Nicht‐invasive Hirnstimulationsverfahren bei chronischen Schmerzen
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel, quasi‐RCT | |
| Participants | Country of study: Egypt Setting: Dept of Neurology, hospital‐based Condition: chronic phantom limb pain Prior management details: unresponsive to various pain medications n = 27, 17 active and 10 sham Age, mean (SD): active group 52.01 (12.7) years, sham group 53.3 (13.3) years Duration of symptoms, mean (SD) months: active group 33.4 (39.3), sham group 31.9 (21.9) Gender distribution: active group 13 M, 4 F; sham group 6 M, 4 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified, number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: M1 stump region Number of treatments: x 5, daily Control type: sham ‐ coil angled away from scalp | |
| Outcomes | Primary: pain VAS (anchors not reported), LANNS When taken: poststimulation session 1 and 5 and at 1 month and 2 months post‐treatment Secondary: none relevant | |
| Notes | AEs: not reported COI: not reported Sources of support: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: not true randomisation Quote: "patients were randomly assigned to 2 groups depending on the day of the week on which they were recruited" |
| Allocation concealment (selection bias) | High risk | Comment: given method of randomisation allocation concealment not viable |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Coil angled away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The second author evaluated these measures blindly, without knowing the type of TMS" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: levels of dropout not reported |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes presented in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | > 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: OA knee Prior management details: not reported n = 41 randomised, 40 analysed Age, mean (SD): active group 60.6 (9.8) years, sham group 59.3 (8.6) years Duration of symptoms: not reported Gender distribution: 19 M, 21 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS 2mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 1 daily for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: 1 d postintervention, 3 weeks postintervention Secondary: WOMAC function score AEs | |
| Notes | Funding source: supported in part by the Claude D. Pepper Older American's Independence Center (P30 AG028740), the Universityof Florida Center for Cognitive Aging and Memory, and NIA Grants K07AG04637 and K01AG050707, and R01AG054077. This Work was also partially supported by VA HSR&D Houston Center for Innovations in Quality, Effectiveness and Safety (CIN# 13‐413), Michael E. DeBakey VA Medical Center, Houston, TX. COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned with a ratio of 1 to 1 to either the active tDCS (n ¼ 20) or sham tDCS group (n ¼ 20) using a covariate adaptive randomization procedure so that the two groups had approximately equal distribution regarding age, gender and race.” |
| Allocation concealment (selection bias) | Low risk | Quote “Allocation concealment was ensured as the randomization codes were released only after all the interventions and assessments were completed.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that participant blinding can be inadequate at intensity of 2 mA. No assessment of blinding success. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No assessment of blinding success. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only one participant withdrew. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | Unclear risk | Comment: 3‐week follow‐up |
| Other bias | Unclear risk | Comment: statistically significant between‐group difference in pain NRS scores at baseline |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management, candidates for invasive MCS n = 14 Age: 31‐66 years; mean 53 (SD 11) Duration of symptoms: mean 6.9 years (SD 4) Gender distribution: 10 M, 4 F | |
| Interventions | Stimulation type: rTMS figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 20 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 2: frequency 1 Hz; coil orientation lateromedial; number of trains 1; duration of trains 26 min, total number of pulses 1600 Condition 3: sham ‐ same as for condition 2 with coil angled away perpendicular to scalp Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition | |
| Outcomes | Primary: VAS 0‐10 cm, anchors "no pain" to "unbearable pain" When taken: immediately poststimulation then daily for 1 week Secondary: none | |
| Notes | Data requested from study authors and received Sources of support: Supported in part by a Grant from the Fondation pour la Recherche Médicale (FRM), France COI: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were consecutively assigned to a randomization scheme generated on the web site Randomization.com (Dallal GE, http://www.randomization.com, 2008). We used the second generator, with random permutations for a 3‐group trial. The randomization sequence was concealed until interventions were assigned." |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment 'suboptimal'. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "To ensure the double‐blind evaluation effects, the physician applying magnetic stimulation was different from the one collecting the clinical data, who in turn was not aware of the modality of rTMS that had been used in each session." |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 participants lost to follow‐up and not accounted for in the data analysis. Given the small sample size it may influence the results |
| Selective reporting (reporting bias) | Low risk | Pain outcomes reported for all participants. Change from baseline figures given; point measures requested from study authors and received |
| Free from carry‐over effects? | Low risk | Comment: a 2‐week washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: France Setting: laboratory‐based Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management, candidates for invasive MCS n = 30 Age: 31‐72 years, mean 55 (SD 10.5) Duration of symptoms: mean 5 years (SD 3.9) Gender distribution: 23 M, 7 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 20 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 2: frequency 20 Hz, coil orientation lateromedial; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 3: sham ‐ same as for active conditions with coil angled away perpendicular to scalp Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition | |
| Outcomes | Primary: 0‐10 NRS (anchors "no pain" to "unbearable pain") When taken: daily for 2 weeks poststimulation Secondary: none | |
| Notes | Data requested from study authors Sources of support: supported in part by a Grant from the Fondation pour la Recherche Médicale (FRM), France COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the order of sessions was randomised (by computerized random‐number generation)" |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The physician who applied the procedure received from a research assistant one sealed envelope containing the order of the rTMS sessions for a given patient. The order remained unknown to the physician collecting clinical data." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2 participants apparently lost to follow‐up and not obviously accounted for in the analysis. However, this is less than 10% and is unlikely to have strongly influenced the results |
| Selective reporting (reporting bias) | Low risk | Comment: medial‐lateral coil orientation condition data not presented but provided by study authors on request |
| Free from carry‐over effects? | Low risk | Comment: a 2‐week washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory‐based Condition: chronic neuropathic pain (mixed) Prior management details: resistant to conventional pharmacological treatment n = 45 Age: 31‐72 years (mean 55) Duration of symptoms: "chronic" Gender distribution: 28 M, 17 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified, number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Stimulation location: M1 hand area Number of treatments: 1 per group Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = unbearable pain When taken: daily for 2 weeks following each stimulation Secondary: none relevant | |
| Notes | AEs: not reported Funding source: charity‐funded COI: declaration ‐ no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less likely to introduce bias in a cross‐over design Quote: "separated into 2 groups determined by the randomization" |
| Adequate blinding of participants? | Unclear risk | Comment: the study authors state "Because the first step of the procedure (motor hotspot and motor threshold determination) that induced motor contractions was identical in placebo and active sessions and the stimulation differed only when intensities below motor threshold were applied, no patient perceived any difference between the 2 types of rTMS" However, the sensation on the scalp may differ and no formal evaluation of blinding presented |
| Adequate blinding of assessors? | Unclear risk | Comment: no mention of blinded assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no mention of dropout/withdrawal |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported for all groups and further data made available upon request to authors |
| Free from carry‐over effects? | Low risk | Comment: 2‐week washout period observed |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory setting Condition: mixed chronic pain, neuropathic and non‐neuropathic Prior management details: therapy‐resistant n = 23, 10 in parallel (6 active, 4 sham), 13 crossed over Age: active‐only group 28‐70 years, sham‐only group 50‐70 years, cross‐over group 41‐70 years Duration of symptoms: chronic 1.5‐25 years (mean 7.4) Gender distribution: 6 M, 17 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ L M1 hand area, cathode right supraorbital Number of treatments: x 5, daily Control type: sham tDCS | |
| Outcomes | Primary: pain VAS 0‐10; VAS anchors 0 = no pain, 10 = the worst pain possible When taken: x 3, daily ‐ averaged for daily pain Secondary: none relevant | |
| Notes | Funding: government funding COI: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed using the order of entrance into the study." Comment: may not be truly random from description |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned though unlikely given the randomisation technique. This is a potentially significant source of bias given that only the parallel results were used in this review due to high levels of attrition after the first phase |
| Adequate blinding of participants? | Low risk | Comment: see above |
| Adequate blinding of assessors? | Low risk | Comment: 1 mA intensity and operator blinded Quote: "The stimulators were coded using a five letter code, programmed by one of the department members who otherwise did not participate in the study. Therefore neither the investigator not the patient knew the type of the stimulation" |
| Incomplete outcome data (attrition bias) | High risk | Comment: the high level of dropout renders the cross‐over results at high risk of bias. This is less of an issue where only the parallel results from the first phase were used ‐ first‐phase data only used in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: while not all outcomes at all time points were included in the study report the authors have provided all requested data |
| Free from carry‐over effects? | Low risk | Comment: participants were excluded if pain had not returned to normal. This, however, represents a threat with regard to attrition bias |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other sources of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: hospital pain units Condition: lumbar radicular pain Prior management details: stable pharmacological treatment for pain and sleep disorders for at least 1 month prior to study n = 36 Age, mean (SD): active group 53.4 (8) years, sham group 51.5 (13) years Duration of symptoms: not reported Gender distribution: 17 F 18 M | |
| Interventions | Stimulation type: rTMS and tDCS (order randomised in active group) Stimulation parameters: rTMS frequency 10 Hz; coil orientation anteroposterior induced current; 80% RMT; number of trains 30; duration of trains 10 s; ITI 20 s; total number of pulses 3000 tDCS: 2 mA intensity, 30 min Stimulation location: M1 contralateral to painful side Number of treatments: 3 stimulation visits on 3 consecutive days for each stimulation type. 3 week washout period. Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = maximal pain imaginable When taken: postintervention Secondary: BPI interference scale AEs | |
| Notes | Funding source: The study received financial support from the Institut National de la Sante´ et de la Recherche Médicale (INSERM) COI: the authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The 2 successive randomisations were prepared by a study nurse not involved in the running of the study or in data analysis, using validated software and a centralised randomisation schedule.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The treatment allocation code was kept in a sealed envelope until the completion of the study.” |
| Adequate blinding of participants? | Unclear risk | Comment: rTMS sham described as controlling for sensory, auditory and visual cues. tDCS 2 mA intensity ‐ evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | tDCS 2 mA intensity ‐ evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis used and low dropout |
| Selective reporting (reporting bias) | High risk | Comment: point estimates for pain scores not provided ‐ only a responder analysis was presented |
| Free from carry‐over effects? | Unclear risk | Comment: the order of active stimulation types was randomised but it is not clear that there were not baseline differences between pre‐rTMS and pre tDCS from the presented data |
| Study Size | High risk | n = 36 |
| Study duration | High risk | Comment: 5 days post intervention was the longest follow up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: unclear Condition: chronic widespread pain Prior management details: not reported n = 19 Age mean (SD): active 54.86 (7.65) years, sham 52.09 (10.02) years Duration of symptoms (months mean (SD)): active group 11 (4.26), sham group 15.64 (6.93) Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified; 120% RMT; number of trains 75; duration of trains 4 s; ITI 26 s; total number of pulses 3000 Stimulation location: L DLPFC Number of treatments: 15 sessions over 4 weeks Control type: sham coil ‐ controls for visual, auditory and scalp sensory cues | |
| Outcomes | Primary: pain NRS 0‐10 anchors not reported When taken: end of treatment period, 1 month following and 3 months following Secondary: pain interference BPI QoL SF‐36 AEs: multiple minor; no clear difference in incidence between active and sham stimulation | |
| Notes | Government‐funded study, manufacturer loaned stimulators COI: funded by the National Institute for Arthritis, Musculoskeletal and Skin Diseases, R21 ART053963 and the Bipolar Illness Fund Neuronetics, Inc. loaned the TMS machine to the study Dr. Avery was a consultant for Neuronetics, Inc. for one day, is a member of the Data and Safety Monitoring Board for Cerval Neuortech, Inc., was on the speakers bureau for Eli Lilly and Takeda, was a consultant for Takeda and received a grant from the National Institute of Mental Health. Dr. Roy‐Byrne is editor for Journal Watch, Depression and Anxiety, and UpToDate and has stock in Valant Medical Systems. None of the other authors has potential COI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the completion of the baseline assessment, patients were randomly assigned to either real TMS or sham stimulation using a computerized randomization program that uses an adaptive randomization and stratification strategy." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Based on the randomization, a "smart card" which determined whether the real TMS or sham coil would be administered was assigned to a particular patient. The card had only a code number that did not reveal the randomization." "The research coordinator blind to the randomization repeated the baseline assessments" Comment: not entirely clear whether the personnel overseeing randomisation was separate from that performing the screening assessment. |
| Adequate blinding of participants? | Low risk | Quote: "... sham stimulation with the electromagnet blocked within the coil by a piece of metal so the cortex was not stimulated. The coils appeared identical. Electrodes were attached to the left side of the forehead for each subject for each session. Those receiving the sham stimulation received an electrical stimulus to the forehead during the sham stimulation. Those receiving the real TMS received no electrical stimulation to the electrodes. Both groups experienced a sensation in the area of the left forehead. In addition, all subjects were given special earplugs and received an audible noise during the stimulation to mask any possible sound differences between the TMS and sham conditions." Comment: optimal sham ‐ controls for visual, sensory and auditory cues Formal testing ‐ blinding appears robust |
| Adequate blinding of assessors? | Low risk | Quote: "The research coordinator blind to the randomization repeated the baseline assessments of pain, functional status, depression, fatigue, and sleep before the 1st and after the 5th, the 10th, and the 15th TMS sessions as well as 1 week, 1 month, and 3 months after the last TMS treatment except for the SF‐36, neuropsychological tests, audiometry and the dolorimetry which were only done at baseline and one week after the 15th TMS session." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "To examine differences in changes in outcomes over time between TMS and comparison group subjects, we estimated random coefficient models following the intent‐to‐treat principle." "11 were randomized to the sham group and 8 were randomized to the TMS group. However, one subject randomized to the TMS had a baseline BIRS score of 4 which was well below the BIRS score of 8 required for randomization. Because of this incorrect randomization, this subject was excluded from the efficacy analyses, but was included in the analysis of side effects. The clinical characteristics of those correctly randomized are in Table 1. One subject in the TMS dropped out after the 10th session because of lack of response and is included in the analyses." Comment: of 2 dropouts from the TMS group, 1 was excluded (reasons given) |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes presented in full in study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Low risk | No other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: MS‐related neuropathic pain Prior management details: concomitant medication intake stable throughout protocol n = 16 Age, mean (SD) 48.9 (10) years Duration of symptoms: mean (SD) 11.8 (9.4) months Gender distribution: 13 F, 3 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25 cm2 electrodes, duration 20 min Stimulation location: anode ‐ L DLPFC, cathode right supraorbital Number of treatments: x 3, daily Control type: sham tDCS | |
| Outcomes | Primary: pain VAS 0 ‐10; VAS anchors not reported When taken: Postintervention, 7 days postintervention Secondary: AEs | |
| Notes | COI: "AC gave expert testimony for CSL Behring, Novartis, received grants from Biogen, Novartis, CSLBehring, GENeuro, Octapharma, and gave lectures for Genzyme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships "that could be construed as potential conflict of interest" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “The randomization schedule was generated by U.P. prior to the beginning of the study using a dedicated software (“true”random number generation without any restriction, stored in a computer until the patient was assigned to the intervention).” |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity, particularly in cross‐over designs. Results of guessing mode of stimulation not reported |
| Adequate blinding of assessors? | Unclear risk | Quote: "Only the performing physician (S.S.A) was aware of the stimulation mode (real or sham tDCS). The evaluators (U.P and M.A.C) and the patients were blind to it.” Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no attrition reported |
| Selective reporting (reporting bias) | Low risk | Comment: results reported in full |
| Free from carry‐over effects? | Unclear risk | Comment: baseline scores for each period not reported. No formal analysis for carry‐over effects presented |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: longest follow‐up 7 days after stimulation |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: South Korea Setting: laboratory Condition: CPSP Prior management details: not reported n = 14 Age, mean (SD): active group 51.1 (3.1) years, sham group 52.3 (2.8) years Duration of symptoms, mean (SD): active group 14.5 (3.2) months, sham group 14.7 (2.7) Gender distribution: 7 M, 7 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 contralateral to painful side, cathode right supraorbital Number of treatments: x 3 per week for 3 weeks Control type: sham tDCS | |
| Outcomes | Primary: pain VAS anchors 0 = no pain, 10 = unbearable When taken: "immediacy", 1 week, 3 weeks (unclear if from end of intervention) Secondary: None relevant | |
| Notes | COI: study authors declared no COI Sources of support: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment procedures |
| Adequate blinding of participants? | Unclear risk | Comment: blinding not reported. Evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding not reported. Evidence that blinding can be inadequate at intensity of 2 mA |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unable to clearly verify if there was any attrition |
| Selective reporting (reporting bias) | Low risk | Comment: adequate reporting of outcomes |
| Study Size | High risk | Comment: total n = 14 |
| Study duration | Unclear risk | Comment: 3‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management n = 8 Age: 40‐82 years; mean 63.3 (SD 5.6) Duration of symptoms: 1‐20 years; mean 8.3 (SD 5.6) Gender distribution: 2 M, 6 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 30 min Condition 1: active tDCS/active TENS Condition 2: active tDCS/sham TENS Condition 3: sham tDCS/sham TENS Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: VAS 0‐10 anchors "no pain" to "worst possible pain" When taken: pre and post each stimulation Secondary: none | |
| Notes | Sources of support: not declared COI: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All the patients received the 3 treatments.... in a randomised order (we used a computer generated randomisation list with the order of entrance)." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Quote: "All evaluations were carried out by a blinded rater" Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2 participants lost to follow‐up. It is unclear how these data were accounted for as there were no missing data apparent in the results tables. However, this may have an impact given the small sample size |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 48‐h washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis Quote: "To analyze whether there was a carryover effect, we initially performed and showed that the baselines for the 3 conditions were not significantly different (P = 0.51). We also included the variable order in our model and this model also showed that order is not a significant term (P = 0.7)." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT; 2 conditions | |
| Participants | Country of study: USA Setting: laboratory Condition: peripheral neuropathic pain Prior management details: not specified n = 4 Age: 33‐58 years; mean 46 (SD 11) Duration of symptoms: 5‐12 years; mean 10.25 (SD 3.5) Gender distribution: 1 M, 3 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation not specified; 100% RMT; number of trains 40; duration of trains 10 s; ITI 20 s; total number of pulses 4000 Stimulation location: L PFC Number of treatments: 3 over a 5‐d period Control type: neuronetics sham coil (looks and sounds identical) | |
| Outcomes | Primary: average daily pain 0‐10 Likert scale, anchors "no pain at all" to "worst pain imaginable" When taken: post‐stimulation for each condition (unclear how many days post) and daily for 3 weeks poststimulation Secondary: none | |
| Notes | AEs: not reported Sources of support: no separate statement provided COI: "Dr. Borckardt receives research funding from the National Institute for Neurological Disorders and Stroke at NIH, Cyberonics Inc, the Neurosciences Institute at MUSC, and is a consultant for Neuropace; however, he has no equity ownership in any device or pharmaceutical company. Dr. George receives research funding from the National Institute for Mental Health, NIDA, and NIAAA at NIH, Jazz Pharmaceuticals, GlaxoSmithKline, and Cyberonics Inc. He is a consultant for Aspect Biomedical, Argolyn, Aventis, Abbott, Bristol‐Meyers Squibb, Cephos, Cyberonics, and Neuropace; however, he has no equity ownership in any device or pharmaceutical company. Dr. Nahas receives research funding from the National Institute for Mental Health at NIH and Cyberonics Ind, and is a consultant for Neuropace. Dr. Kozel receives research funding from the National Institute for Mental Health at NIH and the U.S. Department of Defense. MUSC has filed six patents or invention disclosures in one or more of the authors’ names regarding brain imaging and stimulation." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order (real first or sham first) was randomised" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "Two of the four participants (50%) correctly guessed which treatment periods were real and sham, which is equal to chance. All four of the participants initially said that they did not know which was which, and it was not until they were pushed to "make a guess" that they were able to offer an opinion about which sessions were real and which were sham." Comments: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout |
