Traitement substitutif par enzymes pancréatiques pour les personnes atteintes de mucoviscidose
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind cross‐over trial. Duration: there was a run‐in period (duration not specified) followed by randomisation to 1 of 2 arms. 28 days in each arm. UK based. Home setting. | |
| Participants | 17 patients diagnosed with CF. Age: 18 to 42 years. | |
| Interventions | Group 1: Nutrizyme GR (10000 BP units of lipase). Group 2: Nutrizyme 22 (22000 BP units of lipase). Lipase intake was equivalent to previous intake of patients and was kept constant during the trial. | |
| Outcomes | Weight gain, appetite, stool consistency, stool frequency and FFE. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Low risk | When on Nutrizyme 22, patients took an equal number of placebo capsules and high‐dose enzyme capsules to make the total number of capsules the same as when taking Nutrizyme GR. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | High risk | 1 patient withdrew after the run‐in period; reason was not given. |
| Selective reporting (reporting bias) | High risk | SDs were not presented for the outcome faecal fat excretion; other outcomes were reported in a way, that could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
| Methods | Randomised, double‐blind, 3‐arm parallel, dose‐ranging, multicentre trial. Duration: 29 days. Participants recruited from 26 CF Foundation‐accredited centres in the USA. Home setting. | |
| Participants | 139 patients with previously diagnosed CF and undergoing treatment were screened and 129 enrolled as intention‐to‐treat population. Age: mean (SD) 21.5 (8.5) years. Gender split: 71% were males. | |
| Interventions | Group 1: Altu‐135 5000 units of lipase. Group 2: Altu‐135 25,000 units of lipase. Group 3: Altu‐135 100,000 units of lipase. Doses were not adjusted on basis of weight or food ingested, but were fixed per meal or snack. Lipase, protease & amylase were in a ratio of 1:1:0.15 | |
| Outcomes | CFA, CNA, adverse events, QoL using the CFQ‐R. | |
| Notes | The CFA and CNA were measured at baseline and at 14 days after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants received equal number of unlabelled capsules. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | Low risk | 129 participants were enrolled as intention‐to‐treat population, of whom 12 withdrew (4 due to gastrointestinal adverse events); 117 patients who received at least 1 dose were included in a modified intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | High risk | Trial sponsored and actively supported by Altus Pharmaceuticals. |
| Methods | Randomised, double‐blind, cross‐over trial. Duration: 4 weeks for each treatment arm with a 2 week run‐in period. Single‐centre trial in New Zealand. Home setting. | |
| Participants | 30 children previously diagnosed with CF using clinical and laboratory data. Age: median 10.1 years. Gender split: 17 girls, 13 boys. | |
| Interventions | Group 1: Creon® (lipase 8000 BP, amylase 9000 BP, protease 210 BP). Group 2: Pancrease® (lipase 5000 BP, amylase 3000 BP, protease 350 BP). Participants were started on doses of lipase slightly lower or equivalent to pretrial period. Later they were allowed to adjust according to their requirement. | |
| Outcomes | Mean weight gain, adequate daily intake of energy, fat and nitrogen, stool weight, FFE and nitrogen excretion. | |
| Notes | For the outcomes of interest to the review, the results were given in a descriptive method; means and SDs not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Low risk | Both formulations were prepared in identical opaque capsules from commercial stock. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients withdrew consent and 1 patient was hospitalised due to respiratory exacerbations during the run‐in period. |
| Selective reporting (reporting bias) | High risk | Results were reported in a narrative method and could not be included in analysis |
| Other bias | High risk | Boehringer Ingelheim (NZ) Limited Kali Chemie provided funding and trial materials. |
| Methods | Randomised, open‐label, cross‐over trial. Duration: each arm was for 4 weeks. No run‐in period specified. Single centre in the former East Germany. | |
| Participants | 45 patients with CF. Age: mean 11.8 years. Gender split: 24 boys and 21 girls. | |
| Interventions | Group 1: Pancreon forte (conventional). Group 2: Creon® (acid protected microspheres). | |
