پیوند سلولهای بنیادی هماتوپویتیک اتولوگ پس از شیمیدرمانی با دوز بالا برای سارکومهای بافت نرم غیر‐رابدومیوسارکوم
Appendices
Appendix 1. CENTRAL search strategy
| ID | Search | Hits |
| #1 | MeSH descriptor: [Sarcoma] explode all trees | 704 |
| #2 | MeSH descriptor: [Carcinoma, Small Cell] explode all trees | 748 |
| #3 | MeSH descriptor: [Hemangioendothelioma] explode all trees | 2 |
| #4 | MeSH descriptor: [Mesenchymoma] explode all trees | 2 |
| #5 | MeSH descriptor: [Perivascular Epithelioid Cell Neoplasms] explode all trees | 13 |
| #6 | MeSH descriptor: [Rhabdoid Tumor] explode all trees | 1 |
| #7 | MeSH descriptor: [Gastrointestinal Stromal Tumors] explode all trees | 112 |
| #8 | alveolar soft part sarcoma* | 8 |
| #9 | alveolar soft tissue sarcoma* | 12 |
| #10 | angiosarcoma* | 21 |
| #11 | hemangiosarcoma* | 9 |
| #12 | clear cell sarcoma* | 130 |
| #13 | clear cell tumor* or clear cell tumour* | 1228 |
| #14 | desmoplastic small round cell tumor* or desmoplastic small round cell tumor* | 6 |
| #15 | epithel* sarcoma* | 55 |
| #16 | fibrosarcoma* | 34 |
| #17 | myxofibrosarcoma* | 3 |
| #18 | hemangioendothelioma* | 7 |
| #19 | hemangioendotheliosarcoma* | 1 |
| #20 | intimal sarcoma* | 1 |
| #21 | leiomyosarcoma* | 111 |
| #22 | liposarcoma* | 58 |
| #23 | malignant glomus tumor* or malignant glomus tumour* | 2 |
| #24 | malignant mesenchymoma* | 2 |
| #25 | perivascular epithelioid cell tumor* or perivascular epithelioid cell tumour* | 1 |
| #26 | rhabdoid tumor* or rhabdoid tumour* | 15 |
| #27 | rhabdoid sarcoma* | 7 |
| #28 | synovial sarcoma* | 31 |
| #29 | gastrointestinal stromal tumor* or gastrointestinal stromal tumour* | 263 |
| #30 | malignant peripheral nerve sheath tumour* | 8 |
| #31 | undifferentiated pleomorphic sarcoma* | 10 |
| #32 | MeSH descriptor: [Stem Cell Transplantation] explode all trees | 1861 |
| #33 | MeSH descriptor: [Bone Marrow Transplantation] explode all trees | 1421 |
| #34 | MeSH descriptor: [Transplantation, Autologous] explode all trees | 1528 |
| #35 | MeSH descriptor: [Consolidation Chemotherapy] explode all trees | 41 |
| #36 | autologous transplant* | 4038 |
| #37 | bone marrow rescue | 203 |
| #38 | bone marrow support | 2824 |
| #39 | bone marrow cell | 3820 |
| #40 | stem cell rescue | 211 |
| #41 | stem cell support | 2117 |
| #42 | peripheral blood stem cell | 1487 |
| #43 | high dose chemotherapy | 5860 |
| #44 | intensified chemotherapy | 373 |
| #45 | intensive chemotherapy | 2053 |
| #46 | myeloablative chemotherapy | 234 |
| #47 | dose intensive treatment | 4499 |
| #48 | high dose combination | 11065 |
| #49 | MeSH descriptor: [Randomized Controlled Trial] explode all trees | 157 |
| #50 | randomized controlled trial or randomised controlled trial | 562380 |
| #51 | randomized controlled study or randomised controlled study | 495206 |
| #52 | randomized trial or randomised trial | 564855 |
| #53 | randomized study or randomised study | 498484 |
| #54 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 | 3040 |
| #55 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #42 or #43 or #44 or #45 or #46 or #47 or #48 | 25564 |
| #56 | #49 or #50 or #51 or #52 or #53 | 612136 |
| #57 | #54 and #55 and #56 | 928 |
Limits: publication date from 01 January 2012 to 06 September 2016.
