횡문근 육종을 제외한 연부 육종에 대한 고용량 화학요법 시행 후 자가조혈모세포이식
초록
배경
연부 육종(STS)은 매우 드물고 이질성이 높은 악성 고형종양의 일종이다. 비횡문근 연부 육종(NRSTS)은 횡문근육종을 제외한 모든 연부 육종이 포함된다. 진행중인 국소 또는 전이 질환이 있는 환자들에게 고용량의 화학요법(HDCT) 후에 적용되는 자가조혈모세포이식(HSCT)은 HDCT 관련 심각한 혈액독성에 대해 계획되는 구호 치료법이다. 본 업데이트의 목적은 무작위대조시험(RCT)이 수행되어졌는지의 여부를 판단하고 HDCT 후에 따르는 자가 HSCT가 생존률에 있어 이점을 지니는지에 대해 검증하기 위한 것이다.
목적
소아 및 성인에서 전체 단계의 횡문근 육종을 제외한 연부 육종에 대한 고용량 화학요법(HDCT) 후의 조혈모세포이식의 효과와 안전성을 평가한다.
검색 전략
이번 업데이트에서는 정확도를 향상시키고 무관한 조회수를 줄이기 위해 검색 전략을 검토했다. 검색은 다음의 전자 데이터베이스를 대상으로 실시했다: Cochrane Central Register of Controlled Trials(CENTRAL; 2016년 8호), 2012년˜2016년 9월 6일 PubMed, 2012년˜2016년 9월 26일 Embase. 2012년˜2016년 9월 26일 온라인 임상시험 레지스트리 및 학회 회의록.
선정 기준
연부 육종 및 자가조혈모세포이식을 나타내는 용어가 제목 또는 초록에 포함되어 있는 것을 필수로 했다. 연구 설계는 RCT에 한정했다. 연구 설계는 RCT에 한정했다. 참가자의 80% 이상이 세계 보건기구(WHO) 분류 중 하나의 버전에 포함된 기준에서 악성으로 진단분류된 임상시험을 대상으로 했다. 검색은 소아 및 성인을 포함대상으로 하였으며 연령 제한은 없었다.
자료 수집 및 분석
Cochrane에서 요구되는 표준적인 방법론 절차를 사용하였다. 주요 결과는 전체 생존률과 치료 관련 사망률로 했다.
주요 결과
1,549건의 기록을 확인하였다; 내역은 전자 데이터베이스 85건, 임상시험 레지스트리 45건, 학회 회의록 1,419건이었다. 검색 전략의 재고찰을 실시했지만, 새로운 RCT는 확인되지 않았다. 본 고찰의 이전 버전에서는 대량 화학요법 후 자가조혈모세포이식과 표준 용량의 화학요법을 비교한 1건의 RCT가 확인되었다. 이 임상시험은 이질성이 있다고 여겨지는 19가지의 서로 다른 종양 유형을 지닌 환자 87명을 무작위화한 것이다. 이 중 83명의 데이터를 분석 대상으로 했다.
고찰 대상으로 한 1건의 임상시험에서 3년 생존률은 대량 화학요법군에서 32.7%였던 반면 표준 용량 화학요법군에서는 49.4%였다. 치료군 간에 차이는 없었다(위험비(HR) 1.26, 95% 신뢰구간 (CI) 0.70˜2.29, p=0.44, 1건의 임상시험, 참가자 83명, 근거의 질 높음). 대량 화학요법 전에 완전 반응률이 나타난 하위 그룹은 두 치료군 모두 전체 생존률이 높았다. 3년 전체 생존률은 대량 화학요법군에서 42.8%였던 반면, 표준 용량 화학요법군에서는 83.9%로 표준 용량 화학요법군이 더 우수했다(HR 2.92, 95% CI 1.1˜7.6 , p=0.028, 1건의 임상시험, 참가자 39명).
고찰 대상으로 한 1건의 임상시험에서 연구자는 대량 화학요법 시행 2년 후 치료 관련 백혈병으로 1명이 사망했다고 보고했다. 또한 연구자는 대량 화학요법군의 22명 및 표준 복용량 화학요법군의 51명에서 WHO 등급이 3˜4인 심각한 부작용에 대해서도 검토했다. 대량 화학요법군에서는 위장관 감염, 통증, 무력증에 관련한 독성의 부작용 11건, 표준 용량 화학요법군에서는 부작용 1건이 보고되었다(근거의 질 중간). 이차 암의 발병은 보고되지 않았다. 본 임상시험의 바이어스 위험은 7개 항목 중 3항목이 불분명하였고 4항목은 낮았기 때문에 전체적으로 바이어스 위험은 없는 것으로 판단했다. GRADE에서 3항목은 질이 높다고 판단했고 3항목은 보고되지 않았다.
연구진 결론
바이어스 위험이 불분명한 근거의 질이 중등도에서 높은 정도의 1건의 RCT에서 대량 화학요법(HDCT)의 생존률에 대한 유용성은 인정되지 않았다. 이 치료법은 환자 개인의 상태를 신중하게 고려하는 경우에 제대로 설계된 RCT의 일환으로만 실시되어야 한다.