| Outcomes | Weight gain, height, stool frequency, FFE. | |
| Notes | Outcomes were given in a descriptive method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not given. |
| Selective reporting (reporting bias) | High risk | Narrative results only ‐ could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
| Methods | Randomised, double‐blind, 3‐arm cross‐over trial of 3 different ECMs. Duration: each treatment arm lasted 4 weeks after an initial 2 week run‐in period. Single‐centre trial based in the UK. | |
| Participants | 22 children with CF. Age: 5 ‐ 16 years. Gender split not given. | |
| Interventions | Group 1: Nutrizyme GR. Group 2: Nutrizyme MP. Group 3: Creon®. The preparations were compared in a capsule for capsule basis, even though there was a difference in enzyme content. | |
| Outcomes | Symptom scores, weight gain and CFA. | |
| Notes | Results were given descriptively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | High risk | 4 patients withdrew from study, reasons were not given. |
| Selective reporting (reporting bias) | High risk | Outcomes were reported in a way that could not be included in analysis. |
| Other bias | Unclear risk | Information not given. |
| Methods | Prospective, randomised, open‐label, cross‐over trial. Duration: treatment was for a period of 10 weeks; 2 weeks run in, followed by randomisation to 1 of the 2 arms for 4 weeks, and then cross over to alternative treatment for the next 4 weeks. Multicentre trial at 3 hospitals in the UK. | |
| Participants | 59 children with CF, diagnosed by 2 sweat tests or genotype, had proven pancreatic insufficiency. Age : mean (SD) age of 10 (3.5) years. Gender split: not given. | |
| Interventions | Group 1: ECM (Creon 8000 MS). Group 2: ECMM (Creon 10000 MMS). Dose was lipase for lipase. The median intake of lipase/kg body weight/day was 6689 for Creon 8000 and 8527 for Creon 10000. | |
| Outcomes | FFE, CFA, stool frequency, abdominal pain, patient preference. | |
| Notes | The stool collection for CFA was done only in 1 centre, with 22 patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Stool collection data were from 1 hospital only with 22 patients in an intent‐to‐treat analysis. 54 patients completed the trial, 2 dropped out in run‐in period due to abdominal pain and loose stools; a further 2 dropped out during the ECMM phase (1 due to abdominal pain and loose stools and 1 due to meconium ileus equivalent). The 5th patient dropped out during the ECMM phase due to an appendix abscess considered to be unrelated to treatment. |
| Selective reporting (reporting bias) | High risk | Data on stool frequency and abdominal pain reported in a way that could not be included in the analysis. |
| Other bias | Low risk | Trial appears to be free of other sources of bias. |
| Methods | Randomised, double‐blind, cross‐over trial. Duration: each treatment arm lasted 4 weeks; no run‐in period. Not clear if a single or multicentre trial based in Denmark. | |
| Participants | 11 children with documented CF. Age: 2 ‐ 11 years of age. Gender split: 2 males and 9 females. | |
| Interventions | Group 1: pH sensitive ECM Pancrease (1 ‐ 2 capsules containing 330 FIP‐u protease, 6200 FIP‐u lipase, 3600 FIP‐u amylase/capsule). Group 2: conventional ECM Pancreatin (10 ‐ 35 ml containing 525 FIP‐u trypsin, 12000 FIP‐u lipase, 12750 FIP‐u amylase). Patients were allowed to change doses depending on individual requirements | |
| Outcomes | Symptom scores for stool frequency, consistency, colour, odour and abdominal cramps; weight gain; fat absorption. | |
| Notes | Results reported as medians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Low risk | During both periods placebo preparations were given in the form of capsules or granules. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | Low risk | There were no withdrawals, with all 11 patients completing the study. |
| Selective reporting (reporting bias) | High risk | The results were reported in medians due to which the data could not be included in the analysis. |
| Other bias | Low risk | No other sources of bias were found. |
| Methods | Open‐label randomised cross‐over trial. Duration: 2 consecutive 28‐day treatment periods. Single centre in the UK (Brompton Hospital Adolescent and Adult Cystic Fibrosis Clinic, London). | |
