysis: Analyses were presented for those with complete data (440/459, 96%) (results, paragraph 7, page 1754) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Low (Table 1, page 1752) (ii) Median age: Low (Table 1, page 1752) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Low risk | Subsequent therapies: Low Quote: "In groups A and C, when progression occurred on the FU or Cap regimens, second‐line treatment was considered with the OxFU or OxCap regimens, respectively. Second‐line therapy in groups B and D, and third‐line therapy in all groups, was at the discretion of the physician" (methods, paragraph 9, page 1751) The salvage therapies described in Figure 1, page 1751, are comparable across the arms Risk of bias considerations in a factorial study: Safety ‐ Low Quote: "Tests for interaction between the two treatment factors showed no evidence of an interaction" (Table 4, page 1755) |
| Methods | Randomised controlled trial Phase: II Accrual dates: November 2007 to October 2011 | |
| Participants | No. randomised: 72 Stage/treatment line: Metastatic, first‐line Countries/sites: 1 university hospital and 7 affiliated hospitals in Japan Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age 20‐75 years (median 62/67 years); male (58/63%); PS ECOG 0‐1 (PS ECOG 0: 92/94%) | |
| Interventions | Arm I (FOLFIRI plus bevacizumab (BEV)): BEV 5 mg/kg IV infusion, irinotecan 150 mg/m2, 400 mg/m2 bolus fluorouracil and 2400 mg/m2 infusional fluorouracil (46 hours) (n randomised = 36) Arm II (TEGAFIRI plus BEV): BEV 5 mg/kg IV infusion, irinotecan 150 mg/m2 IV, UFT/LV given oral TDS for 3 weeks, followed by a 7‐day break. LV dose was 75 mg/d for all participants. UFT dose was assigned according to BSA (300 mg/d if BSA < 1.17 m2; 400 mg/d if BSA 1.17 < 1.5 m2; 500 mg/d if BSA 1.5 < 1.83 m2, 600 mg/d if BSA > 1.83 m2) (n randomised = 36) | |
| Outcomes | PFS OS Grade ≥ 3 AEs (CTCAE, version 3.0) Cut‐off date for PFS ‐ 30 June 2015; median follow‐up 27.1 months (IQR 17.8 to 38.1 months) | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: No study funding received Declarations of interest: Merck Serono, Taiho Pharmaceuticals, Yakult Honsha, Chugai Pharmaceuticals, Takeda Pharmaceutical, Otsuka Pharmaceutical, Nippon Kayaku | |
| Notes | Following the approval of BEV in Japan, the study protocol was amended in 2008. Participants were given the option of receiving BEV, and randomisation was stratified by the addition of BEV. 35% (Arm I) and 40% (Arm II) of participants received BEV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done centrally using the minimization method, with stratification by institution, number of metastatic organs (one or more), adhibition of bevacizumab and whether the tumor was unresectable or recurrent" (materials and methods, paragraph 3, page 947) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This open‐label randomized phase II trial..." (materials and methods, paragraph 1, page 947) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Outcome assessment at risk of bias due to lack of blinding (ii) OS: Low |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): Low Quote: "Lesions were measured every 8 weeks with diagnostic imaging, such as computerized tomography or other methods" (methods, paragraph 6, page 948) (ii) Survival (influences PFS/OS): High Assessment for survival following progressive disease was at the discretion of the attending physicians, in both arms (correspondence with Dr. Hirotoshi Hasegawa, received 21 July 2016) (iii) Grade ≥ 3 AEs: Low The schedule of assessment for safety was the same in both arms (correspondence with Dr. Hirotoshi Hasegawa, received 21 July 2016) |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low A similar proportion of participants was not evaluable in each arm (Table 2, page 951) (ii) PFS/OS: Low One participant in each arm had missing survival data; censoring was performed for both of these participants (correspondence with Dr. Hirotoshi Hasegawa, received 21 July 2016) (iii) Toxicity: Low No incomplete toxicity outcome data (correspondence with Dr. Hirotoshi Hasegawa, received 21 July 2016) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low One participant who was randomised to the TEGAFIRI arm did not receive any chemotherapy and was excluded from the primary analyses. However, for the ITT analyses described in results, paragraph 2, page 5, participants were analysed according to the ITT analysis as defined in our review (i.e. kept in the intervention groups to which they were randomised, regardless of whether any intervention was received or which intervention was actually received) (correspondence with Dr. Hirotoshi Hasegawa, received 21 July 2016) Safety analysis: Safety analysis population included participants who received at least 1 dose of the study drugs (Figure 1, page 947) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of arms at baseline | High risk | (i) PS: Low 2% difference in ECOG PS 1 (Table 1, page 948) (ii) Median age: High (Table 1, page 948) 5 year difference (iii) No. organs involved: Low 4% difference in number of participants with multiple sites involved (Table 1, page 948) |
| Other bias | Low risk | Subesequent therapies: Low A similar proportion of participants in both arms received second‐line chemotherapy (Table 1, page 948) Other: Use of BEV ‐ A similar proportion of participants in both arms received BEV (Table 1, page 948). Quote: "No significant difference in PFS and OS was detected by addition of bevacizumab between the two groups" (results, paragraph 2, page 950, and shown in Figure 3, pages 950 and 951) |
| Methods | Randomised controlled trial Phase: III Accrual dates: February 2003 to November 2006 | |
| Participants | No. randomised: 1101 Stage/treatment type: Stage III colon cancer, adjuvant Countries/sites: Japan, 48 sites Setting: Hospital Characteristics (Arm A/B): adjuvant colon cancer; age 20‐75 years (median 61/61 years); male (54/55%); PS ECOG < 0‐1 (PS ECOG 0: 94/95%) | |
| Interventions | Arm A (5‐FU + leucovorin): 3 courses of 5‐FU 500 mg/m2 and l‐LV 250 mg/m2, D1, 8, 15, 22, 29, 36, q8w (n randomised = 550) Arm B (UFT + leucovorin): 5 courses of UFT 300 mg/m2/d and l‐LV 75 mg/d, D1‐28, q5w (n randomised = 551) | |
| Outcomes | DFS OS Grade ≥ 3 AEs (NCI CTC, version 2.0) Median follow‐up: 72.0 months | |
| Study Details | Journal article and abstract, study protocol | |
| Funding sources and declarations of interest | Funding sources: National Cancer Center Research and Development Funds, Ministry Declarations of interest: Taiho, Chugai, Yakult, Pfizer, Sanofi, Novartis, Eli Lilly, Bayer, | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote; "... the patients were randomised ... by the minimisation method of balancing the two arms according to tumour location (i.e. colon versus upper rectum), number of positive lymph node metastases (i.e. ≤ versus >3) and institution" (methods, paragraph 3, page 2233) |
| Allocation concealment (selection bias) | Low risk | Quote: "After confirming the inclusion and exclusion criteria by telephone or fax to the JCOG Data Center, the patients were randomised..." (methods, paragraph 3, page 2233) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The JCOG0205 is a prospective randomised, open‐label, phase III trial..." (results, paragraph 1, page 2234) (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low |
| Schedule of assessment and follow‐up | High risk | (i) Disease recurrence (influences DFS) and (ii) Survival (influences DFS/OS): Low Quote: "Total colonoscopy was performed 1 year after surgery. Chest X‐ray, abdominal ultrasonography or computed tomography (CT) scan and pelvic CT scan or magnetic resonance imaging (MRI) were also performed. The serum tumour markers, CEA and CA19‐9, were examined to check for signs of recurrence every 4 months for first 2 years after random assignment and every 6 months for the next 3 years. After 5 years of follow‐up, patients were followed up annually by physical examination and serum tumour markers until November 2011. Optional CT scans were taken when tumour markers were elevated" (methods, paragraph 5, page 2233) Both groups had the same schedule of assessment and follow‐up (iii) Grade ≥ 3 AEs: High Safety evaluation during the treatment period occurred weekly for the first 6 weeks of each 8‐week cycle for the 5‐FU/l‐LV group and in the 1st and 3rd weeks of every 5‐week cycle for the UFT/LV group (study protocol, pages 27‐28) Therefore, safety evaluations occurred more frequently in the 5‐FU/l‐LV group |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) DFS: Low Quote: "DFS ... was censored at the last day when the patient was alive without any evidence of relapse or secondary cancer" (methods, paragraph 7, page 2233). No evidence of bias related to censoring (ii) OS: Low Quote: "OS ... was censored at the last day when patient was alive" (methods, paragraph 7, page 2233). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low 1101 participants were randomised, however 1092 were eligible and were presented in the analyses for DFS and OS Similar proportions of participants from both arms were ineligible and were excluded from the efficacy analyses (4/550, 0.73% in the 5FU/l‐LV arm, and 5/551 (0.91%) in the UFT/LV arm, respectively) (Fig. 1, page 2234) Safety analysis: Safety analysis population included participants who received any study treatment, regardless of eligibility (Fig. 1, page 2234) |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the study protocol were reported |
| Similarity of arms at baseline | Low risk | (i) PS: Low 1% difference in ECOG 1 (Table 1, page 2235) (ii) Median age: Low 0 years difference in median age (Table 1, page 2235) (iii) TNM stage: Low (Table 1, page 2235) |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: II Accrual dates: July 2005 to August 2008 | |
| Participants | No. randomised: Not specified. "A total of 95 consecutive patients were assessable for response" Stage/treatment line: Metastatic, first‐line Countries/sites: Gruppo Oncologico dell'Italia Meriodionale (GOIM) study Setting: Hospital Characteristics: Metastatic colorectal cancer; age > 18 years; PS ECOG < 2 | |
| Interventions | Arm A (FOLFIRI): irinotecan 180 mg/m2 D1 with LV 100 mg/m2 and IV bolus 5‐FU 400 mg/m2 D1 and D2, and 5‐FU 600 mg/m2 22‐hour infusion, q14d (n randomised not specified) Arm B (XELIRI): irinotecan 250 mg/m2 (200 mg/m2 for participants ≥ 70 years) D1 with capecitabine 1000 mg/m2 bd (750 mg/m2 bd if > 70 years) on D1‐14, q21d (n randomised not specified) | |
| Outcomes | ORR (RECIST, version 1.0) TTP (median) Grade ≥ 3 AEs (NCI criteria, version not specified) No details on median follow‐up None of these outcomes were suitable for inclusion in meta‐analyses | |
| Study Details | Update in journal article | |
| Funding sources and declarations of interest | Funding sources: Gruppo Oncologico dell'Italia Meridionale Declarations of interest: No conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ not specified |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | (i) ORR/TTP: This study was not used for the meta‐analyses of these outcomes (ii) OS: Not an outcome for this study Blinding of participants and personnel was not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | (i) ORR/TTP: This study was not used for the meta‐analyses of these outcomes (ii) OS: Not an outcome for this study Blinding of outcome assessors was not specified |
| Schedule of assessment and follow‐up | Unclear risk | This study was not used for the meta‐analysis of any outcomes Schedule of assessment and follow‐up was not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | This study was not used for the meta‐analysis of any outcomes Information on incomplete outcome data was not specified |
| Incomplete outcome data (ITT analysis) | Unclear risk | This study was not used for the meta‐analysis of any outcomes Information on ITT analysis was not specified |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Similarity of arms at baseline | Unclear risk | This study was not used for the meta‐analysis of any outcomes Information on similarity of arms at baseline was not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: II Accrual dates: June 2005 to June 2008 | |
| Participants | No. randomised: 336 Stage/treatment line: Metastatic, first‐line Countries/sites: Greece, 23 sites Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal cancer; age ≥ 18 years (median 66/67 years); male (62/66%); PS ECOG 0‐2 (PS ECOG 0: 31/30%) | |
| Interventions | Arm A (FOLFIRI‐bevacizumab (BEV)): irinotecan 180 mg/m2 D1, FA 200 mg/m2 D1, 2, IV bolus 5‐FU 400 mg/m2 D1 and 600 mg/m2/d 22‐hour infusion D1, 2, plus BEV 5 mg/kg D1, q14d (n randomised = 168) Arm B (CAPIRI‐BEV): capecitabine 2000 mg/m2 D1‐14, irinotecan 250 mg/m2 D1 and BEV 7.5 mg/kg D1, q21d (n randomised = 168) Treatment continued until PD, unacceptable toxicity, or withdrawal of consent | |
| Outcomes | PFS ORR (RECIST, version 1.0) OS Grade ≥ 3 AEs (NCI CTC, version 3.0) Median follow‐up: 32 months | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: Hellenic Oncology Research Group, University Hospital of Crete Declarations of interest: No conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation covariate‐adaptive randomisation was used (correspondence with Dr. John Souglakos, received 31 July 2012) |
| Allocation concealment (selection bias) | Low risk | Centralised Web‐based randomisation was used (correspondence with Dr. John Souglakos, received 31 July 2012) |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, blinding unlikely (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low Evaluation of response was performed by independent radiologists (correspondence with Dr. John Souglakos, received 31 July 2012) Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low Grade ≥ 3 AEs were reported from the research nurses of participating institutions (correspondence with Dr. John Souglakos, received 31 July 2012) |
| Schedule of assessment and follow‐up | High risk | (i) Response (influences ORR/PFS): Low Quote: "Response to treatment was evaluated every 8 weeks ..." (patients and methods, paragraph 6, page 454) (ii) Survival (influences PFS/OS): Low Following cessation of treatment or disease progression, participants were followed for survival every 3 months in both arms (correspondence with Dr. John Souglakos, received 31 July 2012) (iii) Grade ≥ 3 AEs: High Quote: "During treatment, a CBC with [sic] was performed weekly. In addition, patients were clinically assessed and blood chemistry was performed before each treatment cycle" (patients and methods, paragraph 6, page 454) Cycles were given every 2 weeks in the FOLFIRI‐BEV arm and every 3 weeks in the CAPIRI‐BEV arm (patients and methods, paragraph 3, page 454) Therefore, safety assessments were performed more frequently in the FOLFIRI‐BEV arm |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low 4 participants in the CAPIRI‐BEV arm were not evaluable for response owing to clinical deterioration/early death (correspondence with Dr. John Souglakos, received 31 July 2012) (ii) PFS/OS: Low No missing outcome data (correspondence with Dr. John Souglakos, received 31 July 2012) (iii) Grade ≥ 3 AEs: Low No missing outcome data (correspondence with Dr. John Souglakos, received 31 July 2012) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Analyses were described as performed in an ’intent‐to‐treat’ fashion (correspondence with Dr. John Souglakos, received 31 July 2012) 336 participants were randomised, 168 to FOLFIRI‐BEV and 168 to CAPIRI‐BEV. 167 participants allocated to FOLFIRI‐BEV and 166 to CAPIRI‐BEV, who received treatment, were included in the analysis (excluded 3/336, 0.9%) (Figure 1, page 455) Safety analysis: As for efficacy |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 455) (ii) Median age: Low (Table 1, page 455) (iii) No. of involved organs: Low (Table 1, page 455) |
| Other bias | Low risk | Subsequent therapies: Low Similar proportions in both arms received second‐line oxaliplatin‐based chemotherapy, irinotecan‐based chemotherapy, cetuximab and BEV beyond progression (Table 4, page 457) |
| Methods | Randomised controlled trial Phase: III Accrual dates: November 1998 to November 2001 | |
| Participants | No. randomised: 1987 Stage/treatment type: Stage III colon carcinoma, adjuvant Countries/sites: Multination, 164 centres ‐ North and South America, Europe, Asia (Thailand), Israel, and Australia Setting: Hospital Characteristics (Arm I/II): Resected stage III colon carcinoma; age 18 to 75 years (median 62/63 years); male (54/54%); PS ECOG 0‐1 (PS ECOG 0: 85/85%) | |
| Interventions | Arm I: Eight cycles of oral capecitabine 1250 mg/m2 bd D1‐14, q21d (n randomised = 1004) Arm II: Six cycles of IV bolus leucovorin 20 mg/m2, then fluorouracil 425 mg/m2 D1‐5, q28d (n randomised = 983) | |
| Outcomes | DFS OS Grade ≥ 3 AEs (NCIC CTC, revised May 1991) Median follow‐up: 6.9 years | |
| Study Details | Journal articles | |
| Funding sources and declarations of interest | Funding sources: Hoffman La‐Roche Declarations of interest: Sanofi‐Aventis, Hoffman La‐Roche, Merck, Pfizer, AstraZeneca, Baxter | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, with the use of treatment allocation codes (scratch‐off labels), was stratified by center and performed with a block size of four. The block size was unknown to investigators and monitors" (methods, paragraph 5, page 2698) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label study (correspondence with Dr. Chris Twelves, received 22 August 2012) (i) DFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) DFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low |
| Schedule of assessment and follow‐up | Low risk | (i) Disease recurrence (influences DFS): Low Quote: "Evaluation of efficacy ‐ Patients were assessed every six months for two years after randomization and then yearly" (methods, paragraph 6, page 2698) (ii) Survival (influences DFS/OS): Low Quote as above (iii) Grade ≥ 3 AEs: Low AEs and treatments were recorded throughout chemotherapy and up to 28 days after last study treatment. Safety assessments were performed at weeks 2, 4 (optional), 7, 10, 13, 16, 19, 22, and 25 of treatment. AEs were reported during treatment with trial medication (correspondence with Dr. Chris Twelves, received 22 August 2012) |