PICO
쉬운 말 요약
횡문근 육종을 제외한 연부 육종에 대한 대량 화학요법 후 자가조혈모세포이식
연구의 질문
횡문근 육종을 제외한 연부 육종 환자에서 대량 화학요법(암을 죽이는 약) 후 자가조혈모세포 이식 대 표준 용량 화학요법의 전체 생존률(암으로 진단된 후 또는 치료를 시작하고 모든 원인에 의한 사망까지의 기간)을 비교한 근거에 대해 조사했다. 두 치료법을 비교한 1건의 무작위대조시험(RCT, 둘 이상의 치료 그룹 중 하나에 환자를 무작위로 배정한 임상시험)을 특정했다.
배경
횡문근 육종을 제외한 연부 육종은 희귀 암의 일종이다. 수술 불능(수술 중에 제거할 수 없음) 또는 전이성(암이 신체의 다른 부분에 퍼져있는 상태) 환자는 예후(결과)가 불량하다. 수술 불능(수술 중에 제거할 수 없음) 또는 전이성(암이 신체의 다른 부분에 퍼져있는 상태) 환자는 예후(결과)가 불량하다. 대량 화학요법은 환자의 생존률을 개선할 수 있다고 생각되었다. 하지만 해당 요법은 골수에서 혈액 세포의 생산을 멈추고 위해작용을 할 수 있다. 혈구 수가 지나치게 감소한 경우 대량 화학요법 전에 환자로부터 채취한 줄기 세포(다양한 종류의 세포가 될 수 있는 세포)를 환자 자신에게 다시 넣어 정상 혈구치를 회복할 수 있다. 이것을 자가조혈모세포이식이라고 한다. 연구의 수가 적기 때문에 이러한 치료를 받은 환자가 표준 화학요법을 받은 환자보다 오래 생존하는 것은 증명되지 않았다. 따라서 대량 화학요법 후 자가조혈모세포이식이 표준 용량의 화학요법보다 효과가 있는지 여부를 평가하는 것을 목적으로 했다.
연구 특성
이 근거는 2016년 9월 6일을 현재로 한 것이다. 대량 화학요법과 이식을 실시한 군 38명과 화학요법만 실시한 군 45명을 비교한 1건의 RCT를 특정했다. 공급자에게 혜택이 없는)에서 자금을 지원받았다. 대량 화학요법과 이식을 실시한 군 38명과 화학요법만 실시한 군 45명을 비교한 1건의 RCT를 특정했다. 바이어스 위험은 대체로 낮다고 판단했다(잘 설계되었기 때문에). 참가자들은 다양한 유형의 연부 육종(횡문근 육종 제외)을 가진 18˜65세 환자에게서 약 55개월간 관찰되었다. 치료 기간은 2000년˜2008년이었다. 이 1건의 RCT는 비영리 기관(임상시험에서 좋은 결과가 나왔다 하더라도 자금 공급자에게 혜택이 없는)에서 자금을 지원받았다.
주요 결과
RCT 결과에서 전체 생존률에 대해서는 두 치료군간에 차이가 나타나지 않았다. 치료 관련 사망은 이식군에서 1건이 나타났지만, 화학요법만 시행한 군에서는 관찰되지 않았다. 심각한 비혈액학적(혈액과 관련이 없는) 부작용은 이식군에서 8건, 화학요법만 시행한 군에서 1건 관찰되었다.
근거의 질
데이터의 전반적인 질은 불명확했으며 1건의 RCT에만 기반하고 있었다. 현재 연구의 근거는 횡문근 육종을 제외한 연부 육종 환자에 대한 대량 화학요법 후 자가조혈모세포이식의 사용에 한정되어 있다. 적절하게 설계된 임상시험에 의한 근거가 더 필요하다.
Authors' conclusions
Summary of findings
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
| Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
| Overall survival Follow‐up: median 55 months | 489 per 1000 | 571 per 1000 | HR 1.26 | 83 | ⊕⊕⊕⊕ | ‐ |
| Treatment‐related mortality Follow‐up: 24 months | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊕ | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Progression‐free survival Follow‐up: median 55 months | 756 per 1000 | 849 per 1000 | HR 1.34 | 83 | ⊕⊕⊕⊕ | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Soft tissue sarcomas (STS) are a highly heterogeneous group of rare malignant solid tumors of nonepithelial extraskeletal body tissue and are classified on a histogenetic basis (Weiss 2001). STS have a significant risk of distant metastasis in addition to the potential for locally destructive growth and recurrence. Nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) comprise all STS except rhabdomyosarcoma, which primarily affects children and young adults. In this review, we investigated NRSTS which are categorized as malignant according to the World Health Organization (WHO) classification and included in any of the 2002 first (Fletcher 2002) or the 2013 updated second version (Fletcher 2013). Rhabdomyosarcoma was addressed in the Cochrane Review by Admiraal 2010.
NRSTS usually originate de novo and rarely from benign tumors. In most cases, the pathogenesis is unknown; however, some factors are associated with the development of NRSTS (Weiss 2001). These include exposure to ionizing radiation, environmental carcinogenic substances, oncogenic viruses, and immunologic factors. Genetic factors can also play a role since some inherited diseases such as neurofibromatosis type 1 are associated with a higher risk of NRSTS (Tsao 2000). NRSTS are rare in both children and adults and the distribution of NRSTS differs significantly between children and adults according to Spunt 2006. In the USA, the yearly incidence of STS is 1 per 100,000 population for people 20 years of age or younger and about 7 per 100,000 population for people 20 years of age or older (NCI snapshot 2014). Between 2009 and 2013, the median age at diagnosis of STS, including tumors of the heart, was 59 years (Howlader 2016). Rhabdomyosarcoma represents about 50% of STS in children (Gurney 1997; Miller 1995).