| Selective reporting (reporting bias) | Low risk | Comment: all results reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 3‐week washout period was observed. Presented average pain values were very similar pre‐ each condition |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: specialised pain treatment centre Condition: fibromyalgia Prior management details: stable treatment for more than 1 month before enrolment n = 38 Age, mean (SD): active group 49.1(10.6) years, sham group 47.7 (10.4) years Duration of symptoms, mean (SD): active group 3.7 (4.5) years, sham group 3.6 (3.8) Gender distribution: 37 F, 1 M | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation anteroposterior; 90% RMT; number of trains 20; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: L M1 Number of treatments: 14 sessions. 10 sessions in 2 weeks followed by maintenance phase of 1 session at weeks 4, 6, 8 and 10 Control type: sham coil ‐ did not control for sensory cues | |
| Outcomes | Primary: pain VAS 0 = no pain, 10 = maximal pain imaginable When taken: 2 weeks, 11 weeks Secondary: FIQ AEs | |
| Notes | Funding source: Supported by Inserm (Centre d’Investigation Clinique, CIC, Hôpital de la Conception, Marseille) and AP‐HM (AORC 2008/01) COI: the study authors report no disclosures relevant to the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Individuals were randomized by a computer‐generated list…” |
| Allocation concealment (selection bias) | Low risk | Quote: “...which was maintained centrally so no investigators knew the treatment allocation of any patient.” |
| Adequate blinding of participants? | Unclear risk | Quote: “Sham stimulation was conducted with a sham coil of identical size, color, and shape, emitting a sound similar to that emitted by the active coil. Stimulations were administered by the same technologist.” Comments: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “Patients and clinical raters were blinded to treatment” |
| Incomplete outcome data (attrition bias) | High risk | Quote “All patients completed the induction phase, but 9 (23.7%) were excluded during the maintenance phase (3 in the active rTMS group and 6 in the sham rTMS group)“ Comment: dropout high, ITT analysis used but no information with regards imputation approach taken (or not) |
| Selective reporting (reporting bias) | Low risk | Comment: all results reported clearly and in full |
| Study Size | High risk | Comment: n = 38 |
| Study duration | High risk | Comment: no follow‐up after end of maintenance phase |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: hepatitis C‐related chronic pain Prior management details: not reported n = 28 Age, mean (SD): active group 53.86 (5.76) years, sham group 56.57 (8.52) years Duration of symptoms: not reported Gender distribution: 21 M, 7 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25‐35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 L, cathode right supraorbital Number of treatments: daily, x 5 Control type: sham tDCS | |
| Outcomes | Primary: pain VAS; anchors 0 = no pain, 10 = worst possible pain When taken: end of intervention Secondary: none relevant | |
| Notes | Funding from Brazilian funding agencies: (i) Committee for the Development of Higher Education Personnel (iv) Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre (v) Laboratory of Neuromodulation & Center for Clinical Research Learning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomized numbers in a 1:1 ratio were generated using appropriate software (www.randomization.com) to assign each Participant to either active or sham‐placebo group.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Envelopes were prepared for randomization process and sealed. After subject’s agreement to participate in the trial, one investigator who was not involved with either stimulation or assessments opened the envelope. The allocation concealment was reached since no investigator (stimulators nor accessors) was aware of treatment allocations and had no control over the order of patients randomized.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | Unclear risk | Quote: “Two independent blinded examiners were trained to apply the pain scales and to conduct the psychological tests. Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No assessment of blinding success |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 3 participants dropped out (> 10%) reasons not given. ITT analysis with LOCF |
| Selective reporting (reporting bias) | Low risk | Comment: outcome data adequately reported |
| Study Size | High risk | Comment n = 28 |
| Study duration | High risk | Comment: no follow‐up after immediate postintervention period. |
| Other bias | Low risk | No other bias detected |
| Methods | Partial cross‐over RCT. NB: we only considered first‐phase results therefore we considered the trial as having a parallel design | |
| Participants | Country of study: UK Setting: residential educational centre Condition: post‐SCI pain (unclear whether this was neuropathic or otherwise) Prior management details: unclear n = 30 Age: unclear Duration of symptoms: unclear Gender distribution: unclear | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 10 Hz; pulse width 2 ms; intensity 1 2 μA; duration 53 min Stimulation location: ear clip electrodes Number of treatments: x 2, daily for 4 days Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: 0‐10 VAS 'level of pain', anchors not specified When taken: daily during the treatment period Secondary: none | |
| Notes | COI: no declaration made Sources of support: Laing Foundation (charity) "financial assistance" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method equivalent to picking out of a hat Quote: "Subjects would be randomly assigned into two groups according to their choice of treatment device... The devices were numbered for identification, but neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Allocation concealment (selection bias) | Low risk | Comment: this is achieved through the method of randomisation |
| Adequate blinding of participants? | Low risk | Quote: "neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Adequate blinding of assessors? | Low risk | Quote: "neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3 participants withdrew (not voluntarily) and while the data were not clearly accounted for in the data analysis this constituted 10% of the overall cohort and was unlikely to have strongly influenced the results Quote: "Three of the 30 subjects included were withdrawn from the study after commencement, one of whom developed an upper respiratory infection, and two others were withdrawn from the study because their medication (either H2 antagonist anti‐ulcer or steroidal inhalant) were interacting with the TCET treatment." |
| Selective reporting (reporting bias) | High risk | Comment: pain score values were not provided for any time point |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel randomised clinical trial | |
| Participants | Country of study: Spain Setting: outpatient clinic Condition: fibromyalgia (with major depression) Prior management details: unclear n = 26 Age: active group 47.5 (SD 5.7) years, sham group 54.9 (SD 4.9) years Duration of symptoms: unclear "chronic" Gender distribution: 2 M, 24 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 1 Hz; coil orientation not specified; 110% RMT; number of trains 20; duration of trains 60 s; ITI 45 s; number of pulses 1200 Stimulation location: R DLPFC Number of treatments: up to 20 on consecutive working days Control type: coil angled 45º from the scalp | |
| Outcomes | Primary: Likert pain scale 0‐10, anchors "no pain" to "extreme pain" When taken: 2 weeks, 4 weeks and 8 weeks from commencement of study Secondary: none | |
| Notes | COI: no declaration made Sources of support: IUNICS Institute, Research Institute of Health Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "patients and raters (but not the treating physician) were blind to the procedure" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 participant in each group did not complete the study. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes presented clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Australia Setting: laboratory Condition: knee OA Prior management details: not reported n = 30 Age, mean (SD): active group 59.8 (9.1) years, sham group 64.1 (11.1) years Duration of symptoms mean (SD) years: active group: 7.2 (5.3), sham group 9.0 (7.3) Gender distribution: 10 M, 19 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 1 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 2 weekly for 8 weeks prior to a 30‐min supervised strengthening exercise session. 16 sessions Control type: sham tDCS | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: postintervention Secondary: WOMAC function AEs | |
| Notes | Funding source: Trial funded by Arthritis Australia (The Zimmer Australia Grant). W‐JC (1094434), PWH (1002190), KLB (1058440), MBL (1059116) and SMS (1105040) receive salary support from the National Health and Medical Research Council of Australia, RSH from the Australian Research Council (FT#130100175) and VB from a Western Sydney University Postgraduate Research Award. COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation schedule was concealed in consecutively numbered, sealed opaque envelopes. An investigator not involved in recruitment and assessment prepared and provided the envelopes to the treating physiotherapists who revealed group allocation.” |
| Adequate blinding of participants? | Low risk | Comment: blinding likely maintained at 1 mA intensity |
| Adequate blinding of assessors? | Low risk | Quote: "A single investigator (W‐JC), blinded to the group allocation of the participants, performed participant recruitment, screening, and testing." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 2 (13% dropout from active group), 3 (20%) from control group. ITT analysis with no imputation of missing values. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 30 |
| Study duration | High risk | Comment: postintervention follow‐up only (within 1 week) |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT (to be considered as parallel ‐ first treatment phase only as 2nd unblinded) | |
| Participants | Country of study: USA Setting: pain clinic Condition: fibromyalgia Prior management details: unclear n = 74 Age: 22‐75 years; mean 53 Duration of symptoms: 1‐21 years; mean 7.3 Gender distribution: 4 M, 70 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width unclear; intensity 100 μA; waveform shape modified square wave biphasic 50% duty cycle; duration 60 min Stimulation location: ear clip electrodes Number of treatments: ? daily for 3 weeks Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: 0 ‐5 pain NRS, anchors "no pain" to "worst pain imaginable" When taken: immediately following the 3‐week treatment period Secondary: Oswestry Disability Index When taken: immediately following the 3‐week treatment period | |
| Notes | AEs: not reported COI: no declaration made Sources of support: "Supported by a grant from the Department of Anesthesiology, LSU Health Sciences Center. No financial support was received from the makers of the Alpha‐Stim™; however, Electromedical Products International, Inc. did loan the authors the Alpha‐Stim™ units necessary to do the study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Quote: "All staff, the physicians, and the patient were blind to the treatment conditions." |
| Adequate blinding of assessors? | Low risk | Quote: "All staff, the physicians, and the patient were blind to the treatment conditions." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropout rate not reported |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point |
| Study Size | High risk | Comment: < 50 participants per treatment arm (considered as a parallel trial ‐ 1st phase only) |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 20 Age, mean (SD): active group 41.4 (10.25) years, sham group 44.2 (9.81) years Duration of symptoms, mean (SD) years: active group 4.3 (2.62), sham group 5 (5.04) Gender distribution: all F | |
| Interventions | Stimulation type: tRNS Stimulation parameters: tDCS: 1.5 mA intensity, 20 min (randomly oscillating in frequency range 101‐640 Hz for 10 min, offset set to 0 ma sham ‐ stimulation turned on for 30 s only) Stimulation location: M1 (side not reported) Number of treatments: x 1 daily, 5 days a week for 2 weeks (x 10 sessions) Control type: sham tRNS | |
| Outcomes | Primary: pain NRS anchors not reported When taken: postintervention Secondary: FIQ AEs not reported | |
| Notes | Funding source: not reported COI: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not described |
| Adequate blinding of participants? | Unclear risk | Comment: method of blinding not reported |
| Adequate blinding of assessors? | Unclear risk | Comment: method of blinding not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | High risk | Comment: no numeric reporting of primary outcomes |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: not specified Condition: chronic myofascial pain in the upper body Prior management details: not reported n = 24 Age, mean (SD): active group 45.83 ( 9.63) years, sham group 44.83 (14.09) years Duration of symptoms: not reported Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º from midline, 80% RMT, number of trains 16; duration of trains 10 s; ITI 26 s; total number of pulses 1600 Stimulation location: L M1 Number of treatments: 10 sessions, timescale not specified Control type: sham coil ‐ same sound and appearance and sensation | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: postintervention Secondary: AEs | |
| Notes | Funding source: grants and material support from the following Brazilian agencies: Brazilian Innovation Agency (FINEP), process number 1245/13; Committee for the Development of Higher Education Personnel—PNPD/CAPES, process number 023‐11, and material support; National Council for Scientific and Technological Development—CNPq (grants WC‐301256/2013‐6 and ILST‐ 302345/2011‐6 ); Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul (material support); Postgraduate Research Group at the Hospital de Clınicas de Porto Alegre (grant number 120343 and material support); and Foundation for Support of Research at Rio Grande do Sul (FAPERGS). COI: study authors declared that there was no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer random number generator assigned patients to 1 of 2 groups: rTMS or placebo‐sham using a block randomization strategy.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Before the recruitment phase, opaque envelopes containing the protocol materials were prepared. Each opaque envelope was sealed and numbered sequentially, containing 1 intervention allocation.” |
| Adequate blinding of participants? | Low risk | Quote “we used an inactive rTMS coil (MagPro X100; MagVenture Company, Lucernemarken, Denmark) as a sham method by placing it in the identical area as the active coil. Thus, sham patients underwent similar rTMS experience (including rTMS sound) as those receiving active stimulation.....The patient recorded identical experiences (including sound effects and somatic sensations caused by contraction of the muscles of the scalp) as during active stimulation” Comment: assessment indicates that blinding was successful. |
| Adequate blinding of assessors? | Low risk | Quote “Two independent evaluators who were blinded to the group assignments(W.C. and another) were trained to apply the pain scales and conduct psychophysical and psychological tests.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 dropout |
| Selective reporting (reporting bias) | High risk | Comment: point estimates for outcomes only reported at one time point |
| Study Size | High risk | n = 24 |
| Study duration | Low risk | 12‐week follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: neurology dept Condition: CPSP Prior management details: stable medication for 30 d preceding baseline n = 23 Age, mean (SD): active group 55 (9.67) years, sham group SD 57.8 (11.86) years Duration of symptoms, mean (SD): active group 64.18 (49.27) months, sham group 50.1 (28.04) Gender distribution:active group 45% M, sham group 50% M | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified, 120% RMT, number of trains 25; duration of trains 5 s; ITI 25s; total number of pulses 1250 Stimulation location: L premotor/DLPFC Number of treatments: 10 sessions daily for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors not reported When taken: end of intervention, 1, 2 and 4 weeks postintervention Secondary: AEs, QoL (SF‐36) | |
| Notes | Funding source: study was supported by the Pain Center of the Department of Neurology and by the Transcranial Magnetic Stimulation Laboratory of the Psychiatry Institute, University of Sao Pau COI: the study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Participants were randomly assigned into 2 groups, active stimulation (a‐rTMS) and sham stimulation (s‐rTMS), according to a list automatically generated by an internet‐based tool (www.random.org)” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Quote “Sham stimulation was carried out with a sham coil of identical size color and shape emitting a sound similar to that emitted by the active coil (MC‐P‐B70).” Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “Pain intensity (VAS) was assessed daily, right before and immediately after each rTMS session, from D1 to D10 by an investigator (M.M.) blinded to the type of rTMS patients were receiving. All clinical assessments were performed by a physician and a neuropsychologist (T.L., M.L.M) who were blinded to the type of treatment and had no other role in the study.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1 dropout per group |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | n = 21 |
| Study duration | Unclear risk | Comment: 4‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: "single clinical location" Condition: fibromyalgia Prior management details: FDA‐approved fibromyalgia drugs and centrally active analgesics or stimulants "prohibited". n = 46 Age mean (SD) active 12‐week programme group 55.7 (8.7) active 8‐week programme group 46.6 (10.3), sham group 47.9 (11.2) Duration of symptoms: not reported Gender distribution: reported for completers only 35 F, 3 M | |
| Interventions | Stimulation type: RINCE Stimulation parameters: not reported Stimulation location: parietal region (international 10/20 site PZ),"positioned to create a conduction pathway that includes the primary somatosensory and motor cortex". Number of treatments: Active 12‐week group: 24 treatments of 12 weeks Active 8‐week group: 16 treatments over 8 weeks followed by 8 sham sessions in 4 weeks Sham group: 24 sham sesssions over 12 weeks Control type: nonactivated identical stimulation unit | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst pain imaginable When taken: end of treatment period, 4 weeks post‐treatment Secondary: total FIQ score AEs | |
| Notes | Sources of support: all funding for this study was provided by Cerephex Corporation who manufacture the device. COI: no formal declaration. 5 study authors affiliated to funder ‐ who manufacture the RINCE technology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not clearly established |
| Adequate blinding of participants? | Unclear risk | Quote: “patients cannot feel the RINCE signal and are therefore blinded to receiving treatment or not….no element of hardware or software gave any indication of group assignment” |
| Adequate blinding of assessors? | Unclear risk | Quote: “The investigators were blinded to these codes and no element of hardware or software gave any indication of group assignment, thus maintaining a double blinded sham controlled condition.” |
| Incomplete outcome data (attrition bias) | High risk | Comment: 7/14 participants not analysed in the sham group due to “exposure to unexpected signal source”. These participants not included in sham analysis. Details on how this was confirmed or what the exposure was are not clear. |
| Selective reporting (reporting bias) | High risk | Comment: point estimates with measures of variance not provided for all groups at all time points |
| Study Size | High risk | n = 46, divided into 3 groups |
| Study duration | Unclear risk | Comment: 4‐week follow‐up period |
| Other bias | Unclear risk | Comment: full baseline data not tested and only data with 8 excluded sham participants removed were presented |
| Methods | Parallel RCT | |
| Participants | Country of study: Israel Setting: outpatient department Condition: post‐SCI central neuropathic pain Prior management details: refractory to drug, physical therapy and complementary therapy management n = 12 Age: 44‐60 years; mean 54 (SD 6) Duration of symptoms: > 12 months Gender distribution: 7 M, 4 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 5 Hz; coil orientation not specified; 115% RMT; number of trains 500; duration of trains 10 s; ITI 30 s; total number of pulses 500 reported, likely to have been 25,000 judging by these parameters Stimulation location: M1 ‐ midline Number of treatments: x 10, x 1 daily on consecutive days Control type: sham coil ‐ visually the same and makes similar background noise | |
| Outcomes | Primary: 15 cm 0‐10 VAS pain intensity, anchors "no pain sensation" to "most intense pain sensation" When taken: pre and post each stimulation session Secondary: McGill pain questionnaire When taken: 2‐ and 6‐week follow‐up period | |
| Notes | AEs: not reported Sources of support: supported by the National Association of the insurance companies. COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified Quote: "Patients were randomised into 2 groups that received either real or sham rTMS" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Quote: "Two coils were used; real and sham, both of which were identical in shape and produced a similar background noise." Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation, but did not control for sensory characteristics of active stimulation over the scalp. Given that stimulation was delivered at 110% RMT active stimulation, but not sham, it is likely to have elicited muscle twitches in peripheral muscles |
| Adequate blinding of assessors? | Low risk | Quote: "The patients as well as the person conducting the outcome measurements were blind to the type of treatment received." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 participant withdrew for "logistic reasons". Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: while group means/SD were not presented in the study report, the study authors provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline differences observed in pain intensity levels (higher in active group) |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic temperomandibular disorder Prior management details: pain not adequately controlled by previous therapies for more than 1 year n = 24 Age range, mean (SD): active group 34.8 (13.7) years, sham group 35.6 (16.7) years Duration of symptoms: not reported Gender distribution: all F | |
| Interventions | Stimulation type: HD‐tDCS Stimulation parameters: intensity 2 mA, 4 electrodes arranged at the corners of a 4 x 4 cm square centred over M1 Stimulation location: anode ‐ M1 contralateral to painful side Number of treatments: daily, x 5 Control type: sham tDCS | |
| Outcomes | Primary: pain VAS; anchors not reported ‐ responder analysis only reported When taken: 1‐month follow‐up Secondary: AEs | |
| Notes | Sources of funding: this project was funded by grants from the American Academy of Orofacial Pain and the University of Michigan Rackham Graduate School. Potential undisclosed COI: 1 study author (Biksom) worked for stimulation device manufacturer Soterix | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “participants were randomized to the treatment or placebo group using the Taves covariate adaptive randomization method.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment procedures |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA intensity. Evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | High risk | Comment: study described as single blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no participant dropout |
| Selective reporting (reporting bias) | High risk | Comment: pain outcomes not presented for all follow‐up time points |
| Study Size | High risk | n = 24 |
| Study duration | Unclear risk | 1‐month follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Norway Setting: university hospital Condition: fibromyalgia Prior management details: prescription medication stable for 3 months prior to inclusion n = 50 Age, mean (SD): active group 49/04 (8.63) years, sham group 48.17 (10.56) years Duration of symptoms, mean (SD) sham group 17.73 (7.54) years, sham group 18.50 (11.48) Gender distribution: 47 F, 3 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 side not reported, cathode supraorbital contralateral to anode Number of treatments: daily, x 5 Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors not reported When taken: postintervention, mean 30 days postintervention Secondary: FIQ, SF‐36, AEs | |
| Notes | Sources of funding: study was funded by a grant from the Norwegian Extra Foundation for Health and Rehabilitation through the Norwegian Fibromyalgia Association Study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The codes were associated with the active or sham tDCS condition and randomized using the online Web service www.randomize.org. The ratio of active and sham codes was 1:1.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clearly stated that the sequence generation was separated and concealed |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. Not formal assessment of blinding success |
| Adequate blinding of assessors? | Low risk | Comment: outcomes collected through text message with little potential for assessors to influence process |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: high noncompletion rate for some outcomes and there is not full clarity on how many participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: full reporting of key outcomes |
| Study Size | High risk | n = 50 |
| Study duration | Unclear risk | Comment: follow‐up 30 days postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: unclear Condition: chronic pelvic pain Prior management details: refractory to treatment n = 7 Age: mean 38 years Duration of symptoms: mean 80 months Gender distribution: all F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 dominant hemisphere Number of treatments: 2 Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: VAS overall pain, pelvic pain, back pain, migraine pain, bladder pain, bowel pain, abdomen pain and pain with intercourse. Anchors not specified When taken: daily during stimulation and then for 2 weeks post‐each condition Secondary: none | |