| Participants | 23 patients with CF diagnosed by sweat chloride concentration > 70 mmol/L, evidence of pancreatic insufficiency and symptomatic steatorrhoea. Age: mean (SD) 24.8 (4.2) years. Gender split: 11 males and 12 females. | |
| Interventions | Group 1: ECM (Creon capsules). Group 2: ECT (Pancrex V Forte). Patients received either their usual regimen of ECT or ECM in a ratio of 0.7 capsules for each ECT. Declared lipase of Pancrex V forte to Creon capsules is 0.7:1, protease is 1.6 : 1. | |
| Outcomes | Change in weight, frequency of stools, abdominal pain, FFE and CFA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further information given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients withdrew from study and reasons were given. 1 patient was unable to swallow microsphere capsules and another took more lipase during 1 month than the other. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | Unclear risk | Trial supported by Duphar Laboratories. |
| Methods | Open‐label, randomised, cross‐over trial. Duration: 2 consecutive 28‐day treatment periods. Single centre in the UK. | |
| Participants | 14 patients with CF, diagnosed by sweat chloride > 70 mmol/L and typical pulmonary disease. Age: mean 21.4 years. Gender split: 8 males and 6 females. | |
| Interventions | Group 1: ECM (Creon) with food. Group 2: NECT (Pancrex V) with food and adjuvant cimetidine 40 min before meals. Both contain 8000 BP units of lipase, number of capsules for each individual was same during both treatment periods. | |
| Outcomes | Change in weight, stool frequency, abdominal pain, FFE and CFA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient withdrew due to inability to control frequency of stools. The treatment arm was not specified. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | Unclear risk | Creon® was supplied by Duphar Laboratories. |
| Methods | Randomised, 2‐arm cross‐over trial. Duration: after an 8‐day initial washout each treatment given for a period of 30 days. Single‐centre trial in France. Home setting. | |
| Participants | 17 children with documented CF. Age: 1 ‐ 12.5 years. Gender split was not given. | |
| Interventions | Group 1: ECM (Creon®) (1.2 ‐ 2.4 g/day). Group 2: lyophilised TPE (4 ‐ 8 g/day). | |
| Outcomes | Body weight, FFE, nutritional indicators (body weight to length index, subscapular skin fold, plasma cholesterol, pre‐albumin, retinol, retinol binding protein, zinc and total essential fatty acids), therapeutic tolerance (drug acceptance, alanine amino transferase, prothrombin time, serum bilirubin and uric acid, urinary uric acid excretion). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not given. |
| Selective reporting (reporting bias) | High risk | Change in body weight was incompletely reported and cannot be included in the review. |
| Other bias | Unclear risk | Information not given. |
| Methods | Randomised, double‐blind, double‐placebo cross‐over trial. Duration: 4 weeks for each treatment arm. Single‐centre trial in the UK (London). | |
| Participants | 20 children with CF diagnosed by sweat test sodium greater than 70 mmol/L Age: mean 9.9 years; range 4.1 ‐ 15.3 years. All had steatorrhoea and weight and height above 3rd percentile. The mean weight was 26.0 kg (range 14.2 kg ‐ 50.7 kg) and the mean height was 1.32 metres (range 1.1 metres ‐ 1.63 metres). Gender split not given. | |
| Interventions | Group 1: active ECM plus placebo ECT. Group 2: placebo ECM plus active ECT. Dosage of ECM was calculated to provide equivalent dosage of lipase to ECT. The day‐to‐day dosage of active drug and placebo varied slightly depending on the patients diet. | |
| Outcomes | Change in weight, stool frequency, abdominal pain, FFE. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | Low risk | While taking ECM, patients received a placebo of ECT and while taking ECT, they took a placebo preparation of ECM. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not given. |
| Incomplete outcome data (attrition bias) | High risk | Only 12 paired samples were analysed for FFE. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes are reported. |
| Other bias | High risk | Duphar Ltd, UK supplied pancreatic enzyme supplements and supported the trial. |
| Methods | Randomised, single blind cross‐over trial. Duration: 10 weeks in total, 2‐week run‐in period followed by 4 weeks for each treatment arm. Not clear if multi‐ or single‐centre trial based in the UK. Home setting. | |