Disease progression may be dichotomized into the two categories of limited and extensive disease. Limited disease is typically localized, small‐sized, low‐grade, and operable and is an accessible tumor that has no regional lymph node involvement and no distant metastases. Extensive disease can also be denoted as advanced disease, defined as a localized, large‐sized, and high‐grade tumor that may not be completely removed by surgery, may be invasive, and may have regional lymph node involvement or distant metastases. Both categories differ significantly in terms of prognosis and treatment. Many people with limited disease may be cured by surgery whereas extensive disease is associated with a poor outcome and many people may receive chemotherapy as palliative therapy.
The Tumor, Node, Metastasis (TNM) staging system is developed and maintained by the Union for International Cancer Control (UICC 2009). It combines grade, depth, and size of the tumor as well as regional lymph node involvement and distant metastases and describes the extent of a cancer's spread from stage 0 to IV. It is used by other organizations (AJCC 2016; NCI staging 2015) and combines grade, depth and size of the tumor as well as regional lymph node involvement and distant metastases, and describes the extent of a cancer's spread from stage 0 to IV. According to statistics from the National Cancer Institute, the overall five‐year survival is around 50% (ACS 2016). The overall survival (OS) varies by stage and was estimated at 16% for sarcomas with distant spread and 83% for localized sarcomas (ACS 2016).
The location of the primary tumor can involve any area of the body. The distribution is 40% lower limb and girdle, 20% upper limb and girdle, 20% abdominal sites, 10% trunk, and 10% head and neck (Clark 2005). NRSTS can involve any type of tissue and typically affect muscles, tendons, adipose tissue, blood vessels, and joints (Sondak 2001), and commonly present as a painless mass. The symptoms depend on the anatomical site of origin, the size of the mass, and other aspects. Retroperitoneal sarcomas are most often asymptomatic, until the mass grows large enough to be clinically obvious or presses on vital organs and causes pain (Dileo 2005). People who relapse or experience progressive disease after therapy or metastasis are commonly called high‐risk people because these signs are associated with shorter survival time. Spontaneous recovery from NRSTS is unknown.
Description of the intervention
Surgery is the standard treatment for localized NRSTS (ESMO 2014), and can be curative if distant dissemination is not present (Kotilingam 2006). Chemotherapy is a standard treatment for people with distant metastasis (ESMO 2014), and is regarded mainly as a palliative treatment for high‐risk people who are characterized by inoperable, locally advanced and metastatic disease. Doxorubicin, ifosfamide, gemcitabine, dacarbazine, docetaxel, and trabectedin are used in monotherapy or in combinations (ESMO 2014). Riedel 2012 provides an overview of current systemic therapies and discusses possible novel therapeutic agents and treatment strategies.
Autologous hematopoietic stem cell transplantation (HSCT) is defined as the transplantation of stem cells that have been collected previously from bone marrow or peripheral blood of the same person. High‐dose chemotherapy (HDCT) uses higher doses of chemotherapeutic agents than are usually applied in standard‐dose chemotherapy (SDCT). HDCT may ablate the person's bone marrow reserves and create an absolute requirement for stem cell rescue. Autologous HSCT applied after HDCT or high‐dose radiation is a planned rescue therapy for HDCT‐related severe hematologic toxicity (Banna 2007).
HDCT and autologous HSCT are not standard treatment options; they are an experimental approach mainly used to treat high‐risk people with an unfavorable prognosis (stage IV with distant metastases). HDCT and autologous HSCT may be used in special cases after careful consideration, usually for people who respond well to standard chemotherapy according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Therasse 2000). Independent of the disease status, HDCT and autologous HSCT are hazardous interventions that carry the risk of life‐threatening organ failure. Hematologic adverse events as a result of autologous HSCT are usually manageable but life‐threatening consequences of pancytopenia. They generally affect all patients and include, for example, graft failure, severe infections, and bleeding. Of 23,883 autologous HSCTs that were registered in Europe in 2014 by the European Group for Blood and Marrow Transplantation (EBMT), 19 were undertaken for STS (Passweg 2016).
How the intervention might work
HDCT followed by autologous HSCT was adopted to treat high‐risk people because it was believed that escalating doses in chemotherapy might increase survival by capturing putatively remnant malignant cells and might overcome resistance to SDCT (Banna 2007). HDCT may cause severe hematologic and nonhematologic toxicity and autologous HSCT is a planned rescue therapy for the HDCT‐related demise of hematopoietic stem cells.
Why it is important to do this review
Several authors stated a lack of evidence and the need to conduct RCTs to clarify the relevance of HDCT followed by autologous HSCT in high‐risk people with STS (Blay 2000; Carvajal 2005; Dumontet 1992; Ek 2006; Elias 1998; Kasper 2007; Ladenstein 1997; Pinkerton 1986; Reichardt 2002; Rosti 2002; Schlemmer 2006; Seeger 1991; Woods 1999). Some authors have warned against the use of HDCT followed by autologous HSCT, indicating the possibility of repositioning of malignant cells (Woods 1999). Others have questioned the use of HDCT with reference to the potential existence of refractory cancer stem cells (Banna 2007; Bonnet 1997; Sanchez‐Garcia 2007). In the previous version of this review, we identified and included one RCT (Peinemann 2013). The rationale for this update is to clarify whether additional RCTs have been published or are ongoing.