| Notes | Sources of support: no declaration made COI: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Low risk | Quote: "All other personnel in the study, including the investigators, study coordinators, participants, and their families, and all primary medical caregivers, were blinded." |
| Adequate blinding of assessors? | Low risk | Quote: "All other personnel in the study, including the investigators, study coordinators, participants, and their families, and all primary medical caregivers, were blinded." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | Low risk | Comment: variance measures not presented for group means poststimulation but data provided by study author on request |
| Free from carry‐over effects? | Unclear risk | Comments: pre‐stimulation data not presented and no formal investigation for carry‐over effects discussed |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic pancreatitis pain Prior management details: not specified n = 5 Age: 44 (SD 11) Duration of symptoms: not specified, "chronic" Gender distribution: not specified | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 1 Hz or 20 Hz; coil orientation not specified; 90% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 1600 Stimulation location: L and R SII Number of treatments: 1 for each condition Control type: sham, "specially designed sham coil". No further details | |
| Outcomes | Primary: pain VAS, anchors not specified When taken: after each stimulation session Secondary: none | |
| Notes | COI: no declaration made Sources of support: National Pancreas Foundation/ NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of stimulation was randomised and counterbalanced across patients using a Latin square design." |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment "unclear". Type of sham coil not specified |
| Adequate blinding of assessors? | Low risk | Quote: "Patients were blinded to treatment condition, and a blinded rater evaluated analgesic use, patient's responses in a Visual Analogue Scale (VAS) of pain.... immediately after each session of rTMS." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | High risk | Comment: pain NRS values not provided clearly with measures of variance for any time point for the sham condition |
| Free from carry‐over effects? | Low risk | Quote: "Importantly, baseline pain scores were not significantly different across the six conditions of stimulation... speaking against carryover effect." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: post‐SCI central neuropathic pain Prior management details: refractory to drug management n = 17 Age: mean 35.7 (SD 13.3) years Duration of symptoms: chronic > 3/12 Gender distribution: 14 M, 3 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS 0‐10 cm, anchors "no pain" to "worst pain possible" When taken: before and after each stimulation and at 16‐day follow‐up Secondary: none | |
| Notes | COI: no declaration made Sources of support: support from Harvard Medical School Scholars in Clinical Science programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomisation list generated by a computer using random blocks of six (for each six patients, two were randomised to sham and four to active tDCS) in order to minimize the risk of unbalanced group sizes." |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pre‐generated randomisation list should ensure this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "... we analyzed the primary and secondary endpoints using the intention‐to‐treat method including patients who received at least one dose of the randomised treatment and had at least one post‐baseline efficacy evaluation. We used the last evaluation carried out to the session before the missed session, assuming no further improvement after the dropout, for this calculation." |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain score numerical values not provided clearly in the study report with measures of variance for any time point. On request data were available for the primary outcome at one follow‐up point but not for other follow‐up points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT; 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: unclear n = 32 Age: 53.4 (SD 8.9) years Duration of symptoms: condition 1: 8.4 (SD 9.3) years; condition 2: 10.0 (SD 7.8) years; condition 3: 8.1 (SD 7.5) years Gender distribution: 32 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: condition 1: DLPFC; condition 2: M1; condition 3: sham M1. All conditions contralateral to most painful side or dominant hand Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS 0‐10 cm, anchors not specified When taken: at the end of the stimulation period and at 21‐day follow‐up Secondary: QoL: FIQ | |
| Notes | COI: no declaration made Sources of support: support from Harvard Medical School Scholars in Clinical Science programme/ NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entry into the study and a previous computer‐generated randomisation list, using random blocks of 6 patients (for each 6 patients, 2 were randomised to each group) in order to minimize the risk of unbalanced group sizes." |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pre‐generated randomisation list should have adequately ensured this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "One patient (in the M1 group) withdrew, and the few missing data were considered to be missing at random. We analyzed data using the intent‐to‐treat method and the conservative last observation carried forward approach." |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain score numerical values not provided clearly with measures of variance for most time points in the study report. On request data were available for the primary outcome at 1 follow‐up point but not for other follow‐up points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic visceral pain (chronic pancreatitis) Prior management details: most on continuous opioid therapy, most had received surgery for their pain n = 17, 9 in active group, 8 in sham group Age mean (SD): active group 41.11 (11.27) years, sham group 46.71 (13.03) years Duration of symptoms: > 2 years Gender distribution: 14 F, 3 M | |
| Interventions | Stimulation type: rTMS Stimulation parameters:frequency 1 Hz; coil orientation not specified, number of trains 1; duration of trains not specified; intensity 70% maximum stimulator output, total number of pulses 1600 Stimulation location: SII Number of treatments: 10, x 1 daily (weekdays only) Control type: sham rTMS coil | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = most intense pain imaginable When taken: daily pain logs for 3 weeks pre‐intervention, daily post‐stimulation during intervention period and at 3‐week follow‐up Secondary: none relevant | |
| Notes | COI: no declaration made Sources of support: support from Harvard Thorndike Clinical Research Center/ NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised (using a computer generated list with blocks of 4)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Quote "The sham and real TMS coils looked identical and were matched for weight and acoustic artefact. This sham coil induces a similar tapping sensation and generates the same clicking noise as the real TMS coil, but without induction of a significant magnetic field and secondary current." Comment: sham appears optimal |
| Adequate blinding of assessors? | Low risk | Quote: "The pain evaluation was carried out by a blinded assessor" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropout/withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Comment: reporting of pain scores incomplete across all time points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline values not presented by group for key outcome variables |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: pain clinic Condition: chronic back and neck pain Prior management details: unclear n = 20 Age: 20‐77 years Duration of symptoms: 0.5‐40 years Gender distribution: 9 M, 11 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 77 Hz; pulse width 3.3 ms; intensity ≤ 4 mA; waveform shape biphasic asymmetric; duration 30 min Stimulation location: 3 electrodes, 1 attached to either mastoid process and 1 to the forehead Number of treatments: 8, x 1 daily on consecutive days Control type: "active placebo" units visually indistinguishable. Delivered 50 Hz frequency, intensity ≤ 0.75 mA. Note: may not be inert | |
| Outcomes | Primary: pain VAS, anchors not specified When taken: pre and post each stimulation Secondary: none | |
| Notes | COI: no declaration made Sources of support: grant by Pulse Mazor instruments, Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list." |
| Allocation concealment (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list. At enrolment in the study, the investigator assigned the next random number in that patient’s category. The investigator did not have access to the randomisation list until after the study was completed." |
| Adequate blinding of participants? | Low risk | Quote: "The active placebo device was indistinguishable to the patient and medical team from the real TCES device ‐ it was designed to give the patient the feeling of being treated, inducing an individual sensation of skin numbness or muscle contraction" |
| Adequate blinding of assessors? | Low risk | Quote: "The active placebo device was indistinguishable to the patient and medical team." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values were not provided clearly with measures of variance for most time points in the study report, the study authors have provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: an active placebo that delivers current may not be inert and may bias against between group differences (0.75 mA exceeds the intensity of the active arms of other CES trials) |
| Methods | Parallel RCT | |
| Participants | Country of study: Israel Setting: pain clinic Condition: chronic back and neck pain Prior management details: unclear n = 75 (excluding headache participants) Age: mean 53.9 years, range 22‐82 Duration of symptoms: 0.5‐40 years Gender distribution: 35 M, 40 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 77 Hz; pulse width 3.3 ms; intensity ≤ 4 mA; waveform shape biphasic asymmetric; duration 30 min Stimulation location: 3 electrodes, 1 attached to either mastoid process and 1 to the forehead Number of treatments: 8, x 1 daily on consecutive days Control type: "active placebo" units visually indistinguishable. Delivered 50 Hz frequency, intensity ≤ 0.75 mA. Note: may not be inert | |
| Outcomes | Primary: pain VAS, anchors not specified When taken: pre and post each stimulation; 3 weeks and 3 months following treatment Secondary: none | |
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list" |
| Allocation concealment (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list. At enrolment, the investigator assigned the next random number in that patient’s category. The investigator did not have access to the randomisation list until study completion." |
| Adequate blinding of participants? | Low risk | Quote: "The placebo device was indistinguishable from the active device" |
| Adequate blinding of assessors? | Low risk | Quote: "The investigator did not have access to the randomisation list until study completion" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout is indicated, comparing the results with the number enrolled |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: an active placebo that delivers current may not be inert and may bias against between group differences (0.75 mA exceeds the intensity of the active arms of other CES trials) |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: trigeminal neuralgia Prior management details: stable medication for 6 months prior to study, no invasive procedures prior to study n = 17 Age range: 32‐72 years Duration of symptoms: range 2‐27 years, mean 13 Gender distribution: 7 M, 10 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 40 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 contralateral to painful side, cathode supraorbital contralateral to anode Number of treatments: daily, self‐administered for 14 days Control type: sham tDCS | |
| Outcomes | Primary: pain VAS When taken: postintervention Secondary: AEs | |
| Notes | Study authors' COI statement: "Tim Hagenacker has received research support from Astellas and CSL Behring. Vera Bude, Steffen Naegel have nothing to disclose. Dagny Holle has received research support from Grünental and Allergan. Mark Obermann has received scientific support and/or honoraria from Biogen Idec, Novartis, Sanofi‐Aventis, Genzyme, Pfizer, Teva. He received research grants from Allergan, Electrocore, and the German Ministry for Education and Research (BMBF). Hans‐Christoph Diener has received honoraria for participation in clinical trials, contribution to advisory boards or lectures from Addex Pharma, Allergan, Almirall, AstraZeneca, Bayer Vital, Berlin Chemie, Coherex Medical, CoLucid, Böhringer Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Grünenthal, Janssen‐Cilag, Lilly, La Roche, 3M Medica, Minster, MSD, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, SanofiAventis, and Weber & Weber; received research support from Allergan, Almirall, AstraZeneca, Bayer, Galaxo‐Smith‐Kline, Janssen‐Cilag, and Pfizer. Sources of support: "Headache research at the Department of Neurology in Essen is supported by the German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Adequate blinding of participants? | Unclear risk | Comment: method of blinding not clearly stated |
| Adequate blinding of assessors? | Unclear risk | Comment: method of blinding not clearly stated |
| Incomplete outcome data (attrition bias) | High risk | Comment: 7/17 participants discontinued trial. Details of when not clear. Per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all key outcomes reported |
| Free from carry‐over effects? | Unclear risk | No formal assessment of baseline equivalence reported |
| Study Size | High risk | Comment: n = 17, 10 after attrition |
| Study duration | High risk | Comment: only immediate postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: "professional clinical setting" Condition: fibromyalgia Prior management details: no recent remission of symptoms n = 91 Age: active group 48‐54.7 years, sham group 51‐57 years Duration of symptoms: active group mean 17.12 years, sham group mean 17.5 years Gender distribution: reported for completers only 71 F, 6 M | |
| Interventions | Stimulation type: RINCE Stimulation parameters: current density 0.3 mA/cm2, stimulation duration 11 min, frequency 10 kHz carrier signal delivered at 40 Hz Stimulation location: parietal region (international 10/20 site PZ), ground leads fixed to earlobes Number of treatments: x 2 weekly for 11 weeks Control type: non‐activated identical stimulation unit | |
| Outcomes | Primary: FIQ pain VAS; 0 = no pain, 10 = unbearable pain When taken: end of treatment period Secondary: total FIQ score | |
| Notes | Lead author declared an intellectual property interest in the technology and is a shareholder in a company seeking to develop the technology for commercialisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Quote: "The combined involvement of low driving potentials and high carrier frequencies creates a signal that is subthreshold for perceptibility.....Subjects could not feel the signal regardless of group, and therefore could not tell if they were receiving treatment or not" |
| Adequate blinding of assessors? | Low risk | Quote: "The investigators were blinded to the settings, and no element of hardware or software gave any indication as to which setting had been assigned to the subject." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: per‐protocol analysis used, dropout rate 6/45 (13%) in active group and 8/46 (17%) in sham group |
| Selective reporting (reporting bias) | Low risk | Comment: data reported on all outcomes and supplementary data made available by the study author |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Canada Setting: laboratory Condition: mixed chronic pain (in the over 60s) Prior management details: not reported n = 16 Age, mean (SD): active group 72 (6) years, sham group 71 (8) years Duration of symptoms mean (SD) years: active group 26 (24), sham group 15 (11) Gender distribution: 11 F, 3 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 1 daily for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst imaginable pain When taken: postintervention Secondary: none relevant AEs not reported | |
| Notes | Funding source: G Léonard is supported by the Fonds de Recherche en Santé (FRQ‐S, Montréal, QC, Canada). This project was partially supported by the Neuroscience Centre of Excellence of the Université de Sherbrooke (CeNUS, Sherbrooke, QC, Canada) and an internal start‐up fund from the Research Centre on Aging (Initiatives stratégiques du Centre de recherche sur le vieillissement, Sherbrooke, QC, Canada). COI: study authors report no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization to sham or active tDCS was performed using a random numbers table with a ratio of 1:1, based on order of entry of the participants in the study.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2/8 (25%) in active group withdrew. Data appear to have been excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 14 |
| Study duration | High risk | Comment: 1 week postintervention follow‐up |
| Other bias | High risk | Comment: baseline imbalance in average daily pain |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: chronic low back pain Prior management details: not reported n = 92, relevant to this review 46 Age, mean (SD): active group 51.9 (9.9) years, sham group 54.1 (9.8) years Duration of symptoms mean (SD) months: active group 91.6 (108.3) sham group 69.2 (92.7) months Gender distribution: 10 M, 36 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 3 per week for 4 weeks. 12 sessions in total Control type: sham tDCS | |
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst pain possible When taken: postintervention, 3 months, 6 months Secondary: disability (RMDQ) AEs | |
| Notes | Funding source: none COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated to one of the four treatment groups by means of random‐number‐generating software." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization and allocation concealment were carried out by an external collaborator, not a research participant, who organized patients and their previously allocated treatments in individual opaque envelopes." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2mA. No assessment of blinding success. No formal assessment of blinding success. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 46 |
| Study duration | Low risk | Comment: 6‐month follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 5 conditions | |
| Participants | Country of study: Japan Setting: laboratory Condition: intractable deafferentation pain (mixed central, peripheral and facial) Prior management details: intractable n = 20 Age: 28‐72 years Duration of symptoms: 1.5‐24.3 years, mean 6.4 (SD 6) Gender distribution: 13 M, 7 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 5 Hz; coil orientation not specified; 90% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 500 Stimulation location: condition 1: M1; condition 2: primary sensory cortex; condition 3: pre‐motor area; condition 4: supplementary motor area; condition 5: sham Number of treatments: 1 for each condition Control type: coil angled 45º from scalp with synchronised electrical scalp stimulations to mask sensation | |
| Outcomes | Primary: pain intensity VAS, anchors not specified When taken: 0, 30, 60, 90, 180 min poststimulation Secondary: none | |
| Notes | COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All targets were stimulated in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "The patients were unable to distinguish sham stimulation from actual rTMS, because the synchronized electrical stimulation applied to the forehead made the forehead spasm, as was the case with actual TMS" Comment: sham credibility assessment ‐ suboptimal. Sensory and auditory aspects controlled for but angulation of coil away from the scalp may be visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 20 patients underwent all planned sessions of navigation‐ guided rTMS" |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point but data provided upon request |
| Free from carry‐over effects? | Low risk | Comment: study authors provided requested data. Appears free of carry‐over effects |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Japan Setting: multicentre, laboratory‐based Condition: mixed neuropathic pain Prior management details: pain persisted despite "adequate treatments" n = 70 of whom 64 analysed Age mean (SD): 60.7 (10.6) years Duration of symptoms: 58.2 (10.6) months Gender distribution: 40 M, 24 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 5 Hz; coil orientation parasagittal, number of trains 10; duration of trains 10 s; ITI 50 s, intensity 90% RMT, total number of pulses per session 500 Stimulation location: M1 corresponding to painful region Number of treatments: 10, x 1 daily (consecutive working days) Control type: sham coil | |
| Outcomes | Current daily pain 0‐100 VAS (anchors not reported), SF McGill AEs | |
| Notes | COI: study authors declared no COI Sources of support: "funded by the Japanese Ministry of Health, Labour and Welfare with a Health and Labour Sciences Research Grant. This research was partly supported by Japanese MEXT SRPBS" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the patient enrolment, the independent data center developed a randomization program to assign each patient to one of 2 treatment groups (1:1). A real rTMS period was followed by a sham period in group A, and a real rTMS period came after a sham period in group B. We used Pocock and Simon’s minimization method to stratify treatment groups according to institution, age (< 60 or P60 years), sex, and underlying disease (a cerebral lesion or not), and the Mersenne twister for random number generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "After confirmation of patient eligibility, the data center received a registration form from an assessor who collected questionnaires and assessed adverse events, and then sent an assignment notice to an investigator who conducted the rTMS intervention. Patients were identified by sequential numbers that were assigned by the data center. Patients and assessors were blind to group assignment until the study was completed. The data center was responsible for assigning patients to a treatment group, data management, central monitoring, and statistical analyses." |
| Adequate blinding of participants? | Low risk | Quote: "Realistic sham stimulation [32] was implemented in this study. Ten trains of electrical stimuli at 2 times the intensity of the sensory threshold (one train, 50 stimuli at 5 Hz; inter train interval, 50 s) were delivered with a conventional electrical stimulator through the electrodes fixed on the head. The cortical effect of the cutaneous electrical stimulation was considered to be negligible at this intensity because of the high electrical resistance of the skull and brief duration of the stimulation [32]. A figure‐8 coil, which did not connect to a magnetic stimulator, was placed on the head in the same manner as a real rTMS session. Another coil, which discharged simultaneously with the electrical stimuli, was placed near the unconnected coil to produce the same sound as real rTMS, but not to stimulate the brain." Comment: sham controls for sensory auditory and visual cues |
| Adequate blinding of assessors? | Low risk | Quote: "Patients and assessors were blind to group assignment until the study was completed." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: dropout low (total 6 from recruited 70 participants) Quote: "Seventy patients were enrolled and randomly assigned to 2 groups. Of these patients, one patient never came to the hospital after the registration, and a suicidal wish became apparent before the start of the intervention in another patient. Sixty‐eight patients received the interventions and 64 patients were included in the intention‐to‐treat analysis after excluding 4 patients without any data collection." |
| Selective reporting (reporting bias) | Low risk | Comment: while full numerical means and SDs were not reported for all time points all data were made available upon request to the study authors |
| Free from carry‐over effects? | Low risk | Quote: "To evaluate carry‐over effects, Grizzle’s test for carry‐over effect was applied to the values at day 0 for each period ... Grizzle's test showed no carry‐over effects in VAS and SF‐MPQ" |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: Germany Setting: laboratory Condition: PLP and CNP Prior management details: unclear n = 27 Age: (median) PLP 46.6 years, CNP 51.1 years Duration of symptoms: mean PLP 15.2 (SD 14.8), CNP 3.9 (SD 4.1) years. Gender distribution: 16 M, 11 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 1 Hz; coil orientation not specified; 95% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Condition 2: frequency 5 Hz; coil orientation not specified; 95% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Condition 3: sham frequency 2 Hz; coil orientation not specified; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Stimulation location: M1, contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil; mimics sight and sound of active treatment | |
| Outcomes | Primary: 0‐100 mm VAS pain intensity, anchors "no pain" and "most intense pain imaginable" When taken: pre‐ and post‐stimulation Secondary: none | |
| Notes | Sources of support: no reporting of source of support COI: study authors decare no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | High risk | Comment: 13 of 27 participants did not complete all treatment conditions and this dropout is not clearly accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly and in full |