| Participants | 39 children with symptoms of CF, at least 2 abnormal sweat chloride results and pancreatic insufficiency. Age: median (range) 9.7 (5 ‐ 17) years. Clinical state, as measured by the Shwachman score (100 = normal) ranged from 37 to 91 with a median value of 79. 12 patients were unsuitable for analysis, the remaining 27 children (15 boys and 12 girls) completed the trial. | |
| Interventions | Group 1: ECM Creon® (lipase 8000 BP units, amylase 9000 BP units, protease 210 BP units). Group 2: ECM Pancrease® (lipase 5000 BP units, amylase 2900 BP units, protease 330 BP units). Participants took same number of capsules per day during both treatment periods. | |
| Outcomes | CFA, patient preference, nitrogen excretion, weight change, symptom score for appetite, number, colour and consistency of stools, abdominal pain and general condition. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but further information not given. |
| Allocation concealment (selection bias) | Unclear risk | Information not given. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) | Low risk | Trial medication was issued by pharmacist and order of treatment was not known to the doctor. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding not done. |
| Incomplete outcome data (attrition bias) | High risk | 12 patients (31%) were withdrawn from trial for various reasons and not included in analysis:
|
| Selective reporting (reporting bias) | High risk | Change in weight and symptom scores for abdominal pain, stool frequency were measured but were reported incompletely, so cannot be entered in a meta‐analysis. |
| Other bias | High risk | Corresponding author was financially supported by Cilag Limited (Pancrease). |
CF: cystic fibrosis
CFA: co‐efficient of fat absorption
CFQ‐R: Cystic Fibrosis Questionnaire‐Revised
CNA: co‐efficient of nitrogen absorption
ECM: enteric‐coated microspheres
ECMM; enteric‐coated mini‐microspheres
ECT: enteric‐coated tablets
FFE: faecal fat excretion
NECT: non enteric‐coated tablets
QoL: quality of life
SD: standard deviation
TPE: total pancreatic extracts
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Cross‐over study with PERT for 5 days in each arm. | |
| Doesn't appear to have a control group, PERT dosage increased according to fecal fat levels. | |
| Cross‐over study with PERT for 3 days in each arm. | |
| Cross‐over study with 3 arms of 15 days each. | |
| The study drugs were given for a period of 6 days only after randomisation. | |
| Cross‐over study with each treatment period only 2 weeks. | |
| Crossover study with 2 arms of 2 weeks each. | |
| Crossover study with 2 arms of 7 days each. | |
| Cross‐over study of enzymes given before and during meals; 2 periods of 1 week each. | |
| Cross‐over study with 2 treatment periods of 1 week each. | |
| Study assesses pancreatic enzyme activity. | |
| Study assesses breath tests. | |
| Open label cross‐over study with periods of 1 week each. | |
| Parallel group study for a period of 8 days. | |
| Study compares different methods for assessing exocrine pancreatic function. | |
| Single dose intervention. | |
| Parallel study period of 10 days. | |
| Cross‐over study with 2 periods of 8 days each. | |
| Cross‐over study with 2 periods of 14 days each. Study assessed patient preference, but also assessed clinical symptoms. | |
| Cross‐over study with 4 preparations given for 1 week each. | |
| Crossover study with each intervention given only on a single day. | |
| Cross‐over study with each intervention given only on a single day. | |
| Cross‐over study (assessing coating of PERT) with 2 weeks in each arm. | |
| Placebo‐controlled, parallel study but treatment period for 1 week. | |
| Cross‐over study with 2 arms of 14 days each. | |
| Cross‐over study with 2 weeks in each arm. | |
| Cross‐over study of 4 periods of 14 days each. | |
| Intervention only give for 5 days. | |
| Cross‐over placebo‐controlled trial with 2 periods of 1 week each. | |
| Letter reports cross‐over trial to compare patient preference of different formulations; no other outcomes stated. | |
| Cross‐over study with a total 2‐week study period. | |
| Cross‐over study of 6 treatment (Viokase vs Cotazyme vs Pancrelipase with and without bicarbonate) periods of 3 weeks each. | |
| Study assesses use of bentiromide screening test. | |
| Cross‐over study with 2 treatment periods of 2 weeks each. | |
| Cross‐over study with 2 treatment periods of 14 days each. | |
| Not an RCT or a quasi‐RCT. | |
