大剂量化疗后使用自体造血干细胞移植治疗非横纹肌肉瘤性软组织肉瘤
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Duration: 2000 to 2008 Study design: randomized controlled trial: "This open, multicenter, randomized phase III study [...]". "All patients eligible for preenrollment received the same baseline treatment [...]". "[...] eligible for randomization if they had responded to chemotherapy or, for stable disease, if a complete surgical resection of all disease sites could be carried out. Patients were ineligible for randomization if they had progressed or had only stable disease with no possibility for complete resection of the primary and/or metastatic tumor". "Randomization was stratified by center using a blocked method with block size of four and was carried out centrally". "The intention to treat (ITT)‐modified population included all randomly assigned patients excluding patients found to be ineligible at central histology review." Treatment: number of arms: 2 Follow‐up time: time to event analysis at 3 years with a median follow‐up of 55 months for survivors | |
| Participants | Setting: multicenter trial in 16 centers in France Eligibility criteria: people aged 18 to 65 years with histologically confirmed, inoperable locally advanced or metastatic soft tissues sarcomas; Eastern Cooperative Oncology Group performance status of 0 or 1; normal cardiac, hepatic, and renal function; adequate bone marrow reserve; participants had received no prior chemotherapy or concurrent therapy Exclusions: people for whom it was possible to perform potentially curative locoregional treatments and people with uterine, bone, or digestive tumors Number of participants: 264 participants pre‐enrolled; 207 participants received first 4 of 6 chemotherapy courses:
Age
Gender
| |
| Interventions | All participants received 5 courses of SDCT: doxorubicin 60 mg/m2, ifosfamide 7500 mg/m2, dacarbazine 900 mg/m2, total doses; the 6th course was different between HDCT + autologous HSCT arm and SDCT arm: HDCT + autologous HSCT arm, 6th course:
SDCT arm, 6th course:
| |
| Outcomes | Primary outcomes as defined by the study
Secondary outcomes as defined by the study
| |
| Notes | Financial support: Programme Hospitalier de Recherche Clinique, French Health Ministry (nonprofit organization); French National Federation for Comprehensive Cancer Centers (nonprofit organization). Information about the histologic type of sarcoma designated as "Others" in the article were communicated by personal contact with the first author and listed in Table 1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by center using a blocked method with block size of four and was carried out centrally". We assumed an adequate random sequence generation and judged at low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was carried out centrally, though masking of allocation was not described in full detail. We assumed an adequate allocation concealment. However, we missed a clarifying statement. Therefore, we judged at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Not reported; very likely not possible and not relevant for the reported outcomes of overall survival, treatment‐related mortality, and progression‐free survival. Blinding of participants has no influence on overall survival and treatment‐related mortality, which are defined as primary outcomes of the present review. Therefore, we judged at low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported; very likely not possible and not relevant for the reported outcomes overall survival, treatment‐related mortality, and progression‐free survival. The study was denoted as an "open, multicenter, randomized phase III study". Blinding of outcome assessment has no influence on overall survival and treatment‐related mortality, which are defined as primary outcomes of the present review. Therefore, we judged at low risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | At 36 months from randomization (HDCT versus SDCT), 51 participants had died (24 versus 27) and 25 were at risk (8 versus 17). Of 83 participants (38 versus 45) included in the modified intention‐to‐treat survival analysis, 76 participants (32 versus 44) are accounted for but 7 participants (6 versus 1) may not be explained. The number of participants with missing information was small. The potential influence of this missing information was unclear, therefore we judged at unclear risk of bias. Figure 1 of Bui‐Nguyen 2012 showed that 41 participants were randomized to the HDCT arm, but 22 participants actually received high dose and were evaluated. Figure 1 also showed that 46 participants were randomized to the SDCT arm, but 40 participants actually received standard dose and were evaluated. The potential influence of this missing information was unclear, therefore we judged atn unclear risk of bias. Table 2 of Bui‐Nguyen 2012 showed WHO grades 3/4 toxicity for all randomized participants, 22 in the HDCT arm and 51 in the SDCT arm. There was an inconsistency concerning the number of randomized and evaluated participants between Figure 1 and Table 2. It appeared conflicting that 51 participants were reported to be randomized to the SDCT arm in Table 2, although, according to Figure 1, only 46 participants were randomized and only 40 participants actually received SDCT. Thus, instead of 51 participants, it appeared that only 40 participants were actually eligible to evaluate adverse events after SDCT. |
| Selective reporting (reporting bias) | Low risk | We did not identify any selective outcome reporting. |
| Other bias | Unclear risk | The US Food and Drug Administration sent a warning letter (Reference 15‐HFD‐45‐05‐01) addressed to the first author on 4 May 2015 to inform of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015). The inspection happened after the conclusion of the study included in the present review and may not have been related to the risk of bias. The potential influence of this information was unclear, therefore we judged it at unclear risk of bias. |
HDCT: high‐dose chemotherapy; HSCT: hematopoietic stem cell transplantation; SDCT: standard‐dose chemotherapy; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not intervention of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not study design of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not study design of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not study design of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not intervention of interest | |
| Not intervention of interest | |
| Not population of interest | |
| Not study design of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest | |
| Not population of interest |

Study flow diagram.
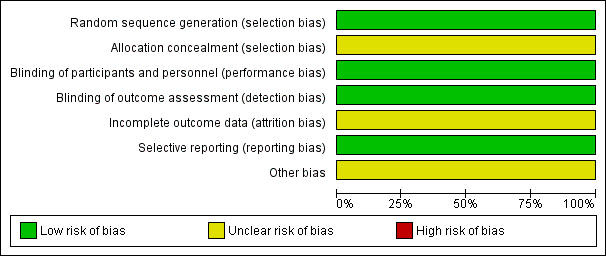
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
| Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
| Overall survival Follow‐up: median 55 months | 489 per 1000 | 571 per 1000 | HR 1.26 | 83 | ⊕⊕⊕⊕ | ‐ |
| Treatment‐related mortality Follow‐up: 24 months | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊕ | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Progression‐free survival Follow‐up: median 55 months | 756 per 1000 | 849 per 1000 | HR 1.34 | 83 | ⊕⊕⊕⊕ | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
| HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization. Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012. *Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification. | ||||