| Free from carry‐over effects? | Low risk | Quote: "The VAS values before the stimulation showed no significant differences in the various types of treatment" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: continued using pharmacological and nonpharmacological therapies. n = 20 Age mean (SD): 46.4 (10.62) years Duration of symptoms: not reported Gender distribution: all F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 15 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 L, cathode right supraorbital Number of treatments: x 1 per week for 10 weeks Control type: sham tDCS | |
| Outcomes | Primary: pain VAS; anchors not reported When taken: postintervention Secondary: FIQ, SF‐36 | |
| Notes | No reporting of sources of support or COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no reporting of concealment procedures |
| Adequate blinding of participants? | Low risk | Quote “Patients, as well as investigator in charge and evaluators, were blind to the nature of applied stimulation” Comment: blinding likely at 1 mA intensity |
| Adequate blinding of assessors? | Low risk | Quote “Patients, as well as investigator in charge and evaluators, were blind to the nature of applied stimulation” Comment: blinding likely at 1 mA intensity |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: attrition not reported |
| Selective reporting (reporting bias) | Low risk | Comment: results reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Unclear risk | Comment: no reporting of baseline comparability |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: post‐SCI pain (neuropathic and non‐neuropathic) Prior management details: not reported n = 31 randomised Age: 22‐77 years Duration of symptoms (months): > 6 months Gender distribution: 22 M, 8 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 contralateral to painful side or on L where pain bilateral Number of treatments: 1 Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: 0‐10 NRS; 0 = no pain, 10 = most intense pain sensation imaginable. An average of current, least, worst and average pain scores When taken: poststimulation Secondary: none relevant | |
| Notes | AEs not reported Government‐funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The remaining 31 individuals were randomly assigned to receive the five procedure conditions in one of five orders, using a Latin square design." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: of 31 randomised there were data from 28 following active tDCS and 27 following sham |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Free from carry‐over effects? | Low risk | Comment: baseline pain levels pre active and sham tDCS session appear equivalent |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Canada Setting: outpatient rehabilitation centre Condition: post‐SCI neuropathic pain Prior management details: almost all participants in various medications n = 18 Age: range 31‐69 years, mean (SD) 50 (9) Duration of symptoms: not reported Gender distribution: 11 M, 5 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º posterolateral, 90% RMT for hand, 110% RMTA for leg, number of trains 40; duration of trains 5 s; ITI 25 s; total number of pulses 2000 Stimulation location: M1 hand or leg area with neuronavigation Number of treatments: single session per condition, 1 session of sham Control type: sham coil ‐ same sound and appearance and sensation | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: immediately poststimulation, 20 min poststimulation Secondary: AEs ‐ though no formal assessment reported | |
| Notes | Funding source: supported by the Canadian Institutes of Health Research (CIHR), Grant Number MOP‐79370. C. Mercier was supported by salary awards from the CIHR and the Fonds de recherche du Québec, Santé (FRQS). F. Jetté was supported by a fellowship from Université Laval and H. B. Meziane by a fellowship from the Réseau Provincial de Recherche en Adaptation‐Réadaptation (REPAR‐FRQS). Support was provided by the Consortium d’Imagerie en Neuroscience et Santé Mentale de Québec (CINQ) for MRI acquisition COI: the study authors declared no potential COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “2 active rTMS sessions (hand/leg M1 area) and 1 sham rTMS session in a randomized, counterbalanced order.” Comment: method of randomisation not described |
| Adequate blinding of participants? | Low risk | Quote “Sham rTMS, using a sham coil (mimicking the noise and scalp sensations), was applied over the hand area using the same parameters |
| Adequate blinding of assessors? | Low risk | Quote “The researcher running the pre‐post assessment (as well as data analysis) was blind relative to the applied rTMS protocol(as was the participant), with the rTMS application being performed by a different researcher |
| Incomplete outcome data (attrition bias) | Low risk | Comment: dropout levels low ‐ 2 in total |
| Selective reporting (reporting bias) | Low risk | Comment: data provided upon author request |
| Free from carry‐over effects? | Unclear risk | Comment: 2‐week washout period observed but no analysis or data presented to confirm baseline comparability |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: immediate poststimulation measurement only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: South Korea Setting: university hospital outpatient setting Condition: post‐SCI central neuropathic pain Prior management details: resistant to drug, physical or complementary therapies n = 11 Age: 33‐75 years, mean 54.8 Duration of symptoms: chronic Gender distribution: 6 M, 5 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation angled 45º posterolaterally; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number pulses 1000 Stimulation location: R M1, hand area Number of treatments: 5, x 1 daily Control type: coil elevated and angled away from the scalp | |
| Outcomes | Primary: NRS average pain over last 24 h, anchors "no pain sensation" to "most intense pain sensation imaginable" When taken: immediately after the 3rd and 5th treatments and 1, 3, 5 and 7 weeks after the end of the stimulation period Secondary: BPI ‐ pain interference (surrogate measure of disability) When taken: as for the NRS | |
| Notes | AEs: not reported COI: studu authors declared no COI Sources of support: supported by the Seoul National University Bundang Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The real and sham rTMS stimulations were separated by 12 weeks and performed in a random order according to the prepared allocation code." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Didnot control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "... a different researcher collected the clinical data; the latter researcher was not aware of the type of rTMS (real or sham)" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no participants withdrew after receiving the first treatment condition |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 12‐week washout period was observed. The pre‐stimulation baseline scores closely match |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT; 3 conditions | |
| Participants | Country of study: Russia Setting: unclear Condition: hip and knee OA Prior management details: unclear n = 64 Age: unclear Duration of symptoms: unclear Gender distribution: unclear | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 11‐15 mA; waveform shape: condition 1 symmetric, condition 2 asymmetric; duration 40 min Stimulation location: appears to be 1 electrode attached to either mastoid process and 1 to the forehead Number of treatments: 5, x 1 daily for 5 consecutive Control type: sham unit ‐ visually indistinguishable from active units | |
| Outcomes | Primary: 0‐10 NRS, anchors "no pain" to "very painful" When taken: unclear. Likely to be pre and post each stimulation session and then daily for 1 week after Secondary: none | |
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "If subjects passed all criteria they were randomly assigned to one of the two active treatments or the sham treatment." Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Adequate blinding of participants? | Low risk | Quote: "The physicians, like all other participants in the study, were unaware of which treatment each subject received." |
| Adequate blinding of assessors? | Low risk | Quote: "The physicians, like all other participants in the study, were unaware of which treatment each subject received." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropout level not specified |
| Selective reporting (reporting bias) | High risk | Comment: it is unclear in the report which time points were reported for primary outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: the reporting of baseline group characteristics is insufficient |
| Methods | Parallel RCT | |
| Participants | Country of study: Egypt Setting: university hospital neurology department Condition: neuropathic pain, mixed central (poststroke) and facial (trigeminal neuralgia) pain Prior management details: refractory to drug management n = 48 Age: poststroke 52.3 (SD 10.3) years, trigeminal neuralgia 51.5 (SD 10.7) years Duration of symptoms: poststroke 39 months (SD 31), trigeminal neuralgia 18 months (SD 17) Gender distribution: 8 M, 16 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified; 80% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: M1 contralateral to the side of worst pain Number of treatments: 5, x 1 on consecutive days Control type: coil elevated and angled away from scalp | |
| Outcomes | Primary: pain VAS, anchors not specified When taken: post 1st, 4th and 5th stimulation session and 15 days after the last session Secondary: none | |
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly assigned to one of the two groups, depending on the day of the week on which they were recruited." Comment: not truly random |
| Allocation concealment (selection bias) | High risk | Comment: the method of sequence generation makes concealment of allocation unlikely |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The second author evaluated these measures blindly—that is, without knowing the type of rTMS" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values were not provided clearly with measures of variance for all time points in the study report, the study authors have provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Egypt Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 40, 36 after attrition Age, mean (SD): active group 31.3 (10.99) years, sham group 33.89 (11.18) years Duration of symptoms, mean (SD) months, active group 6.1 (2.65), sham group 6.05 (2.5) Gender distribution: 34 F, 2 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: L M1 Number of treatments: x 1 daily for 5 days per week for 2 weeks ‐ 10 sessions in total Control type: sham tDCS | |
| Outcomes | Primary: pain VAS anchors not reported When taken: postintervention, 2 weeks and 1 month postintervention Secondary: none relevant AEs | |
| Notes | Funding source: no funding reported COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was given a serial number from a computer generated randomization table, and was placed in the appropriate group after opening the corresponding sealed envelope." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was done using serially numbered closed, opaque envelopes." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 10% dropout per group |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 per group |
| Study duration | Unclear risk | Comment: 1 month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: South Korea Setting: laboratory Condition: chronic painful diabetic polyneuropathy Prior management details: persistent pain after taking medications. Stable doses of analgesics for 2 months prior to commencement n = 72, 60 after dropout, outcome data only given on this 60 Age, mean (SD): active M1 group 59.60 (13.15) years, active DLPFC group 63.5 (8.75) years, sham group 61.6 (10.27) years Duration of symptoms: all participants had had pain for > 2 years Gender distribution: 25 M, 35 F (after dropout) | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25‐35 cm2 electrodes, duration 20 min Stimulation location: group 1: anode ‐ M1, side not specified, group 2 anode DLPFC side not specified, group 3 M1 sham, cathode contralateral supraorbital Number of treatments: daily, x 5 Control type: sham tDCS | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = "worst possible pain" When taken: end of intervention, 2 weeks, 4 weeks Secondary: AEs | |
| Notes | Funding: supported by Eulji University COI: study authors declared no potential COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entry into the study and a computer‐generated randomization chart with random blocks of six patients each." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedure not described |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at intensities of 2 mA, no formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at intensities of 2 mA, no formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | High risk | Comment: 15% dropout, even across groups, analysis appears to be per‐protocol. |
| Selective reporting (reporting bias) | High risk | Comment: point estimates and measures of variance for primary outcome only reported at selected time points |
| Study Size | High risk | Comment: n = 72, 3 groups |
| Study duration | Unclear risk | Comment: 4‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Canada Setting: laboratory Condition: CRPS type I Prior management details: not reported n = 22 Age, mean (SD): active group 40.9 (8.8) years, sham group 52.7 (10.5) years Duration of symptoms, mean (SD) months: active group 36.3 (25.6), sham group 36.6 (25.8) Gender distribution: 14 F, 8 M | |
| Interventions | Stimulation type: tDCS (combined with graded motor imagery) Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 5 weekly for 2 weeks, x 1 weekly for 4 weeks ‐ 14 sessions in total over 6 weeks Control type: sham tDCS (combined with grade motor imagery) | |
| Outcomes | Primary: average pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: 1 month post intervention Secondary: physical function (BPI pain interference), QoL (SF36‐SF) AEs | |
| Notes | Funding source: this study was supported by grants from the Canadian Pain Society (CPS), the Quebec Pain Research Network (QPRN), as well as the Inflammation and Pain Axis and the Faculty of Medicine and Health Sciences from the Université de Sherbrooke COI: the study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: precise method for randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: “order to avoid a potential concealment bias, the randomization sequence was concealed from the investigators, where only an independent research agent held the allocation list.” |
| Adequate blinding of participants? | Low risk | Comment: 2 mA can affect blinding negatively but formal assessment of participant blinding suggests success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: results reported adequately |
| Study Size | High risk | Comment: n = 22 |
| Study duration | Unclear risk | Comment: 1 month postinterventionfollow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Korea Setting: outpatient clinic Condition: fibromyalgia Prior management details: none reported n = 22 Age mean (SD): low‐frequency group 45.6 (9.6) years, high‐frequency group 53 (4.2) years, sham group 51.3 (6.2) years Duration of symptoms (months mean (SD)): low‐frequency group: 47.2 (20.1), high‐frequency group 57.1 (6.4), sham group 44.7 (10.3) Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: Low‐frequency group: frequency 1 Hz; coil orientation not specified, number of trains 2; duration of trains 800 s; ITI 60 s; total number of pulses 1600 High‐frequency group: frequency 10 Hz; coil orientation not specified, number of trains 25; duration of trains 8 s; ITI 10 s; total number of pulses 2000 Stimulation location: right DLPFC (low‐frequency), L M1 (high‐frequency) Number of treatments: 10, x 1 daily (weekdays only) for 2 weeks Control type: sham ‐ coil orientated away from scalp | |
| Outcomes | Primary: 0‐100 mm pain VAS; 0 = none, 100 = an extreme amount of pain When taken: post‐treatment and at 1 month follow‐up Secondary: FIQ | |
| Notes | Comment: no information on AEs given relating to those participants who did not complete all sessions COI: study authors declared no COI Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Coil angled away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not specified |
| Incomplete outcome data (attrition bias) | High risk | Comment: no ITT analysis described ‐ appears to be per protocol. 3/8 in low‐frequency group, 2/5 in high‐frequency group and 2/5 in sham group |
| Selective reporting (reporting bias) | Low risk | Comment: point measures presented in full for all outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: intractable neuropathic pain (mixed central and facial) Prior management details: refractory to drug management n = 14 Age: 34‐80 years, mean 57.2 Duration of symptoms: not specified "chronic" Gender distribution: 6 M, 8 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation not specified; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Stimulation location: M1, contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil used (? inert) | |
| Outcomes | Primary: 0‐10 VAS, anchors not specified When taken: daily for 12 days poststimulation Secondary: none | |
| Notes | COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two different sessions of rTMS separated by 3 weeks at least were randomly performed in each patient." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point in the report but were provided by study authors on request |
| Free from carry‐over effects? | Low risk | Comment: 3/52 washout period makes carry‐over effects unlikely |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central and peripheral) Prior management details: refractory to drug management n = 18 Age: 28‐75 years, mean 54.7 Duration of symptoms: not specified "chronic" Gender distribution: 11 M, 7 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Condition 2: frequency 0.5 Hz; coil orientation posteroanterior; number of trains 1; duration of trains 20 min; total number of pulses 600 Condition 3: sham ‐ same as for condition 1 with sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition | |
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: 5‐10 min poststimulation Secondary: none | |
| Notes | COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To study the influence of the frequency of stimulation, three different sessions of rTMS separated by three weeks at least were randomly performed in each patient" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: the results of some of the planned data analysis (ANOVA of group differences after each condition) not reported. However, adequate data were available for inclusion in the meta‐analysis |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management n = 60 Age: 27‐79 years, mean 54.6 Duration of symptoms: not specified "chronic" Gender distribution: 28 M, 32 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil | |
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: 5 min poststimulation Secondary: none | |
| Notes | COI: study authors declared no COI Sources of support: grant from the ‘Institut UPSA de la douleur’ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "one of the following two protocols was applied in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "ideal sham...which should be performed by means of a coil similar to the real one in shape, weight, and location on the scalp, producing a similar sound and similar scalp skin sensation, but generating no electrical field within the cortex. Such a sham coil has not yet been designed, and at present, the sham coil used in this study is to our knowledge the more valid for clinical trials." Comments: sham credibility assessment ‐ suboptimal |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: unilateral chronic neuropathic pain (mixed central and peripheral) Prior management details: refractory to drug management n = 22 Age: 28‐75 years, mean 56.5 (SD 2.9) Duration of symptoms: 2‐18 years, mean 5.4 (SD 4.1) Gender distribution: 12 M, 10 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Condition 2: frequency 1 Hz; coil orientation posteroanterior; 90% RMT; number of trains 1; duration of trains 20 min; total number of pulses 1200 Condition 3: sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition | |
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: pre‐ and poststimulation Secondary: none | |
| Notes | AEs: not reported COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three sessions of motor cortex rTMS, separated by at least 3 weeks, were performed in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham as Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors only reported for measures of cortical excitability |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: level of dropout not reported and unclear from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point in the study report but were provided by the study authors on request |
| Free from carry‐over effects? | Low risk | Quote: "Post hoc tests did not reveal any differences between the three pre‐rTMS assessments regarding excitability values or pain levels" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management for at least 1 year n = 46 Age: 27‐79 years, mean 54.2 Duration of symptoms: chronic > 1 year Gender distribution: 23 M, 23 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Condition 2: frequency 1 Hz; coil orientation posteroanterior; 90% RMT; number of trains 1; duration of trains 20 min; total number of pulses 1200 Condition 3: sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition | |
| Outcomes | Primary: 0‐10 VAS, anchors not specified When taken: pre‐ and poststimulation Secondary: none | |
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: grant from the ‘Institut UPSA de la douleur’ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three different sessions of rTMS..... were performed in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Low risk | Quote: "In all cases, the examiner was blinded to the type of rTMS administered." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2 participants dropped out but this is < 5% of the cohort. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: results for all outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: outpatient fibromyalgia clinic Condition: fibromyalgia Prior management details: unclear n = 60 Age: 23‐82 years, mean 50 Duration of symptoms: 1‐40 years, mean 11 Gender distribution: 2 M, 58 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; 50% duty cycle; intensity 100 μA; waveform shape biphasic square wave; duration 60 min Stimulation location: ear clip electrodes Number of treatments: 30, x 1 daily for consecutive days Control type: sham unit ‐ indistinguishable from active unit | |
| Outcomes | Primary: 10‐point self‐rating pain scale, anchors not specified When taken: poststimulation (not precisely defined) Secondary: QoL: 0‐10 VAS scale (data not reported) | |
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the subjects were randomly assigned into three separate groups by an office secretary who drew their names, which were on separate sealed slips of paper in a container" |
| Allocation concealment (selection bias) | Low risk | Comment: probably, given the quote above |
| Adequate blinding of participants? | Low risk | Comment: see previous quote |
| Adequate blinding of assessors? | Low risk | Quote: "All subjects, staff, the examining physician and the psychometrician remained blind to the treatment conditions" |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout levels not specified in the report. ITT analysis not discussed in the report |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any time points in the study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Germany Setting: back pain clinic Condition: chronic nonspecific low back pain Prior management details: excluded if had spinal surgery in previous 6 months n = 135 Age range: 26‐64 years, mean (SD) active group 45(9), sham group 44 (10) Duration of symptoms, mean (SD) active group 45 (9) months, sham group 44 (10) Gender distribution: 63 F, 72 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x1 daIly for 5 d Control type: sham tDCS | |
| Outcomes | Primary: pain VAS anchors not reported When taken: end of intervention, 4, 12 and 24 weeks postintervention Secondary: Oswestry Disability Index | |
| Notes | Sources of support: "This study was funded by the Deutsche Forschungsgemeinschaft DFG (MA 1862/10‐1)." Competing interests: "AM, TJ, KL, and AP had financial support from DFG (MA 1862/10‐1) and NeuroImageNord for the submitted work." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We randomised 160 stimulation codes (80 triggering active stimulation, 80 triggering sham stimulation) by custom written software into two separate lists.” |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent researcher created the randomisation lists. To achieve allocation concealment the recruiter provided participants with the next unused stimulation code from the randomised lists. The recruiter had no access to the randomisation list.” |
| Adequate blinding of participants? | Low risk | Quote: “Blinding of participants and the treating physiotherapist was achieved by using a sham paradigm identical to the anodal stimulation procedure…. “ kappa agreement ‐0.120. Comment While 2 mA intensity can be inadequately blinded, assessment suggests blinding successful |
| Adequate blinding of assessors? | Low risk | Comment: while 2 mA intensity can be inadequately blinded, formal assessment suggests blinding successful |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 3 in each group discontinued in stimulation period. ITT approach |
| Selective reporting (reporting bias) | Low risk | Comment: reporting of all core outcomes |
| Study Size | Unclear risk | Comment: n = 67 and 68 per group |
| Study duration | Low risk | Comment: 24‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Colombia Setting: rehabilitation department Condition: phantom limb pain Prior management details: no difference across groups in use of NSAIDS, physical rehabilitation or psychological therapy n = 54 Age, mean (SD): active group 33.1 (6.6) years, sham group 8.2 (6.3) years Duration of symptoms: not reported Gender distribution: 50 M, 4 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45° angle from midline, 90% RMT number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Stimulation location: M1 contralateral to painful side, no neuronavigation Number of treatments: 10 sessions x 1 per work day for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain possible When taken: 15 d and 30 d after treatment Secondary: AEs | |