Objectives
To assess the efficacy and safety of high‐dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (HSCT) for all stages of nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Inclusion criteria
We adopted WHO classification of soft tissue tumors to define the population of people with malignant soft tissue tumors. This classifies soft tissue tumors as benign, intermediate (locally aggressive), intermediate (rarely metastasizing), and malignant. We included all tumor entities classified as malignant in any of the two published versions (Fletcher 2002; Fletcher 2013). This means that an entity is included in the present review, although it was listed in the 2002 version (Fletcher 2002) but not any longer listed in the 2013 (Fletcher 2013) version. This means also that an entity is included in the present review if it was not listed in the 2002 version but was introduced in the 2013 version. Studies were included if least 80% of participants had a diagnosis listed in any version of the WHO classification and classified as malignant, though we did not apply this limitation to rhabdomyosarcoma. We included children and adults with no age limits. Participants were included regardless of the severity of the disease and the clinical staging information, if they received autologous (from either a peripheral or bone marrow source, or both) HSCT.
Exclusion criteria
While the WHO classification of NRSTS includes the Ewing family of tumors, that is extraosseous tumor types, we excluded these as they are primarily bone sarcomas. Because extraosseous types are rarely diagnosed and share common features, they were regarded as one entity with osseous types and were excluded.
Types of interventions
Autologous hematopoietic stem cell transplantation (HSCT), stem cells from a peripheral source or the bone marrow, serving as a rescue therapy usually applied after high‐dose chemotherapy (HDCT) versus standard‐dose chemotherapy (SDCT), which is defined as chemotherapy at a lower dose than HDCT without the need for stem cell rescue.
Types of outcome measures
Primary outcomes
-
Overall survival (OS): the event was death by any cause, from diagnosis or start of HDCT and autologous HSCT.
-
Treatment‐related mortality (TRM): incidence of deaths that were classified as treatment related or the participants died of treatment complications.
Secondary outcomes
-
Disease‐free survival (DFS): time free of disease after diagnosis or start of HDCT and autologous HSCT.
-
Progression‐free survival (PFS): time staying free of disease progression after diagnosis or start of HDCT and autologous HSCT. We provided the definitions if reported in the studies.
-
Event‐free survival (EFS): time staying free of any of a particular group of defined events after diagnosis or start of HDCT and autologous HSCT.
-
Nonhematologic toxicity grade 3 to 4 affecting organs such as gastrointestinal tract, kidney, liver, nervous system, and heart according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE 2016).
-
Secondary neoplasia: as classified by the study authors.
-
Health‐related quality of life measured by validated questionnaires.
Search methods for identification of studies
Electronic searches
We conducted an electronic search in the following medical literature databases. We carefully revised the search strategies to improve precision.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8). The search strategy is shown in Appendix 1. The search dates were limited from 1 January 2012 to 6 September 2016. The search dates of the previous version included references from inception to 5 December 2012.
-
PubMed. The search strategy is shown in Appendix 2. The search dates were limited from 1 January 2012 to 6 September 2016. The search dates of the previous version in MEDLINE included references from inception to 5 December 2012.
-
Embase. We used the search term "soft tissue sarcoma". The search dates were limited from 01 January 2012 to 29 September 2016. The search dates of the previous version in Embase included references from inception to 05 December 2012.
We searched for ongoing trials by scanning the following online registries on 26 September 2016.
-
ClinicalTrials.gov (ClinicalTrials.gov). Search: diagnosis 'soft tissue sarcoma'; intervention 'stem cell transplantation'. Limits: year '2012 to 2016'; study type 'interventional studies'; phase '2' or '3'.
-
WHO International Clinical Trials Registry Platform (ICTRP). Search: diagnosis 'sarcoma'; intervention 'transplantation'. Limits: date of registration '1 January 2012 to 26 September 2016'.
We searched abstracts of annual meeting proceedings issued by the following societies on 26 September 2016:
-
American Society of Clinical Oncology (ASCO): ASCO meetings in 2012 to 2016.
-
American Society of Hematology (ASH): ASH meetings in 2013 to 2015. Search: 'autologous'.
-
Bone Marrow Transplantation (BMT) Tandem Meetings of the American Society for Blood and Marrow Transplantation (ASBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR) (BMT Tandem Meeting 2012; BMT Tandem Meeting 2013; BMT Tandem Meeting 2014; BMT Tandem Meeting 2015).
-
European Society for Blood and Marrow Transplantation (EBMT) (EBMT Meeting 2014; EBMT Meeting 2015; EBMT Meeting 2016). Search: 'sarcoma'.
-
EBMT current study list (EBMT Studies 2016).
-
International Society of Paediatric Oncology (SIOP) (SIOP Meeting 2012; SIOP Meeting 2013). Search: 'sarcoma'.
The search strategies used have been developed and executed by the author team.
Searching other resources
We planned to locate information about trials not registered in electronic databases by searching the reference lists of recently published relevant articles and review articles.
Data collection and analysis
Selection of studies
We endorsed the PRISMA statement, adhered to its principles, and conformed to its checklist (Moher 2009). We retrieved all titles and abstracts by electronic searching, downloaded them, and transferred the bibliographical data into an Excel spreadsheet. We removed duplicates and two review authors (FP, HE) examined the remaining references independently. We excluded those studies that clearly did not meet the inclusion criteria and we documented the reasons for the exclusion of studies. We resolved disagreement by discussion and it was not necessary to consult a third review author (LAS). We considered studies written in languages other than English and asked peers familiar with the particular language and with the principles of study evaluation to translate major methodologic issues. We planned to use the Google Translate 2016 program if required, but this was not necessary.