| Cross‐over study with two treatment periods of 12 days each. | |
| Intervention given only for 6 days. | |
| Cross‐over trial with 2 treatment periods of 5 days each. | |
| Treatment only 6 to 7 days in each arm of the 2‐phase cross‐over trial. | |
| Cross‐over trial with 2 treatment periods of 4 days each. | |
| Cross‐over trial with 2 single interventions on separate days. | |
| Open‐label, cross‐over study with 2 treatment periods of 5 days each. | |
| Cross‐over trial with 2 periods of 6 days each. | |
| Placebo‐controlled trial with a treatment period of 1 day. | |
| Study assesses use of antacids in conjunction with PERT intervention not relevant. | |
| Cross‐over study assessing antibiotic absorption with PERT compared to without in a single intervention. | |
| Cross‐over study with 2 arms of 5 days each. | |
| Cross‐over trial 2 periods of 5 days each. | |
| Not an RCT or quasi‐RCT. | |
| Cross‐over study with 2 treatment periods of 2 weeks each. | |
| Cross‐over study with 2 treatment periods of 1 week each. | |
| Cross‐over study with treatment periods of 2 weeks each. | |
| Study evaluated a breath test used to assess fat malabsorption and not PERT. | |
| Cross‐over study with 2 treatment periods of 2 weeks each. | |
| Cross‐over study with 2 periods of 2 weeks each. | |
| Cross‐over study with 2 treatment periods of 1 week each. | |
| PERT taken for 2‐week period only. | |
| Cross‐over trial with 2 periods of 14 days each. | |
| Study assessing knowledge and education of PERT. | |
| Parallel RCT but duration only 5 ‐ 7 days. | |
| Cross‐over trial with 3 periods of 7 days each. | |
| Cross‐over RCT with 5‐day course of PERT and same for placebo. | |
| Treatment period only 5 days. | |
| Study assesses antibiotic absorption when given with PERT. | |
| Cross‐over study with 2 treatment periods of 1 week each. | |
| Cross‐over study with 2 periods of 8 days each. | |
| Cross‐over study with 2 periods of 7 days each. | |
| Not an RCT or quasi RCT. | |
| RCT with 3 interventions for 2 weeks each. |
PERT: pancreatic enzyme replacement therapy
RCT: randomized controlled trial
vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized, double‐blind, cross‐over trial. Duration: 12 months. UK‐based trial. |
| Participants | 20 patients with CF and chronic distal intestinal obstruction. Mean age: 13.1 years. Gender split: 4 males and 16 females. |
| Interventions | Group 1: low‐dose PERT. Group 2: high‐dose PERT. |
| Outcomes | Weight gain, CFA, episodes of acute distal intestinal obstruction syndrome, abdominal mass, abdominal pain. |
| Notes | Results given in ranges. Await further details from authors. |
| Methods | Possibly randomised, not clear. Duration: 14 months for intervention, control not clear. USA‐based trial. |
| Participants | 20 patients with CF. |
| Interventions | Pancrease (enteric‐coated) ‐ dose 9 to 11 per day ‐ compared with usual supplement (Viokase or Cotazyme) for a period of 14 months in pancrease arm, duration of other usual supplement not given. |
| Outcomes | Body weight, urine uric acid, serum albumin, abdominal symptoms. |
| Notes | Not stated whether patients were randomised or not. Possible extension of excluded study Holsclaw 1979. |
| Methods | Randomised double‐blind cross‐over trial with 3 arms. Duration: 3 months in total, each arm lasted 1 month, not clear if washout period was used. |
| Participants | 11 patients with CF and 1 patient with chronic pancreatitis. All adults. |
| Interventions | Pancrex V Forte: 3 tablets equivalent to 3 g pancreatine BP. Nutrizym: 2 tablets equivalent to 3.2 g pancreatine BP. Nutrizym plus bromelin: 2 tablets equivalent to 3.2 g pancreatine BP also containing 50g bromelin. Nutrizym tablets looked the same whether containing bromelin or not, but not identical to Pancrex V Forte. Patients not allowed to tell outcome assessors how many tablets they were taking. |
| Outcomes | Self‐reported bowel habits, general health and respiratory symptoms (daily diary), FFE. |
| Notes | Combined data given for all patients (CF data not split out). Full trial in French ‐ needs translation. 2 patients who had been on high doses of Pancrex V Forte (9 ‐ 12 per meal) took double the normal preparations in the trial. |
| Methods | Single blind cross‐over and parallel design. Single‐centre trial. |
| Participants | 24 adult CF patients. |