| Notes | Funding source: study was partially supported by a grant from the Colombian Science and Technology Institute (COLCIENCIAS, project code: 6566‐49‐326169). COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a computer‐generated randomization method with a permuted block size of 6 was used to allocate subjects to the sham or active rTMS interventions” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization code was only given to the treating investigator on the first day of treatment session by an independent investigator not involved with any other aspect of the trial.” |
| Adequate blinding of participants? | Low risk | Comment: while sham coil did not control for scalp sensation blinding assessment suggested adequate blinding Quote: “Subjects and investigators did not guess correctly the treatment allocation beyond chance (P = .704; P = .571).” |
| Adequate blinding of assessors? | Low risk | Quote: “All evaluations were performed by an investigator blinded to treatment allocation.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1 participant per group dropped out at 15 days and 2 per group at 30 days. ITT analysis performed Quote “We analyzed the end point of the study using the intention‐to‐treat method including patients who attended at least 1 of the rTMS sessions. The missing data were considered at random, thus we used a regression imputation method to handle this issue.” |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes presented at all follow‐up points |
| Study Size | High risk | Comment: n = 27 per group |
| Study duration | Unclear risk | Comment: 15‐day follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Factorial RCT | |
| Participants | Country of study: Brazil Setting: not specified Condition: chronic myofascial pain syndrome Prior management details: not reported n = 46, of which 23 relevant to this review Age, mean (SD): active group 45.83 (9.63) years, sham group 46.73 (13.09) years Duration of symptoms: not reported Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45° from midline, 80% RMT, number of trains not reported; duration of trains not reported; ITI s not reported; total number of pulses 1600 Stimulation location: L M1 Number of treatments: 10 days of stimulation Control type: sham coil ‐ no details provided | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: at end of intervention Secondary: none relevant | |
| Notes | Funding source: supported by Brazilian funding agencies: National Council for Scientific and Technological Development—CNPq (Dr. I.L.S. Torres, W. Caumo, L.F. Medeiros; J. Dussan‐Sarria, A. Souza, V.L. Scarabelot); Graduate Research Group (GPPG) of Hospital de Clı´nicas de Porto Alegre (Dr W. Caumo— Grant # 100196 and Dr. I.L.S. Torres # 100276); Coordination for the Improvement of Higher Education Personnel—CAPES (A. Deitos); International Cooperation Program—CAPES (n8023/11). COI: authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Participants were randomized to one of the four groups, using a stratified blocked randomization scheme and appropriate statistical Random Allocation Software.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Each envelope was sealed and numbered sequentially and contained the allocated treatment. During the entire protocol timeline, two investigators who were not involved in patient evaluation were responsible for then blinding and randomization procedures” |
| Adequate blinding of participants? | Unclear risk | Quote: “A sham coil was used” Comment: insufficient description to know whether it controlled for all aspects on the experience. No formal assessment of blinding provided |
| Adequate blinding of assessors? | Low risk | Quote: “All participants were instructed not to discuss their group assignment during the treatment sessions or with the project staff collecting outcomes data, all of them were also blind to the group assignments. Independent evaluators’ blind to the group assignments were trained to apply the pain scales and cortical excitability parameter.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: low levels of dropout (2 participants in total) |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain diary data not reported in the results with no clear explanation offered for the omission |
| Study Size | High risk | Comment: group sizes ranged from 11‐12 participants |
| Study duration | High risk | Comment: only follow‐up immediately postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil/USA Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 30 (6 per group) Age, mean (SD): 43.2 (9.8) years Duration of symptoms: not reported Gender distribution: 28 F, 2 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: simulation intensity 2 mA, 20 min duration Stimulation location: Group 1 cathodal M1; Group 2 cathodal supraorbital; Group 3 anodal M1; Group 4 anodal supraorbital; Group 5 sham Number of treatments: 1 session Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst possible pain When taken: immediately poststimulation Secondary: none relevant | |
| Notes | COI: study authors declared no COI Sources of support: NIH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that participant blinding may be suboptimal at this intensity |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts occurred |
| Selective reporting (reporting bias) | High risk | No numerical data provided for any post‐treatment clinical outcome. Data not provided upon request to study authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: excluded if undergoing physical treatment or were on stable pain control medication for "less than 2 months" n = 45 (of which 30 relevant to this review) Age, mean (SD): active group 44.5 (14) years, sham group 48 (11.8) years Duration of symptoms, mean (SD): active group 140.6 (72.2) months, sham group 149.3 (111.1) Gender distribution: 29 F, 1 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x 1 daIly for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = worst pain imaginable When taken: postintervention, 1 month postintervention, 2 months postintervention Secondary: QoL SF‐36 AEs | |
| Notes | Study authors declared that there were no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was performed by a blinded therapist using sealed envelopes for each individual.” Comment: no description of the actual allocation sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was performed by a blinded therapist using sealed envelopes for each individual.” Comment: likely to be a concealed process |
| Adequate blinding of participants? | Unclear risk | Quote: “Participants were blinded to the intervention groups, as were the therapists who performed the evaluation.” Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Quote: “Participants were blinded to the intervention groups, as were the therapists who performed the evaluation.” Comment: Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: ITT analysis using LOCF. Low for postintervention (< 10%) and high for 2/12 follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: adequate reporting of core outcomes |
| Study Size | High risk | n = 45 in 3 groups of which n = 30 relevant to this review |
| Study duration | Low risk | 2‐month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: laboratory Condition: fibromyalgia Prior management details: not reported but concomitant treatments allowed n = 40 Age, mean (SD): active group 51.8 (11.6) years, sham group 49.6 (10) years Duration of symptoms (mean (SD) years): active group 13 (12.9), sham group 14.1 (11.9) Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior, number of trains 15; duration of trains 10 s; ITI 50 s, intensity 80% RMT, total number of pulses 1500 Stimulation location: L M1 Number of treatments: 14, x 1 daily for 5 days, x 1 weekly for 3 weeks, x 1 every two weeks for 6 weeks, x 1 monthly for 3 months Control type: sham coil, did not control for sensory cues | |
| Outcomes | Primary: pain NRS; 0 = no pain, 10 = maximal pain imaginable When taken: day 5, 3 weeks, 9 weeks, 21 weeks, 25 weeks Secondary: BPI interference scale, FIQ | |
| Notes | COI: study authors declared no COI Sources of support: Grants from the ‘‘Fondation APICIL’’ and the ‘‘Fondation de France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to 2 groups...with equal numbers in each group. A study nurse prepared the concealed allocation schedule by computer randomisation of these 2 treatment groups to a consecutive number series; the nurse had no further participation in the trial. Patients were assigned in turn to the next consecutive number." |
| Allocation concealment (selection bias) | Low risk | Comment: see quote above |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ sham coil controls for sound and appearance but not the skin sensation of stimulation |
| Adequate blinding of assessors? | Low risk | Quote: "Both patients and investigators were blind to treatment group. Cortical excitability measurements and transcranial stimulation were performed by an independent investigator not involved in the selection or clinical assessment of the patients." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 25% dropout at long‐term follow‐up but intention‐to‐treat analysis used with BOCF imputation |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric point measures provided for the primary outcome but provided upon request to the authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy Setting: laboratory Condition: neuropathic pain secondary to multiple sclerosis Prior management details: refractory to drug management and medication discontinued over previous month n = 19 Age: 23‐69 years, mean 44.8 (SD 27.5) Duration of symptoms: 1‐10 years, mean 2.79 (SD 2.64) Gender distribution: 8 M, 11 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1, contralateral to painful side Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: 0‐100 mm VAS pain, anchors "no pain" to "worst possible pain" When taken: end of treatment period and x 1 weekly over 3‐week follow‐up Secondary: QoL, multiple sclerosis QoL‐54 scale (MSQoL‐54) When taken: as for primary outcome | |
| Notes | AEs: none COI: no declaration made Sources of support: "Italian National Ministero dell’Universita` e della Ricerca, by the Italian National Ministero della Salute, by the Fondazione Italiana Sclerosi Multipla (FISM) to DC, and by the Agenzia Spaziale Italiana to GB" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomization list generated by a computer." |
| Allocation concealment (selection bias) | Low risk | Comment: likely given that the randomisation list was generated pre‐study |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts observed Quote: "... none of the patients enrolled discontinued the study." |
| Selective reporting (reporting bias) | Low risk | Comment: between‐group means not presented clearly to allow meta‐analysis but data provided on request |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy and Austria Setting: laboratory Condition: below level post SCI, predominantly neuropathic pain Prior management details: > 4/10 pain despite rehabilitation and pharmacological treatment. All participants previously treated with antidepressant, anticonvulsants and analgesics for a minimum period of 6 months n = 12 Age, mean (range): active group 43.7 (26‐56) years, sham group 42.5 (24‐62) years Duration of symptoms: not reported Gender distribution: 9 M, 3 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation AP direction, 120% RMT, number of trains 25; duration of trains 5 s; ITI 25s; total number of pulses 1250 Stimulation location: L PFC (no neuronavigation) Number of treatments: 10 sessions daily x 5 per week for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain VAS anchors not reported When taken: postintervention, 1 month postintervention Secondary: none relevant AEs | |
| Notes | Funding source: no statement provided regarding funding COI: the study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Quote: “Sham stimulation was carried out with a sham coil of identical size color and shape emitting a sound similar to that emitted by the active coil.” Comment: Sham suboptimal ‐ no control for cutaneous sensation associated with stimulation |
| Adequate blinding of assessors? | Low risk | Quote “Pain was assessed by an investigator blinded to the type of rTMS subjects were receiving.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: data reported adequately |
| Study Size | High risk | Comment: n = 12 |
| Study duration | Unclear risk | Comment: 1 month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Thailand Setting: laboratory Condition: neuropathic pain associated with SCI Prior management details: refractory to medication including antidepressants, antiepileptics and opioids n = 20 Age, mean (SD) 44.5 (9.16) years Duration of symptoms: 50.1 (37.05) months Gender distribution: 15 M 5 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to most painful side, cathode supraorbital area contralateral to anode Number of treatments: x 1 session Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the most possible pain When taken: immediately poststimulation Secondary: AEs | |
| Notes | No author declaration of COI made Sources of support "This work was supported by an invitation research grant, Faculty of Medicine, Khon Kaen University, Thailand (Grant number I 55229), the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and Faculty of Social Science, Naresuan University, Phitsanulok, Thailand." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were randomized to receive either active tDCS followed by sham tDCS, or sham tDCS stimulation followed by active tDCS in a 1:1 ratio using a computer generated list of random numbers in blocks of four randomizations.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Low risk | Comment: < 10% dropout rate |
| Selective reporting (reporting bias) | Low risk | Comment: numeric data on pain outcomes not presented in the paper. All data provided by study authors upon request |
| Free from carry‐over effects? | Low risk | Comment: preliminary ANOVA analyses yielded no significant main or interaction effects involving condition order |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: maximum follow‐up 1 week postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: UK Setting: laboratory Condition: mixed refractory neuropathic pain Prior management details: no benefit from medication or other stimulation approaches n = 40 (27 after loss to follow‐up) Age, range: 27‐79 years Duration of symptoms: not reported Gender distribution: 23 M, 17 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation AP direction, 90% RMT, number of trains 20; duration of trains 10 s; ITI 1 min; total number of pulses 2000 Stimulation location: Site A: M1 hotspot, Site B M1 reorganised area, Site C (sham) occipital fissure Number of treatments: 3‐5 sessions per week for 5 sessions Control type: sham active stimulation of occipital fissure | |
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst pain imagined When taken: postintervention, 3 weeks postintervention Secondary: none relevant AEs | |
| Notes | Funding source: research funded by the National Institute for Health Research (NIHR) under Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0110‐20321). COI: Prof. Nurmikko has received travel sponsorship from Nexstim Ltd. None of the other authors report any COI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated to receive three cycles of rTMS in 5 sessions at sites A, B, and SHAM. Randomization order was computer generated." |
| Adequate blinding of participants? | Low risk | Comment: sham was active stimulation of a non target brain area‐ likely indistinguishable from active stimulation |
| Adequate blinding of assessors? | Low risk | Comment: outcomes self‐reported via pain diaries |
| Incomplete outcome data (attrition bias) | High risk | Comment: 40 randomised, 38 received rTMS, 27 included in per‐protocol analysis (33% attrition). Responder analysis n = 33 (17% dropout) Reasons for dropout not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Free from carry‐over effects? | Low risk | Comment: 3 weeks washout period observed. Baseline pain levels for each condition appear equivalent |
| Study Size | High risk | Comment: n = 40, 27 after loss to follow‐up |
| Study duration | Unclear risk | Comment: 3 week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: chronic temporomandibular disorder Prior management details: excluded if received any type of physiotherapy in preceding month n = 32 Age, mean (SD): active group 23.80 (7.3) years sham group 25.5 (6.3) years Duration of symptoms, months mean (SD): active group 29.8 (17.1), sham group 33.7 (22.8) Gender distribution: 3 M, 29 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to painful side, cathode supraorbital area, contralateral to anode Number of treatments: daily sessions for 5 consecutive days. Then twice a week for 3 weeks, up to 10 sessions Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors not reported When taken: 5 months postintervention, no data reported from formal study period Secondary: QoL WHO‐QoL, AEs | |
| Notes | Sources of support: study was carried out without funding COI: study authors decare no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After the first comprehensive evaluation, the secretary of the clinical facility, who was not involved with any other procedures of the study, randomised participants who fulfilled the inclusion criteria for treatment and accepted to participate in the study. Randomisation occurred by the simple random method, in which each subject was invited to remove a small sealed envelope from a larger opaque envelope indicating two treatment groups.” |
| Allocation concealment (selection bias) | Low risk | Quote: “After the first comprehensive evaluation, the secretary of the clinical facility, who was not involved with any other procedures of the study, randomised participants who fulfilled the inclusion criteria for treatment and accepted to participate in the study." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. 15 guessed stimulation condition correctly in active group vs 7 in sham group |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no attrition noted for core follow‐up points |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric reporting of point estimates for most outcomes but data provided upon request |
| Study Size | High risk | Comment: n = 32 |
| Study duration | Unclear risk | Comment: formal follow‐up for 3 weeks postintervention |
| Other bias | Low risk | Commet: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Italy Setting: laboratory n = 25 Condition: neuropathic pain from diabetic neuropathy Prior management details: resistant to standard therapies for at least 1 year Age mean (SD): 70.6 (8.5) years Duration of symptoms (months mean (SD)): not reported Gender distribution: 9 F, 14 M | |
| Interventions | Stimulation type: rTMS using H‐coil Stimulation parameters: frequency 20 Hz; coil orientation H coil, number of trains 30; duration of trains 2.5 s; ITI 30 s, intensity 100% RMT, total number of pulses 1500 Stimulation location: M1 lower limb (deep in central sulcus) Number of treatments: 5 per condition on consecutive days Control type: sham coil, controlled for scalp sensory, auditory and visual cues | |
| Outcomes | Primary: pain VAS 0‐100, no pain to worst possible pain When taken: immediately poststimulation, 3 weeks poststimulation Secondary: none relevant | |
| Notes | COI: 2 authors have links to the manufacturer of the H‐coil Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After enrolment, patients were randomly assigned in a 1:1 ratio to two counterbalanced arms by receiving a sequential number from a computer‐generated random list." |
| Adequate blinding of participants? | Low risk | Quote: "Sham stimulation was delivered with a sham coil placed in the helmet encasing the active rTMS coil. The sham coil produced a similar acoustic artefact and scalp sensation as the active coil and could also mimic the facial muscle activation induced by the active coil. It induced only a negligible electric field inside the brain because its non‐tangential orientation on the scalp and components cancelling the electric field ensured that it rapidly reduced the field as a function of distance" Comment: controlled for visual auditory and sensory aspects of stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: while study described as "double blind" there was no specific mention of blinding assessors |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 2 participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: data not presented by stimulation condition ‐ rather they were grouped by the order in which interventions were delivered. No SDs presented. Data requested |
| Free from carry‐over effects? | Low risk | Comment: 5‐week washout period observed with no difference at T3 |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: MS‐related neuropathic pain Prior management details: stable pharmacological and physical therapies for at least 1 month n = 16 Age, mean (SD) 47.4 (8.9) years Duration of symptoms: not reported for pain Gender distribution: 13 F, 3 M | |
| Interventions | Stimulation type: tRNS Stimulation parameters: Intensity 1 mA, 25 cm2 electrodes, duration 20 min, VARIANCE 650/2 μA Stimulation location: M1 contralateral to most painful side Number of treatments: x 1 daily for 3 days Control type: sham tRNS | |
| Outcomes | Primary: pain VAS, anchors not reported When taken: average for 7 days postintervention Secondary: BPI interference, AEs | |
| Notes | COI: "FP has received grants from neuroConn GmbH, Ilmenau, Germany. The other authors declare no conflict" Sources of support: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Adequate blinding of participants? | Low risk | Quote: "Neither the patients nor the evaluators were aware about the nature of the stimulation block." Comment: assessment of participant blinding integrity suggests success |
| Adequate blinding of assessors? | Low risk | Quote: "Neither the patients nor the evaluators were aware about the nature of the stimulation block." |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2 participants (13%) withdrew and data were excluded |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Free from carry‐over effects? | Unclear risk | Comment: 3‐week washout period observed but no formal assessment of carry‐over effects |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: laboratory Condition: fibromyalgia Prior management details: unclear n = 30 Age: active group: 52.6 (SD 7.8) years, sham group 55.3 (SD 8.9) years Duration of symptoms: active group: 8.1 (SD 7.9), sham group: 10.8 (SD 8.6) Gender distribution: 1 M, 29 F | |
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 25; duration of trains 8 s; ITI 52 s; total number of pulses 2000 Stimulation location: M1 contralateral to painful side Number of treatments: 10, x 1 daily for 10 working days Control type: sham rTMS coil. Mimics sight and sound of active treatment | |
| Outcomes | Primary: 0‐10 NRS of average pain intensity over last 24 h, anchors "no pain" to "maximal pain imaginable" When taken: daily during treatment period and at 15, 30 and 60 days post‐treatment follow‐up Secondary: FIQ When taken: as for primary outcome | |
| Notes | COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients who met all inclusion criteria were randomly assigned, according to a computer‐generated list, to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Quote: "Sham stimulation was carried out with the 'Magstim placebo coil system', which physically resembles the active coil and makes similar sounds." Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation over the scalp |
| Adequate blinding of assessors? | Low risk | Quote: "... investigators were blind to treatment group." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: equal dropout in each group and appropriately managed in the data analysis Quote: "All randomized patients with a baseline and at least one post‐baseline visit with efficacy data were included in the efficacy analyses (intent to treat analysis)." "All the patients received the full course of treatment and were assessed on D15 and D30. Four patients (two in each treatment group) withdrew from the trial between days 30 and 60." |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: CRPS type I Prior management details: refractory to best medical treatment n = 23 Age mean (SD): active group 43.5 (12.1) years, sham group 40.6 (9.9) years Duration of symptoms (months mean (SD)): active group 82.33 (34.5), sham group 79.27 (32.1) Gender distribution: 14 F, 9 M | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior, number of trains 25; duration of trains 10 s; ITI 60 s, intensity 100% RMT, total number of pulses 2500 Stimulation location: M1 contralateral to painful limb Number of treatments: 10, x 1 daily on consecutive weekdays Control type: sham coil ‐ did not control for sensory cues | |
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "most severe pain" When taken: after first and last session then 1 and 3 months post‐treatment Secondary: QoL SF‐36, not reported | |
| Notes | COI: study authors declared no COI Sources of support: University of Sao Paolo, Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: while stated "randomized" the method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: sham suboptimal as it did not control for scalp sensation. Study reported that number who guessed the condition correctly was similar but no formal data or analysis reported |
| Adequate blinding of assessors? | Unclear risk | Comment: study described as "double‐blinded" but assessor blinding not specifically reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 participant dropped out at follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: data presented for primary outcome. While this was not adequate for meta‐analysis it did not really constitute selectivity. No response received to request for full data access |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: CRPS type I Prior management details: drug management ceased for 48 h prior to study n = 10 Age: 29‐72 years, mean 51 Duration of symptoms: 24‐72 months, mean 35 Gender distribution: 3 M, 7 F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation unspecified; 110% RMT; number of trains 10; duration of trains 1.2 s; ITI 10 s; total number of pulses 120 Stimulation location: M1 hand area Number of treatments: 1 for each condition Control type: coil angled 45º away from scalp | |