| Incomplete outcome data (attrition bias) | Low risk | (i) DFS: Low 32/1004 participants in the capecitabine arm and 34/983 in the 5FU/LV arm were lost to follow‐up before the 5‐year date after randomisation (correspondence with Dr. Chris Twelves, received 22 August 2012) (ii) OS: Low 93.4% (capecitabine 640/685; 5‐FU/LV 590/632) of participants with an expected 5‐year follow‐up visit had completed 5 years or longer of survival follow‐up at the clinical cut‐off date on 4 June 2007 (correspondence with Dr. Chris Twelves, received 22 August 2012) (ii) Grade ≥ 3 AEs: Low AEs were reported during treatment with trial medication, and all participants were followed for AEs during this time (correspondence with Dr. Chris Twelves, received 22 August 2012) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The intention‐to‐treat population included all patients who underwent randomization" (methods, paragraph 8, page 2698) Safety analysis: Quote: "The population included in the safety analysis comprised all patients receiving at least one dose of the study drug who were followed up for safety" (methods, paragraph 8, page 2698) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 2699) (ii) Median age: Low (Table 1, page 2699) (iii) TNM stage: Low (Table 1, page 2699) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "Overall, 90% (n = 343) and 87% (n = 350) of patients randomized to capecitabine and 5‐FU/FA, respectively, received ≥1 therapeutic intervention at relapse. A similar proportion of patients received systemic treatments at relapse in the two randomization arms (capecitabine versus 5‐FU/FA); these included 5‐FU (57% versus 49%), oxaliplatin (41% versus 35%), irinotecan (36% versus 41%), raltitrexed (6% versus 6%) and cetuximab (4% versus 5%). Capecitabine, however, was later given to more patients in the 5‐FU/FA versus capecitabine arm (24% versus 14%). Locoregional procedures were carried out at relapse in a similar proportion of patients in the capecitabine versus 5‐FU/FA arms, including radiotherapy (18% versus 19%), partial hepatectomy (9% versus 9%) and laparotomy (9% versus 5%)" (Twelves et al, Annals of Oncology 2011 ‐ results, paragraph 2, page 1191) |
| Methods | Randomised controlled trial Phase: III Accrual dates: Not specified | |
| Participants | No. randomised: 531 Stage/treatment line: Metastatic, first‐line Countries/sites: International Setting: Hospital Characteristics: Advanced colorectal carcinoma; age not specified; KPS ≥ 70% | |
| Interventions | Arm I: eniluracil 11.5 mg/m2 and 5‐FU 1.15 mg/m2 oral bd D1‐28, q35d (n randomised and treated = 268) Arm II: IV 5‐FU 425 mg/m2 + LV 20 mg/m2 D1‐5, q28d (n randomised and treated = 263) | |
| Outcomes | ORR (criteria not specified) PFS OS Grade ≥ 3 AEs (criteria not specified) No details on median follow‐up | |
| Study Details | Abstract | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This multicentre randomised open label phase III study..." (abstract) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Outcome assessment at risk of bias from lack of blinding (ii) OS: Low |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/PFS): Unclear ‐ not specified (ii) Survival (influences PFS/OS): Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Unclear ‐ not specified (ii) PFS/OS: Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Unclear risk | Efficacy analysis: Unclear Not specified Safety analysis: Not specified. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ not specified (ii) Median/mean age: Unclear ‐ not specified (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Accrual dates: October 1996 to February 1998 | |
| Participants | No. randomised: 602 Stage/treatment line: Metastatic, first‐line Countries/sites: Multi‐nation, 59 centres in Europe, Australia, New Zealand, Taiwan, and Israel Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal carcinoma; age ≥ 18 years (median 64.0/63.5 years); male (57/57%); KPS ≥ 70% (median 90/90%) | |
| Interventions | Arm I: capecitabine 1250 mg/m2 bd D1‐14, q21d (n randomised = 301) Arm II: LV 20 mg/m2 then IV bolus 5‐FU 425 mg/m2 D1‐5, q28d (n randomised = 301) | |
| Outcomes | ORR (WHO criteria, 1979) TTP (treated as PFS in this review, based on the definition provided) OS Grade ≥ 3 AEs (NCIC CTC, revised December 1994) No details on median follow‐up | |
| Study Details | Journal articles | |
| Funding sources and declarations of interest | Funding sources: Hoffman La‐Roche Declarations of interest: None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After screening to establish eligibility, patients were randomized to treatment with capecitabine or 5‐FU/LV through a computer‐assisted touch‐tone randomization center. The patients were randomized centrally by country, in blocks of six patients, but with Australia, New Zealand, and Taiwan grouped as a single location" (patients and methods, paragraph 2, page 4098) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was an open‐label, randomized, parallel‐group study ..." (patients and methods, paragraph 2, page 4098) (i) ORR/TTP (treated as PFS): Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/TTP (treated as PFS): Low Quote: "Investigator assessments of tumor response were reviewed by an independent review committee (IRC) composed of radiologists. Members of the IRC were blinded to the treatment received, clinical condition of the patient, and to the investigator’s evaluation and measurements. The IRC‐assessed tumor response solely on the basis of x‐ray or other imaging. Oncologists were available for IRC consultation" (patients and methods, paragraph 6, page 4099) There were both Investigator and blinded IRC response assessments. The absolute ORR was higher for capecitabine than 5‐FU/LV for both of these assessments (results, paragraph 4, page 4100) (ii) OS: Low |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/TTP (treated as PFS)): Low Quote: "Assessments of tumor dimensions and involved sites were performed before the start of treatment and were scheduled during therapy after weeks 6, 12, 18, 24, and 30. Further assessments were performed after weeks 39 and 48 for patients who received prolonged therapy (48 weeks). Follow‐up assessments for disease progression and survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 6, page 4099) (ii) Survival (influences TTP(treated as PFS)/OS): Low Follow‐up assessments for disease progression and survival monitoring were performed every 3 months after the end of treatment" (patients and methods, paragraph 6, page 4099) (iii) Safety: Low Quote: "Safety evaluations were conducted at least monthly until 4 weeks after the last administration of therapy ..." (patients and methods, paragraph 7, page 4099) |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low 11.0% of participants in the capecitabine arm and 12.6% in the 5‐FU/LV arm were missing post‐baseline response data (Table 2, page 4100) (ii) TTP (treated as PFS)/OS: Unclear ‐ not specified (iii) Grade ≥ 3 AEs: Unclear ‐ not specified |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy: Low Quote: "All analyses of efficacy are reported for the all‐randomized population ..." (patients and methods, paragraph 10, page 4099) Safety: Quote: "...all analyses of safety are based on the safety population, which included all patients who received at least one dose of study |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear Presented as mean/median only (Table 1, page 4100) (ii) Median age: Low (Table 1, page 4100) (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
| Methods | Randomised controlled trial Phase: III Accrual dates: February 2009 to March 2011 | |
| Participants | No. randomised: 512 Stage/treatment line: Inoperable locally advanced or metastatic CRC; first‐line (patients with relapse > 180 days after adjuvant chemotherapy were eligible, but prior treatment with oxaliplatin‐containing adjuvant chemotherapy was not permitted) Countries/sites: Japan, 82 institutions Setting: Hospital Characteristics (Arm I/II): Inoperable locally advanced or metastatic colorectal cancer; age 20 to 80 years (median 63/63 years); male (62.4/66.4%); PS ECOG 0‐1 | |
| Interventions | Arm I (mFOLFOX6‐bevacizumab (BEV)): D1 Oxaliplatin 85 mg/m2, LV 200 mg/m2, IV bolus 5‐FU 400 mg/m2 followed by 5‐FU 2400 mg/m2 46‐hour continuous infusion, plus BEV 5 mg/kg D1, q14d (n randomised = 256) Arm II (SOX‐BEV): S‐1 40‐60 mg* oral bd D1‐14 and D1 oxaliplatin 130 mg/m2, plus BEV 7.5 mg/kg D1, q21d *S‐1 doses: 80 mg/d if BSA < 1.25 m2; 100 mg/d if 1.25 m2 ≤ BSA <1.5 m2; 120 mg/d if BSA ≥ 1.5 m2 (n randomised = 256) | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (CTCAE, version 3.0) Median follow‐up: PFS 18.4 months (IQR, 13.1 to 24.9 months); OS 23.4 months (IQR, 19.5 to 29.6 months). Data cut‐off date for PFS ‐ June 30, 2012 | |
| Study Details | Journal article and abstract/poster | |
| Funding sources and declarations of interest | Funding sources: Taiho Declarations of interest: Taiho, Chugai, Pfizer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done centrally with the minimisation method,with stratification by institution and whether postoperative adjuvant chemotherapy had been given" (methods, paragraph 5, page 1279) |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure allocation concealment, a minimisation algorithm with an 80:20 random element was used. The randomisation sequence was generated by a team (EPS Corporation, Tokyo, Japan; independent from the trial sponsor and investigators) who used a validated computer system" (methods, paragraph 5, page 1279) |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "We undertook an open‐label, non‐inferiority, randomised phase 3 trial" (methods, paragraph 1, page 1279) Quote: "Participants, investigators, and data analysts could not be masked to treatment assignment, because we were comparing an oral‐based regimen with an infusional regimen" (methods, paragraph 5, page 1279) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High Quote: "Progressive disease was assessed solely by the investigator in charge of the patient" (methods, paragraph 8, page 1280) (ii) OS: Low |
| Schedule of assessment and follow‐up | High risk | (ii) Response (influences ORR/PFS): Low Quote: "Lesions were measured every 8 weeks with diagnostic imaging (e.g., CT or MRI)" (methods, paragraph 8, page 1280) Quote: "After initiation of study treatment, target and non‐target lesions were assessed every 8 weeks in the same way as at baseline, with the same imaging conditions (e.g., contrast media and slice thickness). The best overall response was identified" (methods, paragraph 9, page 1280) (ii) Survival (influences PFS/OS): Low Same in both arms (correspondence with Aya Takata, received 14 July 2016) (iii) Grade ≥ 3 AEs: High Quote: "... patients who received SOX plus bevacizumab returned to the hospital once every 3 weeks rather than once every 2 weeks for patients who received mFOLFOX6 plus bevacizumab" (discussion, paragraph 1, page 1284) Therefore, participants in the mFOLFOX6 plus bevacizumab arm would have more frequent opportunities to report AEs |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low Among participants with measurable disease, only 7% in the mFOLFOX6‐BEV group and 9% in the SOX‐BEV group were non‐evaluable (Takahari et al, Journal of Clinical Oncology 2013 ‐ poster associated with abstract 3519) (ii) PFS/OS: Low Censoring was noted in the KM curves for PFS/OS (Figure 2, page 1282). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Low No missing outcome data for toxicity |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low 0/256 and 1/256 (0.4%) participants randomised to the SOX + BEV and mFOLFOX6 + BEV arms, respectively, were excluded from the analysis post randomisation owing to ineligibility (Figure 1, page 1280) Safety analysis: Quote: "Patients who received at least one dose of the assigned study drugs were included in analyses of ... safety" (methods, paragraph 12, page 1281) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear. All participants were confirmed to be either PS 0 or 1 when enrolled; however, no information was collected about PS status in the CRF (correspondence with Aya Takata, received 14 July 2016) (ii) Median age: Low (Table 1, page 1281) (iii) No. of involved organs: Low (Table 1, page 1281) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "188 patients given mFOLFOX6 plus bevacizumab and 198 given SOX plus bevacizumab received second‐line treatment. Irinotecan was used as second‐line treatment in 122 (65%) of the 188 patients given mFOLFOX6 plus bevacizumab, oxaliplatin in nine (5%), bevacizumab in 70 (37%), cetuximab in ten (5%), and panitumumab in nine (5%). Irinotecan was used in 116 (59%) of the 198 patients given SOX plus bevacizumab, oxaliplatin in 23 (12%), bevacizumab in 67 (34%), cetuximab in 15 (8%), and panitumumab in nine (5%)" (results, paragraph 7, page 1283) |
| Methods | Randomised controlled trial Phase: II Accrual dates: July 2008 to July 2009 | |
| Participants | No. randomised: 107 Stage/treatment line: Metastatic, first‐line Countries/sites: Japan, 22 institutions Setting: Hospital Characteristics (Arm A/B): Metastatic colorectal cancer; age > 20 years (median 60.5/61.0 years); male (58.9/46.9); PS ECOG < 0‐1 (PS ECOG 0: 87.5/81.6%) | |
| Interventions | Arm A (SOL): S‐1 40‐60 mg bd plus oral LV 25 mg bd D1‐7 and oxaliplatin (L‐OHP) 85 mg/m2 D1, q14d Arm B (mFOLFOX6): D1 oxaliplatin 85 mg/m2, LV 200 mg/m2, IV bolus 5‐FU 400 mg/m2 followed by 5‐FU 2400 mg/m2 46‐hour continuous infusion, q14d | |
| Outcomes | PFS Grade ≥ 3 AEs (CTCAE, version 3.0) ORR (RECIST, version 3.0) OS PFS cut‐off date 31 March 2010; OS cut‐off date 31 January 2012 (median follow‐up 35 months) | |
| Study Details | Journal article and abstract/poster | |
| Funding sources and declarations of interest | Funding sources: Taiho, Yakult Declarations of interest: Taiho, Yakult | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to receive SOL or mFOLFOX6 at a central registration center, using a minimization method with stratification according to disease status (unresectable or recurrent disease) and institution" (patients and methods, paragraph 3, page 570‐1) |
| Allocation concealment (selection bias) | Low risk | Quote as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This randomized, open‐label, phase II study... " (patients and methods, paragraph 4, page 571) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: Low Quote: "Response and PFS were evaluated by an independent review committee (IRC)" (patients and methods, paragraph 6, page 571) (ii) OS: Low |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/PFS): Low Quote: "Tumors were assessed every 6 weeks until disease progression" (patients and methods, paragraph 6, page 571) (ii) Survival (influences PFS/OS): Low The schedule for assessment of survival was the same in both arms (correspondence with Aya Takata, received 22 July 2016) (iii) Grade ≥ 3 AEs: Low Quote: "Physical examinations and laboratory tests ... were repeated every week during the first four cycles of chemotherapy and every 2 weeks from the fifth cycle onward" (patients and methods, paragraph 6, page 571) |
| Incomplete outcome data (attrition bias) | Unclear risk | (i) ORR: Low No missing response data from participants included in the 'full analysis set' (Figure.1, page 572, and Table 2, page 574) (ii) PFS/OS: Low Quote: "Progression‐ free survival (PFS) was defined as the time from randomization to disease progression or death from any cause. Data on patients without documented evidence of progressive disease or death were censored on the date of the last tumor assessment without progression during the protocol treatment" (patients and methods, paragraph 6, page 571). No evidence of bias related to censoring Censoring was also noted on the KM curve for OS (Fig. 2b, page 573). No evidence of bias related to censoring (iii) Grade ≥ 3 AEs: Unclear No information regarding this is available (correspondence with Aya Takata, received 22 July 2016) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low 0/56 (0%) and 2/51 (4%) participants randomised to the SOL and mFOLFOX6 arms, respectively, were excluded from the analysis owing to ineligibility (Fig. 1, page 572) Safety analysis: Low Safety analysis population comprised all participants who were randomised to their respective treatment arms (correspondence with Aya Takata, received 22 July 2016) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Table 1, page 572) (ii) Median age: Low (Table 1, page 572) (iii) No. of involved organs: Low (Table 1, page 572) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "The proportion of patients who received subsequent therapy was slightly higher in the SOL group (100%) than in the mFOLFOX6 group (91.8%). Irinotecan was given to 37 patients (66.1%) in the SOL group and 33 (67.3%) in the mFOLFOX6 group, bevacizumab to 26 patients (46.4%) in the SOL group and 19 (38.8%) in the mFOLFOX6 group, and L‐OHP to 12 (21.4%) in the SOL group and 3 (6.1%) in the mFOLFOX6 group" (results, paragraph 4, page 572) However, of the 12 patients who received post‐treatment containing oxaliplatin in the SOL arm, "7 patients did not discontinue SOL treatment due to disease progression" (Otsuji et al, Journal of Clinical Oncology 2012, poster associated with abstract 586) |
| Methods | Randomised controlled trial Phase: II/III with combined data analysed Accrual dates: January 2006 to January 2008 | |
| Participants | No. randomised: 426 Stage/treatment line: Metastatic, second‐line (irinotecan‐naive) Countries/sites: Japan, 40 institutions Setting: Hospital Characteristics (Arm I/II): Metastatic colorectal cancer; age 20 to 75 years (median 63.0/61.0 years); male (57.7/56.3%); PS ECOG 0‐1 (PS ECOG 0: 75.1/74.2%) | |
| Interventions | Arm I (FOLFIRI): folinic acid 200 mg/m2, irinotecan 150 mg/m2, IV bolus fluorouracil 400 mg/m2 D1 and fluorouracil 2400 mg/m2 46h infusion, D1, 15, q28d (n randomised = 213) Arm II (IRIS): irinotecan 125 mg/m2 D1, 15, and S‐1 (40 mg if BSA < 1.25 m2; 50 mg if BSA 1.25 < 1.5 m2; 60 mg if BSA ≥ 1.5 m2) bd D1–14, q28d (n randomised = 213) Treatment continued until PD, unacceptable toxicity, or participant refusal | |
| Outcomes | PFS OS ORR (RECIST, version 1.0) Grade ≥ 3 AEs (CTCAE, version 3.0) Data collection cut‐off for OS: 29 July 2010, median follow‐up 39.2 months | |
| Study Details | Journal article and abstract | |
| Funding sources and declarations of interest | Funding sources: Taiho Declarations of interest: Daiichi Sankyo, Taiho, Yakult Honsha | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were centrally randomised to receive either FOLFIRI or IRIS using the minimisation method with stratification by institution, prior therapy (with oxaliplatin vs. without oxaliplatin), and performance status (PS; 0 vs. 1)" (patients and methods, paragraph 2, page 154) Quote: "Assignment of patients was concealed from the investigator" (Muro et al, Lancet Oncology 2010, methods, paragraph 3, page 854) |
| Allocation concealment (selection bias) | Low risk | Quotes as above |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Treatment assignment was not masked from the investigators or patients" (Muro et al, Lancet Oncology 2010, methods, paragraph 3, page 854) (i) ORR/PFS: Low Outcome assessment unlikely to be influenced by lack of blinding (ii) OS: Low |
| Blinding of outcome assessment (detection bias) | High risk | (i) ORR/PFS: High "Treatment assignment was not masked from the investigators or patients" (Muro et al, Lancet Oncology 2010, methods, paragraph 3, page 854) The definition of progression for the primary endpoint of PFS included clinical assessment, quote: "Progression was defined when any of the following three events occurred: (1) PD based on the response evaluation criteria in solid tumours (RECIST) version 1.0; (2) clinical progression judged by the investigator; or (3) death from any cause without progression" (patients and methods, paragraph 5, page 155) Outcome assessment at risk of bias from lack of blinding (ii) OS: Low |
