Cama de compañía en la unidad neonatal para la promoción del crecimiento y el desarrollo neurológico en gemelos prematuros estables
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT in a NICU at a university hospital (Iran) | |
| Participants | 105 newborn infants with gestational age of 26 to 34 weeks and postnatal age of less than 20 days who underwent heel blood sampling for blood glucose determination. Study authors did not state the reasons for the odd number recruited. 100 of these infants (50 pairs of twins) received the allocated intervention. Infants who had received sedatives or analgesics, with major congenital malformations, an Apgar scores < 6 at 5 minutes after birth, severe respiratory distress requiring mechanical ventilation, or severe intraventricular haemorrhage were excluded | |
| Interventions | Co‐bedding (cared for in the same incubator) vs separate care (cared for in separate incubators) with an aim to assess their pain response to heel prick. "Newborns in the co‐bedding group were placed side by side in an incubator without any clothing except for diapers so that they could touch each other freely, with each side of the incubator pertaining to one twin. Infants were co‐bedded from 24 hours prior to heel sticks to the end of the study" Although not stated clearly, it appeared that heel prick was performed for all infants | |
| Outcomes | Infant pain response to heel prick, assessed by the Premature Infant Pain Profile (PIPP). Secondary outcome was the amount of salivary cortisol secreted after the heel prick procedure | |
| Notes | Other parameters assessed were duration of crying and highest heart rate during heel prick, which together with the amount of salivary cortisol were not included in our meta‐analysis, as they were not included as pre‐specified outcomes of our review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Materials and methods: "Randomisation was performed using a computer‐generated random number algorithm" |
| Allocation concealment (selection bias) | Low risk | Materials and methods: "Allocation of eligible newborns to the intervention and the control groups was performed using a sealed opaque envelope" |
| Incomplete outcome data (attrition bias) | Low risk | 100 out of 105 infants recruited (95.2%) received the allocated intervention, all of whom were included in the subsequent analysis Five infants (number of twin sets unclear) did not receive co‐bedding as originally allocated and were excluded from the analysis with no reasons stated |
| Selective reporting (reporting bias) | Low risk | Both major outcomes stated in the methods, namely, PIPP and salivary cortisol levels, were reported in sufficient detail in the results |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) | High risk | Study authors stated, "Blood sampling was performed in a standardized manner by expert technicians who could not be blinded to the study" (Materials and methods). Blinding of the other caregivers was highly unlikely in view of differences between the 2 allocated groups in the way the infants were cared for |
| Blinding of outcome assessment (detection bias) | High risk | Study authors stated that "the researchers could not be blinded for the assigned groups" |
| Methods | Randomized repeated measures study at a tertiary level NICU (USA) | |
| Participants | A convenience sample of 37 preterm infants (average 29 weeks' PMA) and 19 parents. Exclusion criteria "included the infants having a known infection, being on mechanical ventilation, and having a combined weight too great to be safely co‐bedded in an incubator." Study authors did not specify the combined weight limit | |
| Interventions | Co‐bedding (cared for in the same incubator) vs separate care (cared for in separate incubators). Study authors stated that only 2 infants were co‐bedded at a time. For triplets, the first 2 infants to achieve physiological stability as determined by the clinicians involved were co‐bedded. In total, 16 infants (8 pairs) were co‐bedded and 21 infants were assigned to separate care. It was unclear how many of these infants were twins and how many were a part of triplets | |
| Outcomes | Main outcomes were physiological parameters, sleep‐wake synchrony of infants, neurobehavioral observations using the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) guidelines, and parental measures including anxiety (measured using Parental State Anxiety Inventory, an 80‐point scale, with higher score indicating higher anxiety level), attachment (measured using Maternal Attachment Inventory, a 112‐point scale, with higher score indicating higher attachment), and satisfaction (measured using Parental Satisfaction Survey, a 55‐point scale, with higher score indicating higher satisfaction level) | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants and sample: "The participants were assigned randomly." No other statements on how random sequence was generated were available |
| Allocation concealment (selection bias) | Unclear risk | Participants and sample: "The participants were assigned randomly." No further statements indicated whether random sequence generation was performed independently from allocation, or how allocation was carried out |
| Incomplete outcome data (attrition bias) | Low risk | All except 1 infant in the control group completed the study. This single infant was withdrawn from the study after sepsis was diagnosed |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods were reported in sufficient detail in the results |
