Риск рака яичников у женщин, принимающих препараты, стимулирующие яичники при недостаточности репродуктивной функции
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | 'Retrospective cohort study'. All women who had sought advice for infertility at 5 large reproductive endocrinology practices in the US, between 1965 to 1988. Identified from clinic records | |
| Participants | All women with primary or secondary infertility were eligible, N = 12,193, median age 30 years exposed and 30 years for unexposed | |
| Interventions | Fertility treatment, dosage and number of cycles reported 3277 (38.4%) received clomiphene, 866 (10.3%) received gonadotropins | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | The median length of follow‐up among participants was 18.8 years (range 1 to 34 years), with more than 80% followed for 15 or more years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All eligible women selected, no history of ovarian cancer at the beginning of the study and all women had at least 1 ovary |
| Confounding | Unclear risk | Adjusted analysis reported with important predictors adjusted for: age at first visit, race, gravidity, cause of infertility, ever breast‐fed, oral contraceptive use, family history of ovarian cancer, hysterectomy, tubal ligation and years of education |
| Performance bias | High risk | Medical record review, no blinding of assessors to exposure status |
| Detection bias | High risk | Information on the development of cancers was obtained from questionnaires, clinic records and cancer registries. Not reported if assessors were blind to exposure status |
| Attrition bias | Unclear risk | Almost all the women (80% 2442/12,193) were followed for 15 or more years |
| Selective reporting (reporting bias) | Low risk | Results for all the fertility drugs investigated were reported |
| Methods | 'Retrospective cohort'. All women who gave birth 1974 to 1976 in 3 major obstetric units in Israel and included in the Jerusalem Perinatal cohort study were linked with the Israel Population Registry and Israel Cancer Registry | |
| Participants | N = 15,426, mean age 27.5 exposed and NR for unexposed | |
| Interventions | Fertility treatment, dosage and number of cycles not reported. Clomiphene citrate (N = 312), human menopausal gonadotrophins (N = 61), other (N = 54), unknown (N = 87). Follow‐up by exposure group not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | 424,193 person‐years follow‐up (median 29 years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Analysis adjusted for: age at first birth, geographic origin, social class, education, parity, mean body mass index, time to conception, ovulation disorders and mechanical treatment |
| Performance bias | High risk | Questionnaires, no blinding of assessors to case‐control status reported |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status used |
| Attrition bias | Low risk | HR estimated and missing data were censored (8%) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Retrospective cohort'. All women who underwent IVF from 1981 to 1992 identified from medical records in 2 fertility clinics, Israel, and linked to Israel National Cancer Registry | |
| Participants | Women who received at least 1 treatment cycle. N = 5026. The mean age at first IVF treatment was 34.0 ± 6.4 years, and mean age at end of follow‐up was 37.5 ± 7.1 years | |
| Interventions | Fertility treatment, number of cycles reported but not dosage. Between 1 to 2 cycles, 663 women, between 3 to 5 cycles, 417 women, more or equal to 6 cycles, 174 women. Length of follow‐up by exposure status not reported, but cancer cases diagnosed within 1 year of IVF treatment were excluded | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | 18,291 women‐years follow‐up, mean follow‐up 3.6 ± 3.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for: place of birth, type of subfertility, number of IVF cycles and pregnancies |
| Performance bias | High risk | Medical record review, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | High risk | 73% (5026/18,291) of women were followed up (mean follow‐up 3.6 ± 3.4 years). Length of follow‐up by exposure status not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Retrospective cohort study'. All women with ovulatory disorders attending 2 IVF clinics from 1963 to 1999 in 2 centres in the UK. Identified from clinic records. Linked to National Health Service Central Register in England and Wales | |
| Participants | N = 7355. Mean age 28.1 years. N = 3196 (44.5%) received fertility drugs | |
| Interventions | Fertility drugs, no dosage and cycles reported (1976 (62%) used clomiphene and 1198 (38%) used clomiphene and HMG) Length of follow‐up by exposure status not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | The mean follow‐up was 21.4 years (89% of the participants were followed up for at least 10 years and 14% for at least 30 years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with ovulatory disorders and at least 1 ovary |
| Confounding | High risk | No adjusted analysis was reported |
| Performance bias | High risk | Medical notes, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Low risk | 7444/9152 (81.3%) followed up, 7355 analysed with complete data. Length of follow‐up by exposure status not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Retrospective cohort' of women who were UK residents attending 1 fertility clinic and who had received at least 1 cycle of fertility treatment from 1975 to 1989. Identified from clinic records. Linked to National Health Service Central Register in England and Wales | |
| Participants | N = 5556, age 20 years or more at the time of treatment, resident in the UK, alive and cancer‐free from 1990. Exposed group (4188, 75%) received drugs to stimulate ovulation, unexposed group did not receive drugs | |
| Interventions | Fertility treatment, number of cycles was reported but no dosage was mentioned. Fewer than 2 cycles 20 (0.5%) women, between 2 to 4 cycles 1246 (30%) women, between 5 to 9 cycles 1770 (42%) women, more and equal to 10 cycles 1152 (28%) women. Follow‐up for women who received ovarian stimulation 32,986 person‐years at risk; for women with no ovarian stimulation 9753 person‐years risk | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | Follow‐up from 1990 to 1997, 43,811 person‐years at risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women attending a single centre with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for: age at first clinical visit, years of first clinical visit, parity, time since first treatment and age at the end of follow‐up |
| Performance bias | High risk | Medical records, no blinding of assessors to exposure status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to case status |
| Attrition bias | High risk | N = 74 women (451 person‐years) excluded as follow‐up restricted to 1990 onwards rather than date of first treatment. These women had died, emigrated or were diagnosed with cancer before 1990. Follow‐up for women who received ovarian stimulation 32,986 person‐years at risk; for women with no ovarian stimulation 9753 person‐years risk |
