Amitriptilina para el dolor neuropático en adultos
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
-
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010c), and arthritis (Moore 2010b), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
-
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010b); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
-
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010b; Moore 2013b; Moore 2014b; Straube 2010; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
-
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Hoffman 2010; Moore 2010d; Moore 2014a).
-
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012bMoore 2012b).
Appendix 2. CENTRAL search strategy (via CRSO)
-
MESH DESCRIPTOR amitriptyline EXPLODE ALL TREES (1002)
-
(am?tr?pt?lin* or amitriptyliini):TI,AB,KY (2074)
-
1 OR 2 (2074)
-
MESH DESCRIPTOR Pain explode all trees (30033)
-
MESH DESCRIPTOR Peripheral Nervous System Diseases explode all trees (2565)
-
MESH DESCRIPTOR Somatosensory Disorders explode all trees (703)
-
MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES (605)
-
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):TI,AB,KY (9635)
-
((neur* or nerv*) and (compress* or damag*)):TI,AB,KY (1930)
-
4 OR 5 OR 6 OR 7 OR 8 OR 9 (38890)
-
3 AND 10 (207)
-
2012 TO 2015:YR (115373)
-
11 AND 12 (32)
Appendix 3. MEDLINE (via Ovid) search strategy
-
Amitriptyline/ (6028)
-
(am?tr?pt?lin* or amitriptyliini).mp. (8111)
-
1 or 2 (8111)
-
exp PAIN/ (314208)
-
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (118087)
-
exp SOMATOSENSORY DISORDERS/ (16640)
-
exp NEURALGIA/ (13991)
-
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (39812)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (49057)
-
4 or 5 or 6 or 7 or 8 or 9 (461007)
-
randomized controlled trial.pt. (386549)
-
controlled clinical trial.pt. (88799)
-
randomized.ab. (284481)
-
placebo.ab. (149366)
-
drug therapy.fs. (1745898)
-
randomly.ab. (201462)
-
trial.ab. (293536)
-
groups.ab. (1288153)
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (3290048)
-
3 and 10 and 19 (739)
-
limit 20 to yr="2012 ‐Current" (100)
Appendix 4. EMBASE (via Ovid) search strategy
-
Amitriptyline/ (34109)
-
(am?tr?pt?lin* or amitriptyliini).mp. (34901)
-
1 or 2 (34901)
-
exp PAIN/ (876555)
-
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (52348)
-
exp SOMATOSENSORY DISORDERS/ (67274)
-
exp NEURALGIA/ (76377)
-
(pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (84841)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (71386)
-
4 or 5 or 6 or 7 or 8 or 9 (1012171)
-
crossover‐procedure/ (41667)
-
double‐blind procedure/ (120544)
-
randomized controlled trial/ randomized controlled trial/ (363694)
-
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1288420)
-
11 or 12 or 13 or 14 (1367733)
-
3 and 10 and 15 (1576)
-
limit 16 to yr="2012 ‐Current" (261)
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study | Treatment (taken at night, unless stated) | Pain outcome | Other efficacy outcome |
| Amitriptyline 75 mg/d = 87 Pregabalin 600 mg/d = 86 Placebo = 81 Treatment taken in divided doses, 3 times daily Titration over first 2 weeks | Participants with ≥ 50% reduction of pain from baseline Amitriptyline = 40/87 Pregabalin = 34/86 Placebo = 24/81 | ||
| Amitriptyline 25 to 125 mg/d = 117 Placebos contained mimicking agents | Both treatments produced substantial pain relief ‐ statistically significant from baseline, but no difference between groups Only physician global reported | Both treatments improved interference with daily activities due to pain, with no difference between groups | |
| Amitriptyline 25 mg twice daily, to 25 mg am and 50 mg night = 28 | No difference between groups in mean pain intensity | ||
| Amitriptyline 10 to 125 mg/d = 44 Titration | Mean data only | ||
| Amitriptyline 12.5 to 200 mg/d = 12 Cross‐over | Significant decrease in mean pain (using VAS) for amitriptyline and amitriptyline + fluphenazine, but not fluphenazine alone or placebo | ||