Appendix 2. PubMed search strategy
| ID | Search |
| #1 | "Sarcoma"[Mesh] |
| #2 | "Carcinoma, Small Cell"[Mesh] |
| #3 | "Hemangioendothelioma"[Mesh] |
| #4 | "Mesenchymoma"[Mesh] |
| #5 | "Perivascular Epithelioid Cell Neoplasms"[Mesh] |
| #6 | "Rhabdoid Tumor"[Mesh] |
| #7 | "Gastrointestinal Stromal Tumors"[Mesh] |
| #8 | alveolar soft part sarcoma* |
| #9 | alveolar soft tissue sarcoma* |
| #10 | angiosarcoma* |
| #11 | hemangiosarcoma* |
| #12 | clear cell sarcoma* |
| #13 | clear cell tumor* or clear cell tumour* |
| #14 | desmoplastic small round cell tumor* or desmoplastic small round cell tumor* |
| #15 | epithel* sarcoma* |
| #16 | fibrosarcoma* |
| #17 | myxofibrosarcoma* |
| #18 | hemangioendothelioma* |
| #19 | hemangioendotheliosarcoma* |
| #20 | intimal sarcoma* |
| #21 | leiomyosarcoma* |
| #22 | liposarcoma* |
| #23 | malignant glomus tumor* or malignant glomus tumour* |
| #24 | malignant mesenchymoma* |
| #25 | perivascular epithelioid cell tumor* or perivascular epithelioid cell tumour* |
| #26 | rhabdoid tumor* or rhabdoid tumour* |
| #27 | rhabdoid sarcoma* |
| #28 | synovial sarcoma* |
| #29 | gastrointestinal stromal tumor* or gastrointestinal stromal tumour* |
| #30 | malignant peripheral nerve sheath tumor* or malignant peripheral nerve sheath tumour* |
| #31 | undifferentiated pleomorphic sarcoma* |
| #32 | "Stem Cell Transplantation"[Mesh] |
| #33 | "Bone Marrow Transplantation"[Mesh] |
| #34 | "Transplantation, Autologous"[Mesh] |
| #35 | "Consolidation Chemotherapy"[Mesh] |
| #36 | autologous transplant* |
| #37 | bone marrow rescue |
| #38 | bone marrow support |
| #39 | bone marrow cell |
| #40 | stem cell rescue |
| #41 | stem cell support |
| #42 | peripheral blood stem cell |
| #43 | high dose chemotherapy |
| #44 | intensified chemotherapy |
| #45 | intensive chemotherapy |
| #46 | myeloablative chemotherapy |
| #47 | dose intensive treatment |
| #48 | high dose combination |
| #49 | "Randomized Controlled Trial" [Publication Type] |
| #50 | randomized controlled trial or randomised controlled trial |
| #51 | randomized controlled study or randomised controlled study |
| #52 | randomized trial or randomised trial |
| #53 | randomized study or randomised study |
| #54 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 |
| #55 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 #42 or #43 or #44 or #45 or #46 or #47 or #48 |
| #56 | #49 or #50 or #51 or #52 or #53 |
| #57 | #54 and #55 and #56 |
Limits: publication date from 01 January 2012 to 06 September 2016.
Appendix 3. Inquiry to trial authors
For this review update, we sent e‐mail inquiries to two authors (Binh Bui‐Nguyen, Jean‐Yves Blay) of the included study (Bui‐Nguyen 2012) regarding clarification of survival data and the US Food and Drug Administration (FDA) warning letter on objectionable conditions and inadequate responses. The warning letter sent by the FDA was addressed to the first author Binh Bui‐Nguyen (Reference 15‐HFD‐45‐05‐01). It informs of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015).
E‐mail sent to Binh Bui‐Nguyen on 3 October 2016, quote: "I am conducting an update of my Cochrane Review and I would like to add some questions. At the moment, I am working on judging the Incomplete outcome data (attrition bias). On page 781, I extracted the following information from section 'Survival outcomes' and from Figure 2: At 36 months from randomization (HDCT versus SDCT), 51 patients had died (24 versus 27) and 25 are at risk (8 versus 17). Of 83 patients included in the modified ITT survival analysis, 76 are accounted for but 7 patients may not be explained. My question: Did I extract correctly? Does 'at the time of analysis' correspond to 36 months after randomization? Do the figures that I extracted or deduced correspond to 36 months after randomization?"
E‐mail sent to Jean‐Yves Blay on 5 October 2016, which contains the above quoted text sent to Binh Bui‐Nguyen and the following additional text, quote: "I also would like to ask about the importance of the attached FDA warning letter on objectionable conditions and inadequate responses. Is there any connection or conflict with the study of Bui‐Nguyen 2012?"
We did not receive any reply by 7 March 2017.

Study flow diagram.
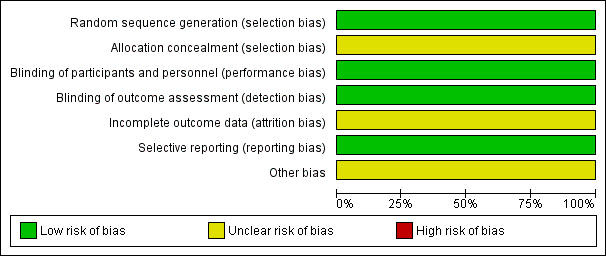
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
| Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
| Overall survival Follow‐up: median 55 months | 489 per 1000 | 571 per 1000 | HR 1.26 | 83 | ⊕⊕⊕⊕ | ‐ |
| Treatment‐related mortality Follow‐up: 24 months | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊕ | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Progression‐free survival Follow‐up: median 55 months | 756 per 1000 | 849 per 1000 | HR 1.34 | 83 | ⊕⊕⊕⊕ | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
| HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization. Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012. *Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification. | ||||