| Selective reporting (reporting bias) | Low risk | All the fertility drugs investigated were reported |
| Methods | 'Case‐control study'. Cases were 195 women with incident epithelial ovarian cancer admitted to the major teaching and general hospitals in 4 centres. Women with borderline tumours were excluded. Controls were 1339 women from the same geographical area and admitted to the same network of hospitals as cases for a wide range of acute non‐neoplastic conditions. Women with hormonal or gynaecological diseases, or bilateral oophorectomy were excluded. From 1992 to 1993. Multicentre in Italy | |
| Participants | Age range for cases was 18 to 75 (median 55) and age range for controls was 19 to 79 years (median 56) | |
| Interventions | Use of 'fertility drugs', drug, dosage and number of cycles not reported | |
| Outcomes | Epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of follow‐up and timing of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | All women admitted with ovarian cancer |
| Confounding | Unclear risk | Factors adjusted for: age, education, parity, medical diagnosis of infertility and length of attempt to first pregnancy |
| Performance bias | High risk | Self reported during an interview, unclear if interviewers were blinded to case‐control status |
| Detection bias | Low risk | Epithelial ovarian cancer by histological diagnosis |
| Attrition bias | Unclear risk | Unclear if exclusions based on incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all the fertility drugs investigated were reported |
| Methods | 'Nested case‐control study'. Women with subfertility problems and referred to all Danish private fertility clinics or hospitals 1963 to 1998, and all women with ICD diagnosis of infertility from the national patient registry (a nationwide register of virtually all discharges for somatic conditions from Danish hospitals since 1977). Linked to civil registration database to obtain date of migration or death. Linked to Danish cancer registry and Danish registry of pathology for ovarian cancer diagnosis. Cases were women with ovarian cancer by 30 June 2006. Controls were randomly selected in 4 age strata and 5 strata according to year of entry to cohort | |
| Participants | Cohort comprised N = 54, 449 women with primary or secondary infertility, N = 176 cases; 1360 controls. Median age at first evaluation of infertility was 30 years (range 16 to 55) and median age at the end of follow‐up was 47 (range 18 to 81) years | |
| Interventions | Fertility drugs, number of cycles reported for each drug but dosage not reported. Gonadotrophins 1 to 4 cycles 18/130, 5 to 9 cycles 7/46 women, more or equal to 10 cycles 1/8. Clomiphene citrate from 1 to 4 cycles 35/226 women, 5 to 9 cycles 15/117 women, more or equal to 10 cycles 8/74 women. HCG between 1 to 4 cycles 31/232 women, 5 to 9 cycles 13/121 women and more or equal to 10 cycles 5/60 women. GnRH between 1 to 4 cycles 14/100 women, 5 to 9 cycles 1/10 women and more or equal to 10 cycles 0 women. Duration of follow‐up by fertility drug not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | 95% of women (54,362) were followed up for a median of 16.0 years (range 0.0 to 42.6 years), with 25% followed for more than 23 years. 957,454 person‐years of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with infertility treated at either a private clinic or public hospital or with a diagnosis of infertility on national disease registry. No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for: parity, number of births, maternal age at birth of first child and maternal age at birth of last child |
| Performance bias | High risk | Medical records, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Low risk | 95% of women had similar length of follow‐up |
| Selective reporting (reporting bias) | Low risk | All the fertility drugs investigated were reported |
| Methods | Retrospective cohort, Sweden, multicentre | |
| Participants | All women who gave birth following IVF treatment during 1982‐2007, identified from all IVF clinics in Sweden and Swedish Medical Birth Register (24,058). A control group comprised 95,775 women recorded in Medical Birth Register. The mean age at first delivery after IVF was 40.3 years | |
| Interventions | There was no clear report of the number of IVF cycles, dosage and type of fertility drugs used | |
| Outcomes | Ovarian cancer by histological diagnosis, Swedish Cancer Registry | |
| Notes | Average follow‐up time was 8.3 years for IVF women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All eligible women selected, no history of ovarian cancer at the beginning of the study and all women had at least 1 ovary |
| Confounding | Unclear risk | Adjustment was made in the analysis for maternal age and year of birth, smoking and parity |
| Performance bias | High risk | Medical record review, no blinding of assessors to case‐control status |
| Detection bias | Unclear risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | High risk | 75% (24,058/95,775) of women were followed up (mean follow‐up time 8.3 years) |
| Selective reporting (reporting bias) | Unclear risk | Dosage, number of IVF cycles and type of drugs used were not reported |
| Methods | Retrospective case‐control study. Multicentre in USA | |
| Participants | Participants were residents in Western Pennsylvania, Eastern Ohio and Western New York state participating in the Hormones and Ovarian cancer Prediction study (national population‐based study). All cases were histologically confirmed to have primary epithelial ovarian cancers diagnosed between 2003 and 2008. Eligible women were at least 25 years old and were within 9 months of initial diagnosis at the time of recruitment. A total of 155 cases. 290 controls were frequency matched to cases (about 2:1) by 5‐year age group and telephone area code through random digit dialling. Women who had undergone a bilateral oophorectomy were ineligible. Trained interviewers collected questionnaire data that included detailed reproductive, gynaecologic and medical histories as well as information about lifestyle and family medical history. Mean age for cases and controls was not reported | |
| Interventions | Fertility drugs used were: raloxifene, danazol, unknown hormone pills, bromocriptine, progesterone and metformin. Fertility drugs doses was not reported. The majority used fertility drugs for less than 12 months (66.7%); mean duration was 11.4 months (range 1 to 134 months). Among the cases 105/155 (67%) were not exposed to fertility drugs and 50/155 (32%) were exposed. Among the controls 192/290 (66%) were not exposed to fertility drugs and 98/290 (34%) were exposed to fertility drugs | |
| Outcomes | Invasive epithelial ovarian cancer by histological diagnosis | |
| Notes | Duration of exposure was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Only live cases included and women who had a confirmed histological diagnosis and returned a questionnaire about exposure |
| Confounding | Low risk | Matched for age at the time of the diagnosis and area of residence. Factors adjusted for age, race, education, tubal ligation, age at menarche, duration of oral contraceptive use, number of live births, duration of breast‐feeding, perineal talc use and family history of breast/or ovarian cancers |