| Schedule of assessment and follow‐up | Low risk | (i) Response (influences ORR/PFS): Low Quote: "Tumours were assessed at baseline (within 1 month before enrolment), 2, 3, and 4 months after enrolment, and every 2 months thereafter until progression" (patients and methods, paragraph 5, page 155) (ii) Survival (influences PFS/OS): Low Same in both arms (correspondence with Aya Takata, received 19 July 2016) (iii) Grade ≥ 3 AEs: Low Quote: "Physical examinations and laboratory tests were performed at baseline and repeated at least every 2 weeks during the treatment" (patients and methods, paragraph 5, page 155) |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low 39/213 (18%) in the FOLFIRI arm and 32/213 (15%) in the IRIS arm did not have evaluable response data (Muro et al, Lancet Oncology 2010, results, paragraph 5, page 856) (ii) PFS/OS: Low Quote: "Progression‐free survival was counted from the date of randomisation to the date when the progressive disease was first confirmed by the investigator’s assessment. For patients without documented progressive disease, data was censored on the date of the last tumour assessment with non‐progression status" (Muro et al, Lancet Oncology 2010, methods, paragraph 9, page 855). No evidence of bias related to censoring Quote: "OS was calculated from the date of randomisation to the date of death from any cause. Surviving patients, including those lost to follow‐up, were censored at the date of last confirmation of survival" (patients and methods, paragraph 6, page 155). No evidence of bias related to censoring (iii) Safety: Low No missing safety outcome data (correspondence with Aya Takata, received 19 July 2016) |
| Incomplete outcome data (ITT analysis) | Low risk | Efficacy analysis: Low Quote: "The intent‐to‐treat (ITT) population consisted of all randomised patients..." (patients and methods, paragraph 7, page 155) Safety analysis: Quote: "Safety was assessed in all patients who received at least one dose of the study drug" (Muro et al, Lancet Oncology 2010, methods, paragraph 11, page 855) |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Low risk | (i) PS: Low (Muro et al, Lancet Oncology 2010, Table 1, page 855) (ii) Median age: Low (Muro et al, Lancet Oncology 2010, Table 1, page 855) (ii) No. of involved organs: Low (Muro et al, Lancet Oncology 2010, Table 1, page 855) |
| Other bias | Low risk | Subsequent therapies: Low Quote: "Third‐line chemotherapy after failure of the protocol treatment in the second‐line therapy was given to 168 (78.9%) patients in the FOLFIRI group and 153 (71.8%) patients in the IRIS group. In these patients, molecularly targeted agents were concomitantly used in 58 (27.2%) patients (bevacizumab, 45; cetuximab, 17) in the FOLFIRI group and 52 (24.4%) patients (bevacizumab, 38; cetuximab, 16) in the IRIS group, and no marked difference in the use of these agents was evident between the two groups" (results, paragraph 2, page 156) |
| Methods | Randomised controlled trial Phase: Not specified Accrual dates: January 2001 to September 2003 | |
| Participants | No. randomised: 43 Stage/treatment line: Metastatic, first‐line or second‐line if no chemotherapy for longer than 6 months Countries/sites: China Setting: Hospital Characteristics: Metastatic colorectal carcinoma; age ≤ 75 years; KPS 0‐1 (unclear) | |
| Interventions | Arm I: irinotecan 90‐125 mg/m2 10‐hour infusion, FA 30 mg/m2 + 5‐FU 425 mg/m2/d 48‐hour continuous infusion, q14d for no less than 6 C (n randomised = 16) Arm II: irinotecan 90‐125 mg/m2 10‐hour infusion, q14d and capecitabine 1250 mg/m2/d for 3 months (n randomised = 27) | |
| Outcomes | ORR (criteria not specified) TTP OS Grade ≥ 3 AEs (criteria not specified) Median follow‐up: 14 months | |
| Study Details | Journal article | |
| Funding sources and declarations of interest | Funding sources: None declared Declarations of interest: None declared | |
| Notes | Original article (in Chinese) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | (i) ORR/TTP: Low Quote (translated from Chinese to English): "According to random double‐blind method ..." (materials and methods, paragraph 1, page 558) This is unclear/unlikely, as no placebo was described in the IV or oral arm for different schedules Outcome assessment unlikely to be influenced by lack of blinding This study was not used for the meta‐analysis of the TTP outcome (ii) OS: This study was not used for the meta‐analysis for this outcome (iii) Grade ≥ 3 AEs: This study was not used for the meta‐analyses of these outcomes |
| Blinding of outcome assessment (detection bias) | High risk | Not specified (i) ORR/TTP: High Outcome assessment at risk of bias if there was lack of blinding This study was not used for the meta‐analysis of the TTP outcome (ii) OS: This study was not used for the meta‐analysis of this outcome (iii) Grade ≥ 3 AEs: This study was not used for the meta‐analyses of these outcomes |
| Schedule of assessment and follow‐up | Unclear risk | (i) Response (influences ORR/TTP): Unclear ‐ not specified. This study was not used for the meta‐analysis of the TTP outcome (ii) Survival (influences OS): Unclear ‐ not specified. This study was not used for the meta‐analysis of this outcome (iii) Grade ≥ 3 AEs: Unclear. This study was not used for the meta‐analyses of these outcomes |
| Incomplete outcome data (attrition bias) | Low risk | (i) ORR: Low The sum of those with CR/PR/SD and PD was the same as the number of participants included in the study for both arms (Table 2, page 558) (ii) TTP/OS: Unclear ‐ not specified. This study was not used for the meta‐analysis of the TTP and OS outcomes (iii) Grade ≥ 3 AEs: Unclear. This study was not used for the meta‐analyses of these outcomes |
| Incomplete outcome data (ITT analysis) | Unclear risk | Efficacy analysis: Unclear ‐ not specified Safety analysis: Not specified. This study was not used for the meta‐analyses of grade ≥ 3 AE outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Similarity of arms at baseline | Unclear risk | (i) PS: Unclear ‐ not specified (ii) Median/mean age: Unclear ‐ not specified (iii) No. of involved organs: Unclear ‐ not specified |
| Other bias | Unclear risk | Subsequent therapies: Unclear ‐ not specified |
For the Characteristics of included studies tables, Outcomes listed are those that the study reported that were of interest in this review, regardless of inclusion in quantitative synthesis. Study details refer to the form of report/s used for this review.
PS: performance status
ECOG: Eastern Cooperative Oncology Group
5‐dFUR: doxifluridine
IV: intravenous
5‐FU / FU: 5‐fluorouracil
ORR: objective response rate
WHO: World Health Organisation
PFS: progression‐free survival
TTP: time to tumour progression
AEs: adverse events
OS: overall survival
LV: leucovorin
KM: Kaplan‐Meier
DFS: disease‐free survival
NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events
NSABP: National Surgical Adjuvant Breast and Bowel Project
CT: computed tomography
MRI: magnetic resonance imaging
WHO: World Health Organisation
CR: complete response
PR: partial response
SD: stable disease
PD: progressive disease
NCI CTC: National Cancer Institute Common Terminology Criteria
TTF: time to treatment failure
UFT: tegafur/uracil
RECIST: Response Evaluation Criteria in Solid Tumours
IRC: independent review committee
ITT: intention‐to‐treat
SICOG: Southern Italy Cooperative Oncology Group
WBC: white blood cell
ASCO: American Society of Clinical Oncology
TTD: Treatment of Digestive Tumors
ECOG CTC: Eastern Cooperative Oncology Group Common Toxicity Criteria
KPS: Karnofsky Performance Scale
LFT: liver function tests
BEV: bevacizumab
FNCLCC: Federation Nationale des Centers de Lutte Contre le Cancer
BP: blood pressure
EPP: Expanded Participation Project
DHHS: Department of Health and Human Services
TME: total mesorectal excision
PME: partial mesorectal excision
T‐CORE: Tohoku Clinical Oncology Research and Education Society
BSA: body surface area
NE: non‐evaluable
EORTC: European Organisation for Research and Treatment of Cancer
FA: folinic acid
GOAM: Gruppo Oncologico Aree Metropolitane
L‐OHP: oxaliplatin
HeCOG: Hellenic Oncology Research Group
CRC: colorectal cancer
AJCC: American Joint Committee on Cancer
SWOG: South‐West Oncology Group
EU: eniluracil
UK MRC: United Kingdom Medical Research Council
MRC CTU: Medical Research Council Clinical Trials Unit
IQR: interquartile range
JCOG: Japan Clinical Oncology Group
CEA: carcinoembryonic antigen
Ca19‐9: cancer antigen 19‐9
GOIM: Gruppo Oncologico dell’Italia Meriodionale
CBC: complete blood count
CRF: case report form
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT, but used a chemotherapy regimen that was not consistent with contemporary practice | |
| This study investigated the role of oral vs IV 5‐dFUR in metastatic CRC, but only in a selected subset of all randomised participants (only those considered to be 5‐FU resistant) | |
| RCT, with cross‐over permitted in only 1 (IV 5‐FU) arm | |
| This study included participants with both gastric and colorectal cancer, and results for participants with CRC were inseparable | |
| RCT with a cross‐over design. Participants in both arms received only 1 cycle of chemotherapy before cross‐over | |
| Study did not state that histologically proven colorectal adenocarcinoma was required for inclusion in the trial, other than for hepatomegaly | |
| This study examined oral capecitabine vs IV calcium folinate/5‐FU in a selected group of randomised participants who had not progressed after 1 cycle of chemotherapy | |
| RCT, but used a chemotherapy regimen that was not consistent with contemporary practice | |
| RCT with a cross‐over design. Participants received only 6 weeks of IV 5FU/LV or two 3‐week cycles of oral capecitabine before cross‐over to the other arm of treatment | |
| Histologically proven adenocarcinoma of the rectum was not confirmed as an inclusion criterion for this study. Mean follow‐up period was only 15 months | |
| RCT with a cross‐over design. Participants in both arms received only 1 cycle of chemotherapy before cross‐over | |
| This study did not compare oral and IV fluoropyrimidines. It compared oral UFT with oral ftorafur after surgery | |
| Histologically proven colorectal adenocarcinoma was not confirmed as an inclusion criterion for this study | |
| Study was closed early owing to poor accrual; no publishable results | |
| Study was ceased early owing to poor accrual, with many elderly patients refusing to have intravenous fluoropyrimidine therapy | |
| No described comparison between 5‐FU and capecitabine arms | |
| No clear comparison between oral and IV fluoropyrimidine described | |
| Histologically proven colorectal adenocarcinoma was not confirmed as an inclusion criterion for this study | |
| RCT with a cross‐over design. Participants in the oral capecitabine arm received only 2 cycles of chemotherapy before cross‐over | |
| RCT, but participants in both Arms A and B received IV fluoropyrimidine (IV 5‐fluorouracil in Arm A and IV Ftorafur in Arm B) | |
| It is unclear if this study was a randomised trial | |
| Histologically proven colorectal adenocarcinoma was not confirmed as an inclusion criterion for this study | |
| Histologically proven colorectal adenocarcinoma was not confirmed as an inclusion criterion for this study | |
| Participants were not randomised to oral vs IV fluoropyrimidine for the induction therapy component of the study, according to the most recent efficacy and safety update for this study | |
| RCT with a cross‐over design. Participants in the oral capecitabine arm received only 1 cycle of chemotherapy before cross‐over, and participants in the IV 5‐FU/LV arm received 2 cycles of de Gramont IV 5‐FU/LV or 1 cycle of Mayo regimen IV 5‐FU/LV (whichever regimen was used routinely in the individual participating centre) before cross‐over |
RCT: randomised controlled trial
IV: intravenous
5‐dFUR: doxifluridine
CRC: colorectal cancer
5‐FU: 5‐fluorouracil
LV: leucovorin
UFT: tegafur/uracil
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Short‐course radiotherapy with concurrent chemotherapy; a single‐center experience |
| Methods | Prospective randomised trial |
| Participants | Target sample size: 150 Stage/treatment type: Distal rectal cancer, neoadjuvant chemoradiation Countries/sites: Russia, single institution ‐ N.N. Blokhin Russian Research Cancer Center, Colorectal Cancer, Moscow, Russian Federation |
| Interventions | Arm I: 5‐FU 425 mg/m2 IV infusion over 24 hours on D1‐5 of radiotherapy Arm II: capecitabine 2000 mg/m2 oral D1‐14 of radiotherapy Arm III: Tegafur 800 mg/m2 oral D1‐21 of radiotherapy Co‐interventions: short‐course 5 × 5 Gy radiotherapy and surgery 2 to 10 weeks after completion of chemo‐radiotherapy |
| Outcomes | Toxicity, tumour regression |
| Starting date | Start: 2011 |
| Contact information | Professor Y Barsukov. N.N. Blokhin Russian Research Cancer Center, Colorectal Cancer, Moscow, Russian Federation |
| Notes | The last informative response from the contact author indicated that the study was ongoing |
| Trial name or title | Bevacizumab + FOLFOX4 or XELOX2 as first‐line treatment in colorectal cancer. Randomized phase 2 study ‐ GOIM 2802 |
| Methods | Prospective open‐label randomised trial |
| Participants | Target sample size: 120 Stage/treatment line: Metastatic, first‐line Countries/Sites: Italy, multiple sites |
| Interventions | Arm A (FOLFOX4 + Bevacizumab (BEV)): 5‐FU 400 mg/m2 bolus D1, D2, 5‐FU 600 mg/m2 infusion D1‐2, Oxaliplatin 85 mg/m2 D1, BEV 5 mg/kg D1. Repeated every 2 weeks Arm B: Capecitabine 2000 mg/m2 orally BD D1‐7, Oxaliplatin 100 mg/m2 D1, BEV 5 mg/kg D1. Repeated every 2 weeks Participants with an objective response or stable disease after 12 cycles will be randomised to: Arm C: 5‐FU 400 mg/m2 bolus D1, D2, 5‐FU 600 mg/m2 infusion D1‐2, BEV 5 mg/kg D1, every 2 weeks; capecitabine 2000 mg/m2 orally BD D1‐7, BEV 5 mg/kg D1. Repeated every 2 weeks. Participants will receive the same fluoropyrimidine used in Arm A or B Arm D: BEV 5 mg/kg, repeated every 2 weeks |
| Outcomes | Primary: ORR Secondary: AEs, OS, TTP |
| Starting date | 2011 |
| Contact information | Dr Evaristo Maiello, Dipartimento di Oncoematologia. U.O. OncologiaI, IRCCS “Casa Sollievo della Sofferenza”, Viale Cappuccini, 71013 San Giovanni Rotondo (FG), Italy |
| Notes | Study ongoing ‐ EU Clinical Trials register https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2010‐022091‐31 (accessed 7 April 2017) |
| Trial name or title | Neoadjuvant chemoradiation with oral capecitabine versus intravenous 5‐fluorouracil and leucovorin in locally advanced carcinoma rectum – a randomized trial |
| Methods | Open label randomised controlled trial Phase: II |
| Participants | Target sample size: ˜100 participants Stage/treatment type: Locally advanced rectal carcinoma, neoadjuvant chemoradiation Countries/sites: India, single institution – Department of Radiotherapy, R. G. Kar Medical College and Hospital, Kolkata |
| Interventions | Arm A (Study, Capecitabine): External beam radiotherapy (EBRT) 50.4 Gy/28 fractions/5.5 weeks with concomitant capecitabine 825 mg/m2 po BD 5 days per week, for the period of EBRT Arm B (Control, 5‐FU‐LV): EBRT 50.4 Gy/28 fractions/5.5 weeks with concomitant 5‐FU 350 mg/m2/d continuous infusion and LV 20 mg/m2 for 5 days every 4 weeks (D1‐5 and D29‐33) Post‐neoadjuvant chemoradiation, participants undergo definitive surgery after 6 weeks. All participants receive adjuvant chemotherapy for 6 months |
| Outcomes | Primary endpoint: Locoregional response Secondary endpoints: Pathological CR, AEs (CTCAE version 4.0) |
| Starting date | January 2011 |
| Contact information | Dr. Abhishek Basu ‐ [email protected] |
| Notes | The last informative response from the contact author indicated that the study was ongoing |
| Trial name or title | A multinational, randomized, Phase III study of XELIRI with/without Bevacizumab versus FOLFIRI with/without Bevacizumab as second‐line therapy in patients with metastatic colorectal cancer |
| Methods | Randomised controlled trial; open label Phase: III |
| Participants | Target sample size: n = 600 Stage/treatment line: Metastatic, second‐line Countries/sites: Japan, South Korea, and China |
| Interventions | Arm I (FOLFIRI +/‐ bevacizumab): bevacizumab 5 mg/kg IV D1, CPT‐11 180 mg/m2 (150 mg/m2 if homozygous for UGT1A1*6 or UGT1A1*28 OR double heterozygous for UGT1A1*6 and UGT1A1*28), l‐LV 200 mg/m2 or dl‐LV 400 mg/m2 IV D1, Bolus 5‐FU 400 mg/m2 IV bolus D1 and Infusional 5‐FU 2400 mg/m2 IV continuous over 46 hours, in a 2‐week cycle Arm II (XELIRI +/‐ bevacizumab): bevacizumab 7.5 mg/kg IV D1, CPT‐11 200 mg/m2 (150 mg/m2 if homozygous for UGT1A1*6 or UGT1A1*28 OR double heterozygous for UGT1A1*6 and UGT1A1*28) IV D1, capecitabine 800 mg/m2 oral BD D1‐15, in a 3 week cycle |
| Outcomes | OS PFS ORR AEs (CTCAE version 4.0) |
| Starting date | Start: December 2013 Estimated completion date: As of August 2015, n = 650 participants had been enrolled. Estimated study completion date is January 2017 (www.clinicaltrials.gov) |
| Contact information | PI: Dr. Kei Muro ‐ kmuro@aichi‐cc.jp |
| Notes | All patients from South Korea and Japan receive concomitant bevacizumab, and the addition of bevacizumab is a stratification factor |
| Trial name or title | Randomised Phase II study of SOX vs mFOLFOX6 as neoadjuvant chemotherapy in patients with resectable rectal cancer |
| Methods | Open‐label randomised controlled trial Phase: II |
| Participants | Target sample size: 110 Stage/treatment type: Resectable rectal cancer, neoadjuvant chemotherapy Countries/sites: Multiple institutions in Japan Setting: Hospital |
| Interventions | Arm I (SOX (S‐1 + L‐OHP)): S‐1 80 mg/m2 oral D 1‐14, L‐OHP 130 mg/m2 D1, in a 3 week cycle until 4 cycles or when discontinuation criteria met Arm II (mFOLFOX6 + L‐OHP): 85 mg/m2 and l‐LV 200 mg/m2 by IV infusion D1, 5‐FU Bolus 400 mg/m2 IV bolus D1 and Infusional 5‐FU 2400 mg/m2 IV continuous over 46 hours, in a 2 week cycle for 6 cycles or when discontinuation criteria met |
| Outcomes | DFS OS AEs (CTCAE v4.0) R0 resection rate Pathological effect |
| Starting date | Start: September 2013 Estimated completion date: August 2020; final data collection date for primary outcome measure (3‐year DFS) is August 2018 (www.clinicaltrials.gov) |
| Contact information | PI: Professor Yoshito Akagi, Kurume University, Japan |
| Notes |
5‐FU: 5‐fluorouracil
IV: intravenous
GOIM: Gruppo Oncologico dell’Italia Meriodionale
BEV: bevacizumab
ORR: objective response rate
AEs: adverse events
OS: overall survival
TTP: time to progression
IRCCS: Institute for Research and Health Care
FG: Foggia
EU: European Union
EBRT: external beam radiotherapy
CR: complete response
CTCAE: Common Terminology Criteria for Adverse Events
CPT‐11: camptothecin‐11
PFS: progression‐free survival
L‐OHP: oxaliplatin
DFS: disease‐free survival
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐free survival Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| Analysis 1.1 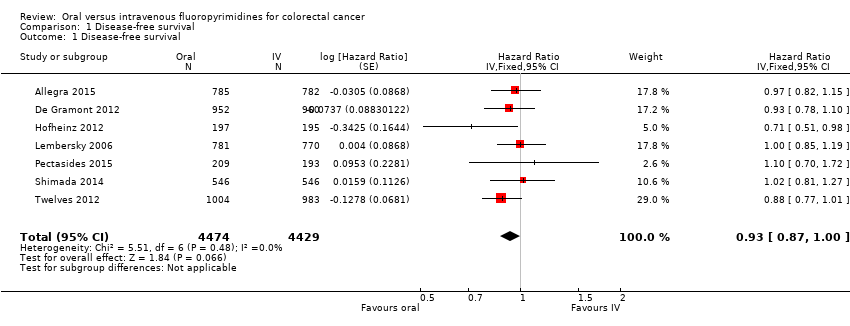 Comparison 1 Disease‐free survival, Outcome 1 Disease‐free survival. | ||||
| 2 Disease‐free survival with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| Analysis 1.2  Comparison 1 Disease‐free survival, Outcome 2 Disease‐free survival with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy. | ||||
| 2.1 Chemotherapy | 5 | 6944 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 2.2 Chemo‐radiotherapy | 2 | 1959 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.78, 1.05] |
| 3 Disease‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 6 | 8511 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.88, 1.02] |