| Interventions | Recombinant acid lipase marketed as MERISPASE® Session 1: all patients received low‐dose pancreatic extract. Session 2: 3 different doses of lipase (MERISPASE®) compared with 84,000 units of pancreatic extract. Session 3: all patients received low doses of CREON®, 3 groups receiving MERISPASE® continued. |
| Outcomes | Safety, tolerance, CFA. |
| Notes | Meristem Therapeutics went out of business in September 2008; clinical trials blocked in phase II. No one appears to be producing or using this agent. |
| Methods | Randomised, double‐blind cross‐over study without placebo. Duration : 28 days in each arm. Not clear if single centre or multicentre, based in Germany. |
| Participants | 16 patients (9 females, 7 males) diagnosed with CF by at least 2 sweat chloride values of ≽ 70 mM and pancreatic insufficient. Age: mean 9.9, range 3 ‐ 27. |
| Interventions | Treatment A: Kreon 25000 (per capsule: 25000 U of lipase, 18000 U of amylase and 1000 U of protease). Treatment B: Panzyrtat (per capsule: 20000 U of lipase, 18000 U of amylase and 1000 U of protease). Both groups received the same number of capsules in each arm. |
| Outcomes | FFE, faecal chymotrypsin, faecal immunoreactive human lipase and serum immunoreactive trypsin. |
| Notes | Mean FFE for both the arms together was given. FFE for individual treatment periods not given. |
| Methods | Open prospective trial; not clear if randomised. Cross‐over trial, each arm 3 months. Consecutive so implies no washout. |
| Participants | 23 patients with CF. Age: range 1.3 ‐ 16.8 years. |
| Interventions | Creon 25000 compared with conventional microsphere preparations. |
| Outcomes | FFE, BMI, height SD score, lean body mass, clinical symptoms (diary), dietary intake, spirometry, Schwachman and Crispin Norman scores, stool frequency. |
| Notes |
BMI: body mass index
CF: cystic fibrosis
CFA: co‐efficient of fat absorption
ECM: enteric‐coated microspheres
FFE: faecal fat excretion
PERT: pancreatic enzyme replacement therapy
SD: standard deviation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 1 Change in weight. | ||||
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Stool frequency Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 2 Stool frequency. | ||||
| 2.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Abdominal pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 3 Abdominal pain. | ||||
| 3.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 4 FFE. | ||||
| 4.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 ECM versus ECT, Outcome 1 Change in weight. | ||||
| 1.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.03, 0.67] |
| 2 Stool frequency Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 ECM versus ECT, Outcome 2 Stool frequency. | ||||
| 2.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.85, ‐0.30] |
| 3 Abdominal pain Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 ECM versus ECT, Outcome 3 Abdominal pain. | ||||
| 3.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.96 [‐12.97, ‐2.94] |
| 4 FFE Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 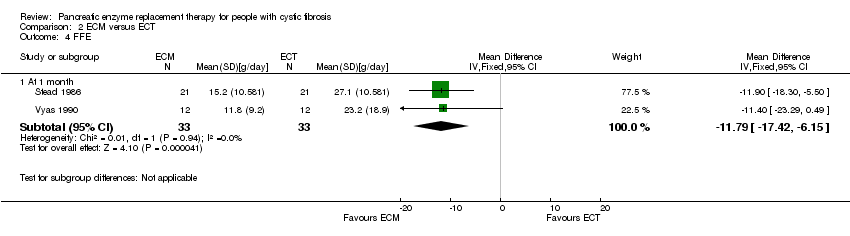 Comparison 2 ECM versus ECT, Outcome 4 FFE. | ||||
| 4.1 At 1 month | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐11.79 [‐17.42, ‐6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 ECM versus ECMM, Outcome 1 FFE. | ||||
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Co‐efficient of fat absorption Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Creon® versus Pancrease®, Outcome 1 Co‐efficient of fat absorption. | ||||
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 ECM versus TPE, Outcome 1 FFE. | ||||
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
![Forest plot of comparison: 2 ECM versus ECT, outcome: 2.2 Stool frequency [number/day].](/es/cdsr/doi/10.1002/14651858.CD008227.pub2/media/CDSR/CD008227/rel0002/CD008227/image_n/nCD008227-AFig-FIG01.png)
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.2 Stool frequency [number/day].
![Forest plot of comparison: 2 ECM versus ECT, outcome: 2.3 Abdominal pain [% days].](/es/cdsr/doi/10.1002/14651858.CD008227.pub2/media/CDSR/CD008227/rel0002/CD008227/image_n/nCD008227-AFig-FIG02.png)
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.3 Abdominal pain [% days].
![Forest plot of comparison: 2 ECM versus ECT, outcome: 2.4 FFE [g/day].](/es/cdsr/doi/10.1002/14651858.CD008227.pub2/media/CDSR/CD008227/rel0002/CD008227/image_n/nCD008227-AFig-FIG03.png)
Forest plot of comparison: 2 ECM versus ECT, outcome: 2.4 FFE [g/day].