Data extraction and management
We extracted the following data as in the previous version.
-
General information on author, title, source, publication date.
-
Study characteristics: trial design, setting, inclusion and exclusion criteria, comparability of participants' characteristics between groups, treatment allocation, blinding, subgroup analysis, length of follow‐up.
-
Participant characteristics: age; gender; number of participants recruited, allocated, affected, analyzed; additional diagnoses; participants lost to follow‐up.
-
Interventions: type of HDCT, source of stem cells, and type of SDCT.
-
Outcomes: OS, TRM, DFS, PFS, EFS including type of event, toxicity, secondary neoplasia, health‐related quality of life.
Assessment of risk of bias in included studies
Two review authors (FP, LAS) independently checked the risk of bias in the included studies using the standard criteria to assess RCTs according to the Cochrane 'Risk of bias' tool (Higgins 2011a). With respect to the previous version, we removed the risk of bias items specifically aimed at checking the risk of bias in nonrandomized studies.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting such as not reporting prespecified outcomes (reporting bias).
-
Other bias.
We applied Cochrane criteria for judging risk of bias (Higgins 2011a). In general, there was a 'low risk' of bias if the bias was unlikely to seriously alter the results, for example, participants and investigators enrolling participants could not have foreseen assignment. There was a 'high risk' of bias if the bias seriously weakened confidence in the results, for example, participants or investigators enrolling participants could possibly have foreseen assignments. There was 'unclear' risk of bias if the bias raised some doubt about the results, for example, the method of concealment was not described or not described in sufficient detail to allow a definite judgment.
Measures of treatment effect
The primary effect measure was the hazard ratio (HR) for time‐to‐event data. If the HR was not directly given in the publication, we planned to estimate HRs according to methods proposed by Tierney 2007, but this was not necessary. We planned to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous outcomes, but this was not applicable. In the case of rare events, we planned to use Peto OR instead, but this was not applicable. We planned to analyze continuous data and to present them as mean differences, if all results were measured on the same scale (e.g. length of hospital stay), but this was not applicable. If this was not the case (e.g. pain or quality of life), we planned to use standardized mean differences, but this was not applicable.
Dealing with missing data
We conformed to Cochrane's principal options for dealing with missing data and analyzed only the available data (Higgins 2011b). If data were missing or only imputed data were reported, we planned to contact trial authors to request data on the outcomes among participants who were assessed.
In the previous version of the review, we contacted the authors of the study by Bui‐Nguyen 2012 by e‐mail (1 December 2012) to ask for missing data about the histologic types that were combined as 'others'. The authors responded and as a consequence we could base the inclusion or exclusion of participant data on the additional data (Table 1). In the current version of the review (September 2016), we sent e‐mail inquiries as shown in Appendix 3 to two authors (Binh Bui‐Nguyen, 3 October 2016; Jean‐Yves Blay, 5 October 2016) of the included study (Bui‐Nguyen 2012) regarding clarification of survival data and Food and Drug Administration (FDA) warning letter on objectionable conditions and inadequate responses (FDA 2015). We did not receive any reply by 7 March 2017.
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization.
Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012.
*Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification.
Assessment of heterogeneity
We had planned to assess heterogeneity between studies by visual inspection of forest plots; by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (I2 statistic) (Higgins 2003); by a formal statistical test of the significance of the heterogeneity (Cochran's Q) (Deeks 2011); and, if possible, by subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We had planned to investigate and report possible reasons if there was evidence of substantial heterogeneity. We had planned to use the random‐effects model with inverse variance weighting for statistical pooling (DerSimonian 1986). We did not pool estimates.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we had planned to assess reporting bias (such as publication bias, time lag bias, multiple (duplicate) publication bias, location bias, citation bias, language bias) by constructing a funnel plot if there were a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis otherwise the power of the tests would be too low to distinguish chance from real asymmetry (Sterne 2011)). We did not assess reporting bias because of the low number of identified studies.
Data synthesis
We analyzed data using Review Manager 5 (RevMan 2014). This was done by one review author (FP) and checked by another review author (LAS). If sufficient, clinically similar studies were available, we had planned to pool their results in meta‐analyses if they used comparable outcome definitions. As we included one RCT, we presented the results descriptively. We had planned to use random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986), but this was not applicable.
For each comparison, we prepared a 'Summary of findings' table using the GRADE profiler software (GRADEpro 2014), in which we presented the following primary outcomes: OS, TRM, DFS, PFS, non‐hematologic toxicity grade 3 to 4 and health‐related quality of life . For each outcome, two review authors (FP, LAS) independently assessed the quality of the evidence by using the five GRADE considerations, that is, study limitations, inconsistency, indirectness, imprecision, and publication bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses based on age, stage, and time period of treatment. However, we found no appropriate data to conduct these analyses.
Sensitivity analysis
We had planned sensitivity analyses to compare the results of studies with low versus high risk of bias. As we included only one study, a sensitivity analysis was not applicable.
Results
Description of studies
Clinical heterogeneity was substantial because tumor entities varied considerably between participants.