| Other bias | High risk | Some imbalance in major characteristics was noted between the 2 assigned groups. Co‐bedded infants had higher PMA at study enrollment than infants in the control group (average 33 weeks PMA in co‐bedding group, 30.6 weeks PMA in control group), and a greater proportion of infants were male (68.3% in the co‐bedded group, 22.7% in the control group) |
| Blinding of participants and personnel (performance bias) | High risk | Although not stated in the paper, blinding for caregivers and for parents appeared highly unlikely. Study authors stated, "Single‐bedded infants received the traditional NICU standard of care. Co‐bedded infants received care using the institution's co‐bedding care protocol," which involved details of infant care, monitoring, and hand hygiene. It was unclear whether the "traditional NICU standard of care" as stated followed the same protocol as the co‐bedded group, other than necessary differences that arose from caring for 1 infant vs 2 in an incubator or crib |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not stated whether the data collector was blinded to the status of the infants |
| Methods | Multi‐centre randomized controlled trial involving 3 tertiary‐level university‐affiliated NICUs in Canada | |
| Participants | Study setting and population: "Twins were considered eligible for recruitment if they were heavier than 1000 g, without major anomalies, requiring at least 1 medically indicated heel lance for blood procurement, and considered medically stable (without infection, indwelling chest tubes or umbilical catheters, or need for mechanical ventilation). Twins were not | |
| Interventions | Co‐bedding (cared for in the same incubator or crib) vs "separate care" (cared for in separate incubators or cribs). All participants also received 1 dose of 24% sucrose 2 minutes before the heel lance and were offered a pacifier. Additional (rescue) doses of 24% sucrose were offered as deemed necessary by the nurse caring for the twins. as per usual care practices | |
| Outcomes | The primary outcome was pain response as measured by the Premature Infant Pain Profile (PIPP), a previously validated 21‐point scale, with higher scores indicating greater pain. Other outcomes included time required for physiological recovery in response to heel lance, determined by length of time for heart rate and oxygen saturation to return to baseline, the need for additional pain relief with 24% sucrose, and adverse outcomes, such as episodes of apnea, bradycardia, infection. and caregiver error | |
| Notes | Study authors adjusted for non‐dependence between twin pairs by using the generalized estimating equation, and for differences in baseline characteristics by using regression techniques (statistical analysis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study design and intervention: Participants were randomized after parental consent by "randomly permuted blocks of 2, 4, or 6, via a computerised off‐site Web site accessed by the principal investigator (M.C‐Y.) or research nurse" |
| Allocation concealment (selection bias) | Low risk | Study design and intervention: Participants were randomized after parental consent by "randomly permuted blocks of 2, 4, or 6, via a computerised off‐site Web site accessed by the principal investigator (M.C‐Y.) or research nurse" |
| Incomplete outcome data (attrition bias) | Low risk | A total of 10 infants (5 from each group) out of 134 did not complete the study. The number of non‐completers was small and was balanced between the 2 groups; reasons for non‐completion (transfer before heel prick and physiological data equipment malfunction) were unlikely to be related to outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods were reported in sufficient detail in the results |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) | High risk | It was not stated whether caregivers and parents were blinded to group assignment, although this appeared highly unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not stated whether the research nurse who collected data on pain score and physiological parameters was blinded to the status of the infants. One statement suggested that the data analyst might be blinded (Statistical analysis: "All data were analysed in a research laboratory off‐site from the NICU") |
| Methods | Randomized controlled trial at a tertiary‐level NICU (USA) | |
| Participants | Thirty‐nine sets of preterm twins born at less than 34 weeks' PMA, who were clinically stable at the time of enrollment | |
| Interventions | Co‐bedding (cared for in the same incubators) vs separate care (cared for in separate incubators). A protocol was drawn for co‐bedding twins enrolled in the study. In the co‐bedding group, twins were placed side by side, initially in an incubator and later in an open crib, when both reached 1700 grams in weight. The same nurse looked after both twins. One blanket was used to lightly swaddle the twins together, allowing the hands to move freely to enable physical contact between the twins. Twins were separated if one or both became unstable. For twin sets in the control group, the only statements concerning their care were these: "The twin sets that were randomised to the control group received routine care in separate beds" (Purpose and methods, paragraph 2), and "Twin sets in both groups were assigned to the same bedside nurse" (Purpose and methods, paragraph 4) | |
