Fenitoína tópica para el tratamiento de las úlceras por presión
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008251.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 febrero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Xiang Yong Hao: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; checked the quality of the statistical analysis; produced the first draft of the review; approved the review prior to submission; and is a guarantor of the review.
Hong Ling Li: designed and coordinated the review; checked the quality of data extraction; analysed or interpreted data; checked quality assessment; checked the quality of the statistical analysis; produced the first draft of the review; approved the review prior to submission; and is a guarantor of the review.
He Su: designed the review; checked the quality of data extraction; analysed or interpreted data; produced the first draft of the review; approved the review prior to submission; and is a guarantor of the review.
Hui Cai: extracted data; undertook quality assessment; analysed or interpreted data; performed statistical analysis; contributed to writing or editing the review; and advised on the review.
Tian Kang Guo: conceived, designed and coordinated the review; analysed or interpreted data; contributed to writing or editing the review; approved the final review prior to submission; and advised on the review
Ruifeng Liu: analysed or interpreted data; checked quality assessment; performed statistical analysis; contributed to writing or editing the review; and advised on the review.
Lei Jiang: analysed or interpreted data; performed statistical analysis; and contributed to writing or editing the review.
Yan Fei Shen: extracted data; undertook quality assessment; checked the quality of the statistical analysis; contributed to writing or editing the review; and advised on the review.
Contributions of editorial base
Nicky Cullum (Coordinating Editor): edited the protocol and advised on methodology, interpretation and protocol content. Approved the final protocol prior to submission.
Jo Dumville (Deputy Co‐ordinating Editor): edited the review and advised on methodology, interpretation and review content. Approved the final review prior to submission.
Sally Bell‐Syer (Managing Editor): co‐ordinated the editorial process for the protocol and advised on methodology, interpretation and content.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process for the review and advised on content.
Ruth Foxlee (Information Specialist): designed the search strategy and edited the search methods section for the protocol.
Reetu Child and Naomi Shaw (Information Specialists): designed the search strategy and edited the search methods section for the review.
Zipporah Iheozor‐Ejiofor (Methodologist): advised on methodology, interpretation and review content.
Ursula Gonthier (Editorial Assistant) edited the references and Plain Language Summary.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
The National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Xiang Yong Hao: none known.
Hong Ling Li: none known.
He Su: none known.
Hui Cai: none known.
Tian Kang Guo: none known.
Ruifeng Liu: none known.
Lei Jiang: none known.
Yan Fei Shen: none known.
Acknowledgements
We would like to thank all the participants and clinical researchers who were involved in the publications we mentioned in this review. We acknowledge the contribution of Yun Tao Ma in advising on part of the review. Thanks also to Cochrane Wounds for the support that they have provided and peer referees Mieke Flour, Liz McInnes, Gill Worthy, Suzanne Hempel, Allen Holloway, Zena Moore, Jane Burch, Ajima Olaghere and Ruth Ropper. Thanks to Elizabeth Royle for copy editing the protocol and the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 22 | Topical phenytoin for treating pressure ulcers | Review | Xiang Yong Hao, Hong Ling Li, He Su, Hui Cai, Tian Kang Guo, Ruifeng Liu, Lei Jiang, Yan Fei Shen | |
| 2010 Jan 20 | Topical phenytoin for treating pressure ulcers | Protocol | Xiang Yong Hao, Tian Kang Guo, Yi Ping Li, Hong Ling Li, Yuan Hui Gu, Hui Cai, Lei Jiang, Ruifeng Liu | |
Differences between protocol and review
-
We added the fact that we searched the following clinical trials registries for ongoing and unpublished studies:
-
Clinical Trials.gov (clinicaltrials.gov),
-
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en),
-
Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn/searchprojen.aspx).
-
-
We added a study flow diagram as recommended by the PRISMA statement to illustrate the process of screening and selecting studies for inclusion in the review (Figure 1) (Liberati 2009).
-
We added a ‘Dealing with missing data' section to the review.
-
We added a ‘Unit of analysis issues’ section in to the review.