Results of the search
For this update we revised the search strategy to improve the precision and reduce the number of irrelevant hits. We identified a total of 1549 items including 85 items from electronic databases, 45 items from study registries, and 1419 items from congress proceedings (Figure 1).

Study flow diagram.
We retrieved 85 records from the electronic literature databases CENTRAL, PubMed and Embase and screened 65 different articles after removal of duplicates. The titles, abstracts or both of all 65 articles did not fulfil the inclusion criteria and we excluded the articles with reasons (Figure 1).
We identified 45 records from study registries and all 45 items did not fulfill the inclusion criteria. We retrieved 39 studies from ClinicalTrials.gov Studies 2016 and 6 studies from ICTRP Studies 2016. We identified a total of 1419 potentially relevant meeting abstracts and all 1419 items did not fulfil the inclusion criteria. We identified 830 abstracts in ASCO Meetings 2012 to 2016, 128 abstracts in ASH Meetings 2013 to 2015, 21 relevant abstracts in BMT Tandem Meeting 2012, BMT Tandem Meeting 2013, BMT Tandem Meeting 2014, BMT Tandem Meeting 2015, and BMT Tandem Meeting 2016, 149 abstracts in EBMT Meeting 2014, EBMT Meeting 2015, EBMT Meeting 2016, 52 current EBMT Studies 2016, and 239 abstracts from SIOP Meeting 2012, SIOP Meeting 2013, SIOP Meeting 2014, SIOP Meeting 2015, and SIOP Meeting 2016.
For the update, we did not identify any additional studies and it was not necessary to contact authors to for missing information.
Included studies
As the updated classification of STS included some changes, we rechecked the extracted data from the previously included RCT which remains included in the this updated version of the review. Two review authors (FP, LAS) independently checked data for study characteristics, participants and interventions, duration of follow‐up, outcomes, and deviations from the protocol. In addition, two review authors (FP, LAS) independently checked the risk of bias. We had planned to resolve differences between review authors by discussion or by appeal to a third review author, but it was not necessary. The update search did not identify any additional RCTs. Therefore, we included one RCT in this update (Bui‐Nguyen 2012). Bui‐Nguyen 2012 randomized 87 participants and included 83 participants in a modified intention‐to‐treat analysis. A detailed description of the study is shown in the Characteristics of included studies table.
Design
Bui‐Nguyen 2012 reported an RCT with two parallel treatment groups, HDCT followed by autologous HDCT versus SDCT. It was an open, multicenter, randomized phase III study. All participants received the same baseline treatment. Participants were eligible for randomization if they had responded to chemotherapy or, for stable disease, if a complete surgical resection of all disease sites could be carried out. Randomization was carried out centrally.
Sample sizes
The trial authors modified the intention‐to‐treat analysis to exclude the data for four participants who were initially randomized but found to be ineligible at central histology review (Bui‐Nguyen 2012). Initially, 87 participants were randomized: 41 in the HDCT arm versus 46 in the SDCT arm but only 83 participants were analyzed in a modified intention‐to‐treat‐analysis: 38 in the HDCT arm versus 45 in the SDCT arm.
Setting
The single included RCT was a French multicenter trial set in 16 different centers (Bui‐Nguyen 2012).
Participants
Bui‐Nguyen 2012 reported a median age of 45.8 years in the HDCT arm and 43.3 years in the SDCT arm. The proportion of males was 58.5% in the HDCT arm and 50% in the SDCT arm. A total of 19 different diagnoses were assigned to the 87 participants. In Table 1, we provide a list of all diagnoses and their incidence among the participants. We clarified the category 'Others' by contacting the trial author.
Interventions
In the study by Bui‐Nguyen 2012, 87 participants received courses one to five of SDCT. Forty‐one participants were randomized to receive HDCT and transplantation of autologous peripheral stem cells as course six in the HDCT arm. Of these, 38 participants were analyzed in a modified intention‐to‐treat analysis. Forty‐six participants were randomized to again receive SDCT as course six. Of these, 45 participants were analyzed in a modified intention‐to‐treat analysis.
Primary outcome
OS was the primary outcome of the review and the study (Bui‐Nguyen 2012). TRM was the primary outcome of the review but was a secondary outcome among adverse events of the study.
Secondary outcomes
PFS and adverse events were secondary outcomes of the review and the study (Bui‐Nguyen 2012). The trial authors evaluated complete remission as a secondary outcome, which was not considered as an outcome in this review.
Excluded studies
We excluded 65 references of the potentially relevant articles with the following reasons (Figure 1):
-
not study type of interest (14);
-
not population of interest (34);
-
not intervention of interest (16).
Excluded studies are described in the Characteristics of excluded studies table.
Risk of bias in included studies
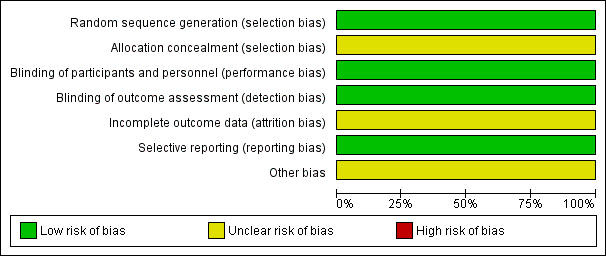
An overview of the risk of bias of Bui‐Nguyen 2012 is shown in Figure 2.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Reporting appeared to be compatible with an adequate random sequence generation and we judged it at low risk of bias. Allocation concealment was not described and it is unclear whether it was carried out adequately, therefore, we judged it at unclear risk of bias.