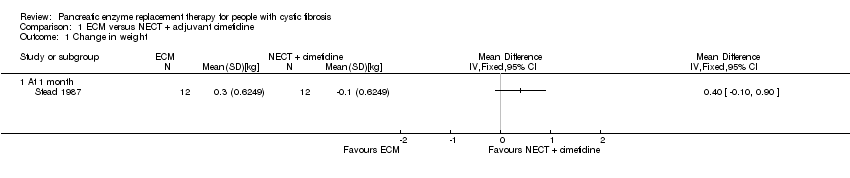
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 1 Change in weight.
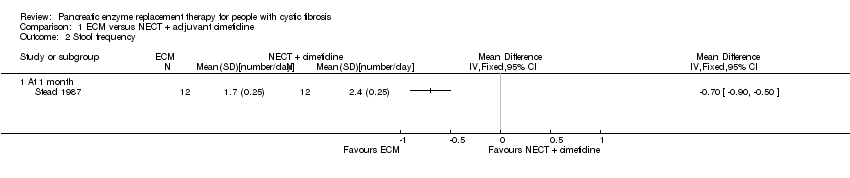
Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 2 Stool frequency.

Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 3 Abdominal pain.

Comparison 1 ECM versus NECT + adjuvant cimetidine, Outcome 4 FFE.

Comparison 2 ECM versus ECT, Outcome 1 Change in weight.
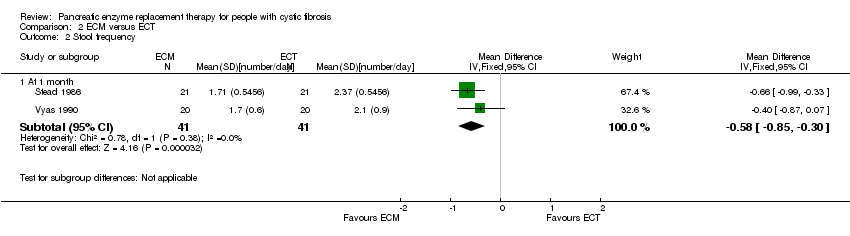
Comparison 2 ECM versus ECT, Outcome 2 Stool frequency.
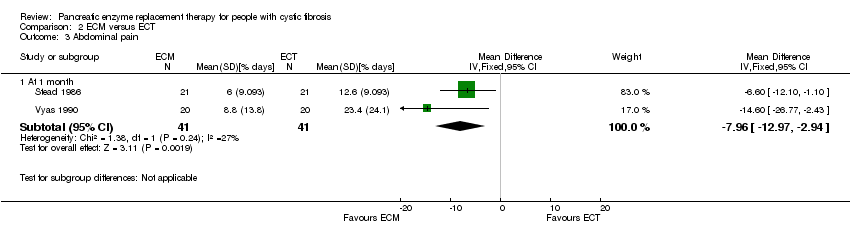
Comparison 2 ECM versus ECT, Outcome 3 Abdominal pain.

Comparison 2 ECM versus ECT, Outcome 4 FFE.

Comparison 3 ECM versus ECMM, Outcome 1 FFE.

Comparison 4 Creon® versus Pancrease®, Outcome 1 Co‐efficient of fat absorption.

Comparison 5 ECM versus TPE, Outcome 1 FFE.
| Term/abbreviation | Definition |
| BMI | body mass index |
| CF | cystic fibrosis |
| CFA | coefficient of fat absorption |
| chyme | the semi‐fluid mass of partly digested food expelled by the stomach into the duodenum |
| DIOS | distal intestinal obstruction syndrome |
| ECM | enteric coated microspheres |
| FFE | faecal fat excretion |
| hyperuricaemia | an excess of uric acid in the blood |
| hyperuricosuria | the presence of excessive amounts of uric acid in the urine |
| Ileocecum | the combined ileum (end of the small intestine) and cecum (start of the large intestine) |
| NECM | non‐enteric coated microspheres |
| PERT | pancreatic enzyme replacement therapy |
| PI | pancreatic insufficiency |
| porcine | relating to or suggesting swine (pigs) |
| RCT | randomised controlled trial |
| steatorrhoea | loss of fat in the stools |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Stool frequency Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Abdominal pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in weight Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.03, 0.67] |
| 2 Stool frequency Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.85, ‐0.30] |
| 3 Abdominal pain Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.96 [‐12.97, ‐2.94] |
| 4 FFE Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 month | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐11.79 [‐17.42, ‐6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Co‐efficient of fat absorption Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FFE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