| Analysis 1.3  Comparison 1 Disease‐free survival, Outcome 3 Disease‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 3881 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 3.2 Bolus intravenous fluoropyrimidine | 3 | 4630 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.86, 1.04] |
| 4 Disease‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| Analysis 1.4  Comparison 1 Disease‐free survival, Outcome 4 Disease‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 5 | 6260 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.83, 0.99] |
| 4.2 UFT/Ftorafur | 2 | 2643 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (curative intent studies) Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| Analysis 2.1 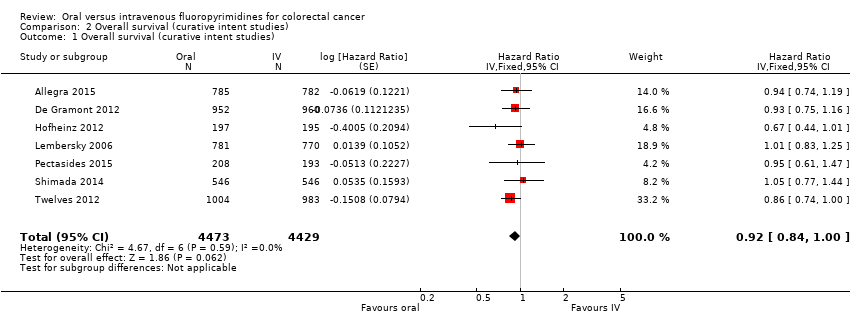 Comparison 2 Overall survival (curative intent studies), Outcome 1 Overall survival (curative intent studies). | ||||
| 2 Overall survival (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| Analysis 2.2 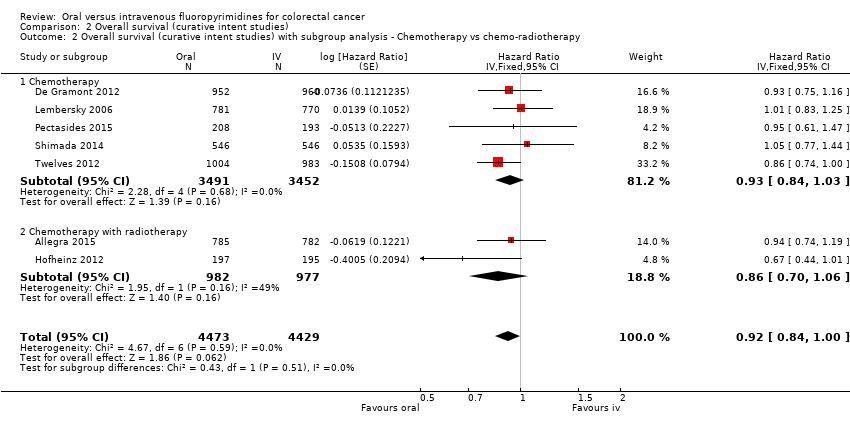 Comparison 2 Overall survival (curative intent studies), Outcome 2 Overall survival (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy. | ||||
| 2.1 Chemotherapy | 5 | 6943 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 2.2 Chemotherapy with radiotherapy | 2 | 1959 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 3 Overall survival (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 6 | 8510 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| Analysis 2.3  Comparison 2 Overall survival (curative intent studies), Outcome 3 Overall survival (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 3880 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.80, 1.09] |
| 3.2 Bolus intravenous fluoropyrimidine | 3 | 4630 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 4 Overall survival (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| Analysis 2.4  Comparison 2 Overall survival (curative intent studies), Outcome 4 Overall survival (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 5 | 6259 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.79, 0.98] |
| 4.2 UFT/Ftorafur | 2 | 2643 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grade ≥ 3 diarrhoea (curative intent studies) Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| Analysis 3.1  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 1 Grade ≥ 3 diarrhoea (curative intent studies). | ||||
| 2 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| Analysis 3.2 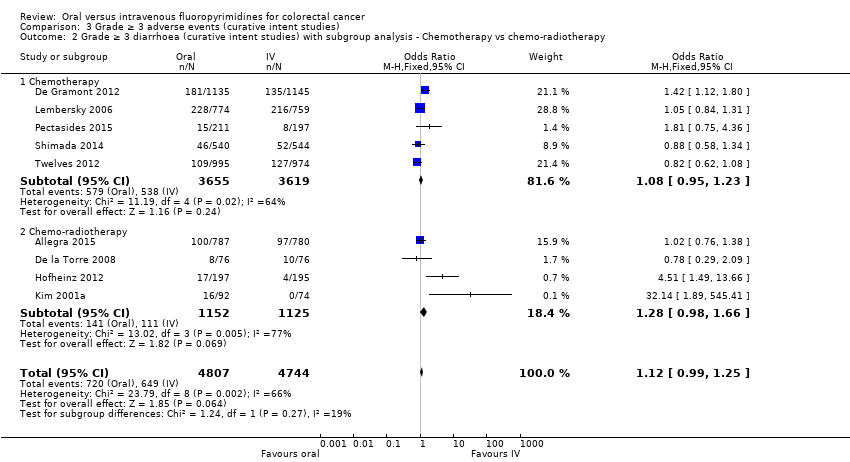 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 2 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy. | ||||
| 2.1 Chemotherapy | 5 | 7274 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 2.2 Chemo‐radiotherapy | 4 | 2277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.98, 1.66] |
| 3 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 8 | 9159 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.23] |
| Analysis 3.3 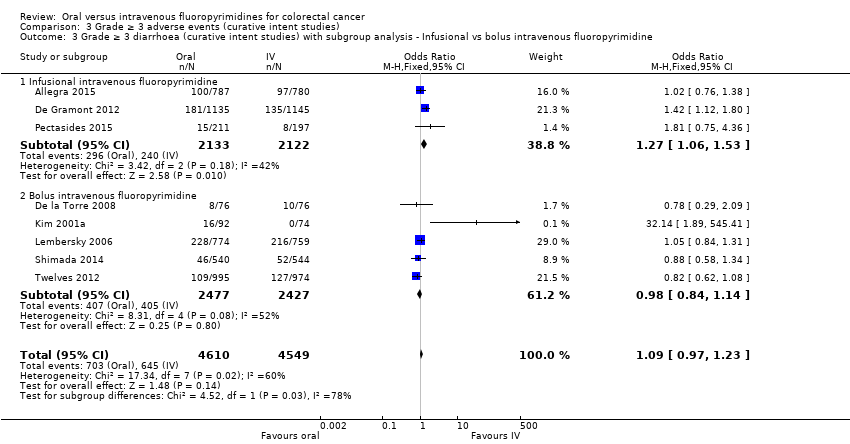 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 3 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 4255 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.06, 1.53] |
| 3.2 Bolus intravenous fluoropyrimidine | 5 | 4904 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| 4 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| Analysis 3.4  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 4 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 5 | 6616 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 4.2 UFT/Ftorafur | 3 | 2769 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 4.3 Doxifluridine | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 32.14 [1.89, 545.41] |
| 5 Grade ≥ 3 hand foot syndrome (curative intent studies) Show forest plot | 5 | 5731 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.59 [2.97, 7.10] |
| Analysis 3.5 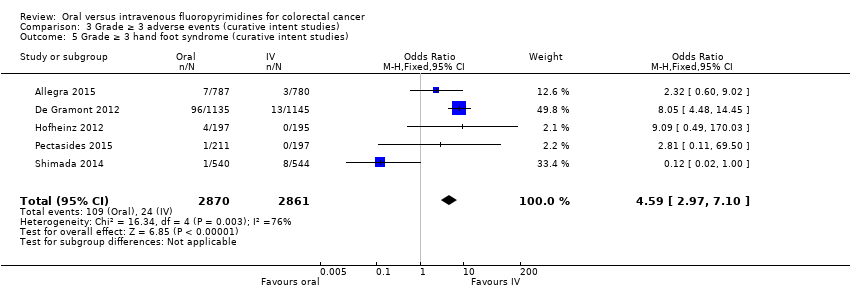 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 5 Grade ≥ 3 hand foot syndrome (curative intent studies). | ||||
| 6 Grade ≥ 3 neutropenia/granulocytopenia (curative intent studies) Show forest plot | 7 | 8707 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.11, 0.16] |
| Analysis 3.6  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 6 Grade ≥ 3 neutropenia/granulocytopenia (curative intent studies). | ||||
| 7 Grade ≥ 3 febrile neutropenia (curative intent studies) Show forest plot | 4 | 2925 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.18, 1.90] |
| Analysis 3.7 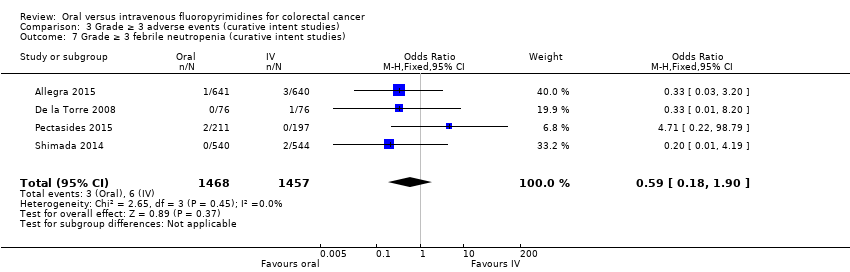 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 7 Grade ≥ 3 febrile neutropenia (curative intent studies). | ||||
| 8 Grade ≥ 3 vomiting (curative intent studies) Show forest plot | 8 | 9385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.34] |
| Analysis 3.8  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 8 Grade ≥ 3 vomiting (curative intent studies). | ||||
| 9 Grade ≥ 3 nausea (curative intent studies) Show forest plot | 7 | 9233 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.97, 1.51] |
| Analysis 3.9  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 9 Grade ≥ 3 nausea (curative intent studies). | ||||
| 10 Grade ≥ 3 stomatitis (curative intent studies) Show forest plot | 5 | 4212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.14, 0.30] |
| Analysis 3.10 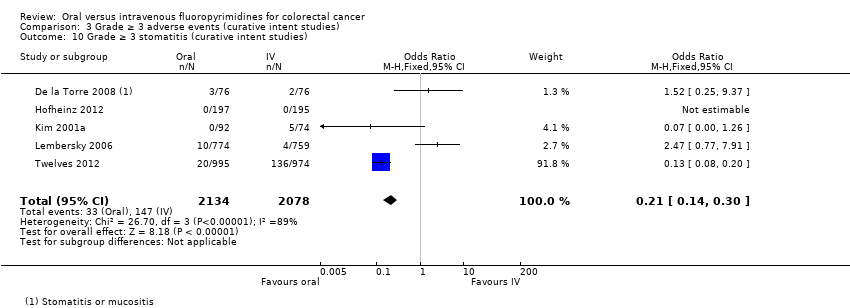 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 10 Grade ≥ 3 stomatitis (curative intent studies). | ||||
| 11 Grade ≥ 3 mucositis (curative intent studies) Show forest plot | 4 | 2233 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.62] |
| Analysis 3.11  Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 11 Grade ≥ 3 mucositis (curative intent studies). | ||||
| 12 Grade ≥ 3 hyperbilirubinaemia (curative intent studies) Show forest plot | 3 | 2757 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.52, 5.38] |
| Analysis 3.12 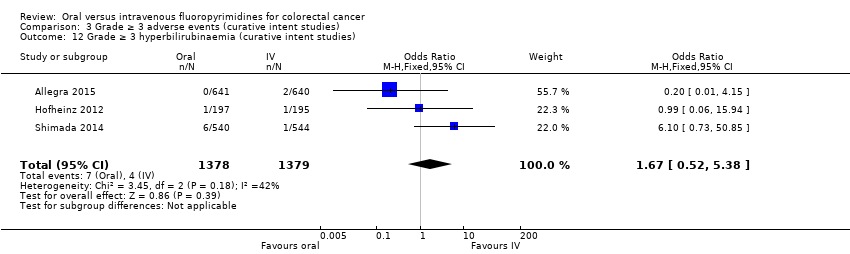 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 12 Grade ≥ 3 hyperbilirubinaemia (curative intent studies). | ||||
| 13 Any grade ≥ 3 adverse events (curative intent studies) Show forest plot | 5 | 7741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| Analysis 3.13 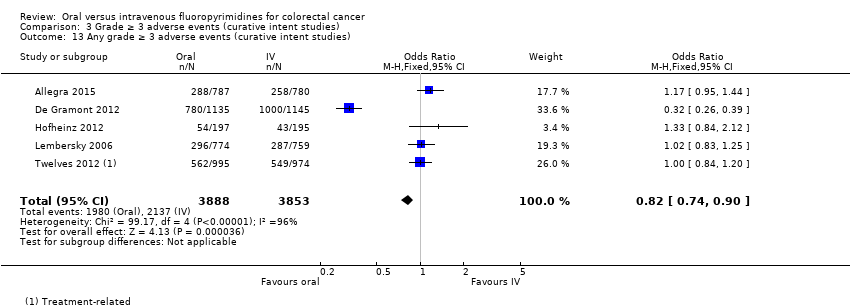 Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 13 Any grade ≥ 3 adverse events (curative intent studies). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression‐free survival Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| Analysis 4.1 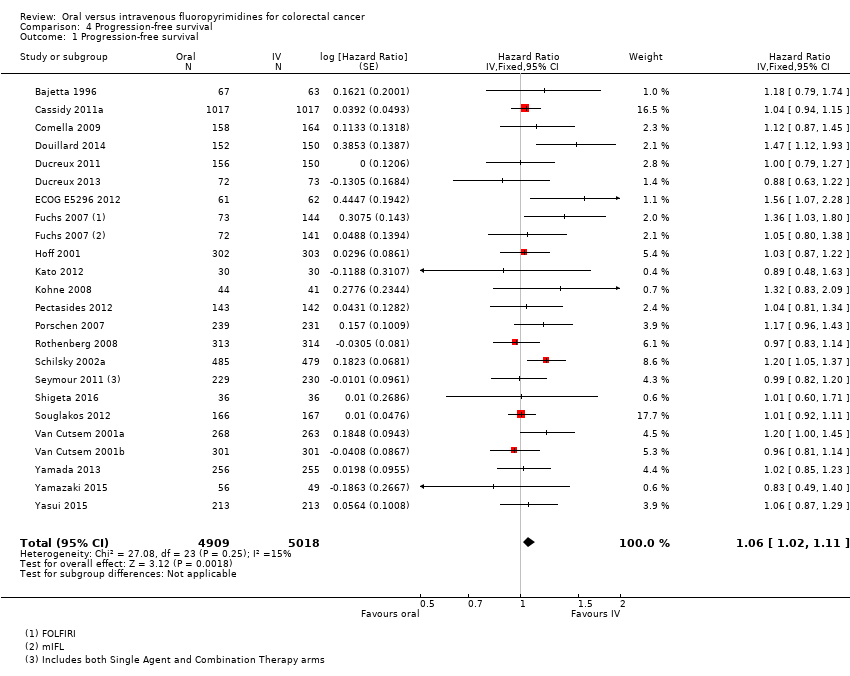 Comparison 4 Progression‐free survival, Outcome 1 Progression‐free survival. | ||||
| 2 Progression‐free survival with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 22 | 9468 | Hazard Ratio (Fixed, 95% CI) | 1.07 [1.03, 1.11] |
| Analysis 4.2 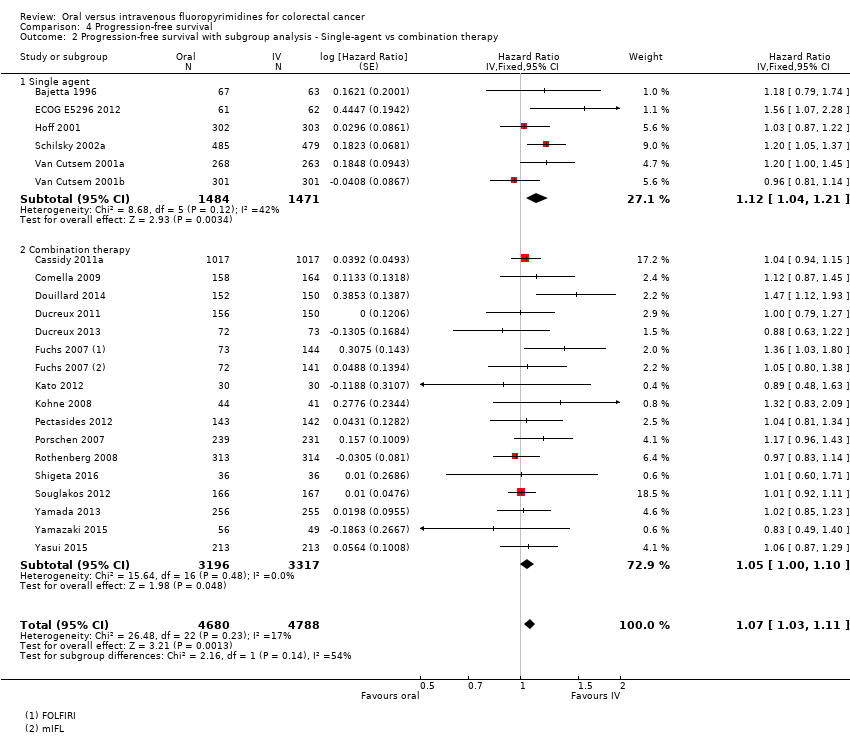 Comparison 4 Progression‐free survival, Outcome 2 Progression‐free survival with subgroup analysis ‐ Single‐agent vs combination therapy. | ||||
| 2.1 Single agent | 6 | 2955 | Hazard Ratio (Fixed, 95% CI) | 1.12 [1.04, 1.21] |
| 2.2 Combination therapy | 16 | 6513 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| 3 Progression‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| Analysis 4.3 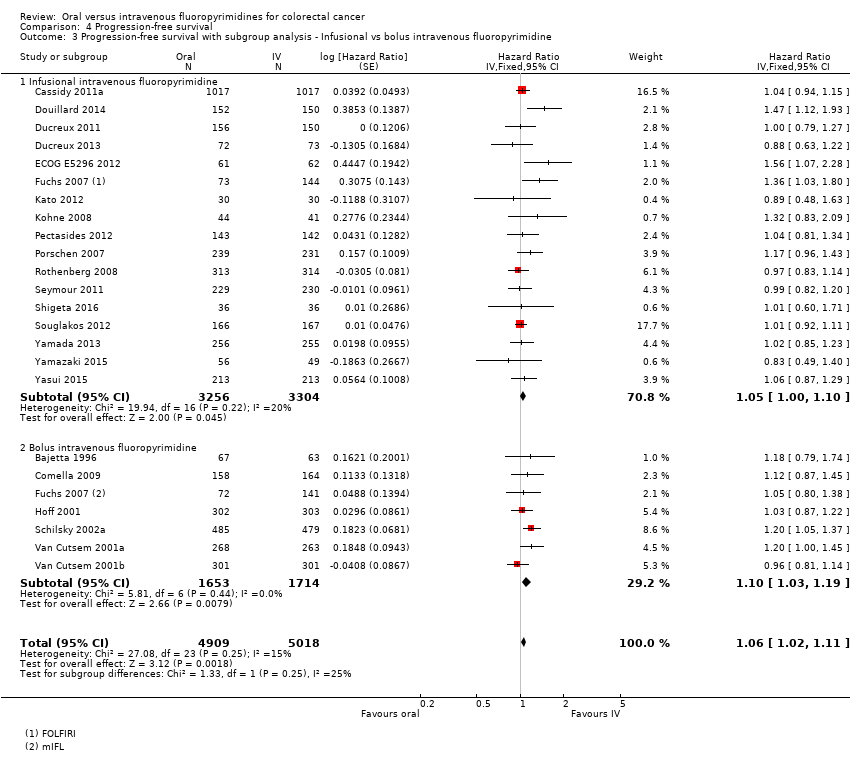 Comparison 4 Progression‐free survival, Outcome 3 Progression‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 17 | 6560 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| 3.2 Bolus intravenous fluoropyrimidine | 7 | 3367 | Hazard Ratio (Fixed, 95% CI) | 1.10 [1.03, 1.19] |
| 4 Progression‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| Analysis 4.4 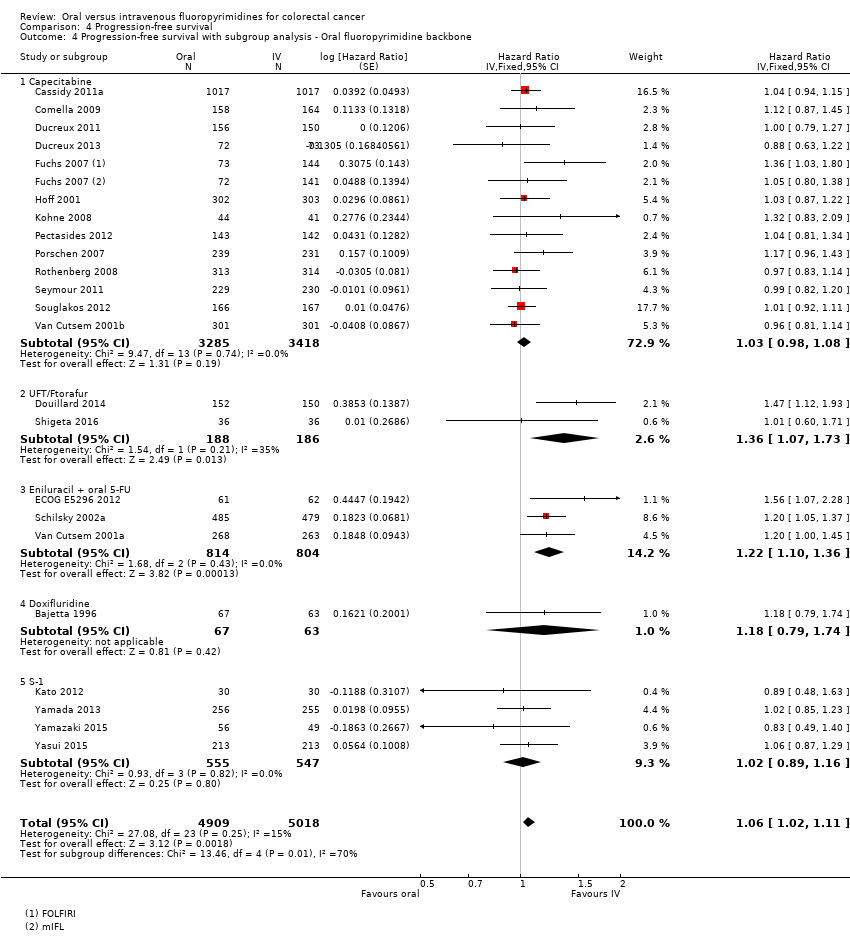 Comparison 4 Progression‐free survival, Outcome 4 Progression‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 13 | 6703 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.98, 1.08] |
| 4.2 UFT/Ftorafur | 2 | 374 | Hazard Ratio (Fixed, 95% CI) | 1.36 [1.07, 1.73] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1618 | Hazard Ratio (Fixed, 95% CI) | 1.22 [1.10, 1.36] |
| 4.4 Doxifluridine | 1 | 130 | Hazard Ratio (Fixed, 95% CI) | 1.18 [0.79, 1.74] |
| 4.5 S‐1 | 4 | 1102 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 5 Progression‐free survival for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 16 | 6513 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| Analysis 4.5 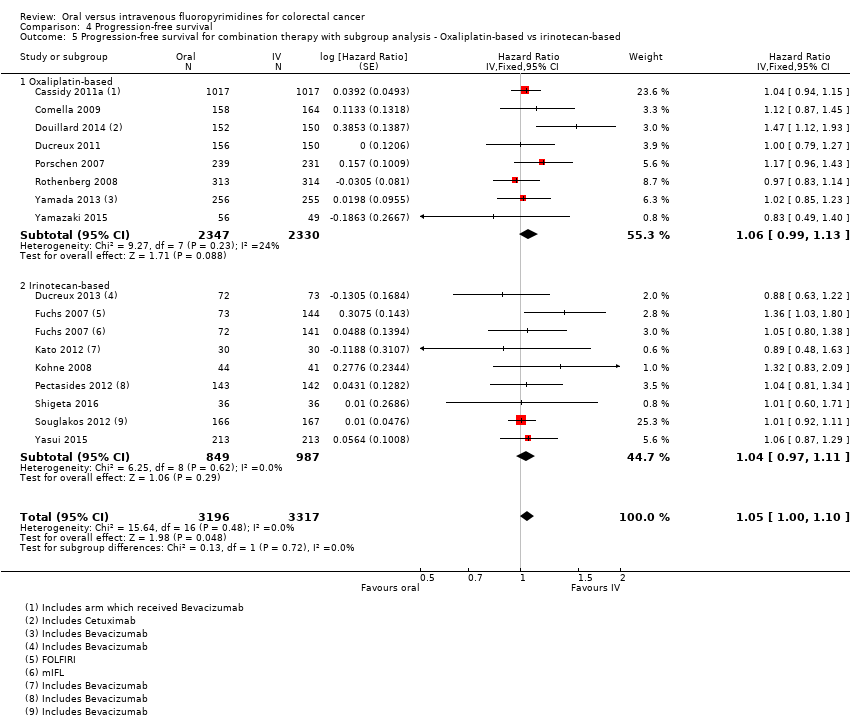 Comparison 4 Progression‐free survival, Outcome 5 Progression‐free survival for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based. | ||||
| 5.1 Oxaliplatin‐based | 8 | 4677 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.99, 1.13] |
| 5.2 Irinotecan‐based | 8 | 1836 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 6 Progression‐free survival for combination therapy with subgroup analysis ‐ with Bev vs no Bev Show forest plot | 14 | 6139 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.98, 1.08] |
| Analysis 4.6  Comparison 4 Progression‐free survival, Outcome 6 Progression‐free survival for combination therapy with subgroup analysis ‐ with Bev vs no Bev. | ||||
| 6.1 With Bevacizumab | 6 | 2033 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 6.2 No Bevacizumab | 9 | 4106 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.99, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (palliative intent studies) Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| Analysis 5.1  Comparison 5 Overall survival (palliative intent studies), Outcome 1 Overall survival (palliative intent studies). | ||||
| 2 Overall survival (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 28 | 11620 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| Analysis 5.2 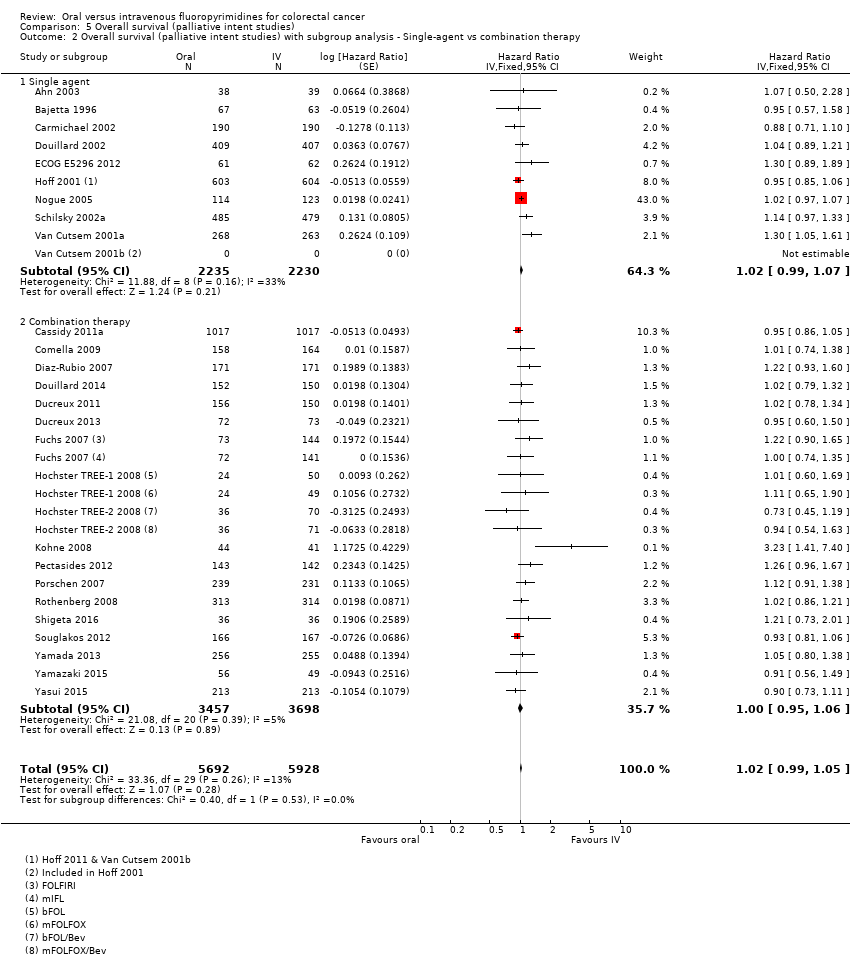 Comparison 5 Overall survival (palliative intent studies), Outcome 2 Overall survival (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy. | ||||
| 2.1 Single agent | 10 | 4465 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.07] |
| 2.2 Combination therapy | 18 | 7155 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 3 Overall survival (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| Analysis 5.3 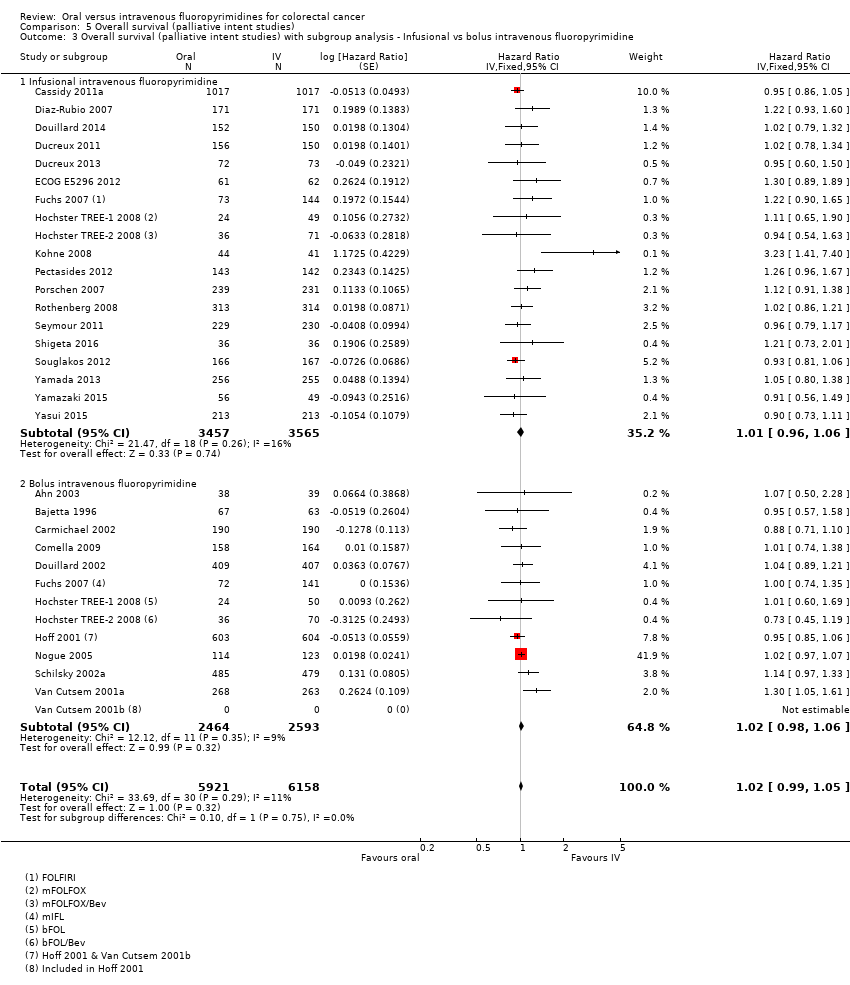 Comparison 5 Overall survival (palliative intent studies), Outcome 3 Overall survival (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 19 | 7022 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 3.2 Bolus intravenous fluoropyrimidine | 13 | 5057 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.98, 1.06] |
| 4 Overall survival (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| Analysis 5.4 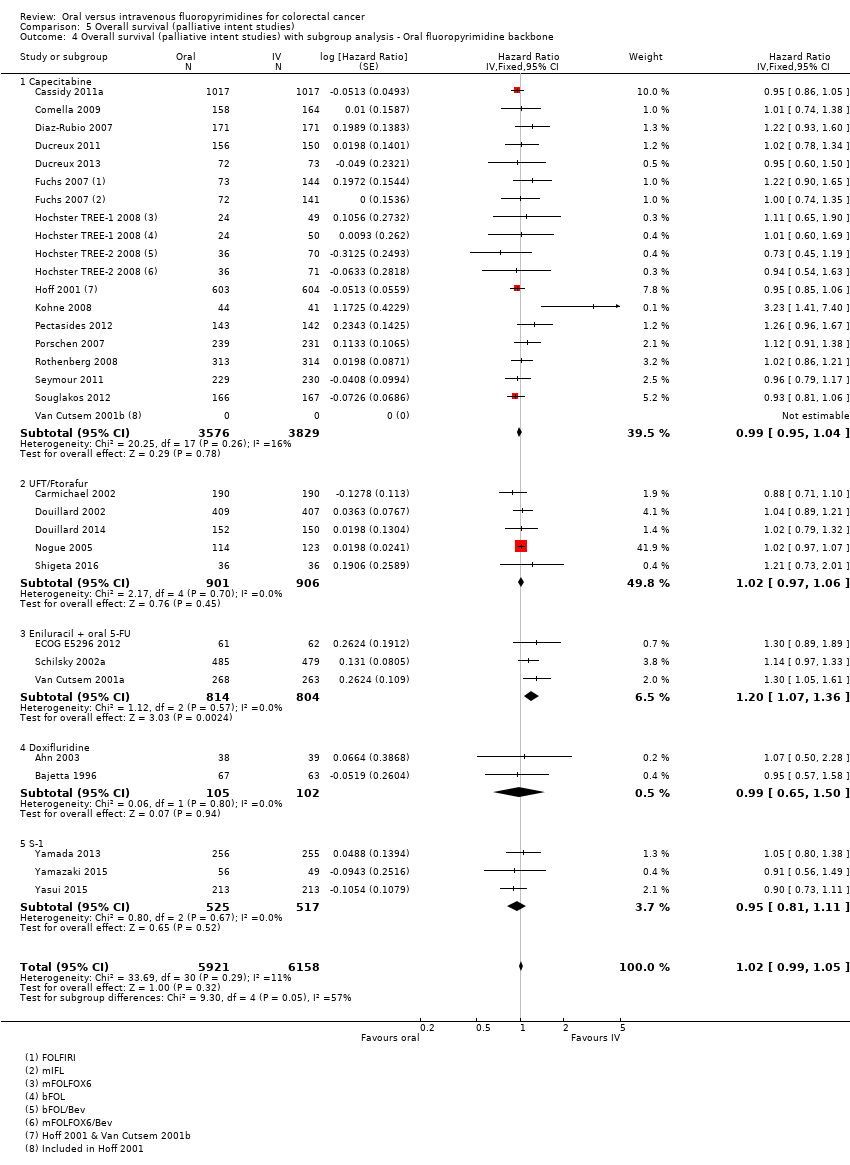 Comparison 5 Overall survival (palliative intent studies), Outcome 4 Overall survival (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 16 | 7405 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.95, 1.04] |
| 4.2 UFT/Ftorafur | 5 | 1807 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1618 | Hazard Ratio (Fixed, 95% CI) | 1.20 [1.07, 1.36] |
| 4.4 Doxifluridine | 2 | 207 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.65, 1.50] |
| 4.5 S‐1 | 3 | 1042 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.81, 1.11] |
| 5 Overall survival (palliative intent studies) for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 18 | 7155 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| Analysis 5.5  Comparison 5 Overall survival (palliative intent studies), Outcome 5 Overall survival (palliative intent studies) for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based. | ||||
| 5.1 Oxaliplatin‐based | 11 | 5379 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 5.2 Irinotecan‐based | 7 | 1776 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to progression Show forest plot | 6 | 1970 | Hazard Ratio (Fixed, 95% CI) | 1.07 [1.01, 1.14] |
| Analysis 6.1  Comparison 6 Time to progression, Outcome 1 Time to progression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ORR Show forest plot | 32 | 11115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| Analysis 7.1  Comparison 7 Objective response rate, Outcome 1 ORR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grade ≥ 3 diarrhoea (palliative intent studies) Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| Analysis 8.1  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 1 Grade ≥ 3 diarrhoea (palliative intent studies). | ||||
| 2 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| Analysis 8.2  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 2 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy. | ||||
| 2.1 Single agent | 10 | 4566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.44] |
| 2.2 Combination therapy | 21 | 7431 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.77, 2.32] |
| 3 Grade ≥ 3 diarrhea (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| Analysis 8.3  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 3 Grade ≥ 3 diarrhea (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 3.1 Infusional intravenous fluoropyrimidine | 21 | 7065 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.74, 2.30] |
| 3.2 Bolus intravenous fluoropyrimidine | 12 | 4932 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.12, 1.53] |
| 4 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| Analysis 8.4  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 4 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 4.1 Capecitabine | 17 | 7382 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.00] |
| 4.2 UFT/Ftorafur | 5 | 1784 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.24, 2.06] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1617 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.38] |
| 4.4 Doxifluridine | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.64, 3.56] |
| 4.5 S‐1 | 4 | 1087 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.19, 5.76] |
| 5 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 20 | 7212 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.75, 2.29] |
| Analysis 8.5 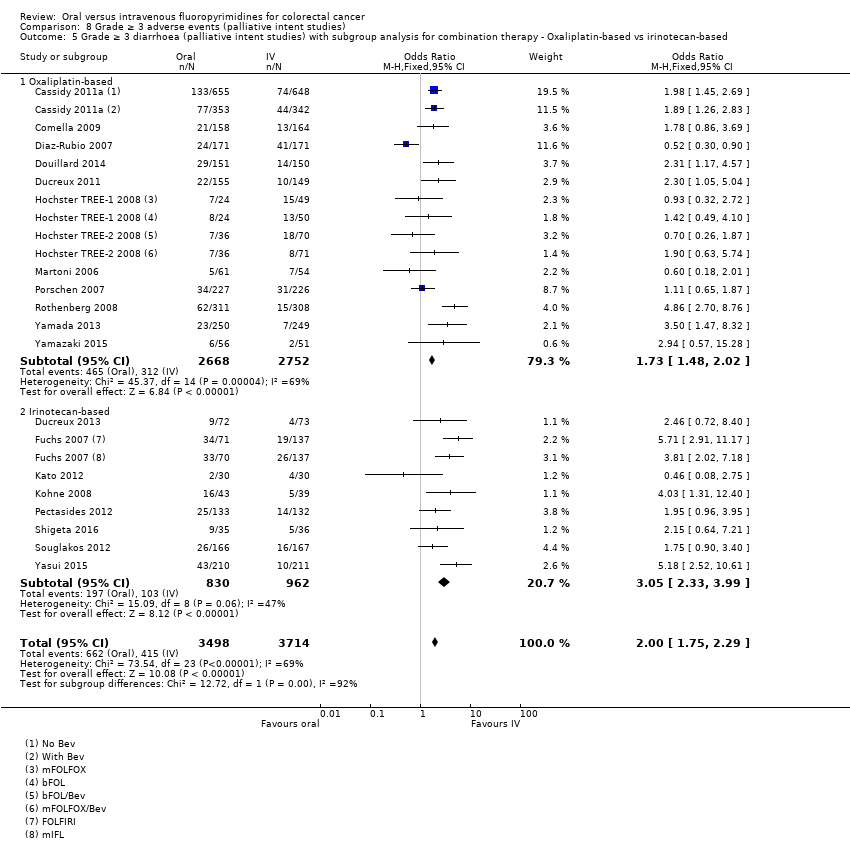 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 5 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based. | ||||
| 5.1 Oxaliplatin‐based | 12 | 5420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.48, 2.02] |
| 5.2 Irinotecan‐based | 8 | 1792 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.33, 3.99] |
| 6 Grade ≥ 3 hand foot syndrome (palliative intent studies) Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| Analysis 8.6 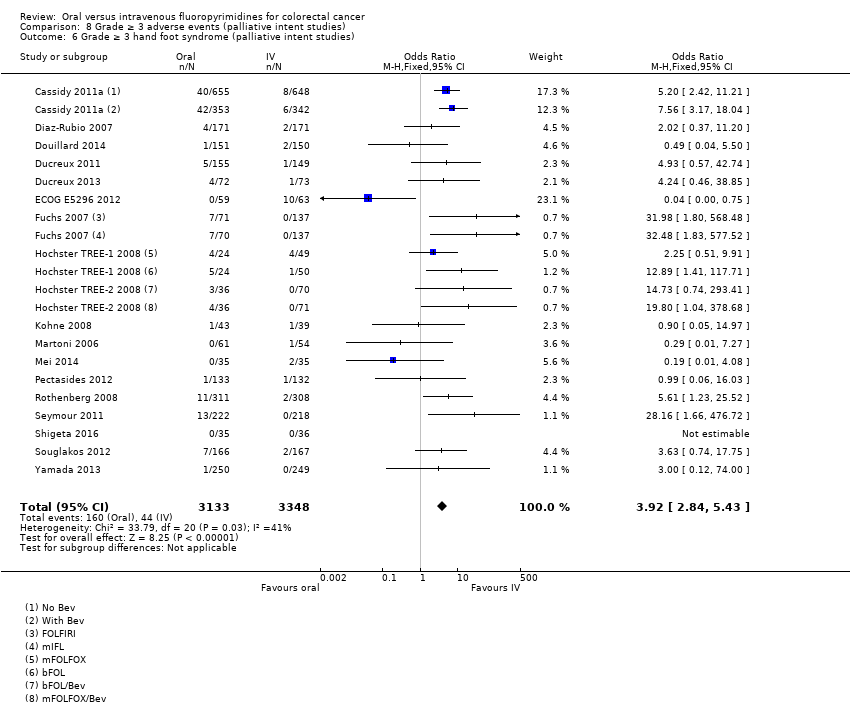 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 6 Grade ≥ 3 hand foot syndrome (palliative intent studies). | ||||
| 7 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.89 [2.82, 5.37] |
| Analysis 8.7  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 7 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy. | ||||
| 7.1 Single agent | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.48, 2.56] |
| 7.2 Combination therapy | 17 | 6138 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.76 [3.32, 6.82] |
| 8 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| Analysis 8.8 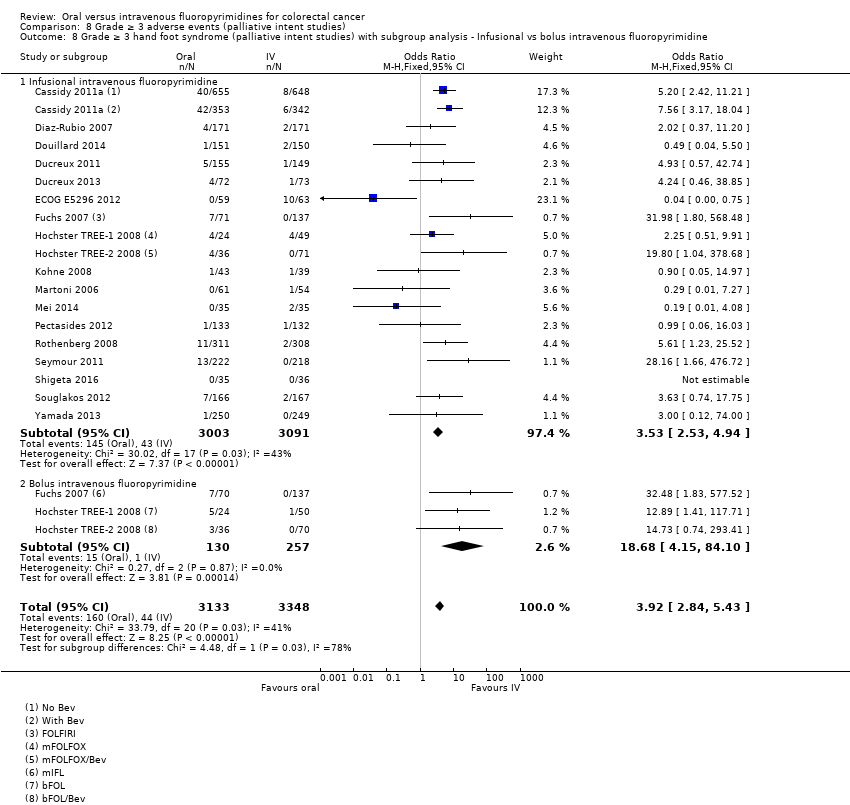 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 8 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine. | ||||
| 8.1 Infusional intravenous fluoropyrimidine | 18 | 6094 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.53 [2.53, 4.94] |
| 8.2 Bolus intravenous fluoropyrimidine | 3 | 387 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.68 [4.15, 84.10] |
| 9 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| Analysis 8.9 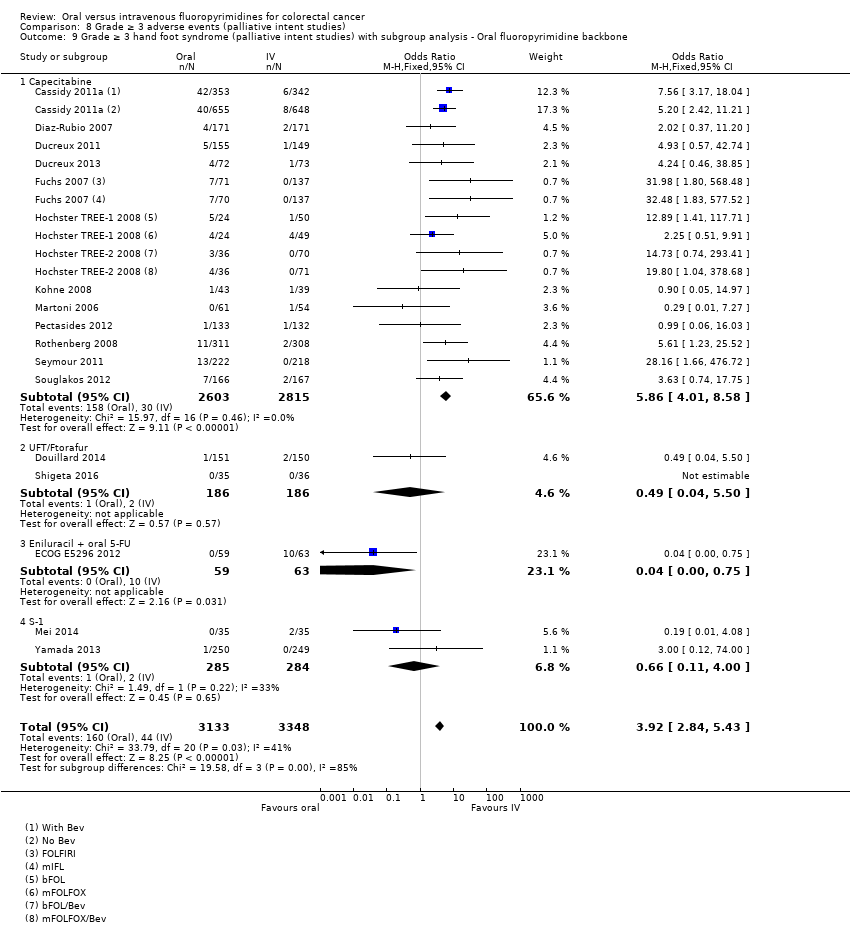 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 9 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone. | ||||
| 9.1 Capecitabine | 13 | 5418 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.86 [4.01, 8.58] |
| 9.2 UFT/Ftorafur | 2 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.50] |
| 9.3 Eniluracil + oral 5‐FU | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.75] |
| 9.4 S‐1 | 2 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 10 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 16 | 5919 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.76 [3.31, 6.83] |
| Analysis 8.10  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 10 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based. | ||||
| 10.1 Oxaliplatin‐based | 10 | 4608 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [3.03, 6.75] |
| 10.2 Irinotecan‐based | 6 | 1311 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.93 [2.52, 13.97] |
| 11 Grade ≥ 3 neutropenia/granulocytopenia (palliative intent studies) Show forest plot | 29 | 11794 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.15, 0.18] |
| Analysis 8.11  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 11 Grade ≥ 3 neutropenia/granulocytopenia (palliative intent studies). | ||||
| 12 Grade ≥ 3 febrile neutropenia (palliative intent studies) Show forest plot | 19 | 9407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.21, 0.36] |
| Analysis 8.12 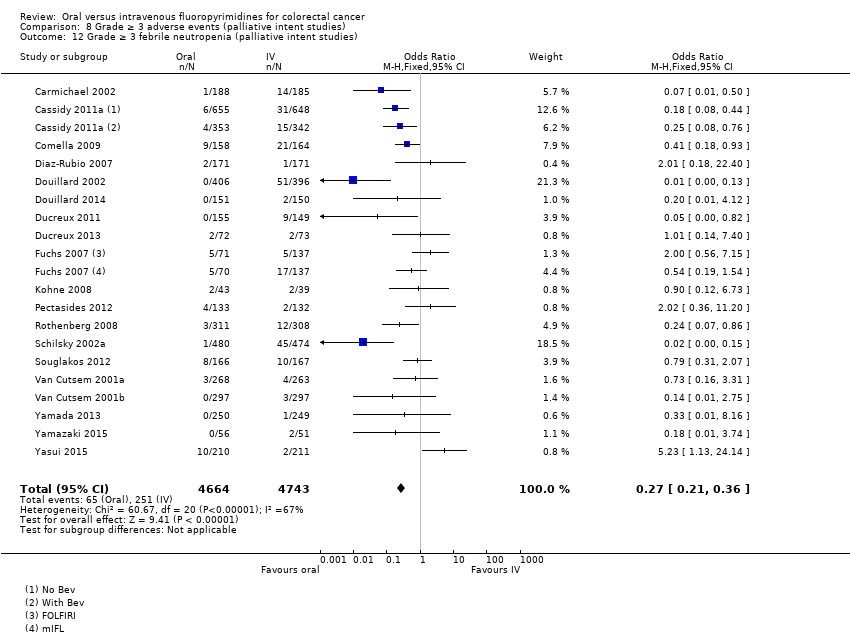 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 12 Grade ≥ 3 febrile neutropenia (palliative intent studies). | ||||
| 13 Grade ≥ 3 vomiting (palliative intent studies) Show forest plot | 23 | 9528 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.40] |
| Analysis 8.13  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 13 Grade ≥ 3 vomiting (palliative intent studies). | ||||
| 14 Grade ≥ 3 nausea (palliative intent studies) Show forest plot | 25 | 9796 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.99, 1.36] |
| Analysis 8.14 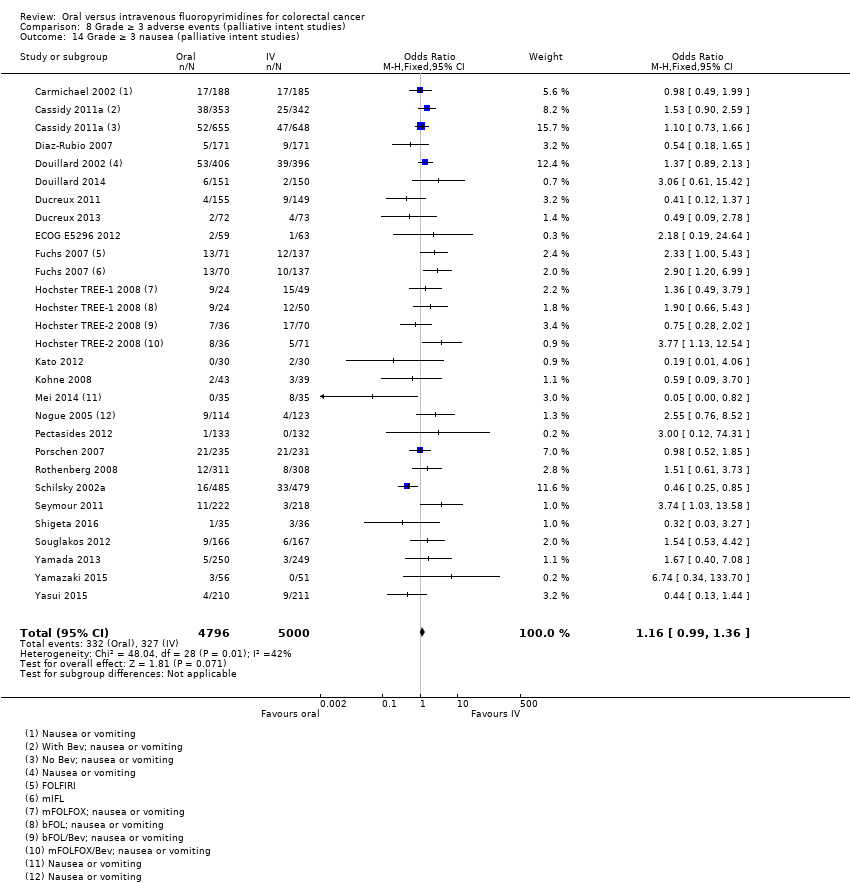 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 14 Grade ≥ 3 nausea (palliative intent studies). | ||||
| 15 Grade ≥ 3 stomatitis (palliative intent studies) Show forest plot | 21 | 8718 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.20, 0.33] |
| Analysis 8.15 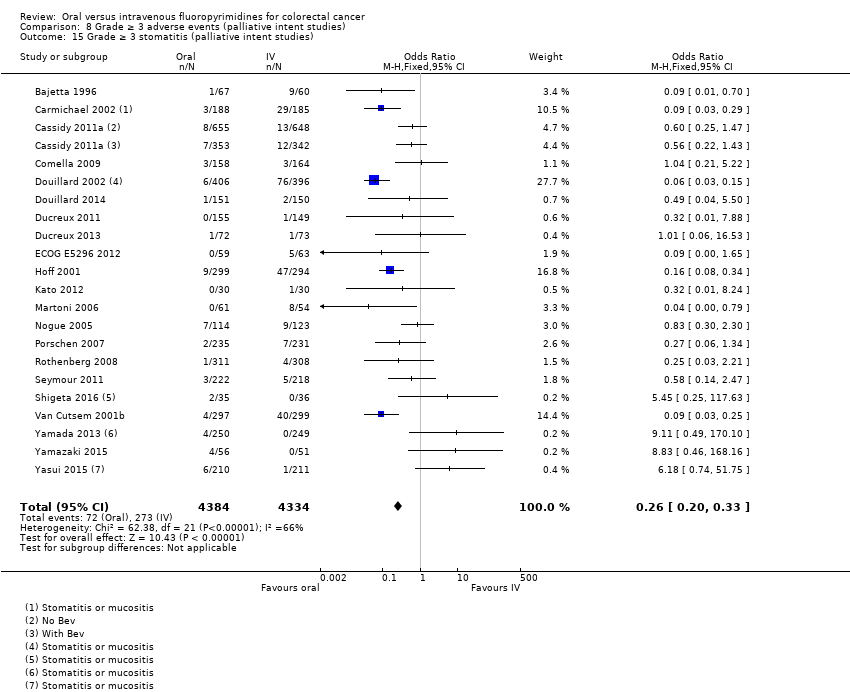 Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 15 Grade ≥ 3 stomatitis (palliative intent studies). | ||||
| 16 Grade ≥ 3 mucositis (palliative intent studies) Show forest plot | 12 | 4962 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.12, 0.24] |
| Analysis 8.16  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 16 Grade ≥ 3 mucositis (palliative intent studies). | ||||
| 17 Grade ≥ 3 hyperbilirubinaemia (palliative intent studies) Show forest plot | 9 | 2699 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.99, 2.64] |
| Analysis 8.17  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 17 Grade ≥ 3 hyperbilirubinaemia (palliative intent studies). | ||||
| 18 Any grade ≥ 3 adverse events (palliative intent studies) Show forest plot | 14 | 5436 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |
| Analysis 8.18  Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 18 Any grade ≥ 3 adverse events (palliative intent studies). | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.1
1 In this graph, the risk of bias for each domain was calculated using the worst assessment documented for that domain in the contributing studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.1
1 In this summary, the risk of bias for each domain was scored using the worst assessment documented for that domain in the study.