| Outcomes | Primary: 0‐10 VAS current pain intensity, anchors "no pain" to "most extreme pain" When taken: 30 s, 15, 45 and 90 min poststimulation Secondary: none When taken: 30 s, 15, 45 and 90 min poststimulation | |
| Notes | AEs: not reported COI: no declaration made Sources of support: "grant from the BMBF (NR. 01EM0102) and by a grant of the Scientific Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computerized random generator, five patients were first assigned to the placebo group (sham rTMS), while the others were treated using verum rTMS" |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: while sham group results not presented in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Quote: "The initial pain intensities (VAS) were similar prior to verum and sham rTMS (Student’s paired t‐test, P = 0.47). The level of intensity was also independent of whether the patients were first subjected to sham or verum rTMS (P > 0.05)." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: postburn neuropathic pain Prior management details: varied n = 3 Age range: 34‐52 years Duration of symptoms: > 6 months Gender distribution: 2 F, 1 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 contralateral to most painful side Number of treatments: 1 per condition Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain ever felt" When taken: before and after stimulation Secondary: none relevant | |
| Notes | COI: study authors declared no COI Sources of support: departmentally funded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized to either active tDCS or sham stimulation." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all 3 participants completed study |
| Selective reporting (reporting bias) | High risk | Comment: no numeric data provided for pain outcomes |
| Free from carry‐over effects? | Unclear risk | Comment: 1‐week washout observed but no data reported for pain outcome so unable to assess this issue |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: rehabilitation clinic Condition: fibromyalgia Prior management details: none reported n = 23 Age mean (SD): active group 58.3 (12.1) years, sham group 52.4 (11.5) years Duration of symptoms, months (mean (SD)): active group 9.9 (11.8), sham group 6.4 (10.3) Gender distribution: all F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 10, x 1 weekly for 10 weeks Control type: sham tDCS (switched off after 30 s stimulation) Both groups received 4 months rehabilitation programme | |
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain" When taken: immediately at end of 4‐month rehabilitation programme Secondary: QoL SF36, FIQ | |
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated simple randomisation method but method not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA used, which may threaten assessor blinding, though formal analysis of blinding appears acceptable |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: while numeric data on the primary outcome not reported in study report the authors made it available upon request |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: there were group imbalances at baseline on the duration of pain, education, age and economic activity |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: outpatient clinic, participants took device home Condition: pain related to Parkinson's disease Prior management details: not reported n = 19 (reduced to 13 through dropout) Age mean (SD): active group 74.7 (7.8) years, sham group 74.4 (8.3) years Duration of symptoms: > 6 months Gender distribution: 15 M, 4 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100 μA; waveform shape not specified; duration 40 min per session Stimulation location: earlobe clips Number of treatments: 42, x 1 daily for 42 days Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: pain VAS 0 ‐10, anchors not reported When taken: at the end of the treatment period Secondary: none | |
| Notes | Sources of support: equipment provided by CES manufacturer as an "unrestricted gift" COI: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Comment: see above comment |
| Adequate blinding of assessors? | Low risk | Comment: participants and the study co‐ordinator were blinded to group assignment and the code sheet indicating which devices were active and which were sham was kept by another person who was not in contact with the participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: > 30% dropout |
| Selective reporting (reporting bias) | Low risk | Comment: mean (SD) pain scores reported for both groups pre‐ and poststimulation |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: pain clinic Condition: chronic pain (mixed musculoskeletal and neuropathic) Prior management details: "intractable" n = 12 Age: 33‐67 years, mean 51.3 (SD 12.6) Duration of symptoms: mean 2.7 (SD 2.4) Gender distribution: 6 M, 6 F | |
| Interventions | Stimulation type: rTMS, circular coil for arm symptoms, double cone coil for leg symptoms Stimulation parameters: frequency 20 Hz; coil orientation not specified; 80% RMT; number of trains 20; duration of trains 2 s; ITI not specified; total number of pulses 800; treatment duration 20 min Stimulation location: M1 (midline) Number of treatments: x 1 for each condition Control type: coil angled 45º away from the scalp | |
| Outcomes | Primary: 0‐100 mm VAS pain intensity, anchors "no pain" to "unbearable pain" When taken: 0, 5, 10 and 20 min post‐stimulation Secondary: none | |
| Notes | Sources of support: supported by Deutsche Forschungsgemeinschaft COI: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sham and active stimulation were given in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation over the scalp and was visually distinguishable. Given that stimulation was delivered at 110% RMT active stimulation, but not sham, likely to have elicited muscle twitches in peripheral muscles |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 participant withdrew due to "headaches". Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Comment: not clearly demonstrated in the study report but clear from unpublished data provided by the study authors (baseline mean group pain scores: active stimulation 65.1 (SD 16), sham stimulation 66.9 (SD 17.4)) |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 4 conditions | |
| Participants | Country of study: Japan Setting: laboratory Condition: neuropathic pain (mixed central and peripheral) Prior management details: intractable n = 13 Age: 29‐76 years, mean 59.4 Duration of symptoms: 2‐35 years, mean 10.2 (SD 9.7) Gender distribution: 7 M, 6 F | |
| Interventions | Stimulation type: rTMS figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation not specified; 90% RMT; number of trains 5; duration of trains 10 s; ITI 50 s; total number of pulses 500 Condition 2: frequency 5 Hz; coil orientation not specified; 90% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 500 Condition 3: frequency 1 Hz; coil orientation not specified; 90% RMT; number of trains 1; duration of trains 500 s; total number of pulses 500 Condition 4: sham, coil angled 45º from scalp with synchronised electrical scalp stimulations to mask sensation Stimulation location: M1 over the representation of the painful area Number of treatments: 1 for each condition | |
| Outcomes | Primary: VAS pain, anchors not specified When taken: 0, 15, 30, 60, 90 and 180 minutes poststimulation Secondary: none | |
| Notes | Sources of support: no declaration made COI: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "rTMS was applied to all the patients at frequencies of 1, 5, and 10 Hz and as a sham procedure in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Sensory and auditory aspects controlled for but angulation of coil away from the scalp may be visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 13 patients participated in all planned sessions of navigation‐guided rTMS" Comment: no dropouts observed |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Comment: not clearly demonstrated in the study report but paired t‐tests on unpublished baseline data provided by the study authors suggest that carry‐over was not a significant issue |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Thailand Setting: laboratory Condition: myofascial pain syndrome (affecting shoulder) Prior management details: stable analgesic use for 3 months preceding study n = 31 Age mean (SD): active group 49.94 (8.25) years, sham group 45.93 (10.24) years Duration of symptoms, mean(SD) active group 5.91 (2.55) months, sham group 45.93 (10.24) Gender distribution: 22 F, 9 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to most painful side, cathode supraorbital area contralateral to anode Number of treatments: x 1 daily for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the most possible pain When taken: post‐treatment, average of daily score in week 1 postintervention, week 2, 3, 4 postintervention. Only responder analysis presented Secondary: QoL WHO‐QoL, data not reported AEs | |
| Notes | COI: "M.P.J. is a consultant to Noninvasive Brain Stimulation Research Group of Thailand. The remaining authors declare no conflict of interest." Sources of support: "Supported in part by Grant Number R21 HD058049 from the National Institutes of Health, National Institute of Child Health and Human Development, Rockville, MD; and National Center for Medical Rehabilitation Research, Rockville, MD." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedures not described |
| Adequate blinding of participants? | Low risk | Comment: “The tDCS device was designed to allow for masked (sham) stimulation. Specifically, the control switch was in front of the instrument, which was covered by an opaque adhesive during stimulation. The power indicator was on the front of the machine, which lit up during the time of stimulation both in active and sham stimulations.” |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 dropout |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric reporting of pain score or QoL point estimates in the paper. All data provided by study authors upon request |
| Study Size | High risk | Comment: n = 31 |
| Study duration | Unclear risk | Comment: 4‐week follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: fibromyalgia Prior management details: naive to TMS n = 20 Age mean (SD): active group 54.2 (8.28) years, sham group 51.67 (18.19) years Duration of symptoms, years mean (SD): active group 12.1 (7.75), sham group 10.10 (12.81) Gender distribution: 84% F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation parasagittal, number of trains 80; duration of trains 5 s; ITI 10 s, intensity 120% RMT, total number of pulses per session 4000 Stimulation location: L DLPFC Number of treatments: 10, x 1 daily (working days) for 2 weeks Control type: sham coil | |
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain" When taken: after 1 and 2 weeks of treatment, then 1 week and 2 weeks posttreatment Secondary: FIQ, BPI function scale | |
| Notes | AEs: no data provided COI: 1 researcher received research grants from the device manufacturer and holds patents for TMS technology Sources of support: Multidisciplinary Clinical, Research Center Grant P60 AR049459 The Office of the Provost and Vice President for Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (random generator software developed by JJB in the Brain Stimulation Laboratory)" |
| Allocation concealment (selection bias) | Low risk | Quote: "A co investigator not directly involved in ratings or treatment released treatment condition to the TMS operator" |
| Adequate blinding of participants? | Low risk | Quote: "A specially designed sham TMS coil is used for all sham conditions that produces auditory signals identical to active coils but shielded so that actual stimulation does not occur. However, subjects do experience sensory stimulation that is difficult to distinguish from real rTMS" Comment: sensory, auditory and visual cues controlled for |
| Adequate blinding of assessors? | Low risk | Quote: "A masked continuous rater assessed patients at baseline, at the end of each treatment week, and at the 2 follow‐up weeks. Importantly the continuous rater did not administer the TMS, minimizing the chances of unmasking due to events during the TMS treatment session." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: full reporting of primary outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Spain Setting: laboratory Condition: post‐SCI neuropathic pain Prior management details: stable pharmacological treatment for at least 2 weeks prior to start of treatment. Unresponsive to medication n = 39 Age mean (SD): 45 (15.5) years Duration of symptoms: not reported Gender distribution: 30 M, 9 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 10, x 1 daily (working days) for 2 weeks Control type: 4 groups, tDCS + visual illusion, sham tDCS + visual illusion, tDCS + control illusion, sham tDCS + control illusion | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = unbearable pain; mean over previous 24 h When taken: end of treatment period, 12 and 24 d post‐treatment Secondary: BPI pain interference scale | |
| Notes | COI: no declaration made Sources of support: "grants from a BBVA Translational Research Chair in Biomedicine, the International Brain Research Foundation (IBRF) and National Institutes of Health grant K 24 RR018875 to A.P.L., the Foundation La Marato´ TV3 (071931) and grant PI082004 and TERCEL funds from the Instituto de Salud Carlos III" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer generated list as randomisation strategy." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA may threaten blinding but assessment of blinding seemed OK |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 3 dropouts, 1 in each group |
| Selective reporting (reporting bias) | Low risk | Comment: all main outcomes reported |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: reference centre for integrated and multidisciplinary treatment for human T‐lymphotropic virus 1 (HTLV‐1) and viral hepatitis Condition: JTLVI‐infected patients with chronic low back or lower limb pain Prior management details: stable pharmacotherapy in the preceding month n = 20 Age, mean (SD): active group 48.8 (11.6) years, sham group 56.2 (14) years Duration of symptoms: not reported Gender distribution: 15 F, 5 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x 1 daIly for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst possible pain When taken: postintervention, responder analysis 30%, 50% pain relief Secondary: AEs | |
| Notes | COI: the study authors declared no COI Sources of support: "G.S.G. was funded by FAPESB, Salvador, BA/Brazil (Fundação de Amparo à Pesquisa do Estado da Bahia) and M.E.M by CAPES, Brasília, DF/Brazil (Coordenação de Aperfeiçoamento Pessoal de Nível Superior)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized using a stratified randomization strategy with pain as the stratification factor.” |
| Allocation concealment (selection bias) | Low risk | Quote “A previously generated randomization list was used to allocate the patients to each stratum, in accordance with the order of their entrance into the study. A researcher who was not involved with assessments or interaction with participants randomized and allocated the patients” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2 dropouts (20%) from sham group, imputation with LOCF |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: tertiary care teaching hospital Condition: neuromuscular pain (excluding fibromyalgia) Prior management details: unclear n = 28 Age: 45‐65 years, mean 55.6 Duration of symptoms: 4‐45 years, mean 15 Gender distribution: 25 M, 3 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width not specified; intensity 10‐600 μA; waveform shape not specified Stimulation location: ear clip electrodes Number of treatments: 12, frequency of treatment not specified Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: VAS 0‐5 pain intensity When taken: pre‐ and post‐ each treatment Secondary: life interference scale, sickness impact profile ‐ Roland Scale When taken: not specified | |
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each subject was randomly assigned to receive either the active or the sham treatment first" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Low risk | Quote: "sham treatment was made possible by having the treatment delivered via a black box" Comment: sham and active stimulators visually indistinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) | High risk | Comment: only 17 participants completed the study and this dropout (over 50%) is not clearly accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly |
| Free from carry‐over effects? | Low risk | Quote: "Note that there were no significant differences in pain ratings pre‐post changes between the active and sham groups" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: participants also received local stimulation to the painful area that may have elicited a therapeutic effect |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: medical centre Condition: post‐SCI pain (not clearly neuropathic) Prior management details: unclear n = 40 Age: 38‐82 years Duration of symptoms: chronic > 6 months Gender distribution: all M | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100‐500 μA; waveform shape not specified; duration 1 h per session Stimulation location: ear clip electrodes Number of treatments: 21, x 1 daily for consecutive days Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: BPI (0‐10 NRS), anchors "no pain" to "pain as bad as you can imagine" When taken: post‐treatment period Secondary: pain interference subscale of BPI When taken: as for primary outcome | |
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The participants were then randomly assigned to either the active or sham CES treatment groups" Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Comment: see quote above |
| Adequate blinding of assessors? | Low risk | Quote: "The investigators,research assistant (RA), and participants were blinded to treatment type until the end of the initial phase." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 2 (5%) participants withdrew from the study. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes presented clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: 4 Veterans Affairs medical centres and 1 private rehabilitation clinic Condition: post‐SCI neuropathic pain Prior management details: not reported n = 105 Age mean (SD): active group 52.1 (10.5) years, sham group 52.5 (11.7) years Gender distribution: 90 M, 15 F | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100 μA; waveform shape not specified; duration 1 h per session Stimulation location: earlobe clips Number of treatments: 21, x 1 daily Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: BPIpain intensity VAS 0‐100, anchors not reported When taken: at end of treatment period Secondary: QoL SF‐12 physical and mental component subscales | |
| Notes | COI: study authors declared no COI Sources of support: funded by Veterans Affairs rehabilitation research and development service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The equipment was set up for a double‐blind study by the manufacturer such that the participants could not differentiate active from sham CES devices. Research staff members who interacted with the participants (e.g. recruited and trained participants, administered questionnaires, followed up by telephone) did not know which devices were sham and which were active. Randomization was achieved by selecting a device from a box initially containing equal numbers of active and sham devices." Comment: whilst unconventional it appeared to avoid a systematic bias |
| Allocation concealment (selection bias) | Low risk | Comment: see quote/comment above |
| Adequate blinding of participants? | Low risk | Comment: stimulation subsensory and units indistinguishable |
| Adequate blinding of assessors? | Low risk | Comment: stimulation subsensory and units indistinguishable |
| Incomplete outcome data (attrition bias) | Low risk | Comment: available case analysis with small loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes fully reported |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline between‐group imbalances on BPI pain interference, SF‐36 pain subscale and coping strategies |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: community rheumatology practices Condition: fibromyalgia Prior management details: not reported but continued stable medication usage n = 57 (46 after dropout) Age mean (SD): active group 51 (10.6) years, sham group 51.5 (10.9) years, usual care group 48.6 (9.8) years Duration of symptoms: not reported Gender distribution: 43 F, 3 M (data reported on completers) | |
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width not specified; intensity 100 μA; waveform shape square wave biphasic, duration 1 h per session Stimulation location: earlobe clip electrodes Number of treatments: x 1 daily for 8 weeks Control type: sham CES unit indistinguishable from active unit | |
| Outcomes | Primary: pain VAS, anchors not reported When taken: at the end of each week of treatment period Secondary: FIQ | |
| Notes | COI: no declaration made Sources of support: University of Virginia. Center for the study of Complementary and Alternative Therapies. Devices loaned by Electromedical Products International | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Comment: identical devices given to sham and active group with subsensory stimulation parameters |
| Adequate blinding of assessors? | Low risk | Comment: participants self‐rated at home |
| Incomplete outcome data (attrition bias) | Low risk | Comment: of 57, 11 did not complete ‐ unclear if ITT analysis employed. However, only 2‐4 per group and balanced, mostly due to assessment burden |
| Selective reporting (reporting bias) | Low risk | Comment: while no numeric data were provided on primary outcomes in the study report, these data were provided upon request to the authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other source of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: Rehabilitation outpatient unit Condition: fibromyalgia Prior management details: no analgesic use for 1 month prior to enrolment n = 51 Age mean (SD): active group 42.4 (78.63) years, sham group 46.5 (8.36) years Duration of symptoms: mean (SD) active group 10.81 (6.31) years, sham group 13.33 (6.65) Gender distribution: 47 F, 4 M | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º angle from the midline, 100% RMT number of trains 30; duration of trains 5 s; ITI 12 s; total number of pulses 1500 Stimulation location: M1 midline, no neuronavigation Number of treatments: 10 sessions daily ‐ unclear whether only work days Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = most severe pain When taken: end of intervention Secondary: WHQoL‐BREF | |
| Notes | Funding source: none reported COI: the study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Randomisation was completed with the help of a software programme that produces random allocation” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: placebo coil did not control for the sensory aspects of stimulation. No formal assessment of blinding success reported |
| Adequate blinding of assessors? | Low risk | Quote: “the investigator who conducted the clinical evaluation received no information about patient admission, randomisation or mode of treatment” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: no suggestion of selective outcome reporting |
| Study Size | High risk | Comment: 25 and 27 participants in each group |
| Study duration | High risk | Comment: only immediate postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: post‐SCI neuropathic pain (sublesion) Prior management details: not reported n = 33 (14 after loss to follow‐up in phase one) Age, mean (SD): active group 51.38 (14.89) years, sham group 51 (10.11) years Duration of symptoms: not reported Gender distribution: 24 M, 9 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x1 daily for 5 days in phase one. Phase 2 not relevant to this review Control type: sham tDCS | |
| Outcomes | Primary: pain VAS anchors 0 = no pain 10 = pain as bad as you can imagine When taken: postintervention, 1 week postintervention, 3 months postintervention Secondary: QoL (PHQ‐9) AEs | |
| Notes | Funding source: this project was supported by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant numbers 90DP0035 and H133N110010). COI: study authors declared no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Incomplete outcome data (attrition bias) | High risk | Comment: while ITT analysis reported with multiple imputation, at the end of phase one, dropout was 57% |
| Selective reporting (reporting bias) | Low risk | Comment: data reported adequately |
| Study Size | High risk | Comment: n = 33 (14 after loss to follow‐up) |
| Study duration | Low risk | Comment: 3‐month follow‐up for phase 1 |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Unclear, likely parallel RCT (for 1 Hz only), 10 Hz data open‐label therefore excluded from this review | |
| Participants | Country of study: USA Setting: not reported, likely laboratory Condition: fibromyalgia Prior management details: "moderate to severe despite current and stable treatment regime" n = unclear, abstract report (Schneider 2012 (see Tzabazis 2013)) stated 45, but full paper stated 16 Age mean (SD): 53.2 (8.9) years Duration of symptoms, years mean (SD): not reported Gender distribution: 14 F, 2 M | |
| Interventions | Stimulation type: rTMS 4‐coil configuration Stimulation parameters: frequency 1 Hz; no of trains not reported; duration of trains not reported; ITI not reported, intensity 110% RMT, total number of pulses per session 1800, stimulation duration 30 min Stimulation location: targeted to the anterior cingulate cortex Number of treatments: 20, x 1 daily (working days) for 4 weeks Control type: sham coil | |