| Outcomes | Weight and apnea, bradycardia, and desaturation (A/B/D) episodes were collected from electronic medical records. Apnea was defined by study authors as cessation of breathing for > 20 seconds, bradycardia as any heart rate < 80 beats per minute, and desaturation as any oxygen saturation level < 85% (Purpose and methods, paragraph 6). If any of these events occurred singularly, they were counted as an individual event. If more than 1 type of event occurred in a cluster, they were counted as a single event Study authors reported adjusted mean weights (after controlling for baseline weights) at weeks 1, 2, and 3, and median number of combined A/B/D episodes | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not mentioned. Correspondence with study authors revealed that the randomisation list was manually created by the first 2 authors, but no detail was provided on the tools used |
| Allocation concealment (selection bias) | Low risk | "Twins were randomised using sealed envelopes that stated either control or experimental group" (Purpose and methods). Correspondence with study authors revealed that sequence allocation was carried out by using "opaque and sealed envelopes" |
| Incomplete outcome data (attrition bias) | Low risk | Study authors mentioned reasons for withdrawal from the study. However, the sample became progressively smaller over the 3 weeks of the study, with loss of participants greater than could be accounted for by withdrawal. Attrition rates differed between the 2 groups over the duration of the study, as by the second week, 13 pairs of twins in the co‐bedding group and 19 pairs in the control groups remained, and by the third week, 10 pairs of twins in the co‐bedding group and 12 pairs in the control group remained. Reasons for this were not directly given but could be inferred from the following statement in Results, paragraph 4: "The data were analysed for the first 3 weeks of enrolment. After this time, the sample size became too small due to discharge home from the hospital" Study authors also explained the reasons why data collection was withheld for some participants during the course of the study, as follows (Results, paragraph 3): "In the co‐bedded group, twins were separated due to complications consisting of conjunctivitis (1 set separated for 2 days); PDA ligation (1 set separated for 1 week); co‐bedding and data collection were resumed once the infants were considered stable based on study guidelines" |
| Selective reporting (reporting bias) | High risk |
Table 1 provides a matrix on the outcomes listed under methods and any additional key outcomes and actual outcomes reported |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) | High risk | Although not stated in the article, blinding of caregivers and parents appeared highly unlikely |
| Blinding of outcome assessment (detection bias) | High risk | Although not stated in the paper, further correspondence with the lead study author confirmed that the research assistant who collected the data was not blinded to the status of the twin pairs |
| Methods | Pilot randomized controlled trial at a tertiary‐level NICU (Canada) | |
| Participants | Six pairs of twins < 2000 grams. It was not stated whether weight criteria referred to weight at birth or at study entry | |
| Interventions | Co‐bedding (cared for in the same incubator) vs separate care (cared for in separate incubators). Twin sets in the co‐bedding group were placed in a common twin incubator (Giraffe, GE Medical, Fairfield, CT) for care. Twins in the control group received standard neonatal care in separate incubators. Each twin set was cared for by the same nurse during each shift. Data for each twin set were recorded over 8 blocks of observational periods over 2 weeks, each lasting 5 hours | |
| Outcomes | Parental self efficacy, parental anxiety, infant "co‐regulation" (defined as synchrony of infant state, heart rate, respirations, temperature, and oxygen saturation ‐ regardless of whether the twins were co‐bedded), infection rate, and incidence of caregiver error A research assistant recorded infants' physiological parameters from their charts and transcribed data onto the Nursing Child Assessment Sleep/Activity Record (NCASAR) for comparison of the record between twin dyads for co‐regulatory activities. In the article, study authors did not define what thresholds were used to define the presence of "co‐regulation" nor was the unit of measurement used for the outcomes of "time in quiet sleep," "time crying," and "co‐regulation" stated. Infection rate was determined via infants' charts and medical records, by 3 measures: incidence of septic workup, treatment with antibiotics, and confirmed incidence of sepsis. Caregiver errors were collected from the nursing quarterly report, rather than recorded through direct observation. Infants were observed for 2 weeks Study authors provided more detailed information through further correspondence on the unit of measurement for the outcomes of "time in quiet sleep," "time crying," and "co‐regulation," as follows: "The same unit of measurement was used for all outcomes. Each 15 minutes for a five hour block of time, infants were observed for 5 minutes. The research assistant would then assign their state for that 5 minute block of time. The outcome refers to the number of times infants were assigned a specific state. Coregulation was assigned if the subjects (twin set) were in the same state or were transitioning to the same state. Transitioning was defined as being only one state apart (e.g.. one twin quiet sleep and one twin active sleep, or one twin drowsy and one twin quiet alert)" This essentially translated into a measurement scale of 0 to 20, and the rating reflected the number of times a twin pair was observed to have the outcome of interest during the 20 observation periods scheduled within the 5‐hour study period. A minimum of 8 five‐hour blocks were completed for each twin pair on a biweekly basis. All ratings in each assigned group were averaged to obtain final estimates | |