Forest plot of disease‐free survival.

Forest plot of comparison: 4 Progression‐free survival with, outcome: 4.4 Progression‐free survival with subgroup analysis ‐ oral fluoropyrimidine backbone.
Funnel plot of progression‐free survival.

Comparison 1 Disease‐free survival, Outcome 1 Disease‐free survival.

Comparison 1 Disease‐free survival, Outcome 2 Disease‐free survival with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy.
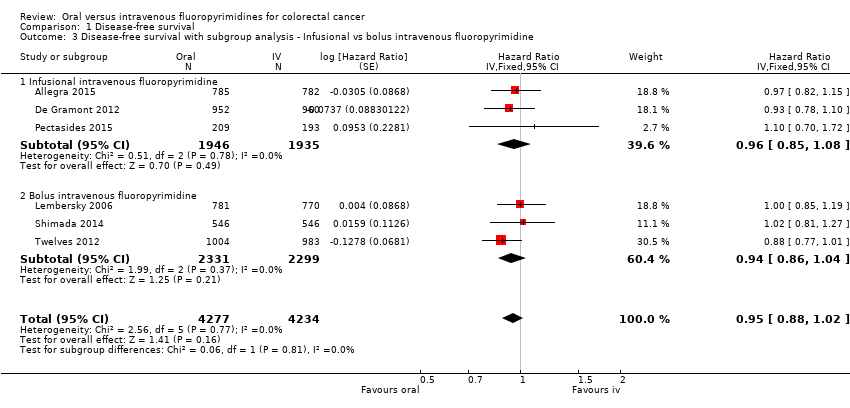
Comparison 1 Disease‐free survival, Outcome 3 Disease‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.

Comparison 1 Disease‐free survival, Outcome 4 Disease‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone.
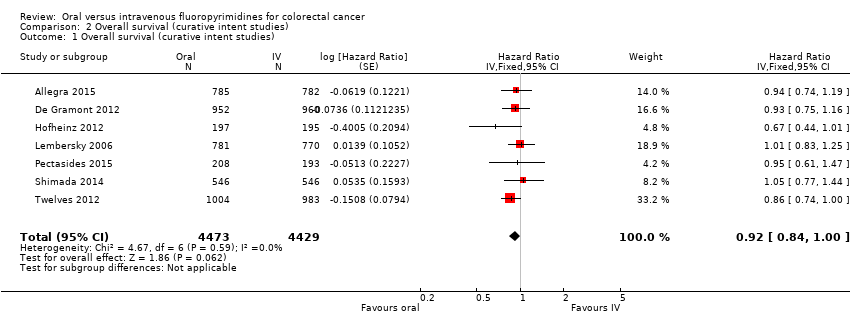
Comparison 2 Overall survival (curative intent studies), Outcome 1 Overall survival (curative intent studies).
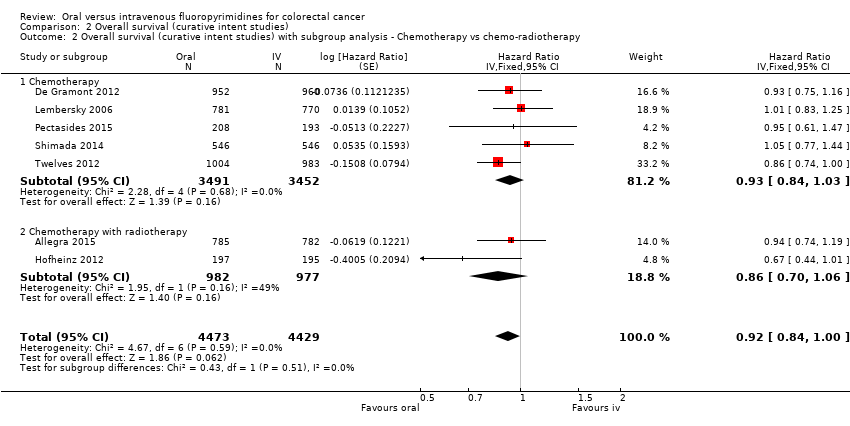
Comparison 2 Overall survival (curative intent studies), Outcome 2 Overall survival (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy.

Comparison 2 Overall survival (curative intent studies), Outcome 3 Overall survival (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.
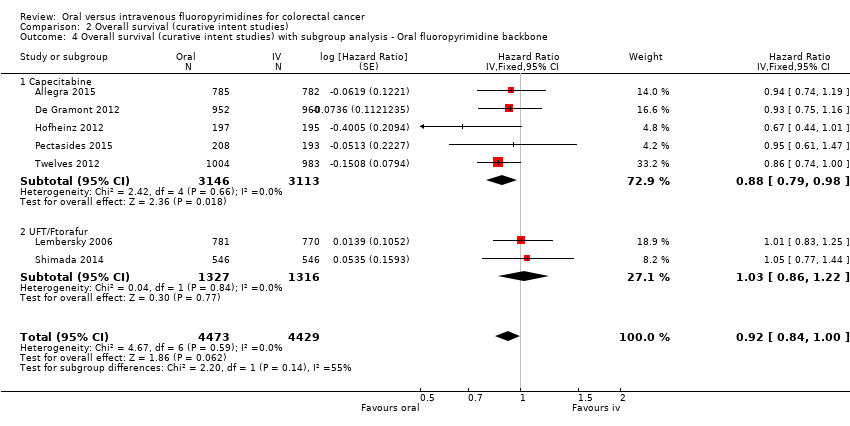
Comparison 2 Overall survival (curative intent studies), Outcome 4 Overall survival (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone.

Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 1 Grade ≥ 3 diarrhoea (curative intent studies).
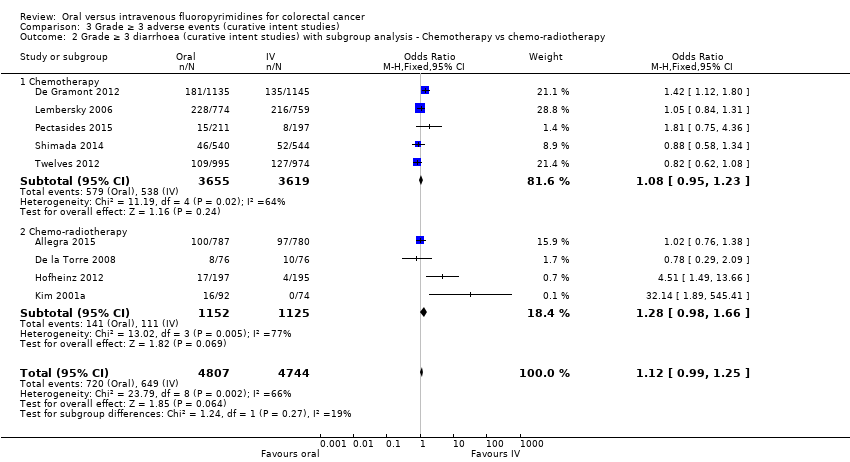
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 2 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy.

Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 3 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.
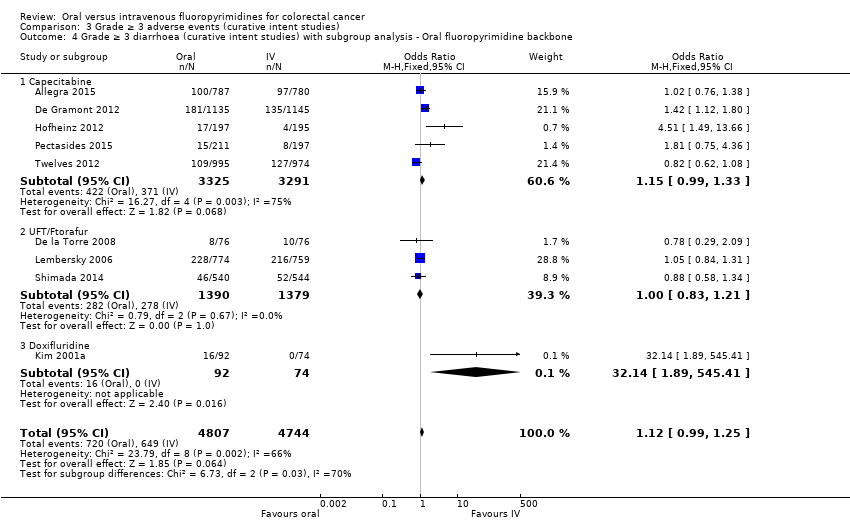
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 4 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone.

Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 5 Grade ≥ 3 hand foot syndrome (curative intent studies).
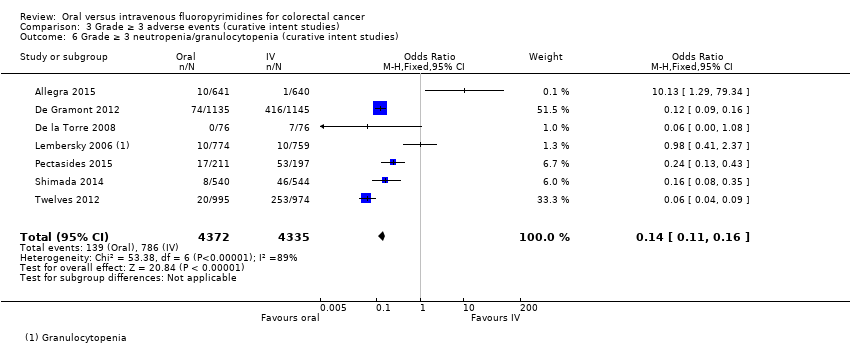
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 6 Grade ≥ 3 neutropenia/granulocytopenia (curative intent studies).
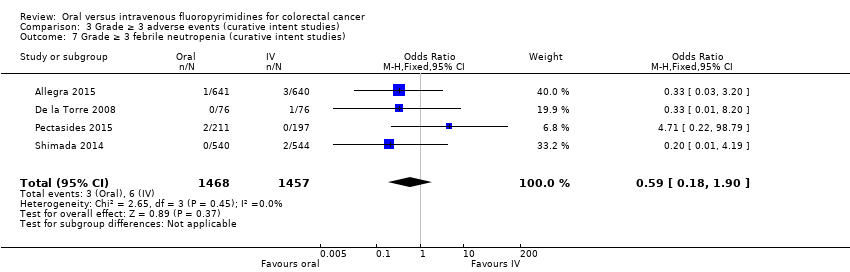
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 7 Grade ≥ 3 febrile neutropenia (curative intent studies).
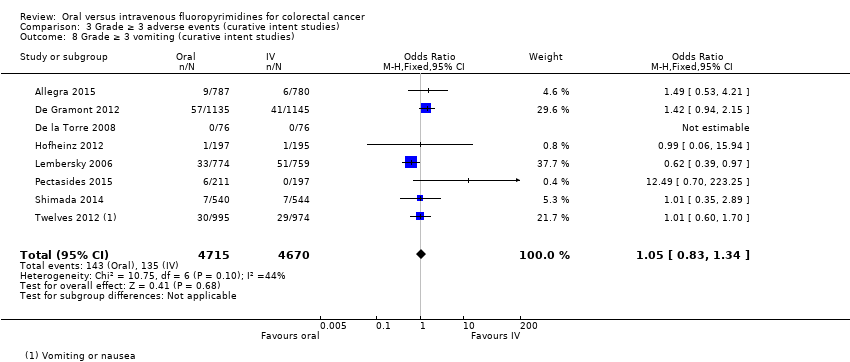
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 8 Grade ≥ 3 vomiting (curative intent studies).
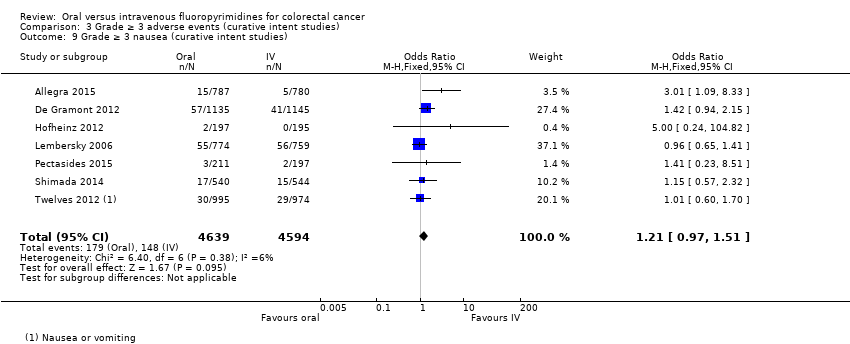
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 9 Grade ≥ 3 nausea (curative intent studies).
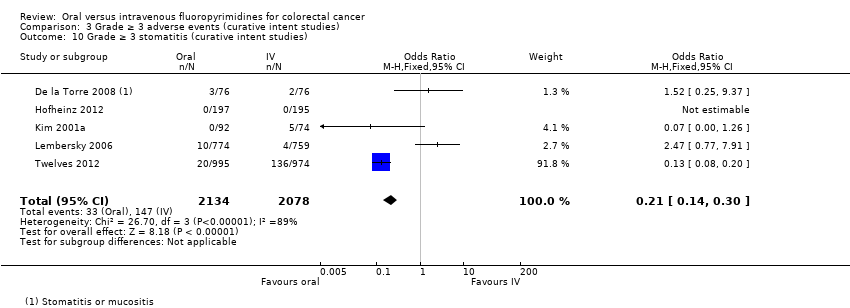
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 10 Grade ≥ 3 stomatitis (curative intent studies).
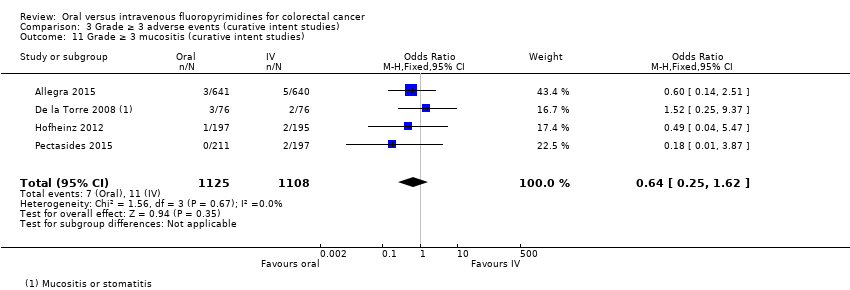
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 11 Grade ≥ 3 mucositis (curative intent studies).
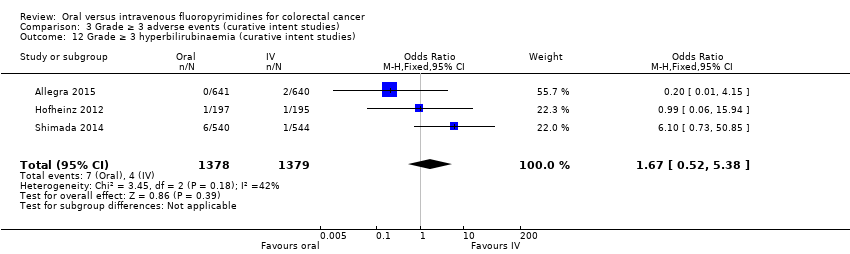
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 12 Grade ≥ 3 hyperbilirubinaemia (curative intent studies).
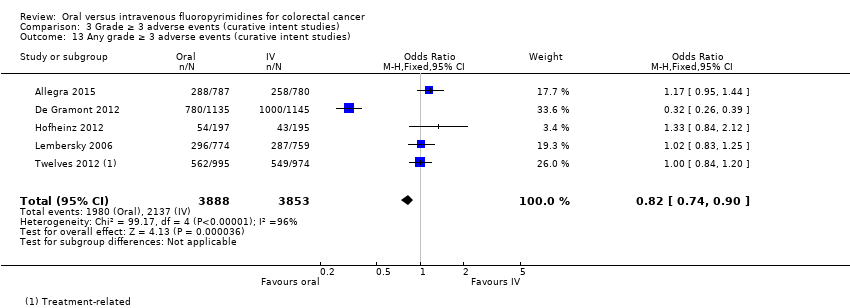
Comparison 3 Grade ≥ 3 adverse events (curative intent studies), Outcome 13 Any grade ≥ 3 adverse events (curative intent studies).