| Performance bias | High risk | Self reported by questionnaire of exposure status. Unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Low risk | 71% (902/1270) of the total cases eligible returned the questionnaire. 97% (1802/1844) of the controls participated to the study |
| Selective reporting (reporting bias) | Low risk | Results for all the drugs investigated were reported |
| Methods | 'Retrospective cohort'. All infertile women who attended 1 IVF clinic and who received at least 1 treatment cycle in Israel from 1984 to 1992 identified from the medical records. Linked to the Israel National Cancer Registry | |
| Participants | N = 1082 with 7002 person‐years follow‐up. The mean age at the first IVF treatment was 32.7 ± 4.8 years, and the mean age at the end of the follow‐up 38.7 ± 5.2 years | |
| Interventions | Fertility drug not reported. 650 women received 1 to 2 cycles of treatment, 323 received 3 to 5 cycles and 109 received more than 6 cycles | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | Mean years of follow‐up 6.5 ± 2.2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary. Women with cancer diagnosed within 1 year of IVF treatment excluded from analyses |
| Confounding | Unclear risk | Factors adjusted for: continent of birth, type of infertility, diagnosis of infertility, number of IVF cycles and treatment outcome (pregnancy or not) |
| Performance bias | High risk | Medical records, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 85% (1082/7002) of women were followed up |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all the fertility drugs investigated and reported |
| Methods | Retrospective cohort, Israel 1964‐1974, only 1 centre | |
| Participants | 2431 subfertile women treated at the Sheba Medical Center compared to the general population | |
| Interventions | Fertility treatment with clomiphene (N = 884), clomiphene and HMG (N = 238) and with HMG (N = 159) | |
| Outcomes | Ovarian cancer by histological diagnosis | |
| Notes | Mean age at the end of the follow‐up 62.7 +/‐ 8.1 years. 88,181 person‐years follow‐up (over 30 years follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women coming to the infertility centre where the study was started. All women had no history of ovarian cancer at the beginning of the study and at least 1 ovary |
| Confounding | Unclear risk | Adjusted analysis was not reported |
| Performance bias | High risk | Medical notes review, no blinding of assessors to exposure status |
| Detection bias | High risk | Information on the development of cancer was obtained from a cancer registry. Not reported whether assessors were blind to exposure status |
| Attrition bias | Low risk | Almost all the women (94%) were followed up (2431/2575) throughout the time |
| Selective reporting (reporting bias) | Unclear risk | Type of drugs used was reported. There was no information about dosage of drugs used and number of cycles |
| Methods | 'Retrospective cohort' of women diagnosed with infertility 1964 to 1974 and who had visited the clinic more than once (2 centres in Israel) identified from medical records. Linked to Israel Cancer Registry | |
| Participants | Women with primary or secondary infertility. N = 2496. Mean age at entry was 28.7, mean age at the end of the follow‐up was 50.0 | |
| Interventions | Fertility treatment, 908 women with clomiphene citrate, 242 women with clomiphene citrate + HMG, 159 women with HMG. No dosage or number of cycles were reported. Women received at least 1 cycle of fertility drugs. Duration of follow‐up by exposure group not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 1) | |
| Notes | 54,413 person‐years follow‐up, mean follow‐up 21.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | High risk | Adjusted analysis not reported |
| Performance bias | High risk | Medical records, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 96% of women followed (2496/54,413) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Prospective case‐control study'. Cases were all women with a first diagnosis of ovarian cancer 1989 to 1994 selected from the Danish Cancer Registry with histological diagnosis and who returned a completed questionnaire with exposure data (N = 684). A random sample of 3 controls per case were selected from the National Person Register, matched by area of residence, age at time of cancer diagnosis, with at least 1 ovary and completed questionnaire from 1989 to 1994. Multicentre in Denmark, but number of centres not reported | |
| Participants | N = 1721 women. Mean age for cases = 47.2 (range 18 to 59). Mean age for controls = 46 (range 19 to 59) | |
| Interventions | Fertility drugs, dosage and number of cycles not reported. 28/684 (20.7%) cases were exposed to infertility drugs and 58/1721 (23.8%) controls were exposed to fertility drugs | |
| Outcomes | Invasive epithelial and non‐epithelial ovarian cancer by histologic diagnosis (see Table 2) | |
| Notes | Duration of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Only live cases included and women who had a confirmed histological diagnosis and returned a questionnaire about exposure |
| Confounding | Unclear risk | Matched for age at time of diagnosis and area of residence. Factors adjusted for: age, menarche, parity, age at first birth, duration of infertility, other causes of infertility, use of oral contraceptive pill, use of intrauterine devices, menopausal status, age at menopause, use of hormonal replacement therapy, age at sterilisation, history of cancer and family history for cancer, smoking and body mass index |
| Performance bias | High risk | Self reported exposure status. Unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 88% questionnaires returned for cases, 79.8% for controls. 80.7% of questionnaires for the cases were valid for analysis and 97% of the questionnaires were valid for the controls |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Case‐control study'. All Danish women < 60 years with histologically confirmed borderline ovarian tumours identified from the Danish Cancer Registry 1989 to 1994 with histological diagnosis and who returned a completed questionnaire with exposure data (N = 263). Random sample of 3 controls per case were selected from the National Person Register, matched by area of residence, age at time of cancer diagnosis and completed a questionnaire. National study in Denmark from 1989 to 1994 | |
| Participants | N = 1721 women with at least 1 ovary. Mean age for cases 43.6 (range 22 to 59). Mean age for controls 46 (range 19 to 59) | |
| Interventions | Fertility drugs, dosage and number of cycles not reported | |
| Outcomes | Borderline ovarian cancer by histologic diagnosis (see Table 2) | |
| Notes | Duration of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Live cases only and women who responded to questionnaire on exposure |
| Confounding | Unclear risk | Case and controls were matched for age at time of diagnosis, area of residence. Factors adjusted for: parity, use of oral contraceptive pill, menopause, use of hormonal replacement therapy and smoking |