| Notes | Unit of analysis issue was that all outcome data were analyzed and reported for each individual infant rather than for each twin pair | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Twins were randomised into two groups" (Design, paragraph 1). No further mention was made of randomization methods in the paper. Study author provided the following additional information: "Twins were randomised using a simple card draw. Randomisation assignment was written on a card and placed in an envelope.The [principal] investigator placed equal assignment cards (7 co‐bedding and 7 separate care) into 14 envelopes. These envelopes were placed in a box" |
| Allocation concealment (selection bias) | Low risk | "Group allocation was assigned randomly using opaque envelopes" (Sample, paragraph 1). Study author provided the following additional information: "The randomisation assignment was placed on the inside of a folded card. This card was then taped shut and placed in a sealed envelope" and "The research assistant blindly selected an envelope from a box after the consent was obtained" |
| Incomplete outcome data (attrition bias) | High risk | It was unclear how many pairs of twins were analyzed for each outcome, and loss of data was not mentioned. Further correspondence with the lead study author revealed that complete data for the whole 8 periods of observation were available for only 2 sets of twins. Another 2 sets of twins were observed for 7 out of 8 periods; 1 set was observed for 4 periods, and another for 2 periods. Reasons for inability to obtain complete outcome data were stated: "Due to early discharge or unplanned transfer to home hospital, not all twin sets had eight five‐hour blocks of analysis" In their final estimates for the outcomes of "time in quiet sleep," "time crying," and "co‐regulated," study authors did not adjust for differential contributions of each twin pair to overall data throughout the study period |
| Selective reporting (reporting bias) | High risk | All outcomes that study authors set out to measure were reported, although the main outcome ‐ parental self efficacy ‐ was not measured. Study authors cited the reason as "Data were insufficient to analyse parental self‐efficacy and parental stress" (Results, paragraph 1) Table 1 provides a matrix on outcomes listed in the methods, any additional outcomes that review authors considered as key outcomes for the study, and actual outcomes reported |
| Other bias | High risk | Further correspondence with the lead study author revealed the ways that major outcomes were reported and their associated risks of bias For the outcomes of "time in quiet sleep," "time crying," and "co‐regulated," the unit of measurement was the number of times infants were observed to be in quiet sleep, crying, or co‐regulated during each period of observation that lasted for 5 hours. A total of 8 five‐hour blocks of observational periods were allocated over the study duration of 2 weeks. For each outcome, the number of episodes observed for each infant throughout the 2‐week study duration was analyzed. Study authors reported mean numbers of episodes (with standard deviations and standard errors) per infant per 5‐hour block of observation for co‐bedded group vs control group From the way the data were analyzed and reported, as detailed above, the study appeared to adopt a repeated‐measures design. Study authors reported overall mean numbers of episodes of each outcome for intervention and control groups; this was an acceptable way of reporting (Higgins 2011). However, 2 concerns arose: Study authors reported the data as per each infant instead of per each twin pair, giving rise to unit of analysis issues; and in the final estimates, no differential weight was given to data collected from each twin pair, as not all twin pairs were observed throughout the 2‐week period (see Incomplete outcome data (attrition bias)) |
| Blinding of participants and personnel (performance bias) | High risk | Although not stated in the article, blinding of caregivers and parents appeared highly unlikely |
| Blinding of outcome assessment (detection bias) | High risk | Although not stated in the article, further correspondence with the lead author confirmed that the research assistant who collected the data was not blinded to the status of the twin pairs |
| Methods | Randomized controlled trial at a tertiary‐level NICU (USA) | |
| Participants | 62 co‐bedded infants (23 pairs of twins, 4 set of triplets, and 1 set of quadruplets) were compared with 59 separately bedded infants (15 pairs of twins, 7 sets of triplets, and 2 sets of quadruplets). All infants were less than 37 weeks' PMA. Birth weight was supposed to be less than 1500 grams, but in the study sample description (Table 4), the birth weight range went up to 2290 g. All infants were non‐ventilated and were not receiving any other forms of intensive care; all had no congenital malformations nor severe neurosensory defects | |