| Amitriptyline 10 to 50 mg/d = 53 Cross‐over | PGIC 50% improvement (efficacy and safety, 100 mm VAS) | No significant difference between groups using median Likert pain and McGill pain Improvements seen from 2nd week onwards | |
| Amitriptyline 10 to 50 mg/d = 20 | In patients who remained in study ≥ 4 weeks Patient global assessment at 14 weeks (5‐point scale) | Patient global using numeric scale showed NSD trend for amitriptyline better than placebo | |
| Amitriptyline 25 to 75 mg/d = 15 Cross‐over | Patient global assessment of PR at end of period (5‐point scale) | Mean PI reduced compared with placebo from 2nd week for amitriptyline, only at 3rd for carbamazepine | |
| Amitriptyline 12.5 to 150 mg/d = 34 | From graph Lorazepam = 2/40 | At baseline 43 patiernts not depressed, 15 depressed (mostly mild). NSD between depressed and non‐depressed for pain relief | |
| Study 1 Cross‐over. Patients could enter other study after completion of first: 38 completed amitriptyline versus desipramine, and 46 completed fluoxetine versus placebo | Global rating of pain relief (6‐point scale) at end of treatment period for completers 'complete' or 'a lot: Amitriptyline = 18/38 Desipramine = 15/38 Fluoxetine = 15/46 Placebo = 10/46 | NSD between amitriptyline and desipramine for mean weekly pain scores | |
| Amitriptyline 50 to 100 mg/d = 30 | Mean pain intensity decreased in all groups over duration of study | Apparent morphine‐sparing effect and improvement in functional capacity. Morphine‐sparing and functional capacity were significantly better with pregabalin than the other treatments. | |
| Amitriptyline 25 to 150 mg/d = 28 (as 3 doses daily) Cross‐over | ≥ 30% PR | Change in average pain from baseline to week 8: NSD between treatments for patients with low depression scores (n = 2 5) Amitriptyline significantly greater than placebo, and NS greater than gabapentin for patients with high depression scores (n = 13) | |
| Amitriptyline 25 to 150 mg/d = 17 | PR at end of treatment (6 weeks) of 'moderate' or better (= ≥ 50% PR) | NSD between treatments for %age change in daily diary VAS from baseline to start of taper NSD between groups for mean final pain category 2.1 to 3.2 (scale 0 to 5) | |
| Amitriptyline 25 to 75 mg/d = 71 (Also included acupuncture treatment arms) | Complete or a lot of relief | Mean changes in PI at weeks 6 and 14, NSD between groups ‐ both improved | |
| Amitriptyline 25 to 75 mg/d = 36 Cross‐over | Patient global at end of each treatment period (5‐point scale) | Responder' = PR 20% from baseline | |
| Amitriptyline = 35 Cross‐over | PI at final or 5th week (none, mild, moderate, no changes) None or mild: Amitriptyline = 15/35 Maprotiline = 12/35 'Effectiveness' (excellent, good, improved but unsatisfactory, no change) Excellent or good: Amitriptyline = 14/35 Maprotiline = 6/35 | NSD between groups for patient estimate of %age improvement in pain Equal sedative scores for groups | |
| Amitriptyline = 33 Cross‐over | Satisfaction with pain relief and tolerable of side effects | NSD between groups for pain VAS NSD between groups for pt estimate of %age improvement in pain |
AE: adverse effect; d: day; NS: non‐significant; NSD: non‐significant difference; PGIC: Patient Global Impression of Change; PI: pain intensity; QoL: quality of life; VAS: visual analogue scale
Appendix 6. Summary of outcomes in individual studies: adverse events and withdrawals
| Study | Treatment (taken at night, unless stated) | Adverse events | Withdrawals |
| Amitriptyline 75 mg/d = 87 Pregabalin 600 mg/d = 86 Placebo = 81 Treatment taken in divided doses, 3 times daily Titration over first 2 weeks | Patients with ≥ 1 AE: Amitriptyline = 59/87 Pregabalin = 57/86 Placebo = 38/81 Amitriptyline = 5/87 Pregabalin = 5/86 (1 death, unrelated) Placebo = 2/81 | All‐cause: Amitriptyline = 23/87, Pregabalin = 24/86, Placebo = 19/81 Pregabalin = 11/86, Placebo = 5/81 LoE: Pregabalin = 7/86, Placebo = 9/81 | |
| Amitriptyline 25 to 125 mg/d = 117 Placebos contained mimicking agents | Amitriptyline ‐ GI, anticholinergic, CNS/neuromuscular, cardiovascular, sedative, skin, other | Not reported | |