| Performance bias | High risk | Self reported (type of treatment ‐ oral/injections) with some checks with the fertility clinics for confirmation. No blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 87.8% questionnaires were returned and they were all selected for cases to analyse; 79.8% questionnaires were returned for controls and all were used for analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Case‐control study'. Cases were women < 75 years diagnosed with invasive ovarian cancer within 1 year of interview and admitted to a major teaching or general hospital in Milan, Italy from 1983 to 1991. Controls were women admitted to the same hospitals where the cases were identified with acute non‐gynaecological, non‐hormonal or non‐neoplastic conditions | |
| Participants | N = 971 cases, age 22 to 74 (median 54 years). N = 2758 controls, age 23 to 74 (median 52 years) | |
| Interventions | Fertility drugs, number of cycles reported but not dosage used per cycle. Fewer than or equal to 6 cycles 1/971 cases and 3/2758 controls. More than or equal to 6 cycles 4/971 cases and 7/2758 controls | |
| Outcomes | Invasive epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of exposure per number of cycles reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographic area |
| Confounding | Unclear risk | Factors adjusted for: age, education, parity, oral contraceptive use, difficulties in conception |
| Performance bias | High risk | Questionnaires, no blinding of assessors to case‐control status used |
| Detection bias | High risk | How the cases were ascertained was not reported and blinding of assessors to exposure status was used |
| Attrition bias | Low risk | Case and controls assessed for exposure and outcome at the same time when admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all the fertility drugs used were investigated |
| Methods | 'Case‐control study'. Cases were women with histologically confirmed borderline ovarian tumours admitted to 1 hospital in Milan, Italy. Controls were women admitted to hospitals serving the same catchment area as the cases lived in with acute non‐gynaecological, non‐hormonal, non‐neoplastic conditions from 1986 to 1991 | |
| Participants | N = 93 cases, age 23 to 64 years. N = 273 controls, age 24 to 64 years | |
| Interventions | Fertility drugs, dosage and number of cycles not reported. 4/93 (4.3%) cases and 0/273 controls exposed to fertility drugs | |
| Outcomes | Borderline ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | States that cases in this report were not included in previous articles on relationship between fertility drugs and ovarian cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographic area |
| Confounding | Unclear risk | Factors adjusted for: age, education, parity, oral contraceptive use and difficulty in conception |
| Performance bias | High risk | Face‐to‐face interview. Blinding unclear |
| Detection bias | High risk | How the cases were ascertained was not reported and no binding of assessors to exposure status |
| Attrition bias | Unclear risk | Case and controls assessed for exposure and outcome at the same time when admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all the fertility drugs used and investigated |
| Methods | 'Case‐control study'. Cases were women with incident histologically confirmed ovarian cancer admitted to the major teaching and general hospitals in 4 geographic regions in Italy (women with borderline tumours were excluded) from 1992 to 1999. Controls were women from the same geographical area and admitted to the same network of hospitals as the cases for a wide range of acute non‐neoplastic conditions (women with hormonal or gynaecological diseases, or bilateral oophorectomy were excluded) | |
| Participants | N = 1031 cases, median age 56, range 18 to 79 years; N = 2411 controls, median age 57, range 17 to 79 years | |
| Interventions | Fertility drugs, dosage and number of cycles not reported. 15/1031 (1.5%) cases were exposed to fertility drugs and 26/2411 (1.1%) controls were exposed to fertility drugs | |
| Outcomes | Epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Length of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographic area |
| Confounding | Unclear risk | Factors adjusted for: age, education, menopausal status, age at menopause, parity, spontaneous miscarriages, termination of pregnancy, oral contraceptive use, family history for ovarian cancer and history of infertility |
| Performance bias | High risk | Structured interviewer‐administered questionnaire and checked with medical records. Unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | How the cases were ascertained has not been specified and it is unclear if blinding of assessors to exposure status was used |
| Attrition bias | Unclear risk | Case and controls assessed for exposure and outcome at the same time as admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
| Methods | 'Retrospective cohort'. All women with infertility attending 1 fertility clinic (Soroka University Hospital), Israel, 1960 to 1984. Identified from medical records. Linked to the National Cancer Registry | |
| Participants | Women with at least 2 recorded visits to the clinic. N = 1197. Mean age at first visit 27.5 ± 5.4 years, mean age at the end of the follow‐up 44.8 ± 6.4 years for cohort. Mean age for exposed at first visit 27.5 ± 5.1, mean age at the end of follow‐up for exposed 27.7 ± 5.8 years, mean age at the first visit for unexposed 27.7 ± 5.8, at the end of the follow‐up 44.8 ± 7.1 years for unexposed | |
| Interventions | Infertility treatment, 0 with clomiphene citrate, 531/780 treated with clomiphene citrate + HMG, 6/780 treated with HMG. 780 (65.2%) exposed to fertility drugs. Duration of follow‐up for women exposed to fertility drugs 18.0 ± 4.9 years, non‐exposed 17.6 ± 5.9 years | |
| Outcomes | Ovarian cancer by histological diagnosis recorded on the National Cancer Registry (see Table 1) | |
| Notes | 21,407 person‐years follow‐up. Mean follow‐up 17.9 ± 5.3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with infertility that attended a particular clinic. No person had ovarian cancer at the start of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for: age and ethnic origin |
| Performance bias | High risk | Medical records, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | Case ascertainment 90% to 95% complete. Missing data by exposure group not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
| Methods | 'Nested case‐control study'. The cohort (N = 3837) comprised women undergoing fertility treatment at participating clinics in Seattle, US from 1974 to 1985. Cases were women with ovarian cancer after enrolment in the study until 1992 identified from cancer registry. Controls were a random selection of women from the cohort stratified by age at enrolment 3:1 for each case within each strata | |
| Participants | Women that had made at least 2 clinic visits and lived in an area covered by the cancer surveillance system. Mean age of women at enrolment 29.7 years | |
| Interventions | Clomiphene dosage and number of cycles not reported | |