Blinding
The study did not address blinding of participants and blinding of outcome assessment. Nevertheless, blinding is not relevant for OS and TRM. Therefore, we judged it at low risk of bias. The previous version of this review did not judge blinding of participants and we added it to the present version. The result of the judgment of blinding of outcome assessment was changed from high risk (blinding not reported) to low risk (blinding not relevant for the primary outcomes).
Incomplete outcome data
The previous version of this review did not judge incomplete outcome data and we added it to this update. From the 87 participants, the data of four participants had not been included in the analysis. At 36 months from randomization (HDCT versus SDCT), 51 participants had died (24 in HDCT arm versus 27 in SDCT arm) and 25 were alive (eight in HDCT arm versus 17 in SDCT arm). Of the 83 participants (38 in HDCT arm versus 45 in SDCT arm) included in the modified intention‐to‐treat survival analysis, 76 participants (32 in HDCT arm versus 44 in SDCT arm) were accounted for but seven participants (six in HDCT arm versus one in SDCT arm) were not adequately explained.
Figure 1 in Bui‐Nguyen 2012 showed that 41 participants were randomized to the HDCT arm, but only 22 participants of these received HDCT and were evaluated. Also 46 participants were randomized to the SDCT arm and 40 participants received SDCT and were evaluated.
Table 2 of Bui‐Nguyen 2012 showed WHO grades 3/4 toxicity for all randomized participants, 22 in the HDCT arm and 51 in the SDCT arm. There was an inconsistency in the number of randomized and evaluated participants between Figure 1 and Table 2. It appeared conflicting that 51 participants were reported to be randomized to the SDCT arm in Table 2, although, according to Figure 1, only 46 participants were randomized and only 40 participants actually received SDCT treatment. Therefore, it appears that only 40 participants were actually eligible to evaluate adverse events after SDCT. As we were unable to contact the trial authors, we could not clarify this issue.
The potential influence of the reported missing information was unclear, therefore we judged it at unclear risk of bias.
Selective reporting
We did not identify any selective reporting and judged it at low risk of bias.
Other potential sources of bias
The previous version of this review did not judge other potential sources of bias and we added it to this update. The FDA sent a warning letter (Reference 15‐HFD‐45‐05‐01) addressed to the first author of the trial on 4 May 2015 to inform of objectionable conditions observed during an inspection at the clinical site between 17 and 19 September 2014 (FDA 2015). The inspection happened after conclusion of the study and may not be related to the risk of bias. The potential influence of this information was unclear, therefore we judged it at unclear risk of bias.
Effects of interventions
Primary outcome
Overall survival
The HR between the survival functions of the HDCT and the SDCT arms in Bui‐Nguyen 2012 was reported as 1.26 (95% CI 0.70 to 2.29; P = 0.44; 1 study, 83 participants; high quality evidence). Therefore, the data did not favor either treatment arm with respect to OS. The trial authors reported the probability of OS at three years postrandomization as 32.7% in the HDCT arm versus 49.4% in the SDCT arm. The trial authors conducted a subgroup analysis of participants who had achieved a complete response before HDCT. The estimated HR for OS of 2.92 (95% CI 1.1 to 7.6; P = 0.028; 1 study, 39 participants) favored the SDCT arm.
Treatment‐related mortality
The trial authors reported a single treatment‐related leukemia death two years after HDCT.
Secondary outcomes
Disease‐free survival
The study did not address DFS.
Progression‐free survival
The HR between the survival functions of the HDCT and the SDCT arms in Bui‐Nguyen 2012 was reported as 1.34 (95% CI 0.81 to 2.20; P = 0.25; 1 study, 83 participants). Therefore, the data did not favor either treatment with respect to PFS. The trial authors reported the probability of PFS at the time point of three years postrandomization of 9.3% in the HDCT arm versus 21.6% in the SDCT arm. The trial authors conducted a subgroup analysis of participants who had achieved a complete response before HDCT. The estimated HR for PFS of 2.87 (95% CI 1.3 to 6.3; P = 0.009; 1 study, 39 participants) favored the SDCT arm.
Event‐free survival
The study did not address event‐free survival.
Nonhematologic toxicity grade 3 to 4
The study evaluated severe adverse events in 22 participants in the HDCT arm and 51 participants in the SDCT arm according to Table 2 in Bui‐Nguyen 2012. The trial authors reported 11 events including digestive‐, infection‐, pain‐, or asthenia‐related toxicity in 22 participants of the HDCT arm and one event in 51 participants of the SDCT arm. However, the study also stated that 40 participants had been randomized to the SDCT arm. We can only assume that the authors mixed participants who were part of the randomization process and other people who were not. As it was not possible to continue a communication with the authors, we could not clarify this issue.
Secondary neoplasia
The study did not address secondary neoplasia.
Health‐related quality of life
The study did not address health‐related quality of life.
Discussion
Summary of main results
In this update, we identified no additional RCTs other than the one RCT that was included in the previous version of this review (Bui‐Nguyen 2012). The data did not favor the HDCT with respect to OS, PFS, or adverse events. The considerable heterogeneity of the tumor entities included in the study may be an important factor as OS may differ between the entities.