| Interventions | Co‐bedding (cared for in the same incubator) vs separate care (cared for in separate incubators) | |
| Outcomes | Average weekly gains in weight, head circumference, and length; feeding advancement, medication errors, infections, thermal insults | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors stated, "A randomized design was used, with experimental (co‐bedded) and control (traditional) conditions," but no other statement was provided on how randomization was achieved. Study authors also stated that "a convenient, consecutive sampling strategy was used to screen all twins and HOM (higher order multiples) according to the inclusion and exclusion criteria" |
| Allocation concealment (selection bias) | Unclear risk | No statement indicated how allocation was carried out |
| Incomplete outcome data (attrition bias) | High risk | For the main outcome of growth, no mention was made of how many pairs of twins were actually analyzed and reasons for loss of data, if any. For outcomes of medication errors, nosocomial infections, sepsis workups initiated, and thermal insults, the number of infants analyzed was reported, and no data were lost |
| Selective reporting (reporting bias) | High risk | Although all outcomes specified in the methods were reported in the results with appropriate units of measurement, standard deviations for outcomes of weekly growth in weight, head circumference, and length, as well as total numbers of participants (sets of twins and higher‐order multiples) for these outcomes were not reported Table 1 provides a matrix on outcomes listed in the methods, additional key outcomes, and actual outcomes reported |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) | High risk | Although not stated in the article, blinding of caregivers and parents appeared highly unlikely |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not stated whether the research assistant who collected the data was blinded to the allocation of twins |
NCASAR: Nursing Child Assessment Sleep/Activity Record
NICU: neonatal intensive care unit
NIDCAP: Newborn Individualized Developmental Care and Assessment Program
PDA: patent ductus arteriosus
PIPP: Premature Infant Pain Profile
PMA: postmenstrual age
RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Commentary on another study, Stainton 2005. Excluded on the basis of article type | |
| Commentary that described development of a guideline on co‐bedding of twins in a NICU with no original study. Excluded on the basis of article type | |
| Study of co‐sleeping between mother and baby, not co‐bedding for twins. Excluded on the basis of the research question | |
| Cross‐sectional study on parents of twins 18 years of age and younger on the sleeping pattern and developmental milestones of the twins, as well as parents’ knowledge and satisfaction with co‐bedding. Excluded on the basis of study design | |
| Retrospective descriptive study examining the incidence of infection in preterm twins over 2 periods: 1997 to 2001, when co‐bedding was introduced as standard practice for preterm twins in the unit; and 1992 to 1996, before co‐bedding was introduced. Excluded on the basis of study design | |
| Prospective cohort study comparing co‐bedded multiples (twins and triplets) (31 infants in total) with historical controls comprising 31 infants of multiple gestations who were matched for PMA and birth weight. Major outcomes assessed were number of episodes of body temperature depression, daily average weight gain, and other physiological parameters. This study was published as an abstract. Excluded on the basis of study design | |
| This record has no abstract, so no information on methods, participants, intervention, and outcomes. It appears very similar to one of the included studies (Lutes 2001), although we could not confirm this, as we have received no reply from study authors to multiple emails sent. Non‐response of study authors led to exclusion of this article on the basis of lack of basic information | |
| Prospective case‐control study on sudden infant death syndrome (SIDS), not on co‐bedding for preterm twins. Excluded on the basis of the research question | |
| Review article describing history and current evidence on benefits and risks of co‐bedding preterm twins. Excluded on the basis of article type | |
| Single‐group study in which 7 pairs of preterm twins were co‐bedded. Data were collected on parental perception of infant behavior and on care before and after the twins were co‐bedded. Excluded on the basis of study design | |
| Prospective study examining breast‐feeding behavior and intake among preterm twins and factors in caregiving practices that might influence them, including co‐bedding of twins. This was not a study involving co‐bedding as an intervention. Excluded on the basis of the research question | |
| Retrospective cohort study comparing co‐bedded and separately bedded twins (n =.71 pairs) and triplets (n.=.3 sets), with discharge weight, number of days on a ventilator, other clinical complications, and proportions co‐bedded after discharge as major outcomes. Excluded on the basis of study design | |