| Outcomes | Ovarian cancer by histological diagnosis recorded in cancer surveillance system (see Table 1) | |
| Notes | 43,438 person‐years of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No person had ovarian cancer at the start of the study and with at least 1 ovary |
| Confounding | Unclear risk | Adjusted analysis presented, but factors adjusted for not reported |
| Performance bias | High risk | Medical records, no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 74.2% of the controls were eligible to be interviewed |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
| Methods | 'Population‐based case‐control study'. From 1994 to 1998 in 3 regions (Atlanta, Georgia; Detroit, Michigan; Seattle, Washington) in the US (cancer registry ‐ local US born, with no history of breast cancer (to match the controls)). Cases identified from cancer registry. Controls randomly selected from the Women's Contraceptive and Reproductive Experiences (CARE) study of breast cancer (English speaking women born in the US, white/black, in 5 geographic regions), age 35 to 64 at reference date | |
| Participants | N = 378 cases; N = 1637 controls. Age range between 35 and 54 for cases and 35 and 64 for controls | |
| Interventions | Fertility drugs, dosage and cycles not reported | |
| Outcomes | Epithelial, non‐epithelial and borderline ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Controls were more likely to have Black ethnicity 27.1% versus 13.5%. Length of follow‐up from exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Only women still alive were selected as cases; controls were matched on geographic area and age |
| Confounding | Unclear risk | Factors adjusted for: study site, race, age, marital status, education, cigarette smoking, age at menarche, oral contraceptive use in months |
| Performance bias | High risk | Information obtained through face‐to‐face interview and not medical records. Interviewers were not blinded to case‐control status |
| Detection bias | High risk | Cancer registry |
| Attrition bias | Unclear risk | Length of follow‐up from exposure not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all the fertility drugs used were investigated |
| Methods | 'Retrospective cohort'. Women with infertility or infertility‐associated disorders attending 3 university hospital fertility clinics in Sweden from 1961 to 1975. Linked to Swedish Cancer Register | |
| Participants | N = 2768, median age 27 (16 to 45) exposed. N = 1615 (58%) unexposed who did not receive hormonal treatment. Median age 27 (16 to 45) | |
| Interventions | Fertility treatment, 389 (34%) with clomiphene citrate; 325 (28%) gonadotrophins and 439 (38%) with clomiphene citrate + HMG. The median follow‐up time for the cohort was 33 years (range 1 to 47 years). Duration of follow‐up by exposure group was not reported | |
| Outcomes | Primary invasive epithelial or borderline ovarian cancer by histological diagnosis obtained from National Cancer Registry (see Table 1) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with at least 1 ovary and no history of ovarian cancer |
| Confounding | High risk | No adjusted analysis reported |
| Performance bias | High risk | Medical record review at the IVF clinics. Any exposure outside the IVF clinics included was unknown. No blinding of assessors to case‐control status reported |
| Detection bias | High risk | Cancer registry, no blinding of assessors to exposure status was reported |
| Attrition bias | Unclear risk | 81% of women were followed up |
| Selective reporting (reporting bias) | Low risk | All the fertility drugs used were reported |
| Methods | 'Population‐based case‐control study' in Israel. Cases were women with invasive and borderline epithelial ovarian cancer reported to registry from 1990 to 1993. Cases were selected from National Cancer Registry (only included living cases). Controls were randomly selected from same telephone dialling code (matched for geographic area) | |
| Participants | N = 164 cases with invasive cancer; N = 36 cases with borderline cancer, N = 408 controls | |
| Interventions | Fertility drugs, dosage and number of cycles were not reported | |
| Outcomes | Primary invasive epithelial or borderline ovarian cancer by histological diagnosis from Israel Cancer Registry (see Table 2) | |
| Notes | Length of follow‐up post‐exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Only women still alive were selected as cases; controls were matched on geographic area and age |
| Confounding | Unclear risk | Factors adjusted for: age, parity, BMI, region of birth, education, family history, interviewer |
| Performance bias | High risk | Self reported during an interview, interviewer not blind to case‐control status |
| Detection bias | High risk | Cancer registry |
| Attrition bias | Unclear risk | 200/287 (70%) of living selected cases interviewed. Length of follow‐up post‐exposure not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if other fertility drugs investigated but not reported |
| Methods | Historical cohort (OMEGA), Netherlands, multicentre (12 hospitals) | |
| Participants | Subfertile women who received at least 1 IVF cycle with ovarian stimulation (19,861) 1983‐1995. The control group comprised subfertile women not treated with IVF (6604) selected from the 4 IVF clinics with a computerised registry of all subfertile women evaluated 1980‐1995 before IVF was a routine procedure. Mean age of IVF‐treated women was 47.5 and for women who did not receive IVF was 49.4 years | |
| Interventions | In the IVF group 32.9% of women had 1 to 2 stimulated IVF cycles, 32.8% had 3 to 4 cycles and 17.5% received 5 or more cycles. Clomiphene/HMG or FSH/HMG stimulation protocols were used until 1988‐1989, whereas stimulation with GnRH agonists became more common after 1990 (from 20% in 1986 to about 90% after 1990). From 1984 to 1994, the number of ampoules of gonadotrophins strongly increased | |
| Outcomes | Ovarian cancer including borderline ovarian tumours by histological diagnosis; linkage with national cancer registry | |
| Notes | Median duration of follow‐up was for the exposed 14.3 years and for the non‐exposed 16.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary. Women in cohort who did not receive IVF were slightly older and had a slightly longer median duration of follow‐up than women who did receive IVF |
| Confounding | High risk | Analysis was adjusted for age at the end of the follow‐up, endometriosis, tubal problems and parity |
| Performance bias | Unclear risk | Information based on medical records and for women without medical record data, information was added from health questionnaire |
| Detection bias | Unclear risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | High risk | Analytic cohort 19,146 IVF treated, 6006 non‐IVF treated. 67.3% responded and consented to future record linkage, 4.3% responders refused, 28.2% non‐responders, 0.2% were deceased at initial approach of IVF group. 40.7% responded and consented to future record linkage, 3.1% responders refused, 55.4% non‐responders, 0.9% were deceased at initial approach of non‐IVF group |