| Amitriptyline 25 mg twice daily, to 25 mg am and 50 mg night = 28 | Pregabalin had highest rate of AEs SAE: 6 (1 death, 5 non‐fatal) | AE: | |
| Amitriptyline 10 to 125 mg/d = 44 Titration | Patients with ≥1 AE: (details for individual events available) | All‐cause: Placebo = 3/40 | |
| Amitriptyline 12.5 to 200 mg/d = 12 Cross‐over | 1 patient in amitriptyline due to AE (excessive sedation) | Amitriptyline worst for dry mouth | |
| Amitriptyline 10 to 50 mg/d = 53 Cross‐over | Total number of events: Lamotrigine = 11 (mainly skin, creatinine) | Lost to follow‐up: Lamotrigine = 0/46 Lamotrigine = 8/46 (rash (3), itching (1), increased creatinine (4)) LoE (titration stopped because no benefit with 2 doses): Amitriptyline = 16/53, Lamotrigine = 22/46 | |
| Amitriptyline 10 to 50 mg/d = 20 | Requiring dose reduction ‐ in patients who remained in trial ≥ 4 weeks: | Exclusion/withdrawal within first 4 weeks: | |
| Amitriptyline 25 to 75 mg/d = 15 Cross‐over | Patients with ≥ 1 AE Most common | 1 participant with carbamazepine had treatment stopped at day 25 due to interaction with warfarin | |
| Amitriptyline 12.5 to 150 mg/d = 34 | Patients with ≥ 1 AE: | AE: LoE: 3 (group not given) | |
| Study 1 Cross‐over. Patients could enter other study after completion of first: 38 completed amitriptyline versus desipramine, and 46 completed fluoxetine versus placebo | In patients taking both drugs Patients with ≥ 1 AE: | AE: | |
| Amitriptyline 50 to 100 mg/d = 30 | Most common were somnolence, | No data | |
| Amitriptyline 25 to 150 mg/d = 28 (as 3 doses daily) Cross‐over | Most commonly reported: | AE: Gabapentin = 5/38, Placebo = 2/38 Medical problem: Amitriptyline = 2/38, Gabapentin = 1/38, Placebo = 1/38 Other: Amitriptyline = 1/38, Gabapentin = 0/38, Placebo = 3/38 | |
| Amitriptyline 25 to 150 mg/d = 17 Desipramine = 93 mg/d, Fluoxetine = 44 mg/d | No usable data | All‐cause Desipramine = 2/15, Fluoxetine = 5/15 (4 were on opioids) | |
| Amitriptyline 25 to 75 mg/d = 71 (Also included acupuncture treatment arms) | Grade 4 AE (serious) | By 14 weeks 35% of patients in either group had discontinued treatment | |
| Amitriptyline 25 to 75 mg/d = 36 Cross‐over | Patients with ≥ 1 AE: | 2 patients did not provide any data for any treatment | |
| Amitriptyline = 35 Cross‐over | Patients with ≥ 1 AE | Excl (added back for efficacy): AE: | |
| Amitriptyline = 33 Cross‐over | Patients with ≥ 1 AE | Patients "left the study" Patients with "intolerable AE ‐ treatment stopped" |
AE: adverse effect; CNS: central nervous system; GI: gastrointestinal; LoE: lack of efficacy; SAE: serious adverse effect

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.
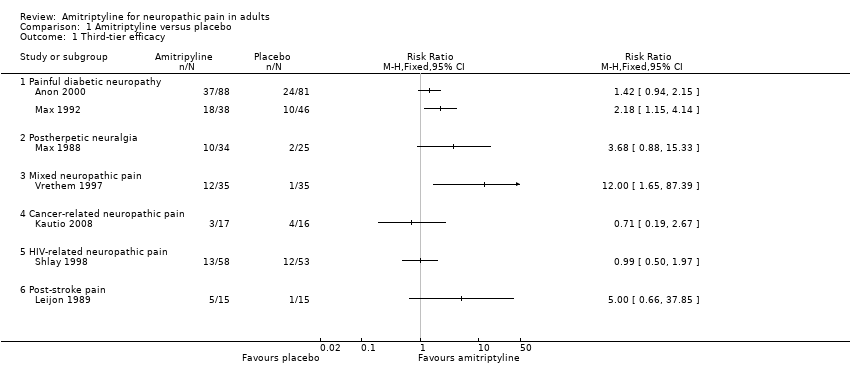
Comparison 1 Amitriptyline versus placebo, Outcome 1 Third‐tier efficacy.

Comparison 1 Amitriptyline versus placebo, Outcome 2 At least 1 adverse event.

Comparison 1 Amitriptyline versus placebo, Outcome 3 All‐cause withdrawal.
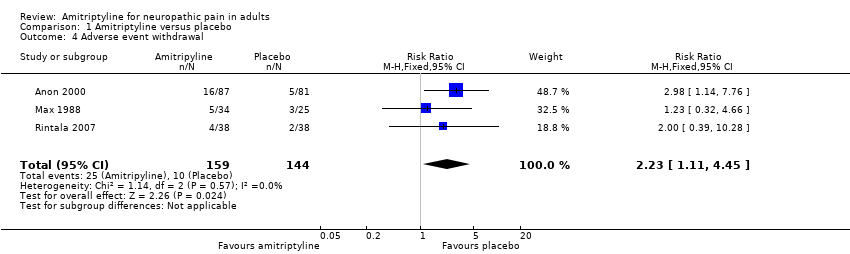
Comparison 1 Amitriptyline versus placebo, Outcome 4 Adverse event withdrawal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Third‐tier efficacy Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Painful diabetic neuropathy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Postherpetic neuralgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Mixed neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Cancer‐related neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 HIV‐related neuropathic pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Post‐stroke pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 At least 1 adverse event Show forest plot | 6 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.32, 1.81] |
| 3 All‐cause withdrawal Show forest plot | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.81, 2.12] |
| 4 Adverse event withdrawal Show forest plot | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.11, 4.45] |