| Prospective cross‐over trial in which 2 groups of twins ‐ 1 group co‐bedded for 24 hours or longer, and the other group with no prior co‐bedding experience ‐ were observed via video recording for 5 consecutive 30‐minute periods, during which each group was co‐bedded and separated for 30 minutes on an alternate basis. Excluded on the basis of the intervention, as we considered that alternation between co‐bedded and separated states every 30 minutes would introduce an element of acute change in the infant environment, in addition to effects of co‐bedding, which in turn would affect infants' neurobehavioral state. Additionally, it was highly likely that estimates at each state would have been "contaminated" by effects of the intervention during the preceding 30‐minute period | |
| Single‐group study in which all 11 pairs of twins were co‐bedded. Data collected included apnea, periodic breathing, bradycardia, and other physiological parameters. Excluded on the basis of study design | |
| Commentary on maternal‐infant co‐sleeping and breast‐feeding in Norwegian, not an original study on co‐bedding of preterm twins. Excluded on the basis of article type |
NICU: neonatal intensive care unit
PMA: postmenstrual age
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Co‐bedding in Daily Weight Gain of Neonate Twins |
| Methods | RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Co‐bedding |
| Outcomes | Primary outcome: daily weight gain of co‐bedded preterm twins vs that of single‐bedded preterm twins |
| Starting date | September 2008 |
| Contact information | Nantes University Hospital (investigator details not provided) |
| Notes |
NICU: neonatal intensive care unit
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of weight gain (in gram/kg of baseline weight/d) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Co‐bedding vs standard care, Outcome 1 Rate of weight gain (in gram/kg of baseline weight/d). | ||||
| 1.1 From study entry to week 1 | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐0.96, 8.96] |
| 1.2 From week 1 to week 2 | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐2.27, 5.07] |
| 1.3 From week 2 to week 3 | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐4.33, 0.13] |
| 1.4 Average from study entry to week 3 | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.60, 2.00] |
| 2 Apnea, bradycardia, or desaturation Show forest plot | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.18, 4.05] |
| Analysis 1.2  Comparison 1 Co‐bedding vs standard care, Outcome 2 Apnea, bradycardia, or desaturation. | ||||
| 3 Episodes in co‐regulated state (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [‐3.44, 5.36] |
| Analysis 1.3  Comparison 1 Co‐bedding vs standard care, Outcome 3 Episodes in co‐regulated state (out of 20 observations). | ||||
| 4 Episodes of crying (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 4.43 [1.72, 7.14] |
| Analysis 1.4  Comparison 1 Co‐bedding vs standard care, Outcome 4 Episodes of crying (out of 20 observations). | ||||
| 5 Episodes in quiet sleep (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 4.58 [1.58, 7.58] |
| Analysis 1.5  Comparison 1 Co‐bedding vs standard care, Outcome 5 Episodes in quiet sleep (out of 20 observations). | ||||
| 6 Neurobehavior: infant pain score following painful procedure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Co‐bedding vs standard care, Outcome 6 Neurobehavior: infant pain score following painful procedure. | ||||
| 6.1 At 30 seconds post heel lance | 2 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.68, ‐0.23] |
| 6.2 At 90 seconds post heel lance | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.14, 1.86] |
| 7 Infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Co‐bedding vs standard care, Outcome 7 Infection. | ||||
| 7.1 Suspected or proven infection (any) | 3 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.30, 2.31] |
| 7.2 Necrotizing enterocolitis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.19, 19.40] |
| 7.3 Conjunctivitis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.15, 6.13] |
| 7.4 Sepsis | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.07, 2.86] |
| 8 Length of hospital stay (days) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐35.23, 25.43] |
| Analysis 1.8  Comparison 1 Co‐bedding vs standard care, Outcome 8 Length of hospital stay (days). | ||||
| 9 Parental anxiety (Parental State Anxiety Inventory) Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.13, 3.93] |
| Analysis 1.9  Comparison 1 Co‐bedding vs standard care, Outcome 9 Parental anxiety (Parental State Anxiety Inventory). | ||||
| 10 Parental attachment (Maternal Attachment Inventory) Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.02, 3.82] |
| Analysis 1.10  Comparison 1 Co‐bedding vs standard care, Outcome 10 Parental attachment (Maternal Attachment Inventory). | ||||
| 11 Parental satisfaction Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐4.49, 3.73] |
| Analysis 1.11  Comparison 1 Co‐bedding vs standard care, Outcome 11 Parental satisfaction. | ||||
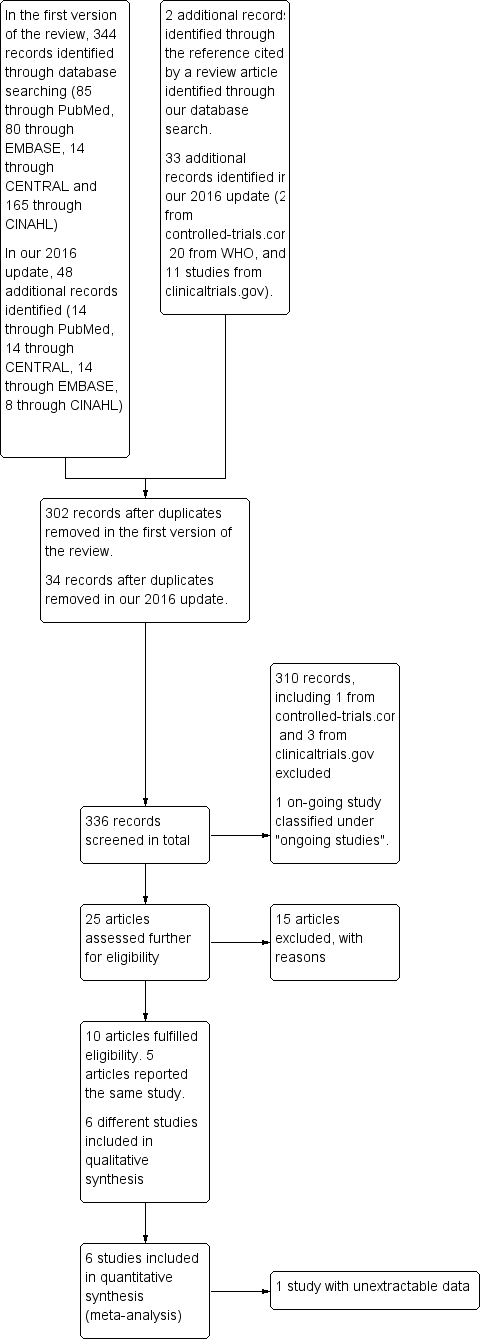
Flow diagram of the review process from initial search to final inclusion of studies.
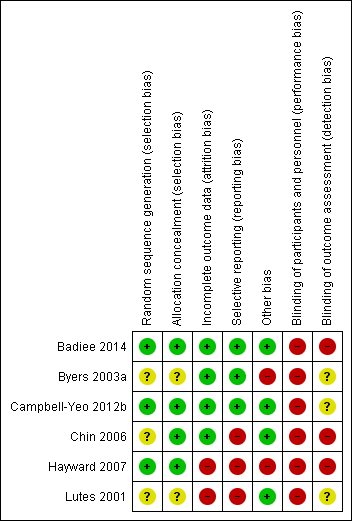
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 Co‐bedding vs separate care, outcome: 1.1 Rate of weight gain (in gram/kg of baseline weight/d).

Forest plot of comparison: 1 Co‐bedding vs separate care, outcome: 1.6 Neurobehavior: infant pain score following painful procedure.
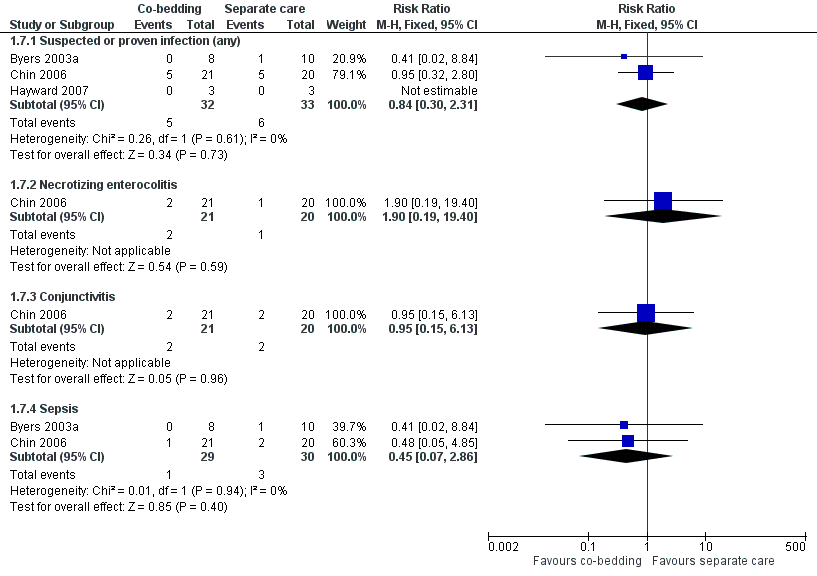
Forest plot of comparison: 1 Co‐bedding vs separate care, outcome: 1.7 Infections.

Comparison 1 Co‐bedding vs standard care, Outcome 1 Rate of weight gain (in gram/kg of baseline weight/d).