| Outcomes | Primary: BPI average pain last 24 h, NRS, anchors not reported When taken: end of treatment, 4 weeks post‐treatment Secondary: FIQ | |
| Notes | COI: 3 study authors have acted as paid consultants to the manufacturer of the stimulation device, of which 2 hold stock in the company and 1 founded the company, is its chief medical officer and has intellectual property rights Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Adequate blinding of participants? | Unclear risk | Comment: no description of blinding of participants for clinical part of study. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation over the scalp |
| Adequate blinding of assessors? | Unclear risk | Comment: no description or mention of blinding assessors for clinical part of study |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no mention of the degree of dropout or how it was managed. However, 45 participants with fibromyalgia reported in the abstract of the same study (Schneider 2012 (Tzabazis 2013)), but only 16 reported in the full paper |
| Selective reporting (reporting bias) | High risk | Comment: no presentation of numeric pain data with measures of variance |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline and demographic data not presented for clinical group |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: not reported Condition: burning mouth syndrome Prior management details: not reported n = 26 Age mean (SD): active group 63.36 (10.78) years, sham group 64.42 (8.35) years Duration of symptoms, mean (SD): active group 61.57 (32.10) months, sham group 65.58 (55.52) Gender distribution: active group 93% F, sham group 92% F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified, 100% RMT, number of trains 10; duration of trains 5 s; ITI 10 s; total number of pulses 3000 Stimulation location: L DLPFC Number of treatments: 10 x 1 daily on work days Control type: sham coil ‐ same sound and appearance and sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = extreme amount When taken: end of stimulation and 15, 30, 60 days after start of treatment Secondary: AEs | |
| Notes | Funding source: no information provided COI: no information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients who met all inclusion criteria were randomly assigned to one of two groups – one given active and the other sham stimulation – using a web‐based randomization generator” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedures not reported |
| Adequate blinding of participants? | Low risk | Comment: sham controls for all aspects of stimulation Quote: “The coil used in the sham group was the same configuration as that used with the real group but shielded so that actual stimulation does not occur. All subjects had ECT electrodes placed under the TMS coil. For those receiving active TMS, the electrodes were disconnected, such that there was no current flowing through during stimulation. In contrast, the electrodes were connected during sham, so participants received a small electrical stimulation through the electrodes, precisely when the TMS was being triggered.” “Ten of 12 (83%) patients in the real group and 4 of 8 (50%) patients in the sham group thought that they were in the real group. There was no significant difference for the belief of the allocated group between two groups (χ2 = 2.54,1, NS), suggesting that blinding for the subjects in this study was kept. The high percentage of correct guessing in the active group is concerning. However, when asked why they guessed the way they did, it was based on whether they had BMS symptom reduction. If this occurred, then they guessed the active group. There were no instances of patient unblinding.” |
| Adequate blinding of assessors? | High risk | Comment: assessor was not blinded to group allocation |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2/14 (14%) randomised did not receive active stim, 4/12 (33%) randomised to sham did not receive sham. Excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Comment: pain intensity data only presented in graphical form without numeric point estimates/precision estimates |
| Study Size | High risk | Comment: combined n = 26 (per protocol = 20) |
| Study duration | Unclear risk | Comment: 7‐week follow‐up |
| Other bias | Low risk | Comment: no other risks of bias detected |
| Methods | Parallel RCT, 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: refractory to medical intervention n = 41 Age: mean 54.8 (SD 9.6) years Duration of symptoms: condition 1: 7.54 (SD 3.93) years; condition 2: 8.39 (SD 2.06) years; condition 3: 8.69 (SD 3.61) years Gender distribution: 0 M, 41 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: condition 1: L DLPFC; condition 2: L M1, condition 3; sham L M1 Number of treatments: 10, x 1 daily on consecutive working days Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS 0‐10 cm, anchors not specified When taken: immediately post‐treatment, averaged over 3 d post‐treatment, 30 and 60 d post‐treatment Secondary: QoL; FIQ | |
| Notes | COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomisation list generated by a computer" |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pregenerated randomisation list should have adequately ensured this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropout occurred |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any post‐treatment time point in the study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: fibromyalgia Prior management details: pain refractory to common analgesics and muscle relaxants n = 18 randomised of which 17 allocated Age mean (SD): 50.3 (8.5) years Duration of symptoms (years) mean (SD): 10.7 (6.8) Gender distribution: 15 F, 3 M | |
| Interventions | Stimulation type: HD‐tDCS Stimulation parameters: intensity 2 mA, duration 20 min, anodal/cathodal/sham 4 x 1‐ring configuration Stimulation location: L M1 Number of treatments: x 1 per condition Control type: sham tDCS | |
| Outcomes | Primary: pain visual numerical scale; 0 = complete absence of pain, 10 = worst pain imaginable When taken: baseline, immediately poststimulation, 30 min poststimulation Secondary: adapted QoL scale for persons with chronic illness (7 points: 1 = terrible, 7 = delighted) | |
| Notes | COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the order of stimulation was counterbalanced and randomly assigned for each individual" Comment: method of randomisation not specified but less likely to introduce bias in a cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only 1 loss to follow‐up and multiple imputation used |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported in full |
| Free from carry‐over effects? | Low risk | Comment: 7‐day washout periods observed. Data similar at baseline |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: chronic abdominal pain with inflammatory bowel disease Prior management details: participants allowed to continue anti‐inflammatory drugs and acute pain medication n = 20 Age, mean (SD) active group 40.6 (12.5) years, sham group 34.4 (13.2) years Duration of symptoms: active group 10 (8.9) years, sham group 34.4 (13.2) Gender distribution: 13 F, 7 M | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to painful side, cathode supraorbital area, contralateral to anode Number of treatments: x 1 daIly for 5 days Control type: sham tDCS | |
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the worst pain possible When taken: postintervention, 1 week postintervention Secondary: inflammatory bowel disease QoL questionnaire AEs | |
| Notes | COI: study authors declared no COI Sources of support: "This study has been supported by the grant “Patientenorientierte Forschung bei CED 2014” of the “Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung e.V.” (Not industry) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed by the unblinded researcher (A.F.) in blocks of 4 generated from a computer‐based random allocation.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Quote: “Randomization was performed by the unblinded researcher (A.F.)” Comment: no apparent steps to conceal allocation |
| Adequate blinding of participants? | Unclear risk | Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: levels of dropout, if any, not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: 1‐week postintervention maximum follow‐up. |
| Other bias | Low risk | Comment: no further bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Australia Setting: laboratory Condition: chronic neuropathic pain post‐SCI Prior management details; none n = 10 Age mean (SD): 56.1 (14.9) years Duration of symptoms: 15.8 (11.3) years Gender distribution: 8 M, 2 F | |
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 5, x 1 daily 5 days Control type: sham tDCS (switched off after 30 s stimulation) | |
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst possible pain" When taken: at end of treatment, 4 weeks post‐treatment Secondary: none relevant | |
| Notes | COI: no declaration made Sources of support: no declaration made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less important for cross‐over design Quote: "A randomized crossover design was used so that all subjects participated in an active treatment (transcranial direct current stimulation) and sham treatment period. Both the subject and the response assessor were blinded to the randomization sequence." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported in full |
| Free from carry‐over effects? | Low risk | Comment: 4‐week washout period observed and data appear free of carry‐over effects |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: not reported Condition: fibromyalgia Prior management details: no improvement in cases of using medical treatment for fibromyalgia for at least 3 months n = 28 Age mean (SD): active group 45.25 (9.33) years, sham group 43 (7.63) years Duration of symptoms, mean(SD): active group 53 (29.15) months, sham group 54.92 (30.44) Gender distribution: all F | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 1 Hz; coil orientation not reported, 90% RMT, number of trains 20; duration of trains 60 s; ITI 45 s; total number of pulses 1200 Stimulation location: L M1, no neuronavigation Number of treatments: 10 sessions, weekdays for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = maximum pain imaginable When taken: end of intervention, 1 month, 3 months Secondary: FIQ AEs | |
| Notes | Funding source: the study authors declared that this study received no financial support COI: no COI was declared by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not outlined Quote: “patients were randomly assigned to be in either a real stimulation group or a sham stimulation group by another clinician” |
| Allocation concealment (selection bias) | Low risk | Quote: “masked clinician evaluated the patients clinically and provided the diagnosis of FM. The patients were randomly assigned to be in either a real stimulation group or a sham stimulation group by another clinician.” |
| Adequate blinding of participants? | Unclear risk | Comment: sham coil did not control for sensory aspects of stimulation. Quote: “Sham stimulation was carried out with the same parabolic coil, which was placed at 90° angles to the motor cortex area” |
| Adequate blinding of assessors? | Low risk | Quote: “A masked clinician evaluated the patients clinically and provided the diagnosis of FM [fibromyalgia]” |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 3 participants dropped out though this exceeds 10% of total number, the group they withdrew from and point of withdrawal were not clear |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Study Size | High risk | N = 28 (per protocol 25) |
| Study duration | Low risk | Comment: 3‐month follow‐up |
| Other bias | Low risk | Comment: no other risk of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: rehabilitation unit Condition: post‐SCI below lesion neuropathic pain Prior management details: pain that is resistant to pharmacological (anticonvulsants, antidepressants, narcotics) and interventional treatments n = 17 Age mean (SD): active group: 40 (5.1) years, sham group 36.94 (8) years Duration of symptoms mean (SD): active group 32.3 (25.9) months, sham group 35.4 (17.9) Gender distribution: all M | |
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation handle pointing posteriorly, number of trains 30; duration of trains 5 s; ITI 25 s; total number of pulses 1500 Stimulation location: M1 midline Number of treatments: daily for 10 weekdays Control type: coil angled away ‐ same sound and appearance, did not control for visual or sensory cues | |
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: end of intervention, 6 weeks, 6 months postintervention Secondary: none relevant | |
| Notes | Funding source: no information reported COI: no information reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer‐generated randomization schedule was used.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: sham condition did not control for visual or sensory aspects of stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “The patients and the researcher evaluating the patients were blinded to type of rTMS.” |
| Incomplete outcome data (attrition bias) | Low risk | Comment: only one participant dropped out |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes adequately reported |
| Study Size | High risk | Comment: n = 16 |
| Study duration | Low risk | Comment: 6‐month follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
AE: adverse event; ANOVA: analysis of variance; BIRS: Gracely Box Intensity Scale (BIRS); BOCF: baseline observation carried forward; BPI: Brief Pain Inventory; CES: cranial electrotherapy stimulation; CNP: central neuropathic pain; COI: conflict of interest; CPSP: central poststroke pain; CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; F: female; FIQ: Fibromyalgia Impact Questionnaire; HD‐tDCS: High definition tDCS; ITI: inter‐train interval; ITT: intention‐to‐treat; L: left; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs pain scale; LOCF: last observation carried forward; M: male; M1: primary motor cortex; MCS: motor cortex stimulation (MCS); NIH: National Institutes of Health; NRS: numerical rating scale; NSAIDS: nonsteroidal anti‐imflammatory drugs; OA: osteoarthritis; PFC: prefrontal cortex; PLP: phantom limb pain; QoL: Quality of Life; R: right; RCT: randomised controlled trial; RINCE: reduced impedance non‐invasive cortical electrostimulation; RMDQ: Roland Morris Disability Questionnaire; RMT: resting motor threshold; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; SII: secondary somatosensory area; SD: standard deviation; TCES: transcranial electrical stimulation; tDCS: transcranial direct current stimulation; TENS: transcutaneous electrical nerve stimulation; TMS: transcranial magnetic stimulation; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The duration of painful symptoms is unclear. May not be exclusively chronic pain | |
| Pain is not measured as an outcome | |
| Inclusion of acute and chronic pain patients | |
| Not clearly a chronic population | |
| Not a study of electrical brain stimulation | |
| Study of acute pain | |
| Study of acute pain | |
| Not clearly a chronic population | |
| Allocation not randomised | |
| A study of postoperative pain. No sham control employed | |
| Not a study of brain stimulation | |
| Uncontrolled long‐term follow‐up data from Hargrove 2012a | |
| Self‐reported pain is not measured | |
| Study not confined to chronic pain | |
| Not clearly a chronic population | |
| Allocation not randomised | |
| Not clearly studying chronic pain | |
| Not clearly a chronic population | |
| Not electrical brain stimulation ‐ magnetic fields unlikely to induce electrical currents | |
| Not clearly a chronic pain population ‐ provoked vestibulodynia | |
| Intervention not designed to alter cortical activity directly by electrical stimulation ‐ a neuro feedback intervention | |
| Not a RCT or quasi‐RCT ‐ no randomisation specifically to treatment group or order | |
| Participants are a mixture of acute and chronic pain patients | |
| Not clearly a chronic population | |
| No sham control employed | |
| No sham control employed | |
| A single case report | |
| Not a study of brain stimulation | |
| Allocation not randomised | |
| Single case study |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel RCT |
| Participants | Post‐polio patients, n = 32 |
| Interventions | tDCS, bi‐anodal, bilateral motor cortex, 1.5 mA, 20 min, daily for 5 days |
| Outcomes | Pain, QoL |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled study, unclear whether randomised |
| Participants | Post‐SCI chronic neuropathic pain, n = 30 |
| Interventions | tDCS motor cortex, 2 mA, 10 sessions |
| Outcomes | Pain intensity |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled trial, unclear whether randomised |
| Participants | Chronic neurogenic orofacial pain, n = 26 |
| Interventions | rTMS motor cortex, frequency unclear, appears to be a single session of stimulation per condition |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled trial, unclear whether randomised, likely to be a cross‐over design |
| Participants | Chronic neurogenic orofacial pain, n = 26 |
| Interventions | rTMS motor cortex, frequency unclear, appears to be a single session of stimulation per condition |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Likely to be a duplicate report of Fricova 2009. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled parallel trial ‐ unclear if randomised |
| Participants | Chronic orofacial pain n = 59 |
| Interventions | rTMS, 10 Hz and 20 Hz, location not clear |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | CRPS type I, n = 18 |
| Interventions | rTMS, 10 Hz, 10 treatment sessions |
| Outcomes | Pain, disability, QoL |
| Notes | Published as conference abstract only. Attempts to contact study author currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Neuropathic orofacial pain, n = 29 |
| Interventions | rTMS, motor cortex, 10 Hz, 5 treatment sessions |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Neuropathic orofacial pain, medication resistant, n = 29 |
| Interventions | rTMS, motor cortex, 10 Hz, 5 treatment sessions |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Likely to be a duplicate report of Klirova 2010. Attempts to contact authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | CRPS type I, n = 25 |
| Interventions | tDCS, motor cortex, 2 mA, 20 min per session, daily for 5 days |
| Outcomes | Pain, QoL, physical activity |
| Notes | Currently published as conference abstract only. Correspondence with study authors ‐ data unavailable as currently being re‐analysed |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 50 |
| Interventions | Low‐frequency rTMS DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Cross‐over RCT |
| Participants | Post‐SCI pain, n = 6 |
| Interventions | tDCS and visual illusion |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Chronic neuropathic pain |
| Interventions | Low‐frequency rTMS, M1 or DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Current and former opioid abusers ‐ pain status unclear. n = 60 |
| Interventions | tDCS M1, number of sessions unclear |
| Outcomes | Not clear whether pain intensity was measured |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Cross‐over RCT |
| Participants | Parkinson's disease with related pain, n = 19 |
| Interventions | rTMS 20 Hz motor cortex, ? whether single session |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Unable to retrieve study report |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 48 |
| Interventions | Low‐frequency rTMS DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Unable to retrieve study report |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 20 |
| Interventions | rTMS, L DLPFC, 10 treatment sessions |
| Outcomes | ? whether pain intensity measured as an outcome |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; FIQ: Fibromyalgia Impact Questionnaire: L: left; M1: primary motor cortex; QoL: quality of life; RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; tDCS: transcranial direct current stimulation; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Investigating the role of transcranial direct current stimulation for pain relief in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia syndrome Myalgic encephalomyelitis/chronic fatigue syndrome |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain, fatigue, FIQ, stimulation condition |
| Starting date | |
| Contact information | Ms Hannah Bereznicki, [email protected] |
| Notes | TRIAL WITHDRAWN |
| Trial name or title | The effectiveness of repetitive transcranial magnetic stimulation in the treatment of fibromyalgia |
| Methods | Parallel RCT |
| Participants | Fibromyalgia |
| Interventions | rTMS to DLPFC 10 Hz sham rTMS |
| Outcomes | Pain severity QoL |
| Starting date | 17 May 2013 |
| Contact information | Dr Bernadette Fitzgibbon, [email protected] |
| Notes | Correspondence with authors 21 December 2016 ‐ data collection ongoing |
| Trial name or title | Modulation of chronic pain perception with noninvasive central and peripheral nervous system stimulation |
| Methods | RCT |
| Participants | Chronic musculoskeletal pain |
| Interventions | Intervention group 1: participants receive tDCS and TENS only Comparator group 1: participants receive tDCS and sham TENS only Comparator group 2: participants receive TENS and sham tDCS only Control group 1: participants receive sham tDCSand sham TENS only |
| Outcomes | Pain VAS WHO‐QOL |
| Starting date | 11 November 2013 |
| Contact information | Prof Allan Abbott, [email protected] |
| Notes | Correspondence with authors 22 December 2016‐ trial did not go ahead due to "changes in project personnel and funding." |
| Trial name or title | The effects of non‐invasive brain stimulation on chronic arm pain |
| Methods | RCT |
| Participants | Neuropathic pain in the upper limb |
| Interventions | tDCS Sham |
| Outcomes | Arm pain Upper limb function |
| Starting date | 16 April 2014 |
| Contact information | A/Prof Gwyn Lewis, [email protected] |
| Notes | Correspondence with authors 21 December 2016, data collection ongoing |
| Trial name or title | The impact of non‐invasive brain stimulation on motor cortex excitability and cognition in chronic lower back pain |
| Methods | RCT |
| Participants | Chronic low back pain |
| Interventions | tDCS Sham |
| Outcomes | Pain, HR‐QoL |
| Starting date | 9 March 2015 |
| Contact information | Dr Andrea Loftus, [email protected] |
| Notes | Correspondence with authors 3 January 2017, data collection ongoing |
| Trial name or title | Safety and feasibility of transcranial direct current stimulation (tDCS) combined with sensorimotor retraining in chronic low back pain: a pilot randomised controlled trial |
| Methods | RCT |
| Participants | Chronic nonspecific low back pain |
| Interventions | tDCS + sensorimotor training sham tDCS + sensorimotor training |
| Outcomes | Pain severity Physical function |
| Starting date | 8 August 2016 |
| Contact information | Dr Siobhan Schabrun, [email protected] |
| Notes | Correspondance with authors 22 December 2016, trial beginning recruitment |
| Trial name or title | |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 118 |
| Interventions | rTMS right DLPFC, low‐frequency, 20 sessions |
| Outcomes | Unclear whether self‐reported pain scores were collected |
| Starting date | |
| Contact information | |
| Notes | Published as conference abstract only. Correspondance with study authors ‐ paper currently in press awaiting publication |
| Trial name or title | Transcranial magnetic stimulation induced motor evoked potential in the expression of brain‐derived neurotrophic factor BDNF, pathological pain and quality of life in patients with spinal cord injury |
| Methods | Parallel RCT |
| Participants | Post‐SCI pain, n = 60 |
| Interventions | rTMS |
| Outcomes | Pain, QoL |
| Starting date | 01 July 2017 |
| Contact information | Dr Shi Jiajia [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effect of transcranial magnetic stimulation on pain modulation status in fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia |
| Interventions | rTMS |
| Outcomes | Pain |
| Starting date | 01 September 2013 |
| Contact information | Dr Rathmi Mashur, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | |
| Methods | Parallel RCT |
| Participants | Mixed chronic pain |
| Interventions | tDCS, M1, DLPFC, number of sessions not clear |
| Outcomes | Pain, QoL |
| Starting date | |
| Contact information | |
| Notes | Published as conference abstract only. Correspondence with study authors ‐ study ongoing |
| Trial name or title | The effect of transcranial direct current stimulation (t‐DCS) On the P300 component of event‐related potentials in patients with chronic neuropathic pain due to CRPS or diabetic neuropathy |
| Methods | Cross‐over RCT |
| Participants | Chronic neuropathic pain due to CRPS or diabetic neuropathy |
| Interventions | tDCS or sham, 2 mA, 20 min, x 1 session, location not specified |
| Outcomes | Pain intensity |
| Starting date | February 2009 |
| Contact information | Dr Pesach Schvartzman, [email protected] |
| Notes | Contact in 2010 ‐ study ongoing, recent attempts to contact for update unsuccessful |
| Trial name or title | Occipital transcranial direct current stimulation in fibromyalgia |
| Methods | Cross‐over RCT |
| Participants | Fibromyalgia |
| Interventions | tDCS or sham, parameters not specified |
| Outcomes | Pain VAS and FIQ |
| Starting date | July 2009 |
| Contact information | Dr Mark Plazier, [email protected] |
| Notes | Attempts to contact study authors currently unsuccessful |
| Trial name or title | Application of transcranial direct current stimulation in patients with chronic pain after spinal cord injury |
| Methods | Parallel RCT |
| Participants | Chronic pain after SCI, proposed n = 60 |
| Interventions | tDCS 2 mA, 10 sessions |
| Outcomes | Pain VAS, QoL |
| Starting date | April 2010 |
| Contact information | Dr Felipe Fregni, [email protected], Kayleen Weaver, [email protected] |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Transcranial direct current stimulation for chronic pain relief |
| Methods | Cross‐over RCT |
| Participants | Chronic pain patients, proposed n = 100 |
| Interventions | tDCS, motor cortex, 2 mA, daily for 5 days |
| Outcomes | Pain relief |