| Selective reporting (reporting bias) | Unclear risk | 10,343/19,146 (54%) at 10 years follow‐up and 7621/19,146 (40%) at more than 15 years of follow‐up |
| Methods | 'Retrospective cohort'. Women who registered with at least 1 of 10 participating clinics in Australia before 1994. 30% before 1986, 70% 1986 to 1996. Linked to cancer registry | |
| Participants | Women who received at least 1 IVF treatment. N = 29,700, median age 31 (range 18 to 50) in exposed, median 30 (range 18 to 53) in unexposed | |
| Interventions | Fertility treatment used, 1182 (6.9%) with clomiphene citrate, 6543 (38.2%) with clomiphene citrate + HMG, 1464 (8.5%) with HMG, 11,153 (65%) with HMG + GnRH agonist, 1771 (8.6%) with other treatments NR. Dosage NR. 6346 (37.0%) with 1 cycle, 3712 (21.6%) with 2 cycles, 5157 (30.1%) between 3 and 5 cycles, 1933 (11.3%) more than 6 cycles. 134,240 person‐years follow‐up in exposed, 96,794 person‐years in unexposed. Median follow‐up in exposed 7 (range < 1 to 21) years; in unexposed median 10 (< 1 to 22) years | |
| Outcomes | Invasive ovarian cancer by histological diagnosis from the Victoria Cancer Registry (see Table 1) | |
| Notes | 80% of the cohort sample was followed up until 1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No person had ovarian cancer at the start of the study and had at least 1 ovary |
| Confounding | High risk | No adjusted analysis reported and groups were not matched or balanced for confounding factors at baseline |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | 81% exposed and 72% unexposed were followed up |
| Selective reporting (reporting bias) | Low risk | All the drugs used were reported |
| Methods | Retrospective cohort, Finland 1996‐1998, single centre | |
| Participants | Subfertile women (N = 9175) who purchased drugs for IVF between 1996‐1998 and their age and residence‐matched controls randomly selected from the general population register (N = 9175) | |
| Interventions | Fertility treatment but dosage, number of cycles and type of drugs used was not reported | |
| Outcomes | Ovarian cancer by histological diagnosis, Finnish cancer registry | |
| Notes | Mean follow‐up time for exposed subfertile women was 7 years and 9 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Cases were age and residence matched with controls and further adjusted for socioeconomic position and marital status |
| Performance bias | High risk | Medical record review, no blinding of assessors to exposure status |
| Detection bias | Unclear risk | Information on the development of the cancers was obtained from the medical notes and cancer registry. Not reported if assessors were blind to exposure status |
| Attrition bias | Low risk | All women (9175) were followed up for 7 years and 9 months |
| Selective reporting (reporting bias) | Unclear risk | The fertility drugs used were not reported |
amp: ampoule
BMI: body mass index
FSH: follicle‐stimulating hormone
GnRH: gonadotrophin‐releasing hormone
HCG: human chorionic gonadotrophin
HMG: human menopausal gonadotrophin
HR: hazard ratio
ICD: International Classification of Diseases
IVF: in vitro fertilisation
NR: not reported
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| General article about risk factors for ovarian cancer | |
| General article | |
| Review article | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| General article in infertility and risk of ovarian cancer | |
| Case report | |
| Case series (fewer than 30 patients) | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility | |
| General article on infertility and risk of ovarian cancer | |
| Case series (fewer than 30 patients) | |
| General article on infertility and risk of ovarian cancer | |
| Case report | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility | |
| Review article | |
| Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs | |
| Review article | |
| Review article | |
| Review article | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility and risk of ovarian cancer | |
| Cohort study about other risk factors (no infertility or infertility drugs included) for ovarian cancer | |
| Review article | |
| Unpublished data. Abstract not fully informative about risk calculated by authors | |
| Case‐control study evaluating risk of borderline ovarian cancer. Controls were women treated for benign ovarian pathology requiring surgery. Crude estimates only presented, no attempt at controlling for confounding. No details on how ovarian cancer was confirmed | |
| Review article on infertility and ovarian cancer | |
| Review article on infertility and risk of ovarian cancer | |
| Case series (fewer than 30 patients) | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| Pooled analysis of 3 European case‐control studies | |
| Case series (fewer than 30 patients) | |
| Review article | |
| Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| Case series (fewer than 30 patients) | |
| Research article | |
| Review article | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Collaborative analysis of 12 US case‐control studies | |
| Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs | |
| General article on infertility and risk of ovarian cancer | |
| Collaborative analysis of 12 US case‐control studies | |
| Cohort study on reproductive factors and risk of breast cancer | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of breast cancer | |
| Review article | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility and ovarian cancer | |
| General article on infertility | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| Review article | |
| Review article | |
| Cohort study on reproductive factors and risk of breast cancer | |
| Meta‐analysis of only some of the cohort studies published on the risk of ovarian cancer in women treated with ovulation stimulation drugs | |
| General article on infertility and ovarian cancer | |
| Review article | |
| General article on infertility and risk of ovarian cancer | |
| General article on infertility and risk of ovarian cancer | |
| Article on sensitivity and specificity of possible ovarian cancer screening | |
| General article on infertility/article in Chinese (abstract in English) | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Pooled analysis of case‐control studies | |
| Pooled analysis of case‐control studies | |
| Review article | |
| General article on the risk of ovarian cancer | |
| General article on infertility and risk of ovarian cancer | |
| Review article on infertility and risk of ovarian cancer | |
| Review article on infertility and risk of ovarian cancer | |
| Research article | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| General article on infertility/article in Swedish (abstract in English) | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| General article on infertility and ovarian cancer | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Reported only risk of ovarian cancer in infertile women but not treated with ovarian stimulating drugs | |
| Review article | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| General article on infertility | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of breast cancer | |