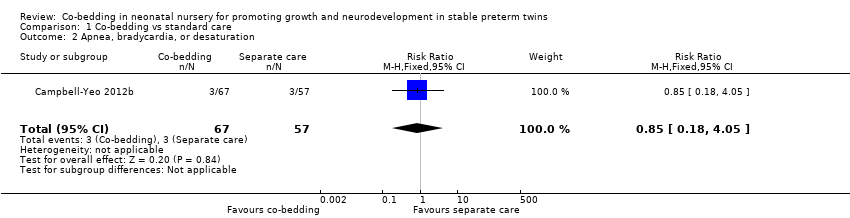
Comparison 1 Co‐bedding vs standard care, Outcome 2 Apnea, bradycardia, or desaturation.
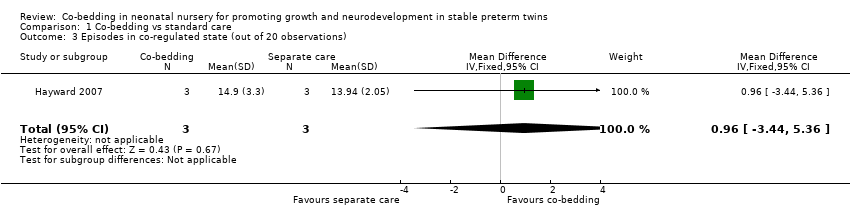
Comparison 1 Co‐bedding vs standard care, Outcome 3 Episodes in co‐regulated state (out of 20 observations).

Comparison 1 Co‐bedding vs standard care, Outcome 4 Episodes of crying (out of 20 observations).
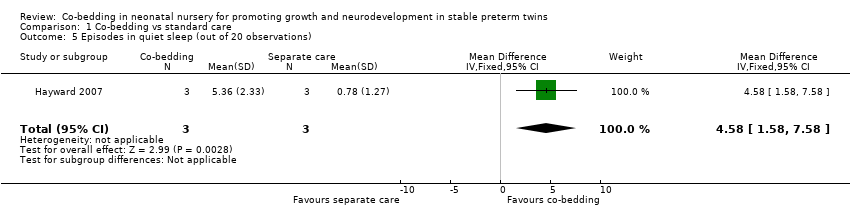
Comparison 1 Co‐bedding vs standard care, Outcome 5 Episodes in quiet sleep (out of 20 observations).
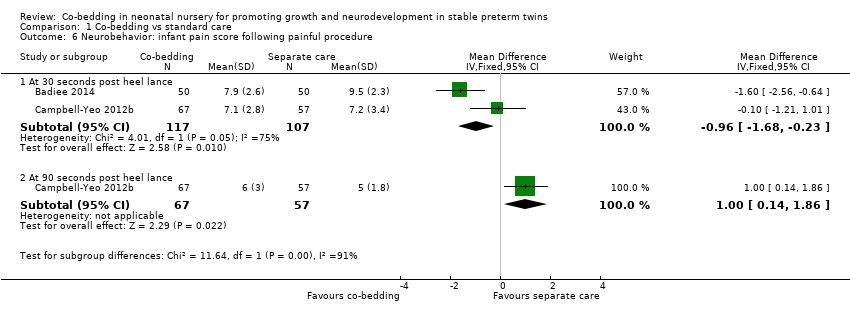
Comparison 1 Co‐bedding vs standard care, Outcome 6 Neurobehavior: infant pain score following painful procedure.

Comparison 1 Co‐bedding vs standard care, Outcome 7 Infection.
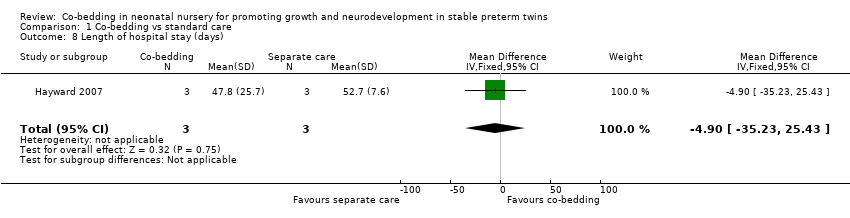
Comparison 1 Co‐bedding vs standard care, Outcome 8 Length of hospital stay (days).

Comparison 1 Co‐bedding vs standard care, Outcome 9 Parental anxiety (Parental State Anxiety Inventory).
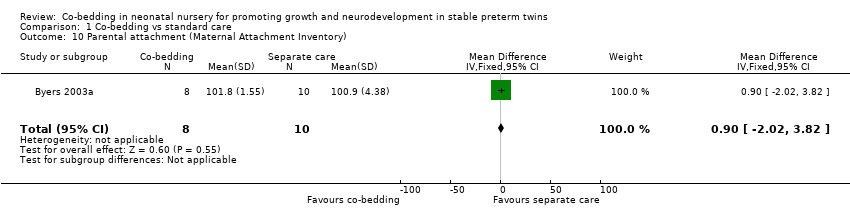
Comparison 1 Co‐bedding vs standard care, Outcome 10 Parental attachment (Maternal Attachment Inventory).