-
For assessment of reporting biases, we updated it as follows “In future, if 10 or more studies are included for meta‐analysis, visual asymmetry of funnel plots will be assessed to identify any potential reporting or publication bias (Sterne 2011).”
-
We added 'Summary of findings' tables with GRADE ratings.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
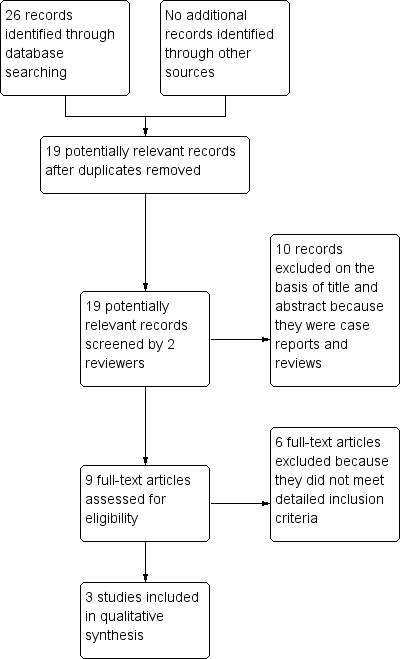
Study flow diagram.
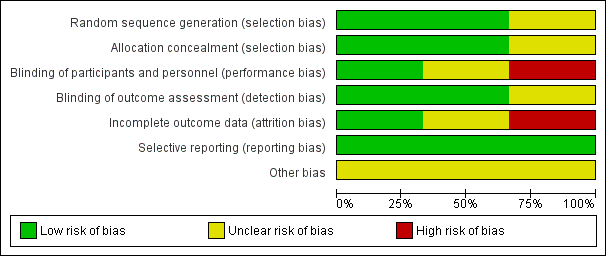
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
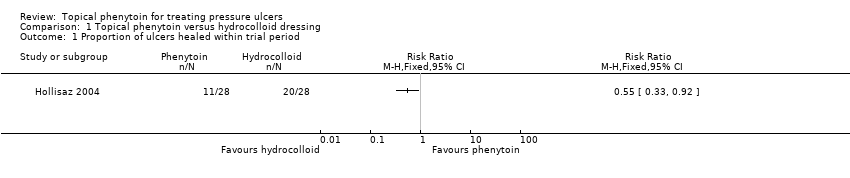
Comparison 1 Topical phenytoin versus hydrocolloid dressing, Outcome 1 Proportion of ulcers healed within trial period.

Comparison 2 Topical phenytoin versus simple dressing, Outcome 1 Proportion of ulcers healed within trial period.
| Topical phenytoin compared with hydrocolloid dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes Intervention: topical phenytoin Comparison: hydrocolloid dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with hydrocolloid dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | 714 per 1000 | 393 per 1000 (236 to 657) | RR 0.55 (0.33 to 0.92) | 56 (1 study) | ⊕⊕⊝⊝ | |
| Adverse events | See comments | See comments | Not estimable | 84 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded two levels: serious limitation (risk of bias), serious imprecision (small number of events ) | ||||||
| Topical phenytoin compared with triple antibiotic ointment for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: nursing homes Intervention: topical phenytoin Comparison: triple antibiotic ointment | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with triple antibiotic ointment | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | See comments | See comments | This outcome was not reported for this comparison | |||
| Adverse events | See comments | See comments | Not estimable | 28 (1 study) | No adverse drug reactions or interactions were detected in the included RCT. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical phenytoin compared with a simple dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes, and a hospital Intervention: topical phenytoin Comparison: a simple dressing | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with a simple dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | This outcome was not reported for this comparison | |||
| Proportion of ulcers healed within trial period (eight weeks) | 296 per 1000 | 394 per 1000 (187 to 824) | RR 1.33 (0.63 to 2.78) | 55 (1 study) | ⊕⊝⊝⊝ very low1 | |
| Adverse events | See comments | See comments | Not estimable | 81 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | This outcome was not reported for this comparison. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded three levels: once for serious limitation (risk of bias), and twice for very serious imprecision (small number of events and a wide confidence interval which includes the possibility of both increased healing and reduced healing) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