Overall completeness and applicability of evidence
The search was comprehensive and we considered the risk of not detecting an RCT (either published or ongoing) to be very small. The participants included in the trial were recruited from 2000 to 2008 and, considering the advancement in medicine, the results may not be applicable to the current treatment of people with NRSTS.
Quality of the evidence
Using the 'Risk of bias' tool for randomized studies we judged an overall unclear risk of bias. We judged a low risk of bias for four items (selection bias, performance bias, detection bas, and reporting bias) and unclear for the remaining three items. Each tumor entity may carry an individual risk profile and, therefore, ideally should be evaluated separately. However, the frequency of the population and the intervention of interest is tiny. In 2014, only 19 autologous HSCTs indicated for STS were registered by the European Group for Blood and Marrow Transplantation (Passweg 2016). We presented estimates in the summary of findings Table for the main comparison for outcomes that mainly constitute death as the endpoint. These outcomes included OS, TRM, and PFS. The lack of blinding did not result in judging a high risk of bias. In summary of findings Table for the main comparison, we assigned high quality with respect to those outcomes using GRADE criteria. Other outcomes were not reported or were not adequately reported. The authors reported a secondary analysis carried out to investigate the effects of surgery. According to the authors, "Overall, there were no survival differences observed (HR = 0.63, 95% CI 0.35‐1.12), according to the performance of surgery (54.3%) or not (33.2%)." We were unable to determine the number of participants in this subgroup. Therefore, we did not include the information in the 'Results' section.
Potential biases in the review process
Strengths: the search strategy was broad and it is very likely that the search identified all relevant studies. We contacted authors to request additional data.
Limitations: heterogeneity of the tumor entities and the time period of treatment may limit the conclusions that may be drawn from the data.
Agreements and disagreements with other studies or reviews
We agree with Kasper 2005 and would like to extend the views that the use of HDCT followed by autologous HSCT for locally advanced or metastatic adult STS is highly experimental, might be even be less effective than SDCT, and should not be performed outside of RCTs.

Study flow diagram.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
| Autologous hematopoietic stem cell transplantation following high‐dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcoma | ||||||
| Patient or population: people with non‐rhabdomyosarcoma soft tissue sarcoma Settings: specialized hospital Intervention: autologous hematopoietic stem cell transplantation following HDCT Comparison: SDCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| SDCT | Autologous HSCT following HDCT | |||||
| Overall survival Follow‐up: median 55 months | 489 per 1000 | 571 per 1000 | HR 1.26 | 83 | ⊕⊕⊕⊕ | ‐ |
| Treatment‐related mortality Follow‐up: 24 months | See comment | See comment | Not estimable | 83 | ⊕⊕⊕⊕ | 1 event 2 years after HDCT and 0 events after SDCT |
| Disease‐free survival | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Progression‐free survival Follow‐up: median 55 months | 756 per 1000 | 849 per 1000 | HR 1.34 | 83 | ⊕⊕⊕⊕ | ‐ |
| Non‐hematologic toxicity grade 3 to 4 | See comment | See comment | Not estimable | ‐ | See comment | Not adequately reported, people from within and without the randomization were mixed in the control arm. |
| Health‐related quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Sarcoma type | Sarcoma type 'Others' | Both arms | HDCT arm | SDCT arm |
| Leiomyosarcoma | ‐ | 16 | 7 | 9 |
| Liposarcoma | ‐ | 10 | 6 | 4 |
| Synovial sarcoma | ‐ | 9 | 2 | 7 |
| Angiosarcoma | ‐ | 6 | 2 | 4 |
| Malignant peripheral nerve sheath tumor | ‐ | 2 | 1 | 1 |
| Clear cell sarcoma | ‐ | 1 | 1 | 0 |
| Desmoplastic small round cell sarcoma | ‐ | 1 | 0 | 1 |
| Rhabdomyosarcoma | ‐ | 9 | 4 | 5 |
| Malignant fibrous histiocytoma | ‐ | 16 | 8 | 8 |
| Extraskeletal osteosarcoma | ‐ | 1 | 0 | 1 |
| Melanoma* | ‐ | 1 | 1 | 0 |
| 'Others' | Leiomyosarcoma | 1 | 1 | 0 |
| Fibrosarcoma | 1 | 1 | 0 | |
| Myofibrosarcoma | 1 | 0 | 1 | |
| Undifferentiated sarcoma | 2 | 1 | 1 | |
| Desmoplastic small round cell sarcoma | 2 | 2 | 0 | |
| Gastrointestinal stromal tumor | 1 | 0 | 1 | |
| Malignant Triton tumor | 1 | 0 | 1 | |
| Unclassified sarcoma | 1 | 1 | 0 | |
| Myoepithelioma* | 2 | 2 | 0 | |
| Endometrial stromal sarcoma* | 3 | 1 | 2 | |
| Total | 87 | 41 | 46 | |
| Not listed in the WHO classification | 6 | 4 | 2 | |
| HDCT: high‐dose chemotherapy; SDCT: standard‐dose chemotherapy; WHO: World Health Organization. Bui‐Nguyen: the table lists the sarcoma types assigned to each individual of all randomized participants of the study by Bui‐Nguyen 2012. *Soft tissue sarcomas: tumor entities not listed in either versions of the WHO classification (Fletcher 2002; Fletcher 2013), or soft tissue tumors not categorized as malignant are italicized. Myoepithelioma is categorized as an intermediate soft tissue tumor. Melanoma and endometrial stromal sarcoma are not listed in the classification. | ||||