| General article on infertility and risk of ovarian cancer | |
| Review article | |
| A meta‐analysis on risk of ovarian cancer and women treated with ovarian stimulating drugs for infertility | |
| General article on infertility and ovarian cancer | |
| General article on infertility and ovarian cancer | |
| Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| General article on infertility and ovarian cancer | |
| General article on infertility and ovarian cancer | |
| Review article | |
| Article in Bulgarian/review article | |
| Case series (fewer than 30 patients) | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Review article | |
| Sub‐set of larger cohort described in Venn 1999 | |
| Review article | |
| Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer | |
| Review article | |
| Review article | |
| Commentary/letter | |
| Case series (fewer than 30 patients) | |
| Review article |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Borderline ovarian cancer Show forest plot | 5 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Infertility drugs versus no infertility drug, Outcome 1 Borderline ovarian cancer. | ||||
| 1.1 Any infertility drug | 4 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clomiphene | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Clomiphene + gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Invasive ovarian cancer Show forest plot | 16 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Infertility drugs versus no infertility drug, Outcome 2 Invasive ovarian cancer. | ||||
| 2.1 Any infertility drug | 12 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clomiphene | 8 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Clomiphene + gonadotrophin | 6 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Gonadotrophin | 6 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 GnRH | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
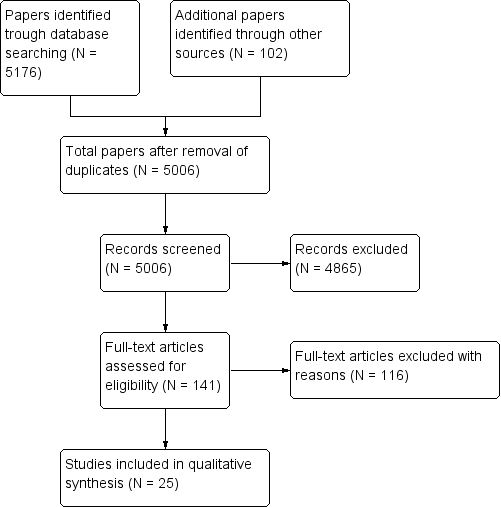
Identification and selection of studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Infertility drugs versus no infertility drug, outcome: 1.2 Invasive ovarian cancer.

Forest plot of comparison: 1 Infertility drugs versus no infertility drug, outcome: 1.2 Borderline ovarian cancer.

Comparison 1 Infertility drugs versus no infertility drug, Outcome 1 Borderline ovarian cancer.

Comparison 1 Infertility drugs versus no infertility drug, Outcome 2 Invasive ovarian cancer.
| Cohort studies | |||||||
| Study | Ovarian cancer type | Total number of exposed cases versus unexposed | Drug, dose and number of cycles | Crude estimate (95% CI) | Adjusted estimate RR (95% CI) | Factors adjusted for | Standardised incidence ratio (SIR) |
| Modan 1998 | Invasive | 1309 versus 1187 | Fertility drugs | — | — | — | 1.6 (0.8 to 2.9) |
| Clomiphene citrate/HMG | — | — | — | Not calculated | |||
| Clomiphene citrate | — | — | — | 2.7 (0.97 to 5.8) | |||
| HMG | — | — | — | Not calculated | |||
| Potashnik 1999 | Invasive | 780/417 | Fertility drugs | — | — | — | 0.91 (0.10 to 3.27) |
| Venn 1999 | Invasive | 20,656 versus 9044 | Fertility drugs | — | — | — | Any drug: 0.88 (0.42 to 1.84) 1 to 2 cycles IVF: 1.26 (0.41 to 3.90) 3 to 5 cycles: 0.71 (0.10 to 5.03) > 6 cycles: 2.00 (0.3 to 14.2) |
| Clomiphene citrate | — | — | — | 2.46 (0.35 to 17.5) | |||
| Clomiphene citrate + HMG | — | — | — | 0.77 (0.19 to 3.07) | |||
| HMG | — | — | — | 1.14 (0.16 to 8.10) | |||
| HMG + GnRH | — | — | — | 0.48 (0.07 to 3.38) | |||
| Doyle 2002 | Invasive | 4188 versus 1231 | Fertility drugs | — | Hazard rate ratio 0.59 (0.12 to 3.00) | Age at first clinical visit, years of first clinical visit, parity, time since first treatment and age at the end of follow‐up | — |
| Dor 2002 | Invasive | 1254 versus 3772 | Fertility drugs | — | — | — | 1 to 2 IVF cycles: 0.98 (0.45 to 1.86) 3 to 5 IVF cycles: 1.21 (0.52 to 2.39) > 6 cycles: 0 (0 to 1.20) |
| Lerner‐Geva 2003 | Invasive | 1082 versus 5920 | Fertility drugs | — | — | — | 5.0 (1.02 to 14.6) |
| Brinton 2004 | Invasive | 4143 versus NR | Clomiphene citrate | Rate ratio 0.82 (0.4 to 1.5) | 1 to 900 mg rate ratio: 0.94 (0.4 to 2.3) 901 to 2250 mg: 0.71 (0.2 to 2.0) > or equal to 2251 mg: 0.80 (0.3 to 2.1) < 6 cycles: 0.85 (0.4 to 1.7) 6 to 11 cycles: 0.44 (0.1 to 1.9) > or equal to 12 cycles: 1.54 (0.5 to 5.1) | Age, calendar time, area of residence, parity at first visit | — |
| Gonadotrophins | Rate ratio 1.09 (0.4 to 2.8) | Dosage (amps) 1 to 24: rate ratio 1.36 (0.3 to 5.7) > or equal to 25: 0.96 (0.3 to 3.1) 1 to 2 cycles: 0.95 (0.2 to 3.9) > or equal 3 cycles: 1.21 (0.4 to 3.9) | Age, calendar time, area of residence, parity at first visit | — | |||
| Gonadotrophins + clomiphene citrate | — | Rate ratio 1.02 (0.3 to 2.8) | Age, calendar time, area of residence, parity at first visit | — | |||
| Calderon‐Margalit 2009 | Invasive | 929 versus 14,463 | Fertility drugs | HR 0.61 (0.08 to 4.42) | Age | — | |
| Clomiphene citrate | HR 0.98 (0.14 to 7.11) | Age | — | ||||
| Sanner 2009 | Invasive | 1153 versus 1615 | Fertility drugs | — | — | — | 1.19 (0.54 to 2.25) |
| Borderline | Fertility drugs | — | — | — | 2.62 (1.35 to 4.58) | ||
| Invasive | Gonadotrophins | RR 3.55 (1.23 to 10.24) | 5.21 (1.67 to 16.20) (a) 5.28 (1.70 to 16.47) (b) | a) Age and indication b) Pregnancy during follow‐up | 2.29 (0.84 to 4.97) | ||
| Borderline | Gonadotrophins | RR 0.95 (0.11 to 8.11) | 1.11 (0.12 to 10.17) (a) 1.12 (0.12 to 10.32) (b) | a) Age and indication b) Pregnancy during follow‐up | 1.88 (0.05 to 10.45) | ||
| Invasive | Clomiphene citrate | RR 1.12 (0.24 to 5.29) | 1.52 (0.3 1,7.79) (a) 1.57 (0.32,7.62) (b) | a) Age and indication b) Pregnancy during follow‐up | 0.92 (0.11 to 3.32) | ||
| Borderline | Clomiphene citrate | RR 2.70 (0.64 to 11.28) | 3.06 (0.69 to 13.68) (a) 3.25 (0.72 to 14.51) (b) | a) Age and indication b) Pregnancy during follow‐up | 4.59 (0.95 to 13.42) | ||
| Invasive | Clomiphene citrate + gonadotrophins | RR 0.48 (0.06 to 3.80) | 0.72 (0.09 to 6.00) (a) 0.74 (0.09 to 6.22) (b) | a) Age and indication b) Pregnancy during follow‐up | 0.36 (0.01 to 2.00) | ||
| Borderline | Clomiphene citrate + gonadotrophins | RR 2.28 (0.55 to 9.54) | 2.70 (0.58 to 12.65) (a) 2.90 (0.62 to 13.55) (b) | a) Age and indication b) Pregnancy during follow‐up | 3.99 (0.82 to 11.67) | ||
| Dos Santos Silva 2009 | Invasive | 3194 versus 3976 | Fertility drugs | — | 1.42 (0.53 to 3.99) | Age, calendar time, area of residence, parity at first visit | 1.10 (0.57 to 1.93) |
| Kallen 2011 | Invasive | 24,058/1,394,061 | Fertility drugs | — | 2.09 (1.39 to 3.12) | Year of delivery after IVF, age and smoking | |
| Van Leeuwen 2011 | Invasive | 19,146/6006 | Fertility drugs | — | — | — | 1.35 (0.91 to 1.92) Excluding the first year: 1.30 (0.86 to 1.88) |
| HMG/FSH | — | — | — | 1 to 40 ampoules: 1.25 (0.41 to 2.93) 41 to 80: 1.21 (0.39 to 2.83) > 81: 1.58 (0.68 to 3.11) | |||
| IVF | — | 1.14 (0.54 to 2.41) (a) 1.51 (0.65 to 3.54) (b) 2.26 (0.78 to 6.55) (c) | a) Age, endometriosis b) > 1 year follow‐up c) > 10 years follow‐up | 1 to 2 cycles: 1.35 (0.68 to 2.42) 3 to 4 cycles: 1.19 (0.57 to 2.18) > 5 cycles: 1.41 (0.57 to 2.90) | |||