Comparison 4 Progression‐free survival, Outcome 1 Progression‐free survival.
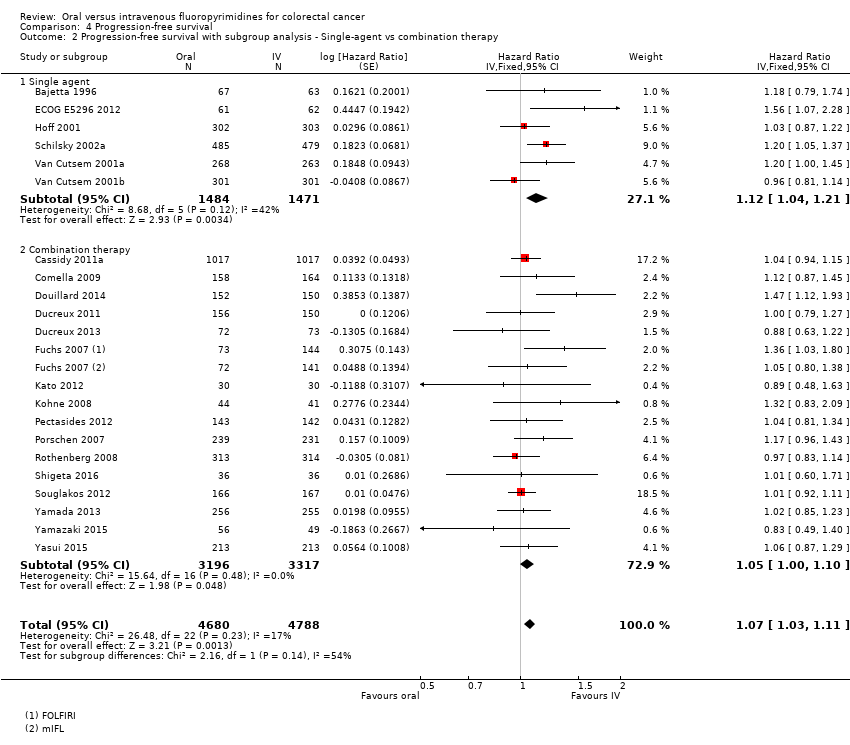
Comparison 4 Progression‐free survival, Outcome 2 Progression‐free survival with subgroup analysis ‐ Single‐agent vs combination therapy.

Comparison 4 Progression‐free survival, Outcome 3 Progression‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.

Comparison 4 Progression‐free survival, Outcome 4 Progression‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone.

Comparison 4 Progression‐free survival, Outcome 5 Progression‐free survival for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based.
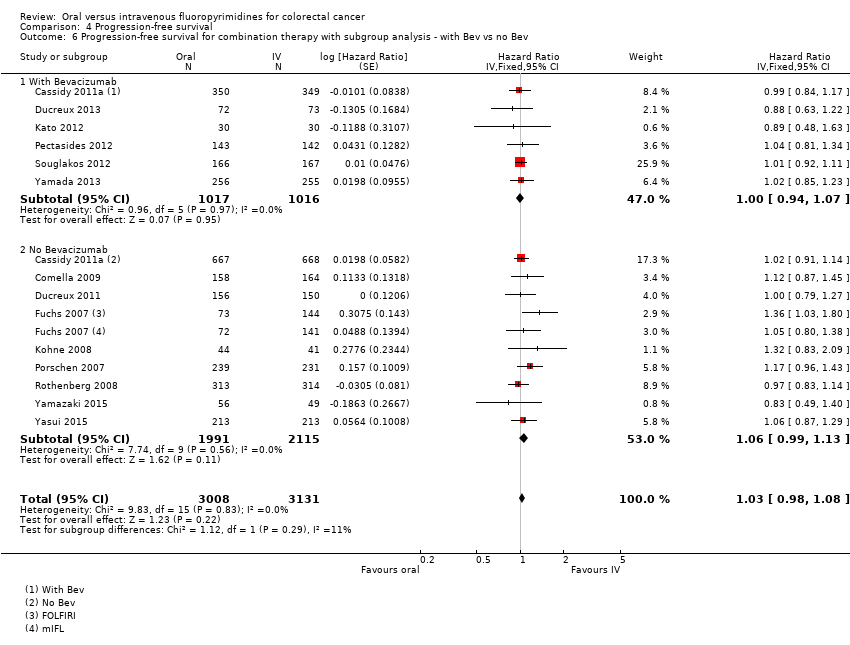
Comparison 4 Progression‐free survival, Outcome 6 Progression‐free survival for combination therapy with subgroup analysis ‐ with Bev vs no Bev.
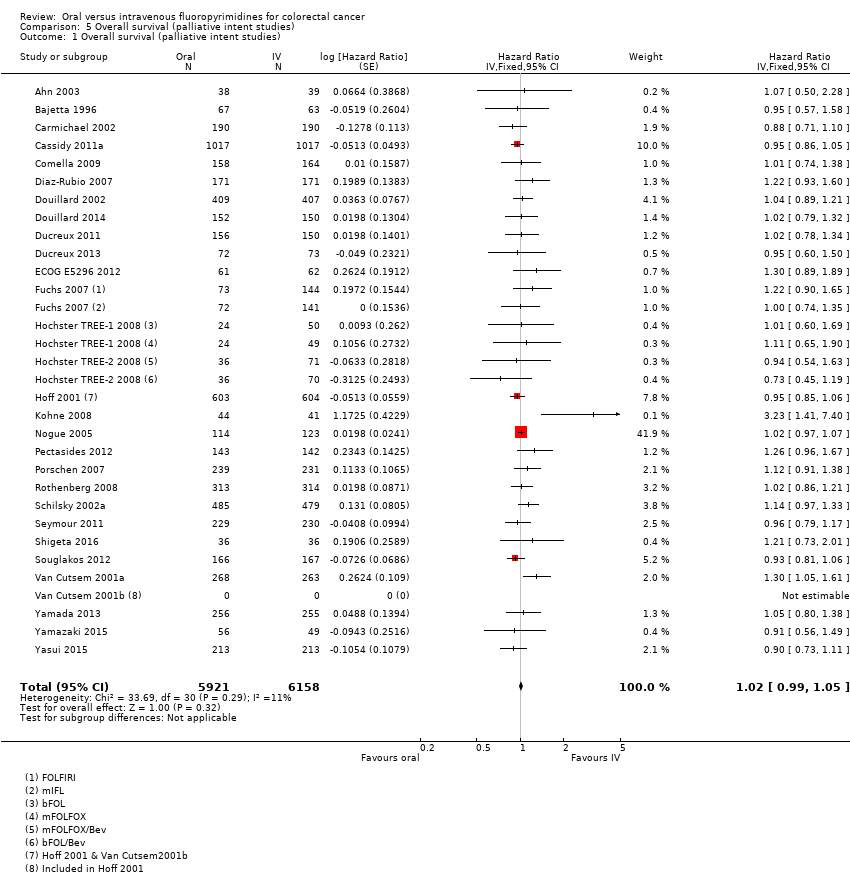
Comparison 5 Overall survival (palliative intent studies), Outcome 1 Overall survival (palliative intent studies).

Comparison 5 Overall survival (palliative intent studies), Outcome 2 Overall survival (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy.

Comparison 5 Overall survival (palliative intent studies), Outcome 3 Overall survival (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.
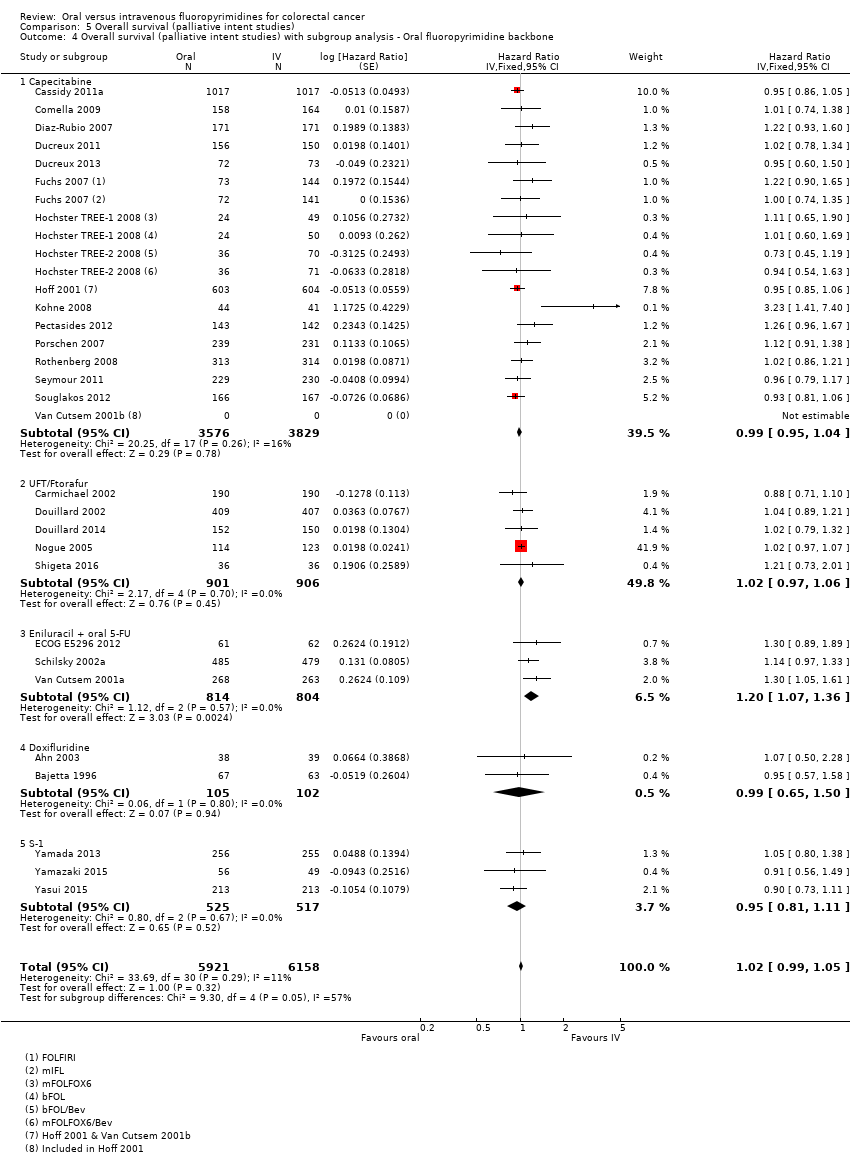
Comparison 5 Overall survival (palliative intent studies), Outcome 4 Overall survival (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone.
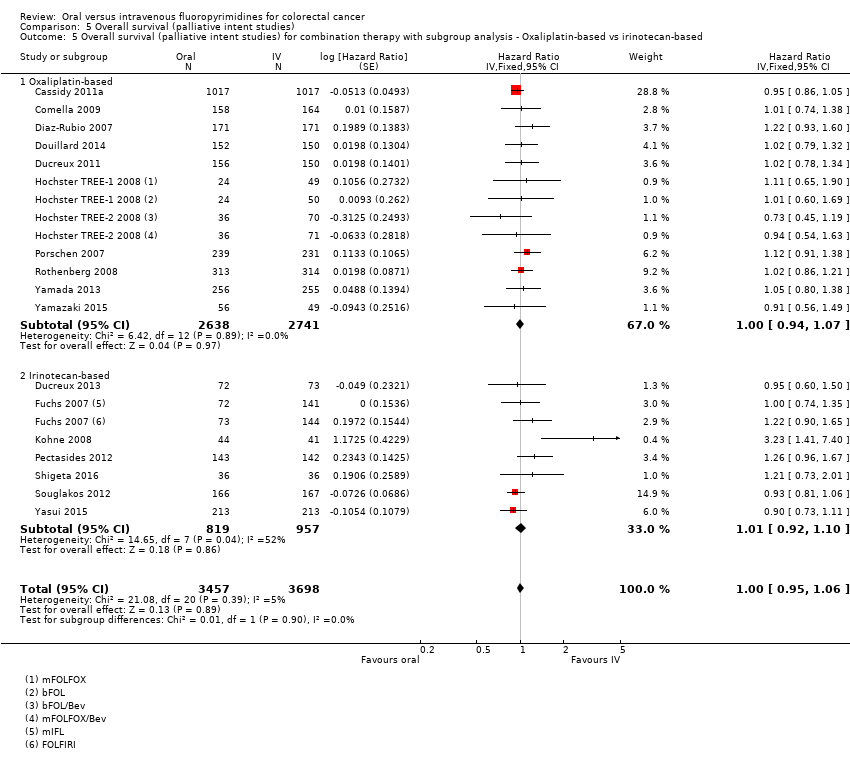
Comparison 5 Overall survival (palliative intent studies), Outcome 5 Overall survival (palliative intent studies) for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based.
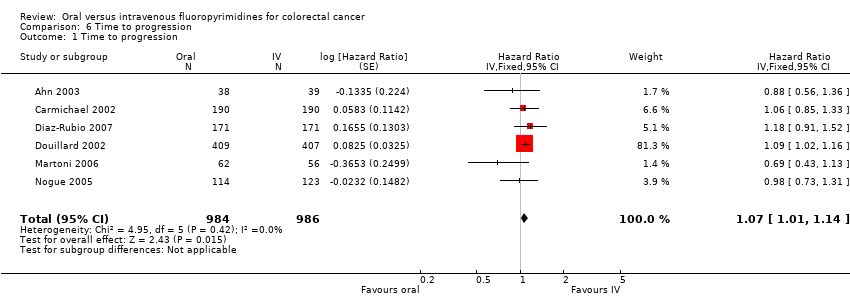
Comparison 6 Time to progression, Outcome 1 Time to progression.
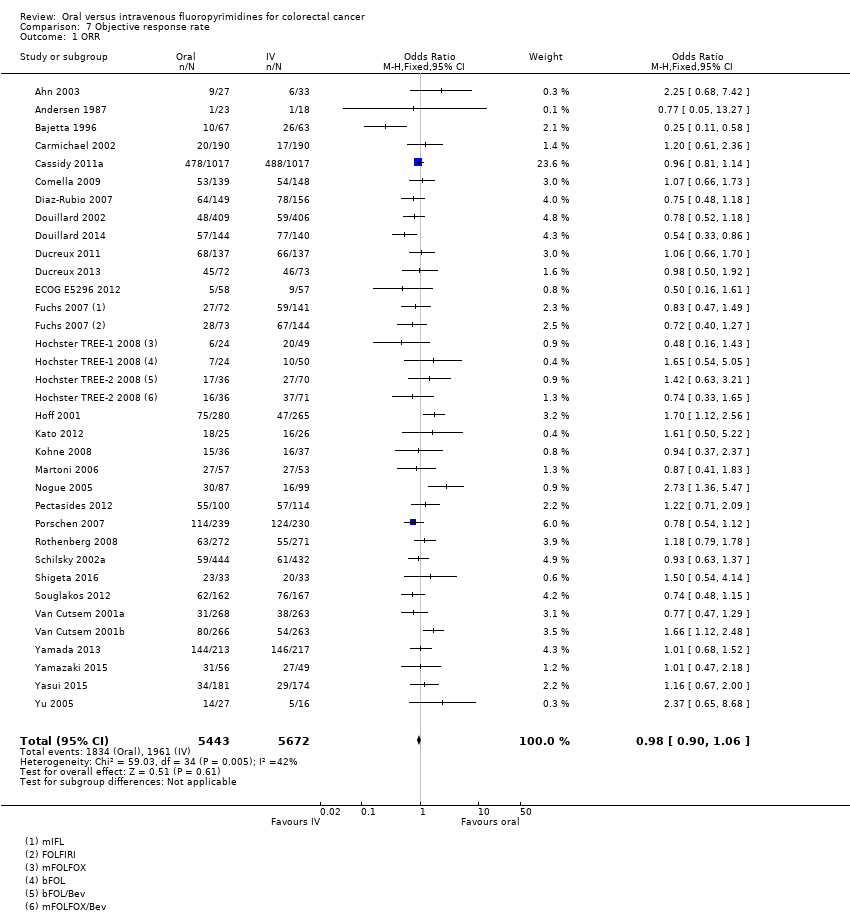
Comparison 7 Objective response rate, Outcome 1 ORR.
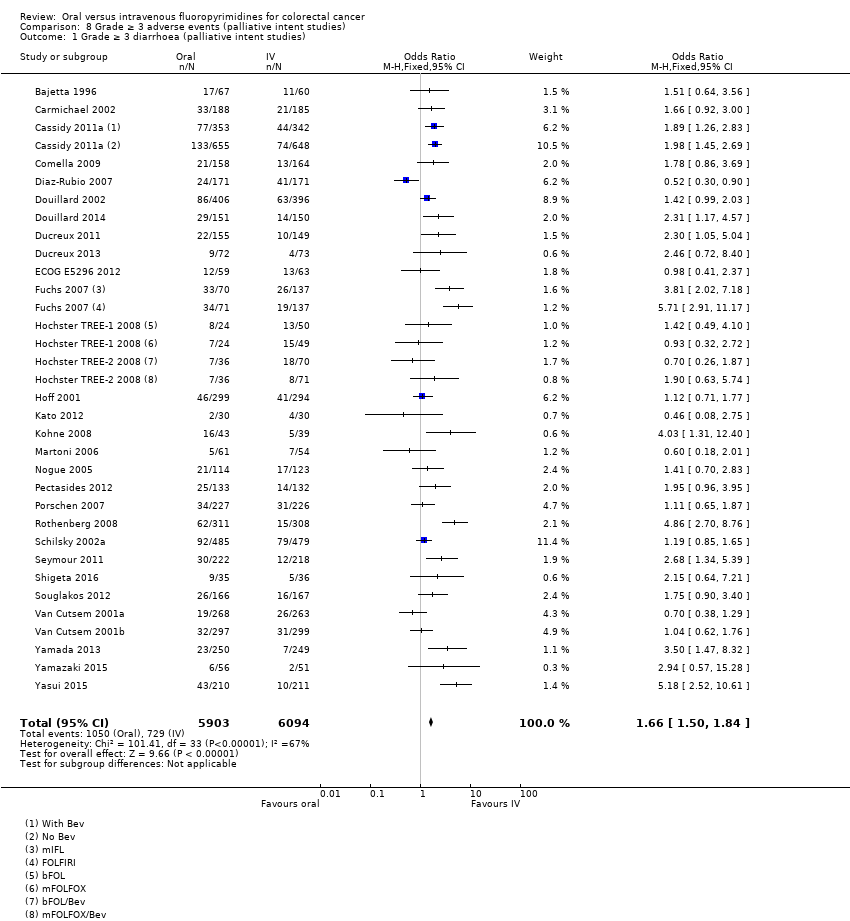
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 1 Grade ≥ 3 diarrhoea (palliative intent studies).

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 2 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy.

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 3 Grade ≥ 3 diarrhea (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.
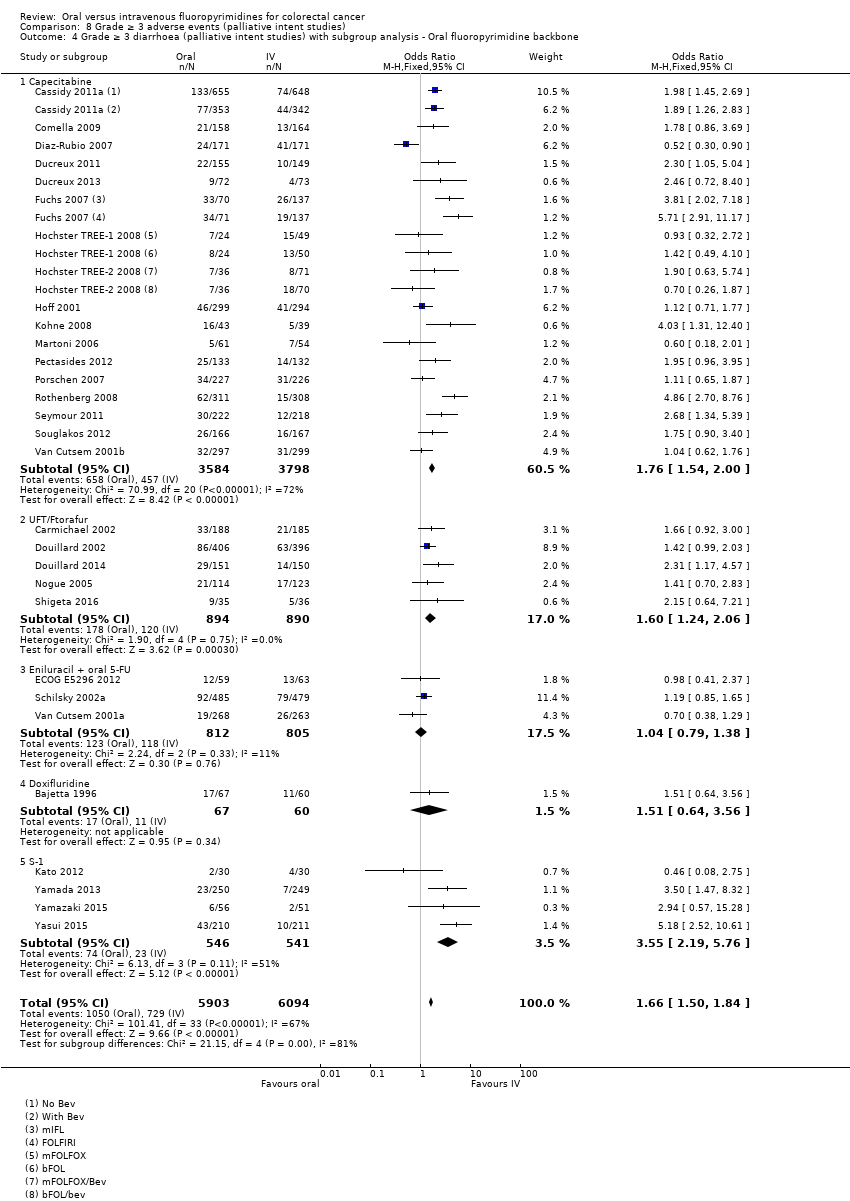
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 4 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone.
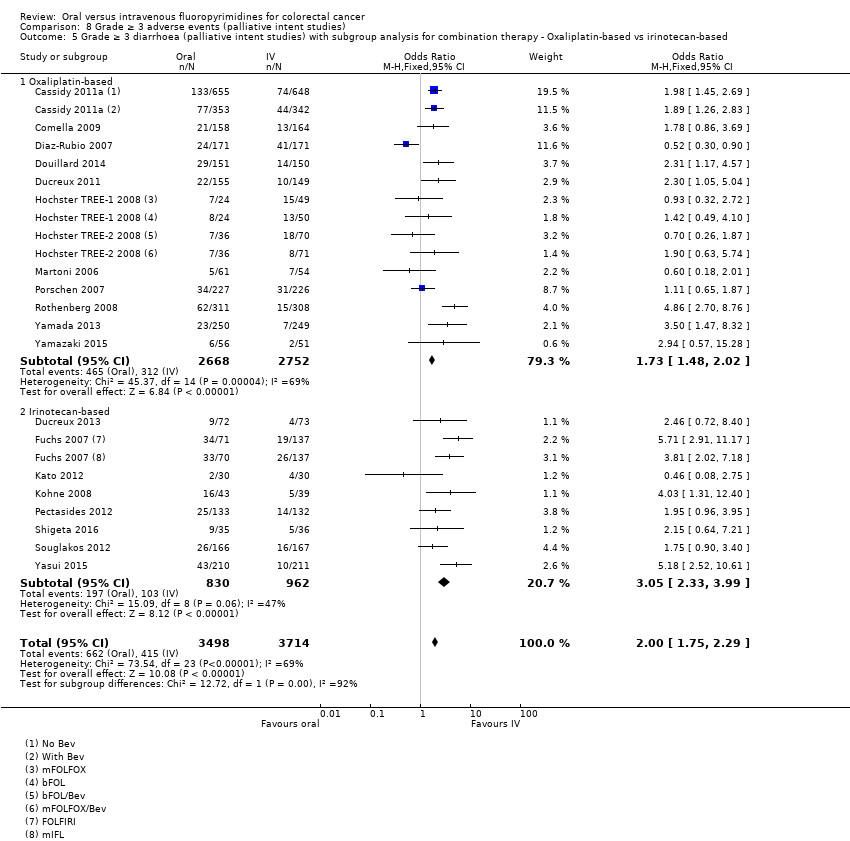
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 5 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based.