Comparison 1 Co‐bedding vs standard care, Outcome 11 Parental satisfaction.
| Co‐bedding versus separate care for promoting growth and neurodevelopment in stable preterm twins | ||||||
| Patient or population: stable preterm twins with growth and neurodevelopment promoted | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Separate care | Co‐bedding | |||||
| Rate of weight gain (in grams/kg of baseline weight/d) ‐ average from study entry to week 3 | Mean rate of weight gain (in grams/kg of baseline weight/d) ‐ average from study entry to week 3 in control groups was | Mean rate of weight gain (in grams/kg of baseline weight/d) ‐ average from study entry to week 3 in intervention groups was | 18 | ⊕⊕⊝⊝ | ||
| Apnea, bradycardia, or desaturation | Study population | RR 0.85 | 124 | ⊕⊕⊝⊝ | ||
| 53 per 1000 | 45 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 45 per 1000 | |||||
| Episodes in co‐regulated state (out of 20 observations) Scale from 0 to 20 | Mean number of episodes in co‐regulated state (out of 20 observations) in control groups was | Mean number of episodes in co‐regulated state (out of 20 observations) in intervention groups was | 6 | ⊕⊝⊝⊝ | ||
| Infections ‐ suspected or proven infections (any) | Study population | RR 0.84 | 65 | ⊕⊝⊝⊝ | ||
| 182 per 1000 | 153 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 84 per 1000 | |||||
| Length of hospital stay (days) | Mean length of hospital stay (days) in control groups was | Mean length of hospital stay (days) in intervention groups was | 6 | ⊕⊝⊝⊝ | ||
| Parental satisfaction Scale from 0 to 55 | Mean parental satisfaction in control groups was | Mean parental satisfaction in intervention groups was | 18 | ⊕⊕⊕⊝ | ||
| Neurobehavior: infant pain score following painful procedure ‐ at 30 seconds post heel lance Scale from 0 to 21 | Mean neurobehavior: infant pain score after painful procedure ‐ at 30 seconds post heel lance in control groups was | Mean neurobehavior: infant pain score after painful procedure ‐ at 30 seconds post heel lance in intervention groups was | 224 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded 1 level for study limitations (owing to unclear method of sequence generation, non‐blinding, and presence of selective outcome reporting) fDowngraded 1 level for study limitations (owing to non‐blinding of care personnel and outcome assessors) | ||||||
| Study ID | Outcomes listed in the methods | Additional key outcomes relevant to the study | Actual outcomes reported |
|
| This study assessed specifically physiological measures in the short term. Growth parameters were reported, but as "mean daily weight," which was not suitable for meta‐analysis. Additionally, physiological measures were reported in the form of average figures, such as average "highest activity heart rate," not as episodes of apnea, bradycardia, or desaturation, which are more clinically relevant |
| |
|
| This study assessed specifically infants' response to pain; growth‐related outcomes such as weight gain and length of hospital stay were not included in the outcomes. The only outcome that was relevant to this review was the pain response, which we considered as a form of neurobehavior |
| |
|
|
|
| |
|
|
|
(Study authors stated, "Data were insufficient to analyse parental self‐efficacy and parental stress" ‐ Results, paragraph 1, lines 1 to 3) | |
| No outcome was listed in the methods. However, the following 3 major outcomes were stated in the purpose and hypothesis section
|
|
| |
|
| Like Campbell‐Yeo 2012b, this study assessed specifically infant response to pain; growth‐related outcomes were not assessed | The only outcome included in this review is PIPP score |
| Study ID | Study period | Median combined A/B/D episodes | P value | |
| Co‐bedded group | Control group | |||
| Week 1 | 4.5 | 7 | 0.2 | |
| Week 2 | 6 | 12 | 0.8 | |
| Week 3 | 2.5 | 8 | 0.4 | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rate of weight gain (in gram/kg of baseline weight/d) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 From study entry to week 1 | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐0.96, 8.96] |
| 1.2 From week 1 to week 2 | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐2.27, 5.07] |
| 1.3 From week 2 to week 3 | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐4.33, 0.13] |
| 1.4 Average from study entry to week 3 | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.60, 2.00] |
| 2 Apnea, bradycardia, or desaturation Show forest plot | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.18, 4.05] |
| 3 Episodes in co‐regulated state (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.96 [‐3.44, 5.36] |
| 4 Episodes of crying (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 4.43 [1.72, 7.14] |
| 5 Episodes in quiet sleep (out of 20 observations) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 4.58 [1.58, 7.58] |
| 6 Neurobehavior: infant pain score following painful procedure Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 30 seconds post heel lance | 2 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.68, ‐0.23] |
| 6.2 At 90 seconds post heel lance | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.14, 1.86] |
| 7 Infection Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Suspected or proven infection (any) | 3 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.30, 2.31] |
| 7.2 Necrotizing enterocolitis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.19, 19.40] |
| 7.3 Conjunctivitis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.15, 6.13] |
| 7.4 Sepsis | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.07, 2.86] |
| 8 Length of hospital stay (days) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐35.23, 25.43] |
| 9 Parental anxiety (Parental State Anxiety Inventory) Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.13, 3.93] |
| 10 Parental attachment (Maternal Attachment Inventory) Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐2.02, 3.82] |
| 11 Parental satisfaction Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐4.49, 3.73] |