| Borderline | Fertility drugs | — | — | — | 1.93 (1.31 to 2.73) Excluding the first year: 1.76 (1.16 to 2.56) | ||
| HMG/FSH | — | — | — | 1 to 40 ampoules: 1.75 (0.57 to 4.08) 41 to 80: 2.03 (0.74 to 4.42) > 81: 1.69 (0.62 to 3.69) | |||
| IVF | — | 6.38 (2.05 to 19.84) (a) 4.23 (1.25 to 14.33) (b) 2.26 (0.46 to 11.05) (c) | a) For age, tubal problems and parity b) > 1 year follow‐up c) > 10 years | 1 to 2 cycles: 1.70 (0.97 to 3.74) 3 to 4 cycles: 1.99 (1.22 to 4.14) > 5 cycles: 1.45 (0.47 to 3.38) | |||
| Yli‐Kuha 2012 | Invasive | 9175/9175 | IVF | — | 2.75 (0.69 to 9.63) 2.25 (0.59 to 8.68) excluding first year after treatment | Adjusted for marital status and socioeconomic position | — |
| Borderline | 1.68 (0.31 to 9.27) 2.25 (0.59 to 8.68) excluding first year after treatment | Adjusted for marital status and socioeconomic position | — | ||||
| Lerner‐Geva 2012 | Invasive | 2431/NR | Clomiphene citrate | — | — | — | 1.33 (0.57 to 2.63) |
| Clomiphene citrate + HMG | — | — | — | NR | |||
| HMG | — | — | — | 0.74 (0.01 to 4.12) | |||
| FSH: follicle‐stimulating hormone | |||||||
| Case‐control studies | ||||||
| Study | Ovarian cancer type | Total number of cancer cases/controls | Drug | Crude OR (95% CI) | Adjusted odds ratio (95% CI) | Factor adjusted for |
| Rossing 1994 | Invasive/borderline | 135 / NR | Clomiphene citrate | Rate ratio 11.00 (1.50 to 80.67) | Rate ratio > 1 year 11 (1.5 to 8.2) | Parity |
| — | Rate ratio > 1 year 7.2 (1.2 to 43.9) | Age | ||||
| — | Rate ratio < 1 year 0.7 (0.1 to 4.6) | Age | ||||
| — | Rate ratio < 1 year 0.8 (0.1 to 5.7) | Parity | ||||
| HCG | — | Rate ratio 1.0 (0.2 to 4.1) | Age | |||
| — | Rate ratio 1.0 (0.2 to 4.3) | Parity | ||||
| Franceschini 1994 | Invasive | 195 / 1339 | Fertility drugs | 0.8 (0.2 to 3.7) | 0.7 (0.2 to 3.7) | Age, area of residence, education, |
| Sushan 1996 | Invasive | 68 / 77 | Fertility drugs | 1.78 (0.97 to 3.27) | 1.31 (0.63 to 2.74) | Age, parity, BMI, region of birth, education, family history, interviewer |
| Borderline | 26 / 77 | Fertility drugs | 5.03 (2.04 to 12.22) | 3.52 (1.23 to 10.09) | Age, parity, BMI, region of birth, | |
| Invasive | Clomiphene citrate | 1.32 (0.57 to 3.01) | 0.88 (0.33 to 2.34) | Age, parity, BMI, region of birth, | ||
| Borderline | Clomiphene citrate | 1.62 (0.25 to 7.87) | 1.28 (0.25 to 6.87) | Age, parity, BMI, region of birth, | ||
| Invasive | HMG | 3.95 (1.33 to 12.2) | 3.19 (0.86 to 11.82) | Age, parity, BMI, region of birth, | ||
| Borderline | HMG | 14.58 (3.82 to 55.91) | 9.38 (1.66 to 52.08) | Age, parity, BMI, region of birth, | ||
| Invasive | HMG/clomiphene citrate | 1.97 (1.03 to 3.77) | 1.42 (0.65 to 3.12) | Age, parity, BMI, region of birth, | ||
| Borderline | HMG/clomiphene citrate | 4.86 (1.81 to 12.79) | 3.08 (0.98 to 9.69) | Age, parity, BMI, region of birth, | ||
| Parazzini 1997 | Invasive | 971 / 2758 | Fertility drugs | 0.5 (0.1 to 3.6) 0.6 (0.1 to 3.5) | 1.1 (0.4 to 3.3) < 6 cycles 0.7 (0.1 to 7.9) 1.0 (0.2 to 3.8) | Age, education, OCP, parity |
| Mosgaard 1997 | Invasive | 684 / 1721 | Fertility drugs | Nulliparous 0.80 (0.92to 5.58) | Nulliparous 0.83 (0.35 to 2.01) 0.56 (0.24 to 1.29) | Age, residence, use of OCP, menopausal status, previous cancer, family history, HRT, BMI |
| Clomiphene citrate | Nulliparous 0.69 (0.23 to 1.96) | Nulliparous 0.67 (0.23 to 1.96) 1.11 (0.4 to 3.06) | Age, residence, use of OCP, menopausal status, previous cancer, family history, HRT, BMI | |||
| Clomiphene citrate + HCG | Nulliparous 1.99 Parous 0.24 | Nulliparous 1.12 (0.32 to 3.96) 0.56 (0.12 to 2.7) | Age, residence, use of OCP, menopausal status, previous cancer, family history, HRT, BMI | |||
| HMG + HCG | Nulliparous 1.06 Parous 0.54 | Nulliparous 0.82 (0.18 to 3.71), 0.5 (0.10 to 2.47) | Age, residence, use of OCP, menopausal status, previous cancer, family history, HRT, BMI | |||
| Parazzini 1998 | Borderline | 92 / 273 | Fertility drugs | 27.5 (1.5 to 51.60) | — | — |
| Mosgaard 1998 | Borderline | 231 / 1721 | Fertility drugs | 2.27 (1.30 to 3.96) | 2.19 (1.24 to 3.85) | Age and residence |
| Clomiphene citrate | Nulliparous 0.71 | Nulliparous 0.80 (0.19 to 3.38) 1.93 (0.56 to 6.59) | Age, residence, use of OCP, use of HRT, smoking | |||
| Clomiphene citrate + HCG | Nulliparous 5.20 | Nulliparous 3.01 (0.73 to 12.33) | Age, residence, use of OCP, use of HRT, smoking | |||
| HCG + HMG | Nulliparous 1.95 | Nulliparous 0.91 (0.14 to 6.13) 1.43 (0.28 to 7.19) | Age, residence, use of OCP, use of HRT, smoking | |||
| Parazzini 2001 | Invasive | 1031 / 2411 | Fertility drugs | 1.3 (0.7 to 2.5) 0.6 (0.7 to 2.5) Parous | — | — |
| Rossing 2004 | Invasive | 378 / 1634 | Fertility drugs | — | Nulliparous 1.0 (0.4 to 2.8) | Age, race, study site, duration of use of OCP (parous ‐ also number of births) |
| Clomiphene citrate | — | Nulliparous 1.2 (0.4 to 3.5) 0.8 (0.4 to 1.6) | Age, race, study site, duration of use of OCP (parous ‐ also number of births) | |||
| HMG/clomiphene citrate/gonadotrophins | — | Nulliparous 1.0 (0.4 to 3.0); | Age, race, study site, duration of use of OCP (parous ‐ also number of births) | |||
| Jensen 2009 | Invasive | 626 / 615 | Clomiphene citrate | Rate ratio 1.28 (0.79 to 2.07) | Rate ratio: 1.14 (0.79 to 1.64) 1 to 4 cycles: 1.27 (0.83 to 1.94) 5 to 9 cycles: 1.03 (0.57 to 1.86) Equal or > 10 cycles: 0.92 (0.42 to 2.02) | Age, parity |
| Gonadotrophins | Rate ratio 0.85 (0.44 to 1.64) | Rate ratio: 0.83 (0.50 to 1.37) 1 to 4 cycles: 0.74 (0.41 to 1.33) 5 to 9 cycles: 1.09 (0.49 to 2.44) Equal or > 10 cycles: 0.96 (0.09 to 10.30) | Age, parity | |||
| HCG | Rate ratio 0.95 (0.57 to 1.58) | Rate ratio: 0.89 (0.62 to 1.29) 1 to 4 cycles: 0.96 (0.62 to 1.48) 5 to 9 cycles: 0.86 (0.47 to 1.57) Equal or > 10 cycles: 0.70 (0.28 to 1.80) | Age, parity | |||
| GnRH | Rate ratio 0.71 (0.32 to 1.54) | Rate ratio: 0.80 (0.42 to 1.51) 1 to 4 cycles: 0.81 (0.42 to 1.56) 5 to 9 cycles: 0.68 (0.09 to 5.38) | Age, parity | |||
| Kurta 2012 | Invasive | 155 / 290 | Fertility drugs | 1.87 (0.53 to 6.65) < 6 months: 0.92 (0.48 to 1.74) > 6 months: 0.75 (0.42 to 1.34) | 0.57 (0.31 to 1.05) parous 0.47 (0.09 to 2.53) nulliparous | — |
| Clomiphene citrate | 0.87 (0.49 to 1.56) | — | — | |||
| Gonadotrophins | 0.51 (0.20 to 1.32) | — | — | |||
| Gonadotrophins + Clomiphene citrate | 0.94 (0.37 to 2.42) | — | — | |||
| BMI: body mass index | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Borderline ovarian cancer Show forest plot | 5 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Any infertility drug | 4 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clomiphene | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Clomiphene + gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Invasive ovarian cancer Show forest plot | 16 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Any infertility drug | 12 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clomiphene | 8 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Clomiphene + gonadotrophin | 6 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Gonadotrophin | 6 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 GnRH | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