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 6 Grade ≥ 3 hand foot syndrome (palliative intent studies).
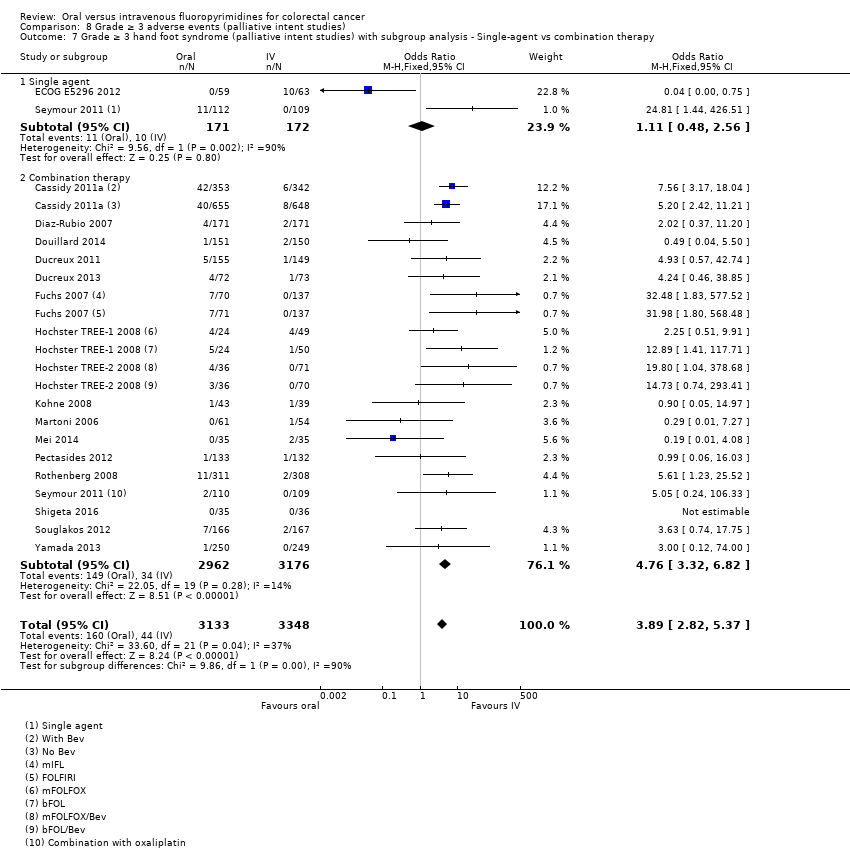
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 7 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy.

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 8 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine.
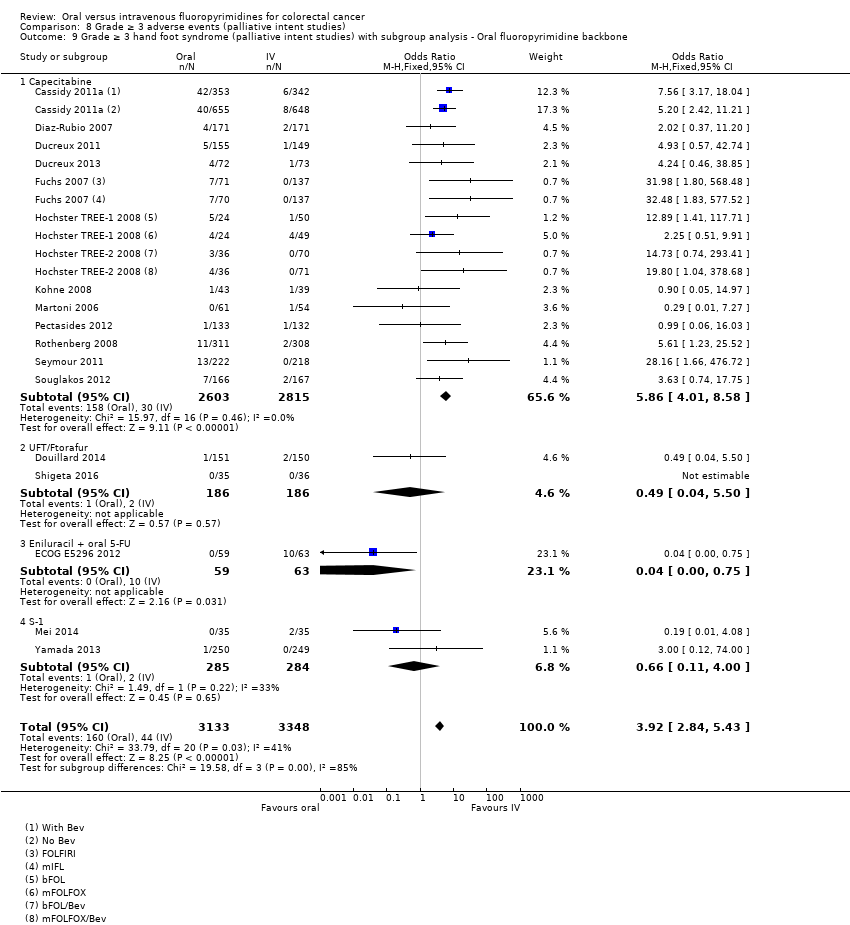
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 9 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone.
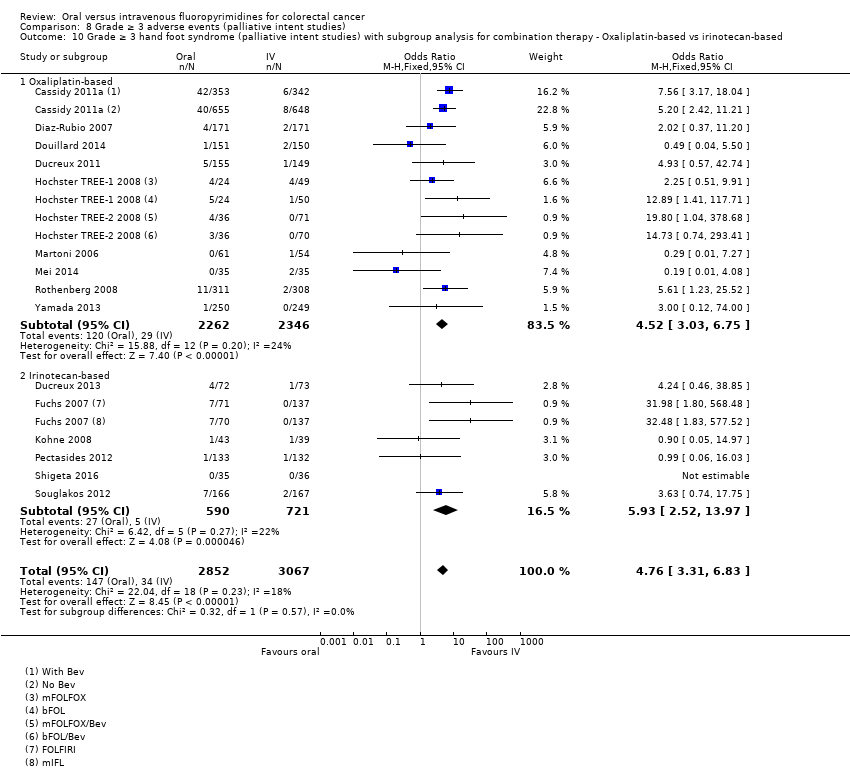
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 10 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based.
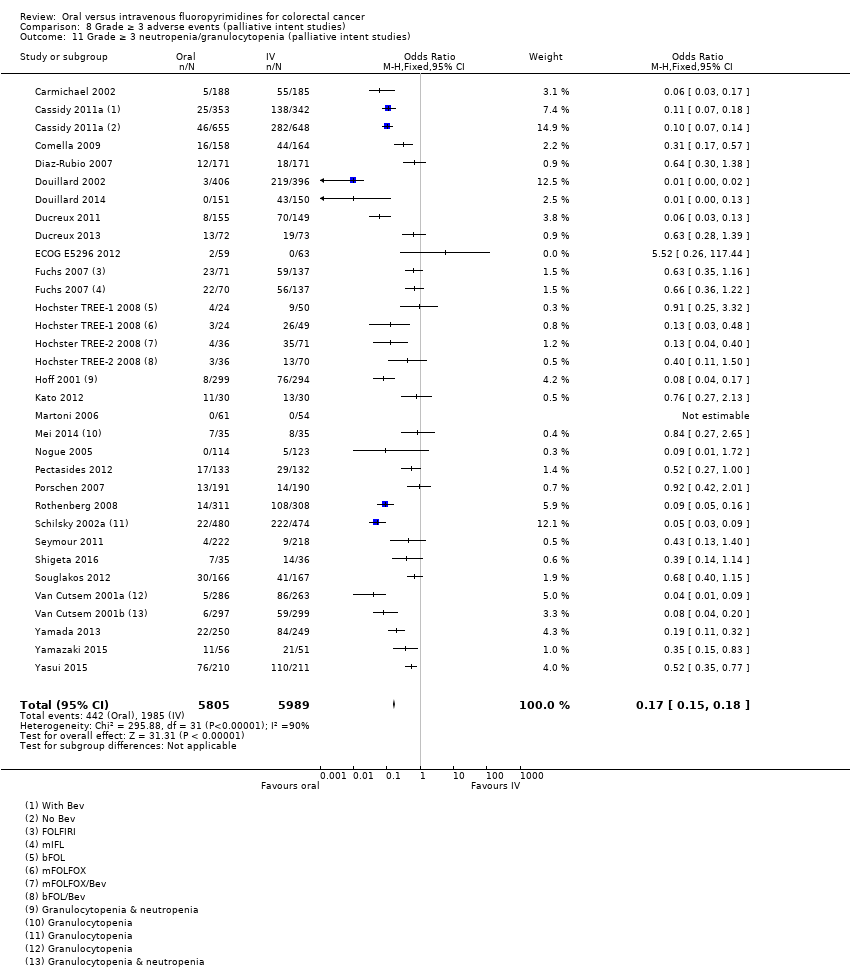
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 11 Grade ≥ 3 neutropenia/granulocytopenia (palliative intent studies).
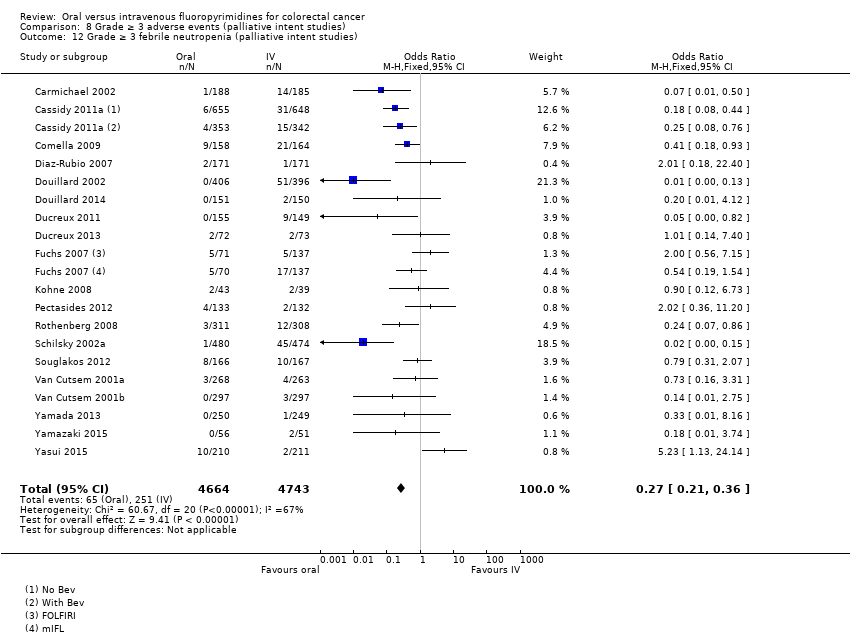
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 12 Grade ≥ 3 febrile neutropenia (palliative intent studies).

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 13 Grade ≥ 3 vomiting (palliative intent studies).
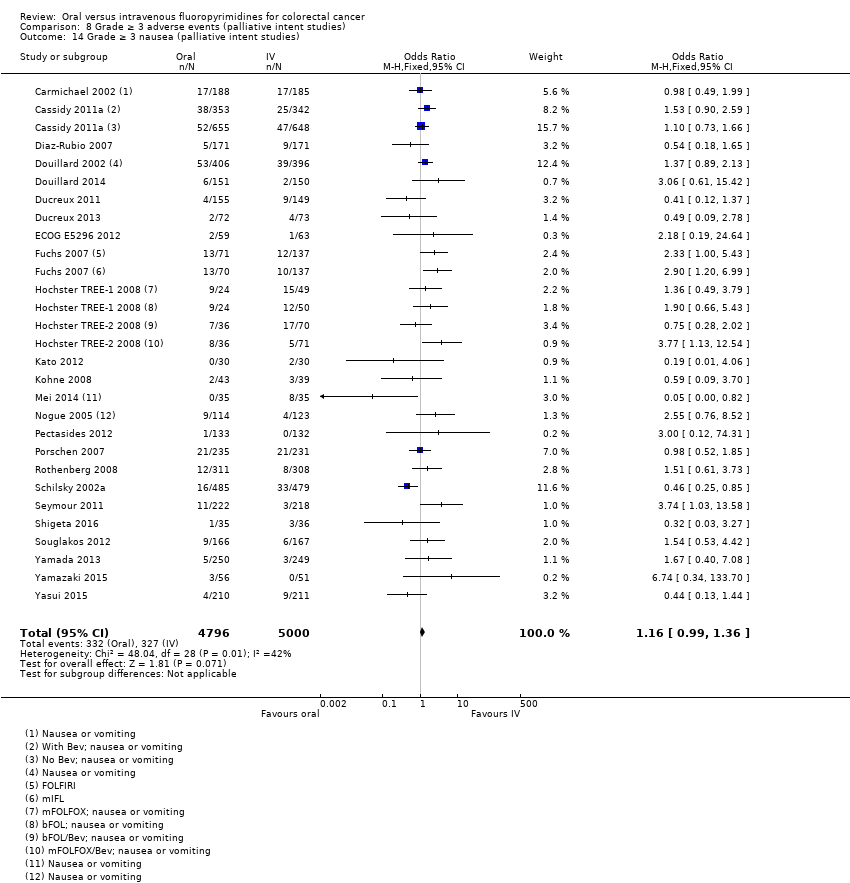
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 14 Grade ≥ 3 nausea (palliative intent studies).
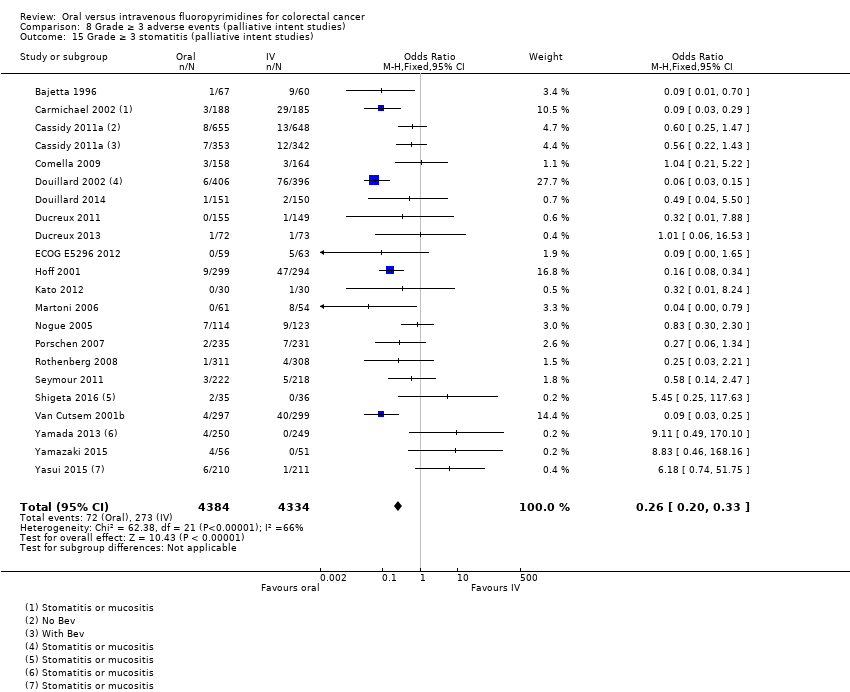
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 15 Grade ≥ 3 stomatitis (palliative intent studies).
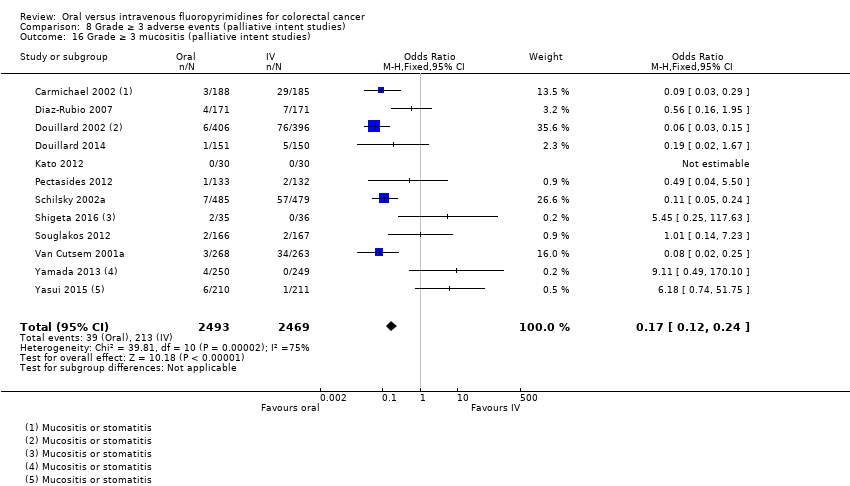
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 16 Grade ≥ 3 mucositis (palliative intent studies).

Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 17 Grade ≥ 3 hyperbilirubinaemia (palliative intent studies).
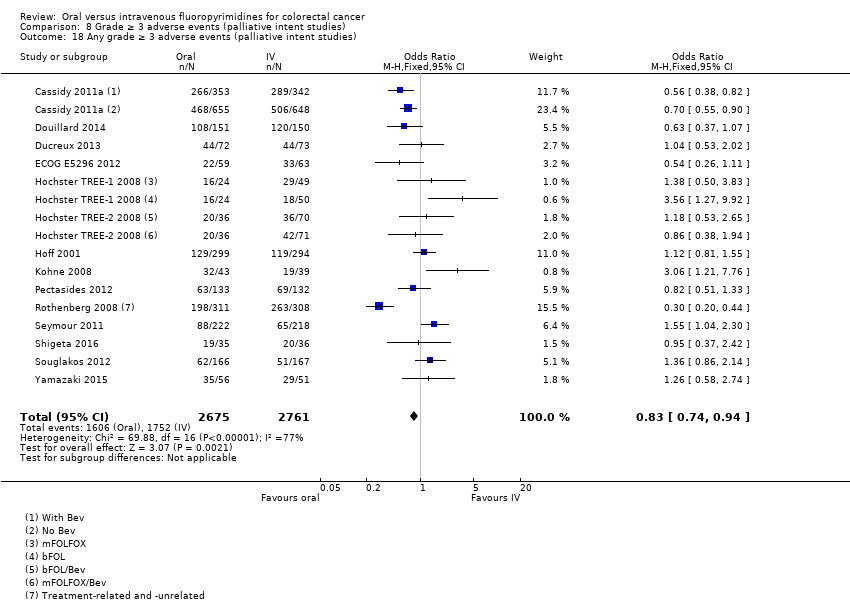
Comparison 8 Grade ≥ 3 adverse events (palliative intent studies), Outcome 18 Any grade ≥ 3 adverse events (palliative intent studies).
| Oral compared with intravenous fluoropyrimidines for colorectal cancer ‐ Patients treated with curative intent | |||||
| Patient or population: Patients treated with curative intent for colorectal cancer with neoadjuvant and/or adjuvant chemotherapy Setting: Hospital Intervention: Oral fluoropyrimidines Comparison: Intravenous fluoropyrimidines | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk* | Corresponding risk** | ||||
| Intravenous fluoropyrimidines | Oral fluoropyrimidines | ||||
| Disease‐free survival | 313 per 1000a | 291 per 1000 (272 to 313) | HR 0.93 | 8903 | ⊕⊕⊕⊝ |
| Overall survival | 222 per 1000c | 204 per 1000 (186 to 222) | HR 0.92 (0.84 to 1.00) | 8902 (7 RCTs) | ⊕⊕⊕⊕ |
| Grade ≥ 3 diarrhoea | 137 per 1000d | 153 per 1000 (135 to 171) | OR 1.12 | 9551 | ⊕⊝⊝⊝ |
| Grade ≥ 3 hand foot syndrome | 8 per 1000d | 37 per 1000 (24 to 57) | OR 4.59g | 5731 | ⊕⊕⊝⊝ |
| Grade ≥ 3 neutropenia/granulocytopenia | 181 per 1000d | 25 per 1000 (20 to 29) | OR 0.14 (0.11 to 0.16) | 8087 (7 RCTs) | ⊕⊕⊕⊝ |
| *The basis for the assumed risk is provided in footnotes. **The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Pooled estimates from fixed‐effects meta‐analysis are reported in the table | |||||
| GRADE Working Group grades of evidence | |||||
| aThe assumed risk for disease‐free survival was based on the 3‐year disease‐free survival rate in the control group from studies in the meta‐analysis (68.7%) bDowngraded by one level owing to a high risk of bias in included studies. cThe assumed risk for overall survival was based on the 5‐year overall survival rate in the control group from studies in the meta‐analysis (77.8%) dThe assumed risk for each grade ≥ 3 AE was the mean risk in the control group from studies in the meta‐analysis eDowngraded by one level owing to inconsistency of results that was supported by non‐overlapping CIs, high I2 values, and statistically significant heterogeneity of effect estimates fDowngraded by one level owing to imprecision gRandom‐effects estimate, OR 2.36 (95% CI 0.52 to 10.74). Pooled effect estimate was sensitive to the meta‐analysis model used | |||||
| Oral compared with intravenous fluoropyrimidines for colorectal cancer ‐ Patients treated with palliative intent | |||||
| Patient or population: Patients treated with palliative intent for inoperable advanced or metastatic colorectal cancer with chemotherapy Setting: Hospital Intervention: Oral fluoropyrimidines Comparison: Intravenous fluoropyrimidines | |||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk* | Corresponding risk** | ||||
| Intravenous fluoropyrimidines | Oral fluoropyrimidines | ||||
| Progression‐free survival | 398 per 1000a | 422 per 1000 (406 to 442) | HR 1.06 | 9927 | ⊕⊕⊕⊝ |
| Overall survival | 336 per 1000c | 343 per 1000 (333 to 353) | HR 1.02 (0.99 to 1.05) | 12,079 (29 RCTs) | ⊕⊕⊕⊕ |
| Grade ≥ 3 diarrhoea | 120 per 1000d | 199 per 1000 (180 to 221) | OR 1.66 | 11,997 | ⊕⊕⊝⊝ |
| Grade ≥ 3 hand foot syndrome | 13 per 1000d | 51 per 1000 (37 to 71) | OR 3.92 | 6481 | ⊕⊕⊕⊝ |
| Grade ≥ 3 neutropenia/granulocytopenia | 331 per 1000d | 56 per 1000 (50 to 60) | OR 0.17 (0.15 to 0.18) | 11,794 (29 RCTs) | ⊕⊕⊝⊝ |
| *The basis for the assumed risk is provided in footnotes. **The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Pooled estimates from fixed‐effects meta‐analysis are reported in the table | |||||
| GRADE Working Group grades of evidence | |||||
| aThe assumed risk for progression‐free survival was based on the 6‐month progression‐free survival rate in the control group from studies in the meta‐analysis (60.2%) bDowngraded by one level owing to a high risk of bias in included studies cThe assumed risk for overall survival was based on the 12‐month overall survival rate in the control group from studies in the meta‐analysis (66.4%) dThe assumed risk for each grade ≥ 3 AE was the mean risk in the control group from the studies in the meta‐analysis eDowngraded by one level owing to inconsistency of results that was supported by non‐overlapping CIs, high I2 values, and statistically significant heterogeneity of effect estimates | |||||
| Treatment setting | Study ID | Phase | Treatment type | Treatment arm/s (oral), n randomised | Treatment arm/s (IV), n randomised | IV arm: bolus vs Infusional |
| Neoadjuvant | Rectal | |||||
| III | Fluoropyrimidine combined with RT | Capecitabine (Grp 2), n = 146 Capecitabine (Grp 5), n = 326 Capecitabine + oxaliplatin (Grp 6), n = 330 | 5‐FU (Grp 1), n = 147 5‐FU (Grp 3), n = 330 5‐FU + oxaliplatin (Grp 4), n = 329 | Infusional | ||
| III | Fluoropyrimidine combined with RT | UFT (Tegafur/Uracil) + LV with RT, n = 78 | 5‐FU + LV with RT, n = 77 | Bolus | ||
| Neoadjuvant/ Adjuvant | Rectal | |||||
| III | Fluoropyrimidine combined with RT | Capecitabine with RT, n = 197 ∙ Adjuvant cohort: n = 116 ∙ Neoadjuvant cohort: n = 81 | 5‐FU with RT, n = 195 ∙ Adjuvant cohort: n = 115 ∙ Neoadjuvant cohort: n = 80 | Bolus and infusional | ||
| Adjuvant | Rectal | |||||
| ND | Fluoropyrimidine combined with RT (after completion of 2C of fluoropyrimidine alone) | 5‐dFUR + LV, n = 92 | 5‐FU + LV, n = 74 | Bolus | ||
| Colon | ||||||
| III | Combination chemotherapy ‐ Oxaliplatin + Bevacizumab (BEV) | BEV‐XELOX, n = 952 | BEV‐FOLFOX4, n = 960 | Infusional | ||
| III | Fluoropyrimidine alone | UFT + LV, n = 805 | 5‐FU + LV, n = 803 | Bolus | ||
| III | Fluoropyrimidine alone | UFT + LV, n = 551 | 5‐FU + LV, n = 550 | Bolus | ||
| III | Fluoropyrimidine alone | Capecitabine, n = 1004 | 5‐FU + LV, n = 983 | Bolus | ||
| Colorectal | ||||||
| III | Combination chemotherapy ‐ fluoropyrimidine + oxaliplatin | CAPOX (capecitabine + oxaliplatin), n = 197 | mFOLFOX6, n = 211 | Infusional | ||
| IV: intravenous RT: radiotherapy 5‐FU: 5‐fluorouracil UFT: tegafur/uracil LV: leucovorin ND: no data available 5‐dFUR: doxifluridine BEV: bevacizumab | ||||||
| Oral fluoropyrimidine backbone | Study ID | Phase | Treatment line | Treatment arm/s (Oral), n randomised | Treatment arm/s (IV), n randomised | IV arm: Bolus vs Infusional |
| Capecitabine | III | First | Capecitabine, n = 302 | 5‐FU + LV, n = 303 | Bolus | |
| III | First | Capecitabine, n = 301 | 5‐FU + LV, n = 301 | Bolus | ||
| Doxifluridine (5‐dFUR) | II | First | 5‐dFUR + LV, n = 38 | 5‐FU + LV, n = 39 | Bolus | |
| II | First | 5‐dFUR + LV, n = 67 | 5‐dFUR + LV, n = 63 | Bolus | ||
| Eniluracil + oral 5‐FU | III | First | Eniluracil/Oral 5‐FU, n = 61 | 5‐FU, n = 64 | Infusional | |
| III | First | Eniluracil/Oral 5‐FU, n = 488 | 5‐FU + LV, n = 493 | Bolus | ||
| III | First | Eniluracil/Oral 5‐FU, n = 268 | 5‐FU + LV, n = 263 | Bolus | ||
| Ftorafur/tegafur (FT) | ND | First | Ftorafur, n = 30 | 5‐FU, n = 30 | Bolus | |
| Unclear; described as Phase IV in abstracts | First | FT + LV, n = 114 | 5‐FU + LV, n = 123 | Bolus | ||
| Ftorafur + uracil (UFT) | III | First | UFT + LV, n = 190 | 5FU + LV, n = 190 | Bolus | |
| III | First | UFT + LV, n = 409 | 5‐FU + LV, n = 407 | Bolus | ||
| IV: intravenous 5‐FU: 5‐fluorouracil LV: leucovorin 5‐dFUR: doxifluridine ND: no data available FT: tegafur UFT: tegafur + uracil | ||||||
| Chemotherapy | Study ID | Phase | Study design ‐ other details | Treatment line | Treatment arm/s (Oral), n randomised | Treatment arm/s (IV), n randomised | IV arm: Bolus vs Infusional |
| Oxaliplatin | Combination with capecitabine | ||||||
| III | 2 × 2 factorial ‐ following protocol amendment | First | XELOX alone, n = 317 | FOLFOX‐4 alone, n = 317 | Infusional | ||
| XELOX + Placebo, n = 350 | FOLFOX‐4 + Placebo, n = 351 | Infusional | |||||
| XELOX + BEV, n = 350 | FOLFOX‐4 + BEV, n = 350 | Infusional | |||||
| III | First | OXXEL (Capecitabine + oxaliplatin), n = 158 | OXAFAFU (5‐FU/LV + Oxaliplatin), n = 164 | Bolus | |||
| III | First | XELOX, n = 174 | FUOX (5‐FU + Oxaliplatin), n = 174 | Infusional | |||
| III | First | XELOX, n = 156 | FOLFOX‐6, n = 150 | Infusional | |||
| ND | First | CapeOx, n = 50 | mFOLFOX6, n = 50 | Infusional | |||
| bFOL, n = 50 | Bolus | ||||||
| ND | First | CapeOx + BEV, n = 74 | mFOLFOX6 + BEV, n = 75 | Infusional | |||
| bFOL + BEV, n = 74 | Bolus | ||||||
| II | First | XELOX, n = 62 | pviFOX, n = 56 | Infusional | |||
| III | First | CAPOX, n = 242 | FUFOX, n = 234 | Infusional | |||
| III | Second | XELOX, n = 313 | FOLFOX‐4, n = 314 | Infusional | |||
| ND | 2 × 2 factorial, cross‐over (only from no oxaliplatin to oxaliplatin) | First | Capecitabine or OxCap, n = 229 ∙ Capecitabine, n = 115 ∙ OxCap, n = 114 | 5‐FU or OxFU, n = 230 ∙ 5‐FU, n = 115 ∙ OxFU, n = 115 | Infusional | ||
| Combination with Ftorafur/uracil (UFT) | |||||||
| II | First | UFOX + Cetuximab, n = 152 | FOLFOX4 + Cetuximab, n = 150 | Infusional | |||
| Combination with S‐1 | |||||||
| ND | First | SOX, n = 35 | FOLFOX4, n = 35 | Infusional | |||
| III | First | SOX‐BEV, n = 256 | mFOLFOX6‐BEV, n = 256 | Infusional | |||
| II | First | SOL (S‐1 + oxaliplatin + oral LV), n = 56 | mFOLFOX6, n = 51 | Infusional | |||
| Irinotecan | Combination with capecitabine | ||||||
| II | First | XELIRI + BEV, n = 72 | FOLFIRI + BEV, n = 73 | Infusional | |||
| III | 3 × 2 factorial (Period 1) | First | CapeIRI + Celecoxib/Placebo, n = 145 | FOLFIRI + Celecoxib/Placebo, n = 144 | Infusional | ||
| mIFL + Celecoxib/Placebo, n = 141 | Bolus | ||||||
| III | 2 × 2 factorial | First | CAPIRI + Celecoxib/Placebo, n = 44 | FOLFIRI + Celecoxib/Placebo, n = 41 | Infusional | ||
| III | First | XELIRI + BEV, n = 143 | FOLFIRI + BEV, n = 142 | Infusional | |||
| II | First | XELIRI, n = ND | FOLFIRI, n = ND | Infusional | |||
| II | First | CAPIRI + BEV, n = 168 | FOLFIRI + BEV, n = 168 | Infusional | |||
| ND | First and second | Capecitabine + Irinotecan, n = 27 | 5‐FU + Irinotecan, n = 16 | Infusional | |||
| Combination with Ftorafur/uracil (UFT) | |||||||
| II | First | TEGAFIRI (UFT, leucovorin, irinotecan) ± BEV, n = 35 | FOLFIRI ± BEV, n = 36 | Infusional | |||
| Combination with S‐1 | |||||||
| II | First and second | Sequential IRIS‐BEV, n = 30 | mFOLFIRI‐BEV, n = 30 | Infusional | |||
| II/III | Second | IRIS (Irinotecan + S‐1), n = 213 | FOLFIRI, n = 213 | Infusional | |||
| IV: intravenous BEV: bevacizumab ND: no data available UFT: tegafur/uracil | |||||||
| Setting | Related | Related and unrelated | Not specified |
| Patients treated with curative intent for CRC with neoadjuvant and/or adjuvant chemotherapy | |||
| Patients treated with palliative intent for inoperable advanced or metastatic CRC with chemotherapy
|
| ||
| CRC: colorectal cancer | |||
| Study ID | Outcome | |||||||||||
| Efficacy | Grade ≥ 3 AE | |||||||||||
| DFS | OS | Diarrhoea | HFS | Neutropenia/ granulocytopenia | Febrile neutropenia | Vomiting | Nausea | Stomatitis | Mucositis | Hyperbilirubinemia | Any | |
| X | X | X | X | X | X | X | X | X | X | X | ||
| X | X | X | X | X | X | X | X | |||||
| Oa | Oa | X | Ob | X | X | X | Xc | Xc | ||||
| X | X | X | X | X | X | X | X | X | X | |||
| X | X | |||||||||||
| X | X | X | X | X | X | X | X | |||||
| X | X | X | X | X | X | X | X | X | ||||
| X | X | X | X | X | X | X | X | X | ||||
| X | X | X | Ob | X | Xd | Xd | X | Oe | X | |||
| X: Study contributed to the pooled effect estimate for the outcome O: Study reported the outcome but did not contribute to the pooled effect estimate for the outcome aInsufficient follow‐up time ‐ median 22 months in each arm (< 3 years) bAssessed grade ≥ 3 HFS using criteria not considered to be sufficiently similar to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (versions 2.0 to 4.0) cReported combined data for grade ≥ 3 stomatitis and mucositis dReported combined data for grade ≥ 3 vomiting and nausea eAssessed grade 3 ≥ hyperbilirubinaemia using criteria not considered to be sufficiently similar to NCI CTCAE (versions 2.0 to 4.0 and 1981) and World Health Organisation (WHO) (1981 version) AE: adverse event DFS: disease‐free survival OS: overall survival HFS: hand foot syndrome | ||||||||||||
| Study ID | Outcome | |||||||||||||
| Efficacy | Grade ≥ 3 AE | |||||||||||||
| PFS | TTP | OS | ORR | Diarrhoea | HFS | Neutropenia/ granulocytopenia | Febrile neutropenia | Vomiting | Nausea | Stomatitis | Mucositis | Hyperbilirubinemia | Any | |
| X | X | X | Oa | Oa | Oa | Oa | ||||||||
| Ob | X | |||||||||||||
| X | X | X | X | X | ||||||||||
| X | X | X | X | X | X | Xc | Xc | Xd | Xd | Oe | ||||
| X | X | X | X | X | X | X | Xc | Xc | X | X | ||||
| X | X | X | X | X | X | X | ||||||||
| X | X | X | X | X | X | X | X | X | X | X | ||||
| X | X | X | X | Of | X | X | Xc | Xc | Xd | Xd | Oe | |||
| X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| X | X | X | X | X | X | X | X | X | X | |||||
| X | X | X | X | X | X | X | X | X | X | X | ||||
| X | X | X | X | X | X | X | X | X | X | X | ||||
| X | X | X | X | X | X | X | X | X | ||||||
| Ob | X | X | X | X | X | Xc | Xc | X | ||||||
| Ob | X | X | X | X | X | Xc | Xc | X | ||||||
| X | X | X | X | Og | X | X | X | X | X | |||||
| X | X | X | X | X | X | X | X | X | ||||||
| X | X | X | X | X | X | X | X | X | ||||||
| X | X | X | X | X | X | X | ||||||||
| Oh | X | X | Xc | Xc | ||||||||||
| X | X | X | X | X | Xc | Xc | X | |||||||
| X | X | X | X | X | X | X | X | X | X | X | ||||
| X | X | X | X | X | X | X | X | |||||||
| X | X | X | X | X | X | X | X | X | X | Oi | X | |||
| X | X | X | X | Og | X | X | X | X | X | |||||
| X | X | Oj | X | X | X | X | X | X | X | |||||
| X | X | X | X | X | X | X | X | Xd | Xd | X | X | |||
| Ob | Oa | Oa | Oa | |||||||||||
| X | X | X | X | X | X | X | X | X | X | |||||
| X | X | X | X | X | X | X | ||||||||
| X | X | X | X | Og | X | X | X | X | ||||||
| X | X | X | X | X | X | X | X | X | Xd | Xd | X | |||
| X | X | X | X | X | X | X | X | X | ||||||
| X | X | X | X | X | X | X | Xd | Xd | ||||||
| Ob | Ob | X | Oa | Oa | Oa | Oa | Oa | |||||||
| X: Study contributed to the pooled effect estimate for the outcome O: Study reported the outcome but did not contribute to the pooled effect estimate for the outcome aUnclear number of participants assessed for outcomes in both arms bHazard ratios could not be estimated either directly or indirectly from the provided information cReported combined data for grade ≥3 vomiting and nausea dReported combined data for grade ≥3 stomatitis and mucositis eAssessed grade ≥ 3 hyperbilirubinaemia using Common Toxicity Criteria (CTC), version not specified fAssessed grade ≥ 3 HFS using CTC, version not specified gAssessed grade ≥ 3 HFS using criteria not considered to be sufficiently similar to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (versions 2.0 to 4.0) hORR reported after 2 cycles of chemotherapy iAssessed grade ≥3 hyperbilirubinaemia using criteria not considered to be sufficiently similar to NCI CTCAE (versions 2.0 to 4.0 and 1981) and World Health Organisation (WHO) (1981 version) jORR reported 12 to 14 weeks after start of treatment AE: adverse event PFS: progression‐free survival TTP: time to progression OS: overall survival ORR: objective response rate HFS: hand foot syndrome | ||||||||||||||
| Risk of bias assessment | ||
| Low | Unclear | High |
| No studies | No studies | |
| Risk of bias assessment | ||
| Low | Unclear | High |
| Sensitivity analyses for PFS outcome | Original analysis: (effect estimatea, fixed | Sensitivity analysis: (effect estimatea, fixed |
| Excluding studies with 'High' risk of bias | 1.06 (1.02 to 1.11) | 1.01 (95% CI 0.96 to 1.07) |
| Excluding Seymour 2011 study (frail and elderly study population) | 1.06 (1.02 to 1.11) | 1.07 (1.03 to 1.11) |
| Excluding second‐line studies in patients treated with palliative intent for inoperable or metastatic colorectal cancerb | 1.06 (1.02 to 1.11) | 1.07 (1.03 to 1.12) |
| aEffect estimates presented as inverse‐variance hazard ratios for time‐to‐event outcomes, and Mantel‐Haenszel odds ratios for adverse events bAnalysis excluding Kato 2012, Rothenberg 2008, Yasui 2015, and Yu 2005. Kato 2012 and Yu 2005 included patients receiving first‐ or second‐line treatment PFS: progression‐free survival CI: confidence interval | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐free survival Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 2 Disease‐free survival with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 2.1 Chemotherapy | 5 | 6944 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 2.2 Chemo‐radiotherapy | 2 | 1959 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.78, 1.05] |
| 3 Disease‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 6 | 8511 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.88, 1.02] |
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 3881 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 3.2 Bolus intravenous fluoropyrimidine | 3 | 4630 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.86, 1.04] |
| 4 Disease‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 7 | 8903 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 4.1 Capecitabine | 5 | 6260 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.83, 0.99] |
| 4.2 UFT/Ftorafur | 2 | 2643 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (curative intent studies) Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| 2 Overall survival (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| 2.1 Chemotherapy | 5 | 6943 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 2.2 Chemotherapy with radiotherapy | 2 | 1959 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 3 Overall survival (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 6 | 8510 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 3880 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.80, 1.09] |
| 3.2 Bolus intravenous fluoropyrimidine | 3 | 4630 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 4 Overall survival (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 7 | 8902 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| 4.1 Capecitabine | 5 | 6259 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.79, 0.98] |
| 4.2 UFT/Ftorafur | 2 | 2643 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grade ≥ 3 diarrhoea (curative intent studies) Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| 2 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Chemotherapy vs chemo‐radiotherapy Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| 2.1 Chemotherapy | 5 | 7274 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 2.2 Chemo‐radiotherapy | 4 | 2277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.98, 1.66] |
| 3 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 8 | 9159 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.23] |
| 3.1 Infusional intravenous fluoropyrimidine | 3 | 4255 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.06, 1.53] |
| 3.2 Bolus intravenous fluoropyrimidine | 5 | 4904 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| 4 Grade ≥ 3 diarrhoea (curative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 9 | 9551 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.99, 1.25] |
| 4.1 Capecitabine | 5 | 6616 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 4.2 UFT/Ftorafur | 3 | 2769 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 4.3 Doxifluridine | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 32.14 [1.89, 545.41] |
| 5 Grade ≥ 3 hand foot syndrome (curative intent studies) Show forest plot | 5 | 5731 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.59 [2.97, 7.10] |
| 6 Grade ≥ 3 neutropenia/granulocytopenia (curative intent studies) Show forest plot | 7 | 8707 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.11, 0.16] |
| 7 Grade ≥ 3 febrile neutropenia (curative intent studies) Show forest plot | 4 | 2925 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.18, 1.90] |
| 8 Grade ≥ 3 vomiting (curative intent studies) Show forest plot | 8 | 9385 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.34] |
| 9 Grade ≥ 3 nausea (curative intent studies) Show forest plot | 7 | 9233 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.97, 1.51] |
| 10 Grade ≥ 3 stomatitis (curative intent studies) Show forest plot | 5 | 4212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.14, 0.30] |
| 11 Grade ≥ 3 mucositis (curative intent studies) Show forest plot | 4 | 2233 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.62] |
| 12 Grade ≥ 3 hyperbilirubinaemia (curative intent studies) Show forest plot | 3 | 2757 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.52, 5.38] |
| 13 Any grade ≥ 3 adverse events (curative intent studies) Show forest plot | 5 | 7741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression‐free survival Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| 2 Progression‐free survival with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 22 | 9468 | Hazard Ratio (Fixed, 95% CI) | 1.07 [1.03, 1.11] |
| 2.1 Single agent | 6 | 2955 | Hazard Ratio (Fixed, 95% CI) | 1.12 [1.04, 1.21] |
| 2.2 Combination therapy | 16 | 6513 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| 3 Progression‐free survival with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| 3.1 Infusional intravenous fluoropyrimidine | 17 | 6560 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| 3.2 Bolus intravenous fluoropyrimidine | 7 | 3367 | Hazard Ratio (Fixed, 95% CI) | 1.10 [1.03, 1.19] |
| 4 Progression‐free survival with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 23 | 9927 | Hazard Ratio (Fixed, 95% CI) | 1.06 [1.02, 1.11] |
| 4.1 Capecitabine | 13 | 6703 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.98, 1.08] |
| 4.2 UFT/Ftorafur | 2 | 374 | Hazard Ratio (Fixed, 95% CI) | 1.36 [1.07, 1.73] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1618 | Hazard Ratio (Fixed, 95% CI) | 1.22 [1.10, 1.36] |
| 4.4 Doxifluridine | 1 | 130 | Hazard Ratio (Fixed, 95% CI) | 1.18 [0.79, 1.74] |
| 4.5 S‐1 | 4 | 1102 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 5 Progression‐free survival for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 16 | 6513 | Hazard Ratio (Fixed, 95% CI) | 1.05 [1.00, 1.10] |
| 5.1 Oxaliplatin‐based | 8 | 4677 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.99, 1.13] |
| 5.2 Irinotecan‐based | 8 | 1836 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 6 Progression‐free survival for combination therapy with subgroup analysis ‐ with Bev vs no Bev Show forest plot | 14 | 6139 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.98, 1.08] |
| 6.1 With Bevacizumab | 6 | 2033 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 6.2 No Bevacizumab | 9 | 4106 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.99, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (palliative intent studies) Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 2 Overall survival (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 28 | 11620 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 2.1 Single agent | 10 | 4465 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.07] |
| 2.2 Combination therapy | 18 | 7155 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 3 Overall survival (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 3.1 Infusional intravenous fluoropyrimidine | 19 | 7022 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 3.2 Bolus intravenous fluoropyrimidine | 13 | 5057 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.98, 1.06] |
| 4 Overall survival (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 29 | 12079 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 4.1 Capecitabine | 16 | 7405 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.95, 1.04] |
| 4.2 UFT/Ftorafur | 5 | 1807 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1618 | Hazard Ratio (Fixed, 95% CI) | 1.20 [1.07, 1.36] |
| 4.4 Doxifluridine | 2 | 207 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.65, 1.50] |
| 4.5 S‐1 | 3 | 1042 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.81, 1.11] |
| 5 Overall survival (palliative intent studies) for combination therapy with subgroup analysis ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 18 | 7155 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 5.1 Oxaliplatin‐based | 11 | 5379 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 5.2 Irinotecan‐based | 7 | 1776 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to progression Show forest plot | 6 | 1970 | Hazard Ratio (Fixed, 95% CI) | 1.07 [1.01, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ORR Show forest plot | 32 | 11115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grade ≥ 3 diarrhoea (palliative intent studies) Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| 2 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| 2.1 Single agent | 10 | 4566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.44] |
| 2.2 Combination therapy | 21 | 7431 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.77, 2.32] |
| 3 Grade ≥ 3 diarrhea (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| 3.1 Infusional intravenous fluoropyrimidine | 21 | 7065 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.74, 2.30] |
| 3.2 Bolus intravenous fluoropyrimidine | 12 | 4932 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.12, 1.53] |
| 4 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 30 | 11997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.50, 1.84] |
| 4.1 Capecitabine | 17 | 7382 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.00] |
| 4.2 UFT/Ftorafur | 5 | 1784 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.24, 2.06] |
| 4.3 Eniluracil + oral 5‐FU | 3 | 1617 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.38] |
| 4.4 Doxifluridine | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.64, 3.56] |
| 4.5 S‐1 | 4 | 1087 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.19, 5.76] |
| 5 Grade ≥ 3 diarrhoea (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 20 | 7212 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.75, 2.29] |
| 5.1 Oxaliplatin‐based | 12 | 5420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.48, 2.02] |
| 5.2 Irinotecan‐based | 8 | 1792 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.33, 3.99] |
| 6 Grade ≥ 3 hand foot syndrome (palliative intent studies) Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| 7 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Single‐agent vs combination therapy Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.89 [2.82, 5.37] |
| 7.1 Single agent | 2 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.48, 2.56] |
| 7.2 Combination therapy | 17 | 6138 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.76 [3.32, 6.82] |
| 8 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Infusional vs bolus intravenous fluoropyrimidine Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| 8.1 Infusional intravenous fluoropyrimidine | 18 | 6094 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.53 [2.53, 4.94] |
| 8.2 Bolus intravenous fluoropyrimidine | 3 | 387 | Odds Ratio (M‐H, Fixed, 95% CI) | 18.68 [4.15, 84.10] |
| 9 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis ‐ Oral fluoropyrimidine backbone Show forest plot | 18 | 6481 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.84, 5.43] |
| 9.1 Capecitabine | 13 | 5418 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.86 [4.01, 8.58] |
| 9.2 UFT/Ftorafur | 2 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.50] |
| 9.3 Eniluracil + oral 5‐FU | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.75] |
| 9.4 S‐1 | 2 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 10 Grade ≥ 3 hand foot syndrome (palliative intent studies) with subgroup analysis for combination therapy ‐ Oxaliplatin‐based vs irinotecan‐based Show forest plot | 16 | 5919 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.76 [3.31, 6.83] |
| 10.1 Oxaliplatin‐based | 10 | 4608 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [3.03, 6.75] |
| 10.2 Irinotecan‐based | 6 | 1311 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.93 [2.52, 13.97] |
| 11 Grade ≥ 3 neutropenia/granulocytopenia (palliative intent studies) Show forest plot | 29 | 11794 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.15, 0.18] |
| 12 Grade ≥ 3 febrile neutropenia (palliative intent studies) Show forest plot | 19 | 9407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.21, 0.36] |
| 13 Grade ≥ 3 vomiting (palliative intent studies) Show forest plot | 23 | 9528 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.40] |
| 14 Grade ≥ 3 nausea (palliative intent studies) Show forest plot | 25 | 9796 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.99, 1.36] |
| 15 Grade ≥ 3 stomatitis (palliative intent studies) Show forest plot | 21 | 8718 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.20, 0.33] |
| 16 Grade ≥ 3 mucositis (palliative intent studies) Show forest plot | 12 | 4962 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.12, 0.24] |
| 17 Grade ≥ 3 hyperbilirubinaemia (palliative intent studies) Show forest plot | 9 | 2699 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.99, 2.64] |
| 18 Any grade ≥ 3 adverse events (palliative intent studies) Show forest plot | 14 | 5436 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.94] |