| Starting date | November 2010 |
| Contact information | Dr Silvio Brill, Tel Aviv Sourasky Medical Centre |
| Notes | Correspondence with study authors: study ongoing |
| Trial name or title | Exploration of parameters of transcranial direct current stimulation in chronic pain |
| Methods | Parallel RCT |
| Participants | Chronic pain following traumatic SCI, n = 60 |
| Interventions | tDCS or sham, 2 mA, motor cortex, 20 min, x 1 daily for 5 days |
| Outcomes | Pain |
| Starting date | April 2010 |
| Contact information | Dr Felipe Fregni, [email protected]; Kayleen Weaver, [email protected] |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Effects of transcranial direct current stimulation and transcranial ultrasound on osteoarthritis pain of the knee |
| Methods | Parallel RCT |
| Participants | Chronic knee OA pain, n = 30 |
| Interventions | tDCS or sham, 20 min, 2 mA, motor cortex, 5 sessions |
| Outcomes | Pain |
| Starting date | January 2011 |
| Contact information | Dr Felipe Fregni, [email protected]; Kayleen Weaver, [email protected] |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Effects of transcranial direct current stimulation in chronic corneal pain |
| Methods | Cross‐over RCT |
| Participants | Chronic corneal pain |
| Interventions | tDCS, active or sham, 1 session of each, parameters not reported |
| Outcomes | Pain VAS |
| Starting date | January 2012 |
| Contact information | Dr Felipe Fregni, [email protected]; Kayleen Weaver, [email protected] |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Assessment and treatment patients with atypical facial pain through repetitive transcranial magnetic stimulation |
| Methods | Parallel RCT |
| Participants | Atypical facial pain, n = 40 |
| Interventions | rTMS or sham, parameters not reported, 5 sessions |
| Outcomes | Pain VAS |
| Starting date | March 2011 |
| Contact information | Ricardo Galhardoni |
| Notes | Correspondence with study authors: study near completion |
| Trial name or title | Effect of cranial stimulation and acupuncture on pain, functional capability and cerebral function in osteoarthritis |
| Methods | Parallel RCT |
| Participants | Chronic OA pain, n = 80 |
| Interventions | 4 groups, real tDCS + electroacupuncture sham; sham tDCS + electroacupuncture sham, sham tDCS + electroacupuncture, real tDCS + electroacupuncture tDCS 2 mA motor cortex. All single session |
| Outcomes | Daily pain intensity, WOMAC |
| Starting date | January 2012 |
| Contact information | Dr Wolnei Caumo, [email protected] |
| Notes | Correspondence with study authors: study ongoing |
| Trial name or title | The effects of transcranial direct current stimulation on central pain in patients with spinal cord injury |
| Methods | RCT |
| Participants | Central neuropathic pain post‐SCI |
| Interventions | tDCS Sham |
| Outcomes | Pain, average 24 h Pain interference |
| Starting date | March 2008 |
| Contact information | Hyung‐Ik Shin, Associate Professor, Seoul National University Bundang Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effects of transcranial direct current stimulation (tDCS) on neuropathic symptoms following burn injury |
| Methods | RCT |
| Participants | Burn injury |
| Interventions | tDCS Sham |
| Outcomes | Pain QoL |
| Starting date | January 2013 |
| Contact information | Dr Felipe Fregni, [email protected] |
| Notes | Contact with authors unsuccessful |
| Trial name or title | tDCS for the management of chronic visceral pain in patients with chronic pancreatitis (tDCS) |
| Methods | RCT |
| Participants | Chronic pancreatitis pain |
| Interventions | tDCS Sham |
| Outcomes | Pain QoL |
| Starting date | March 2013 |
| Contact information | Steven Freedman, MD PhD |
| Notes | Contact with study author 20 December 2016 ‐ stated all results published but did not respond to request to identify the published paper. Trial register record implies the study was withdrawn prior to enrolment |
| Trial name or title | tDCS effects on chronic low back pain |
| Methods | RCT |
| Participants | Chronic nonspecific low back pain, n = 45 |
| Interventions | Real‐tDCS + back school Sham tDCS + back school |
| Outcomes | Pain |
| Starting date | January 2012 |
| Contact information | Sofia Straudi, MD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Functional neuroimaging in fibromyalgia patients receiving tDCS |
| Methods | RCT |
| Participants | Fibromyalgia, n = 34 |
| Interventions | tDCS + pregabalin Sham tDCS + pregabalin |
| Outcomes | Pain FIQ WH‐QoL |
| Starting date | March 2013 |
| Contact information | Wolnei Caumo, MD, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Deep rTMS in central neuropathic pain syndromes (DRTMS) |
| Methods | RCT |
| Participants | Central pain, n = 90 |
| Interventions | rTMS double cone coil rTMS H‐coil Sham rTMS |
| Outcomes | Pain VAS |
| Starting date | March 2011 |
| Contact information | Daniel Ciampi, MD, PhD, [email protected] |
| Notes | Correspondence with authors 22 December 2016, data collection complete, analysis ongoing |
| Trial name or title | Investigation of the efficacy of tDCS in the treatment of complex regional pain syndrome (CRPS) Type 1 |
| Methods | RCT |
| Participants | CRPS type 1, n = 22 |
| Interventions | tDCS + GMI |
| Outcomes | sham tDCS + GMI |
| Starting date | April 2013 |
| Contact information | Yannick Tousignant‐Laflamme, PT Ph.D, Université de Sherbrooke |
| Notes | Correspondence with study authors ‐ manuscript under review for publication |
| Trial name or title | Long‐term effects of transcranial direct current stimulation (tDCS) on patients with phantom limb pain (PLP) |
| Methods | Cross‐over RCT |
| Participants | Phantom limb pain, n = 24 |
| Interventions | Anodal tDCS Cathodal tDCS Sham TDCS |
| Outcomes | Pain AEs |
| Starting date | May 2015 |
| Contact information | Itzhak Siev‐Ner, MD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Analgesic dffect of repetitive transcranial magnetic stimulation (rTMS) for central neuropathic pain in multiple sclerosis (STIMASEP) |
| Methods | RCT |
| Participants | Central neuropathic pain due to multiple sclerosis, n = 66 |
| Interventions | rTMS Theta burst TMS Sham rTMS |
| Outcomes | Pain |
| Starting date | February 2014 |
| Contact information | Patrick Lacarin placarin@chu‐clermontferrand.fr |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Transcranial magnetic stimulation for low back pain |
| Methods | Cross‐over RCT |
| Participants | Chronic low back pain |
| Interventions | rTMS ? comparator |
| Outcomes | Pain |
| Starting date | January 2014 |
| Contact information | Sean Mackey, Chief, Division of Pain Medicine, Stanford University |
| Notes | Contact with study authors unsuccessful. Register record states this study was withdrawn prior to enrolment. Reasons not given |
| Trial name or title | The effect of tDCS in the treatment of chronic pelvic pain associated with endometriosis (tDCS) |
| Methods | Parallel RCT |
| Participants | Painful endometriosis, n = 30 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain AEs QoL |
| Starting date | June 2014 |
| Contact information | Wolnei Caumo, MD, PhD, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Efficacy of transcranial magnetic stimulation (TMS) in central post stroke pain (CPSP) |
| Methods | RCT |
| Participants | Central poststroke pain, n = 20 |
| Interventions | Navigated rTMS |
| Outcomes | Pain intensity QoL AEs |
| Starting date | June 2013 |
| Contact information | Eija Kalso, PhD, Helsinki University Central Hospital |
| Notes | Register record notes "The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years." Correspondence with study authors 05 January 2017: data analysis ongoing |
| Trial name or title | Effects of tDCS and tUS on pain perception in OA of the knee |
| Methods | Parallel RCT |
| Participants | OA of the knee, n = 28 |
| Interventions | Active tDCS + active tUS Sham tDCS + sham tUS |
| Outcomes | Pain AEs QoL |
| Starting date | March 2015 |
| Contact information | Felipe Fregni, Principal Investigator, Spaulding Rehabilitation Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Repetitive transcranial magnetic stimulation in central neuropathic pain |
| Methods | Cross‐over RCT |
| Participants | Central neuropathic pain, n = 50 |
| Interventions | rTMS Sham rTMS |
| Outcomes | Pain VAS, average and responder analysis |
| Starting date | November 2015 |
| Contact information | Charles Quesada, Roland Peyron |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | A novel non invasive brain stimulation based treatment for chronic low back pain (CLBP) |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 80 |
| Interventions | tDCS/tACS stimulation Sham tDCS |
| Outcomes | Pain |
| Starting date | May 2015 |
| Contact information | Dr Silviu Brill, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | The effects of cognitive behavioral therapy and transcranial current stimulation (tDCS) on chronic lower back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 120 |
| Interventions | tDCS of DLPFC + CBT Sham tDCS + CBT |
| Outcomes | Pain |
| Starting date | January 2015 |
| Contact information | Jeffrey Borckardt, Professor, Medical University of South Carolina |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Optimizing rehabilitation for phantom limb pain using mirror therapy and transcranial direct current stimulation (tDCS) |
| Methods | Factorial RCT |
| Participants | Chronic phantom limb pain, n = 132 |
| Interventions | Active tDCS and active mirror therapy Active tDCS and sham mirror therapy Sham tDCS and active mirror therapy Sham tDCS and sham mirrory therapy |
| Outcomes | Pain QoL (short version SF‐36) AEs |
| Starting date | July 2015 |
| Contact information | Dr Felipe Fregni [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Non invasive brain stimulation treatment for CLBP (NIBSTCLBP) |
| Methods | Cross‐over RCT |
| Participants | Chronic low back pain, n = 60 |
| Interventions | tDCS Sham tDCS "partially active‐ first 2.5 weeks will receive sham treatment followed by active" |
| Outcomes | Pain Disability |
| Starting date | January 2016 |
| Contact information | Iftach Dolev, PhD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Home‐based transcranial direct current stimulation in fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 32 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain Functional capacity |
| Starting date | January 2016 |
| Contact information | Wolnei Caumo [email protected] Aline Brietzke [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Adjunctive transcranial direct current stimulation (tDCS) |
| Methods | Parallel RCT |
| Participants | Chronic pain, n = 36 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain Physical activity |
| Starting date | January 2016 |
| Contact information | Alok Madan, PhD [email protected] Gladys Jimenez, PhD [email protected] |
| Notes | Correspondance with study authors 20 December 2016 ‐ data collection ongoing |
| Trial name or title | Imaging the effects of rTMS on chronic pain |
| Methods | Parallel RCT |
| Participants | Chronic neuropathic pain, n = 60 |
| Interventions | Active rTMS, prefrontal Sham rTMS |
| Outcomes | Pain QoL |
| Starting date | March 2016 |
| Contact information | Diana Martinez, MD, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | The effects of CBT and (tDCS) on fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 72 |
| Interventions | tDCS + CBT Sham tDCS + CBT |
| Outcomes | Pain QoL |
| Starting date | November 2014 |
| Contact information | Jeffrey Borckardt, Ph.D. [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effects of tDCS and tUS on pain perception in OA of the knee |
| Methods | Parallel RCT |
| Participants | OA knee, n = 64 |
| Interventions | Active tDCS/active tUS Sham tDCS/sham tUS |
| Outcomes | Pain |
| Starting date | September 2016 |
| Contact information | Felipe Fregni, Spaulding Rehabilitation Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Transcranial direct current stimulation for chronic low back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 60 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain |
| Starting date | November 2014 |
| Contact information | Butler Hospital, individual not specified |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | tDCS for chronic low back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 40 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain |
| Starting date | October 2013 |
| Contact information | Frederick Burgess, MD, PhD Benjamin Greenberg, MD, PhD Providence VA Medical Center |
| Notes | Correspondence with study authors 21 December 2017, study in progress |
| Trial name or title | tDCS associated with peripheral electrical stimulation for pain control in individuals with sickle cell disease (tDCS/PES_SCD) |
| Methods | Parallel RCT |
| Participants | Sickle cell disease, n = 80 |
| Interventions | ss‐tDCS (active) plus PES (active) ss‐tDCS (active) plus PES (simulated) ss‐tDCS (simulated) plus PES (active) ss‐tDCS (simulated) plus PES (simulated) sc‐tDCS (active) plus PES (active) sc‐tDCS (active) plus PES (simulated) sc‐tDCS (simulated) plus PES (active) sc‐TDCS (simulated) plus PES (simulated) |
| Outcomes | Pain Function |
| Starting date | March 2016 |
| Contact information | Prof. Abrahão F Baptista, [email protected] Tiago S. Lopes, Sr, [email protected] |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Analgesic effect of non invasive stimulation: transcranial direct current stimulation of opercular‐insular cortex |
| Methods | Parallel RCT |
| Participants | CRPS, n = 40 |
| Interventions | tDCS of operculo‐insular cortex |
| Outcomes | Pain |
| Starting date | November 2016 |
| Contact information | luis.garcia‐larrea@univ‐lyon1.fr |
| Notes |
| Trial name or title | TMS for complex regional pain syndrome |
| Methods | Parallel RCT |
| Participants | CRPS, n = 40 |
| Interventions | Theta‐burst rTMS |
| Outcomes | Pain |
| Starting date | 24 April 2017 |
| Contact information | |
| Notes |
| Trial name or title | Effectiveness of transcranial direct current stimulation combined with kinesiotherapy in patients with chronic temporomandibular disorders (TMJ): clinical, randomized, double‐blind, placebo controlled trial |
| Methods | Parallel RCT |
| Participants | Chronic temporomandibular pain |
| Interventions | tDCS + kinesiotherapy Sham tDCS + kinesiotherapy |
| Outcomes | Pain |
| Starting date | December 2013 |
| Contact information | Maitê de Freitas, [email protected] |
| Notes | Correspondence with study authors 31 December 2016 ‐ study report under peer review for publication |
AE: adverse events; CBT: cognitive behavioural therapy; CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; FIQ: Fibromyalgia Impact Questionnaire; GMI: graded motor imagery; HR‐QoL: health‐related quality of life; OA: osteoarthritis; PES: peripheral electrical stimulation; QoL: quality of life; RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; tACS: transcranial alternating current stimulation; tDCS: transcranial direct current stimulation; TENS: transcutaneous electrical nerve stimulation; tUS: transcranial ultrasound; VAS: visual analogue scale; WHO‐QOL: World Health Organization‐QoL; WOMAC: Western Ontario and McMaster Universities Arthritis Index
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 27 | Std. Mean Difference (Fixed, 95% CI) | ‐0.22 [‐0.29, ‐0.16] | |
| Analysis 1.1  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 1 Pain: short‐term follow‐up. | ||||
| 1.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | 0.13 [‐0.03, 0.28] | |
| 1.2 High‐frequency ≥ 5 Hz | 25 | Std. Mean Difference (Fixed, 95% CI) | ‐0.30 [‐0.37, ‐0.23] | |
| 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies Show forest plot | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| Analysis 1.2 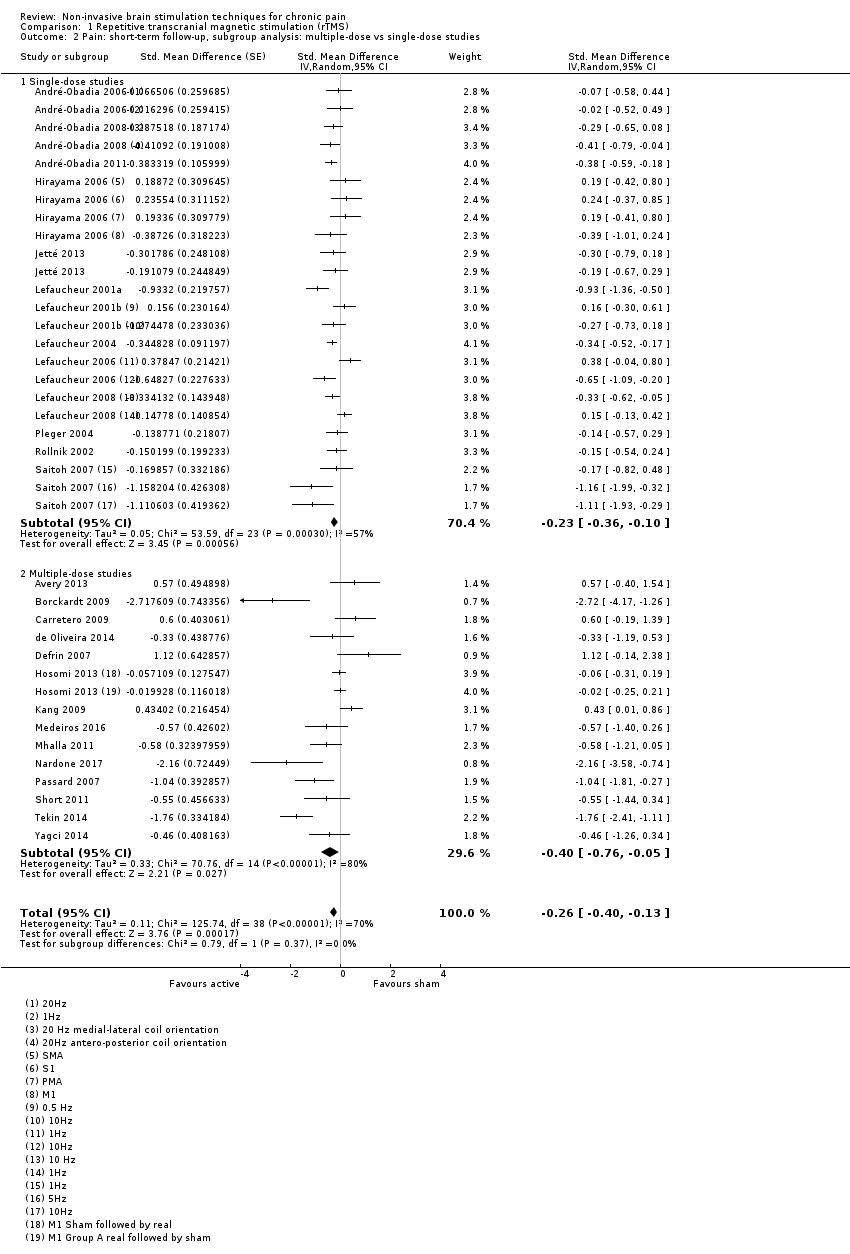 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies. | ||||
| 2.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.10] | |
| 2.2 Multiple‐dose studies | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.76, ‐0.05] | |
| 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only Show forest plot | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.13] | |
| Analysis 1.3 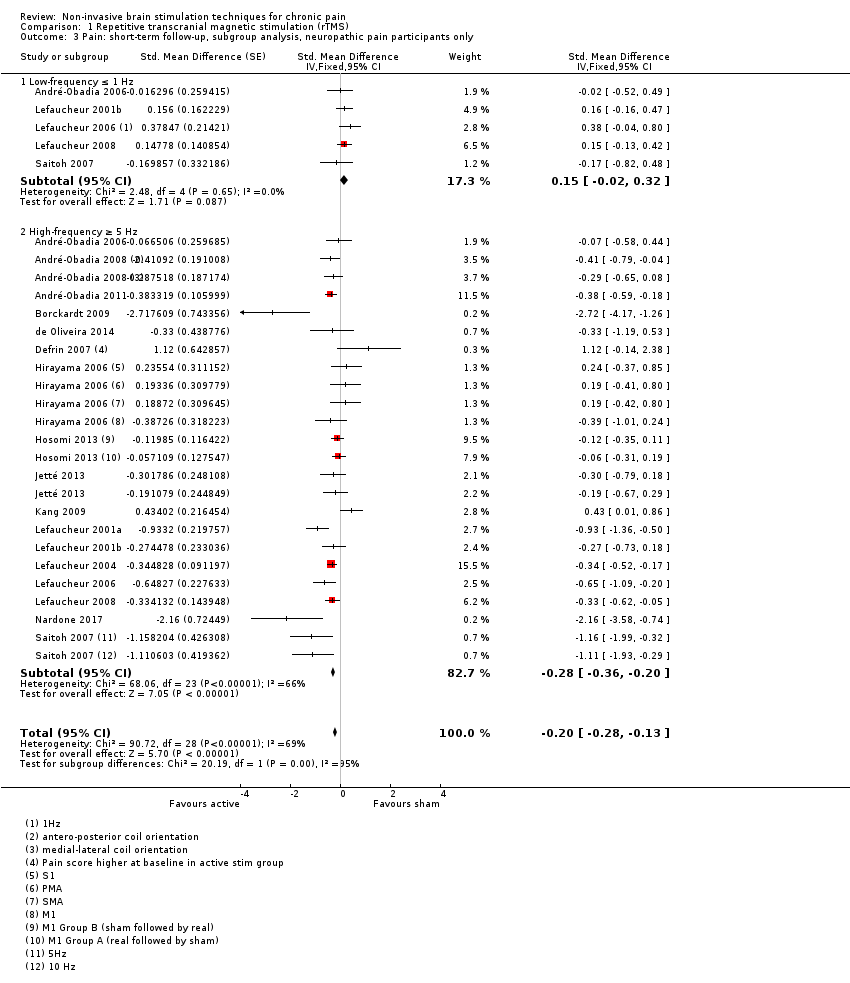 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only. | ||||
| 3.1 Low‐frequency ≤ 1 Hz | 5 | Std. Mean Difference (Fixed, 95% CI) | 0.15 [‐0.02, 0.32] | |
| 3.2 High‐frequency ≥ 5 Hz | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.28 [‐0.36, ‐0.20] | |
| 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only Show forest plot | 8 | Std. Mean Difference (Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.17] | |
| Analysis 1.4  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only. | ||||
| 4.1 Low‐frequency ≤ 1 Hz | 1 | Std. Mean Difference (Fixed, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 4.2 High‐frequency ≥ 5 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | ‐0.56 [‐0.81, ‐0.31] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.51, ‐0.22] | |
| Analysis 1.5  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded. | ||||
| 5.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.49, ‐0.27] | |
| 5.2 Multiple‐dose studies | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.73, 0.05] | |
| 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up Show forest plot | 29 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.40, ‐0.14] | |
| Analysis 1.6 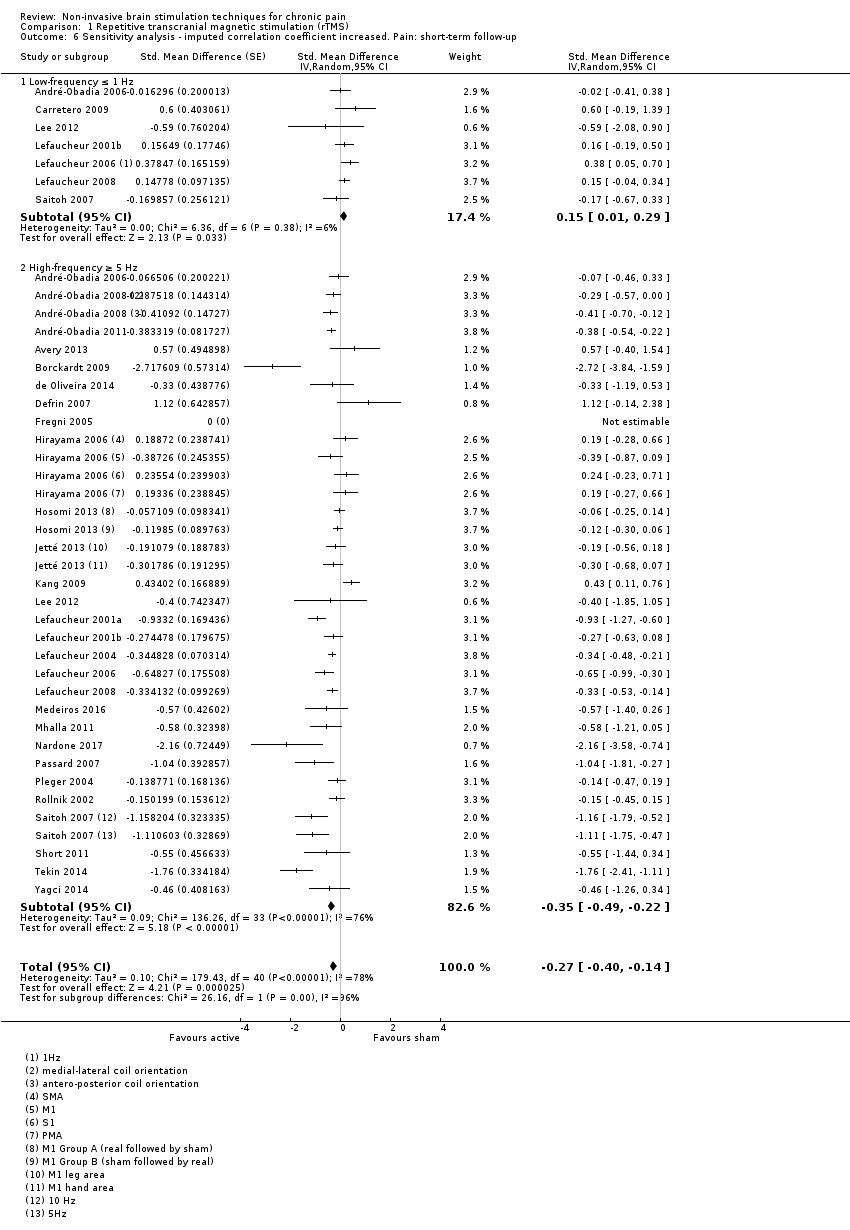 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up. | ||||
| 6.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.15 [0.01, 0.29] | |
| 6.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.49, ‐0.22] | |
| 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up Show forest plot | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| Analysis 1.7  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up. | ||||
| 7.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 7.2 High‐frequency ≥ 5 Hz | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.49, ‐0.19] | |
| 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] | |
| Analysis 1.8 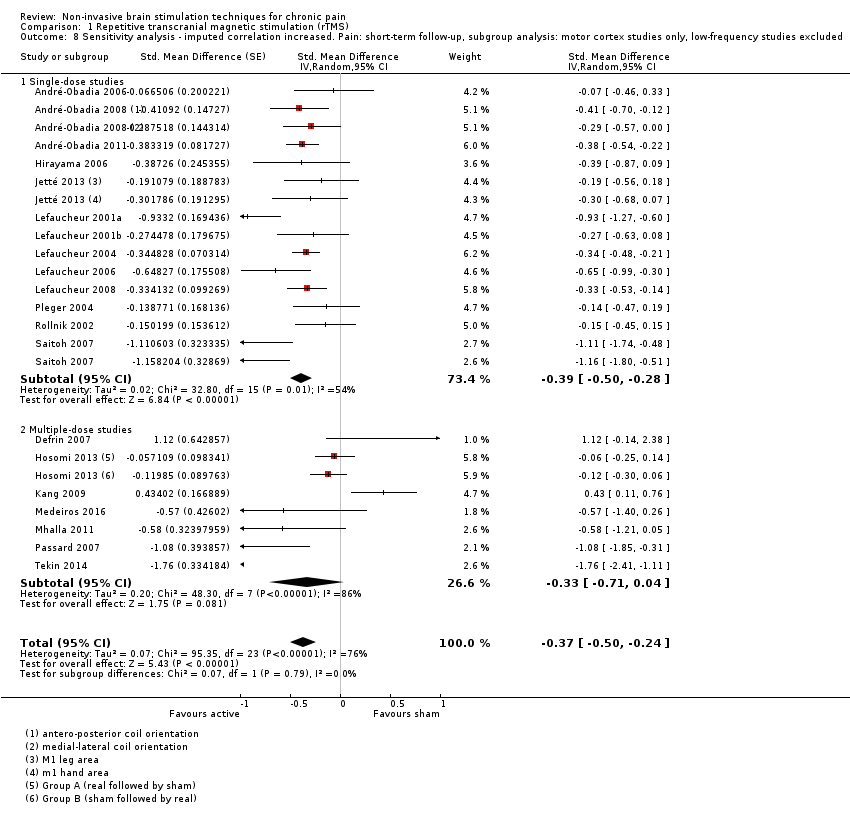 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded. | ||||
| 8.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.50, ‐0.28] | |
| 8.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.71, 0.04] | |
| 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.52, ‐0.22] | |
| Analysis 1.9 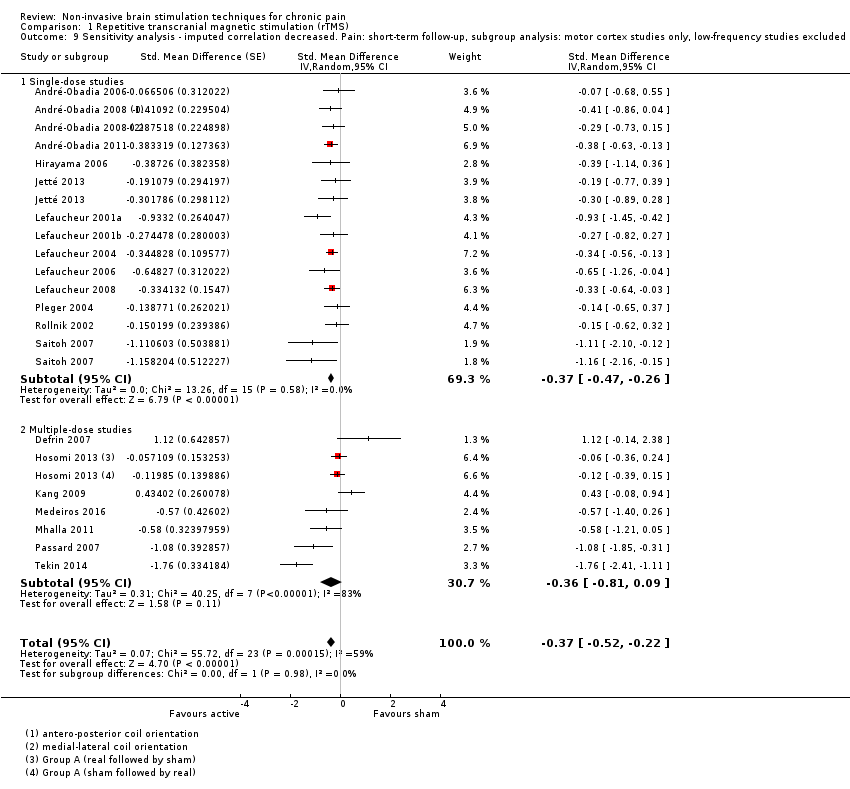 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded. | ||||
| 9.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.47, ‐0.26] | |
| 9.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.81, 0.09] | |
| 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 31 | Std. Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.34, ‐0.20] | |
| Analysis 1.10  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up. | ||||
| 10.1 Low‐frequency ≤ 1 Hz | 10 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.07, 0.22] | |
| 10.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Fixed, 95% CI) | ‐0.36 [‐0.44, ‐0.29] | |
| 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 24 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.55, ‐0.26] | |
| Analysis 1.11  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded. | ||||
| 11.1 Single‐dose studies | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.46, ‐0.24] | |
| 11.2 Multiple‐dose studies | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐0.91, ‐0.15] | |
| 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.48, 0.15] | |
| Analysis 1.12 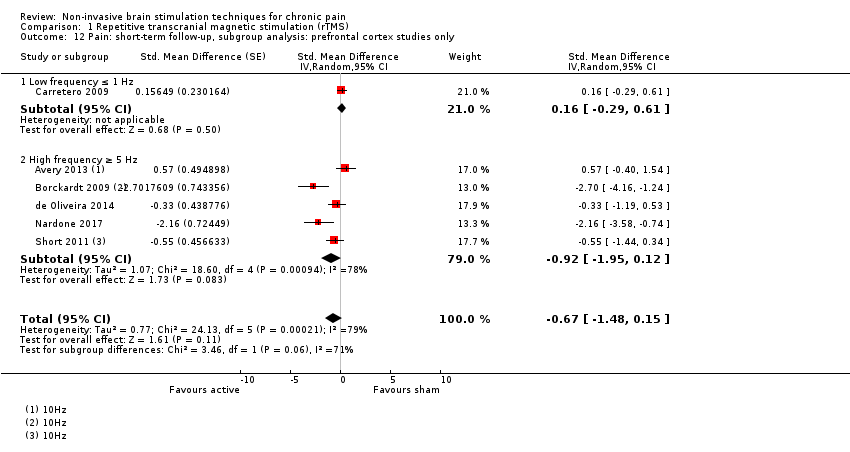 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only. | ||||
| 12.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 12.2 High frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.92 [‐1.95, 0.12] | |
| 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| Analysis 1.13 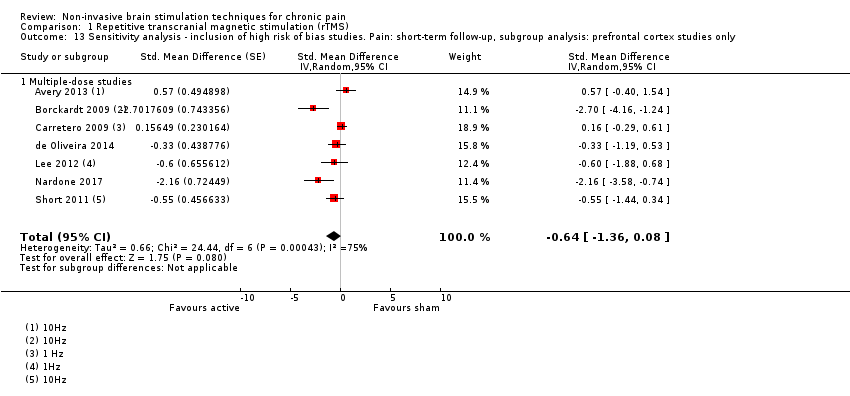 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only. | ||||
| 13.1 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 14 Pain: short term responder analysis 30% pain reduction Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.17, 3.80] |
| Analysis 1.14 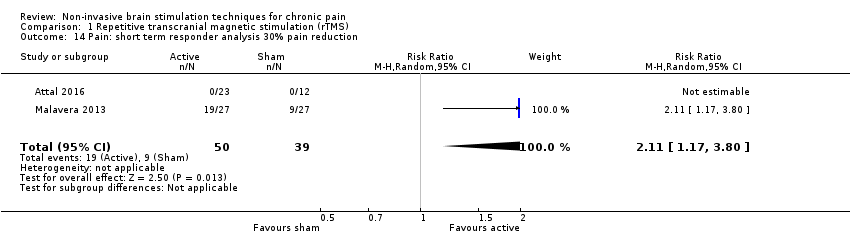 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 14 Pain: short term responder analysis 30% pain reduction. | ||||
| 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐1.01, 0.17] | |
| Analysis 1.15  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up. | ||||
| 16 Pain: medium‐term follow‐up Show forest plot | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.61, 0.05] | |
| Analysis 1.16  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 16 Pain: medium‐term follow‐up. | ||||
| 16.1 Low‐frequency ≤ 1 Hz | 2 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.41, 0.69] | |
| 16.2 High‐frequency ≥ 5 Hz | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.73, 0.00] | |
| 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] | |
| Analysis 1.17 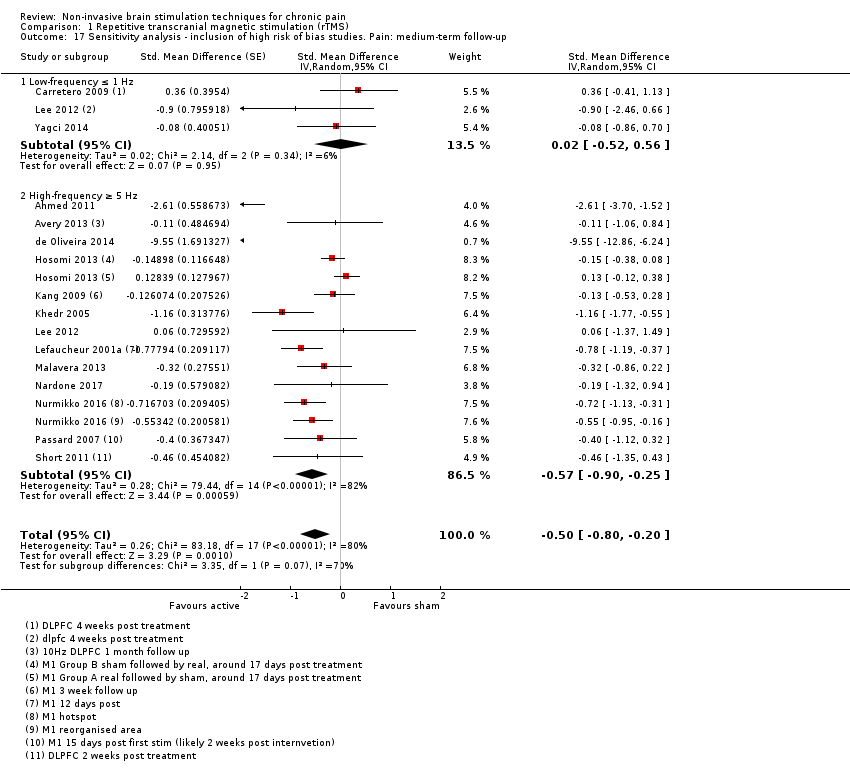 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up. | ||||
| 17.1 Low‐frequency ≤ 1 Hz | 3 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.52, 0.56] | |
| 17.2 High‐frequency ≥ 5 Hz | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐0.90, ‐0.25] | |
| 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.46, 0.02] | |
| Analysis 1.18 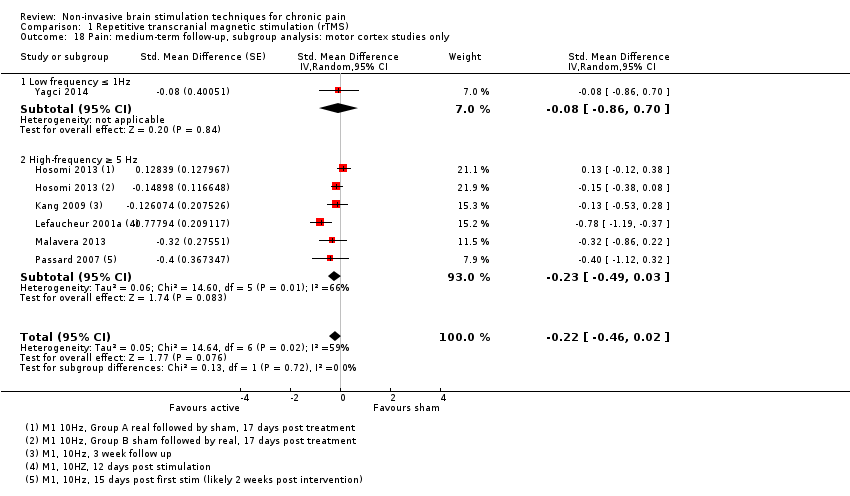 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only. | ||||
| 18.1 Low frequency ≤ 1Hz | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.86, 0.70] | |
| 18.2 High‐frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.49, 0.03] | |
| 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐1.08 [‐2.49, 0.32] | |
| Analysis 1.19  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only. | ||||
| 19.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.36 [‐0.41, 1.13] | |
| 19.2 High‐frequency ≥ 5 Hz | 4 | Std. Mean Difference (Random, 95% CI) | ‐1.74 [‐3.66, 0.19] | |
| 20 Pain: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.44, 0.17] | |
| Analysis 1.20 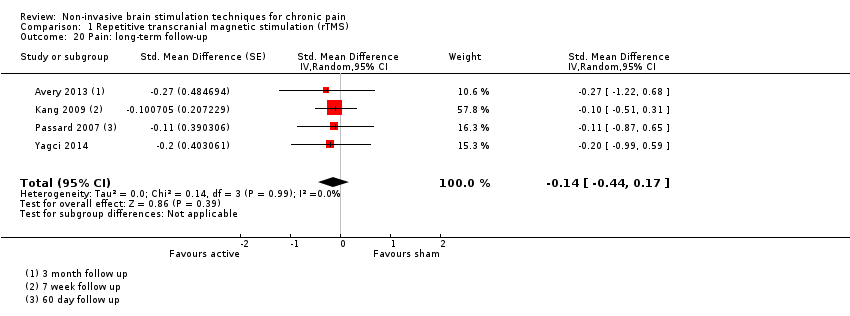 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 20 Pain: long‐term follow‐up. | ||||
| 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.89, 0.10] | |
| Analysis 1.21  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up. | ||||
| 22 Disability: short‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.87, 0.29] | |
| Analysis 1.22  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 22 Disability: short‐term follow‐up. | ||||
| 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.72, 0.12] | |
| Analysis 1.23  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up. | ||||
| 24 Disability: medium‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐1.07, 0.33] | |
| Analysis 1.24  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 24 Disability: medium‐term follow‐up. | ||||
| 25 Pain: short term responder analysis 50% pain reduction Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.03, 3.47] |
| Analysis 1.25 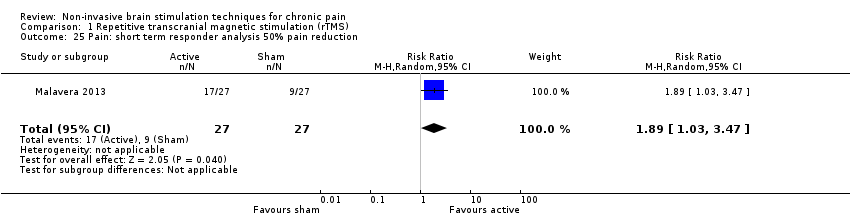 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 25 Pain: short term responder analysis 50% pain reduction. | ||||
| 26 Disability: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.62, 0.16] | |
| Analysis 1.26 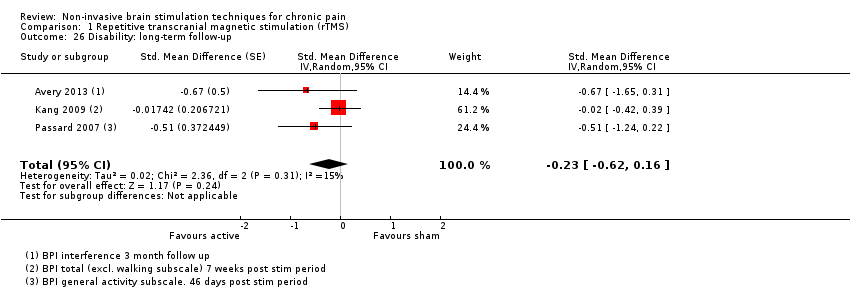 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 26 Disability: long‐term follow‐up. | ||||
| 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.87, 0.05] | |
| Analysis 1.27  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up. | ||||
| 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐15.04, ‐6.55] |
| Analysis 1.28 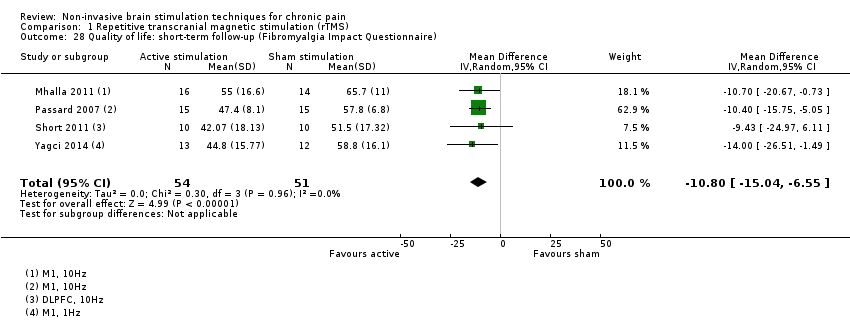 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire). | ||||
| 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐11.49 [‐16.73, ‐6.25] |
| Analysis 1.29  Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire). | ||||
| 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 5 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐8.93 [‐13.49, ‐4.37] |
| Analysis 1.30 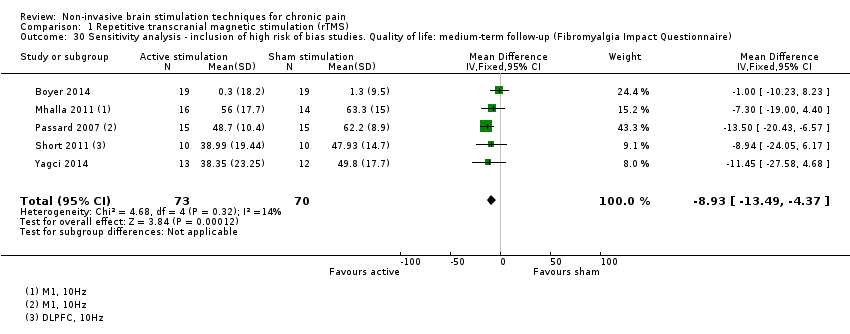 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire). | ||||
| 31 Quality of life: long‐term follow‐up Show forest plot | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐6.78 [‐13.43, ‐0.14] |
| Analysis 1.31 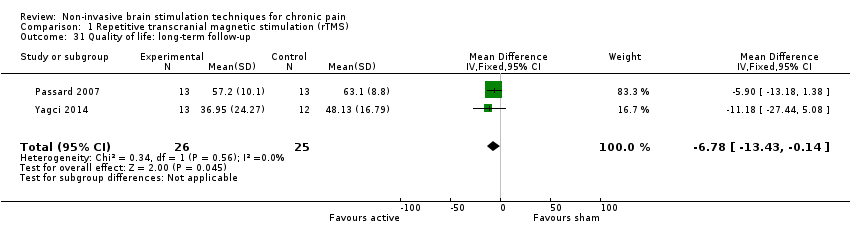 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 31 Quality of life: long‐term follow‐up. | ||||
| 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up Show forest plot | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐13.84, ‐3.33] |
| Analysis 1.32 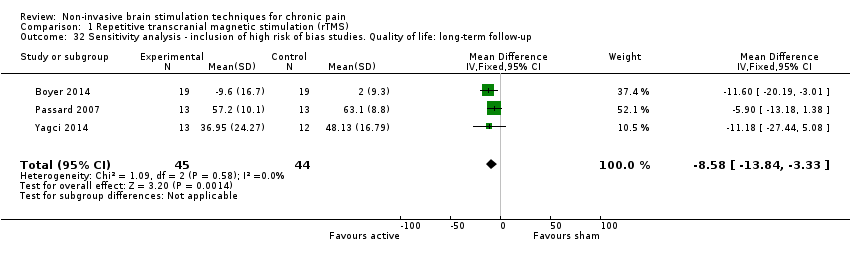 Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.48, 0.01] |
| Analysis 2.1  Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 1 Pain: short‐term follow‐up. | ||||
| 2 Quality of life: short term follow up Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 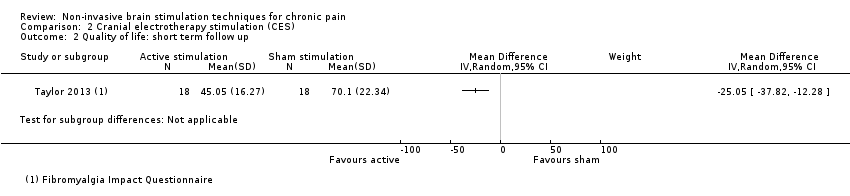 Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 2 Quality of life: short term follow up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.63, ‐0.22] | |
| Analysis 3.1  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 1 Pain: short‐term follow‐up. | ||||
| 1.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 1.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.77, ‐0.25] | |
| 2 Pain: short‐term sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.62, ‐0.23] | |
| Analysis 3.2 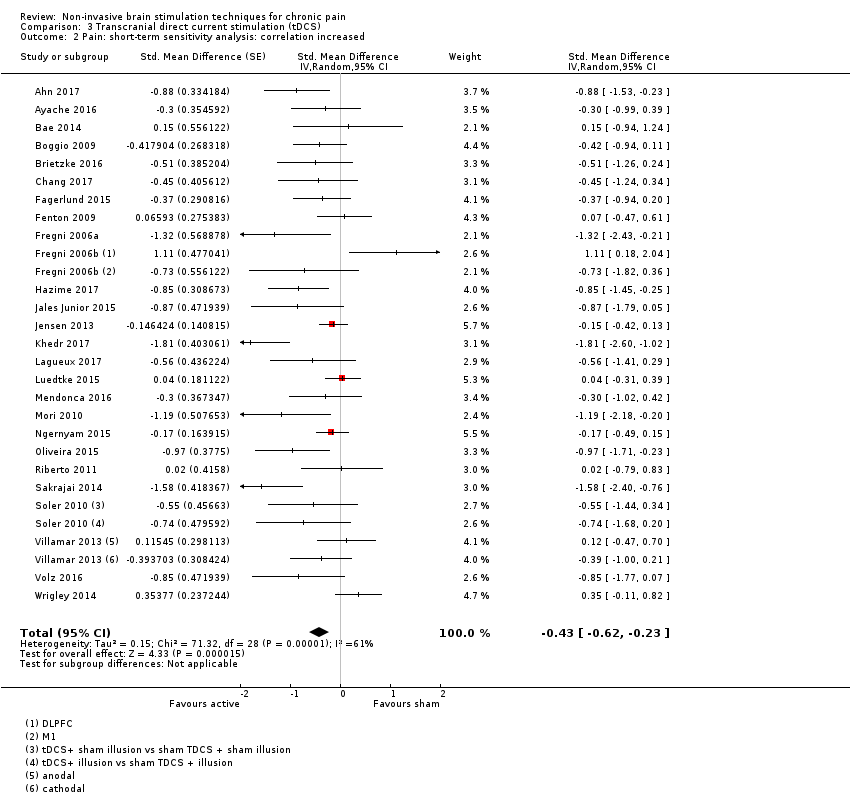 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 2 Pain: short‐term sensitivity analysis: correlation increased. | ||||
| 3 Pain: short‐term sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.64, ‐0.23] | |
| Analysis 3.3  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 3 Pain: short‐term sensitivity analysis: correlation decreased. | ||||
| 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies Show forest plot | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.67, ‐0.29] | |
| Analysis 3.4 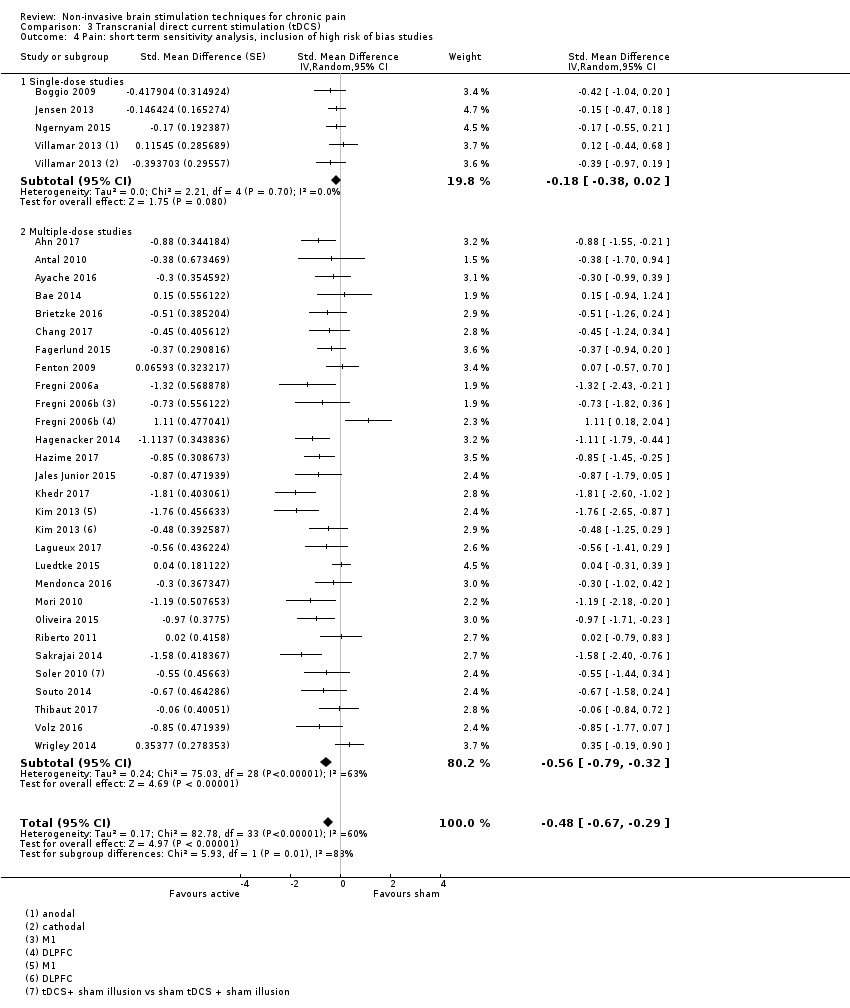 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies. | ||||
| 4.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 4.2 Multiple‐dose studies | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.79, ‐0.32] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.67, ‐0.28] | |
| Analysis 3.5 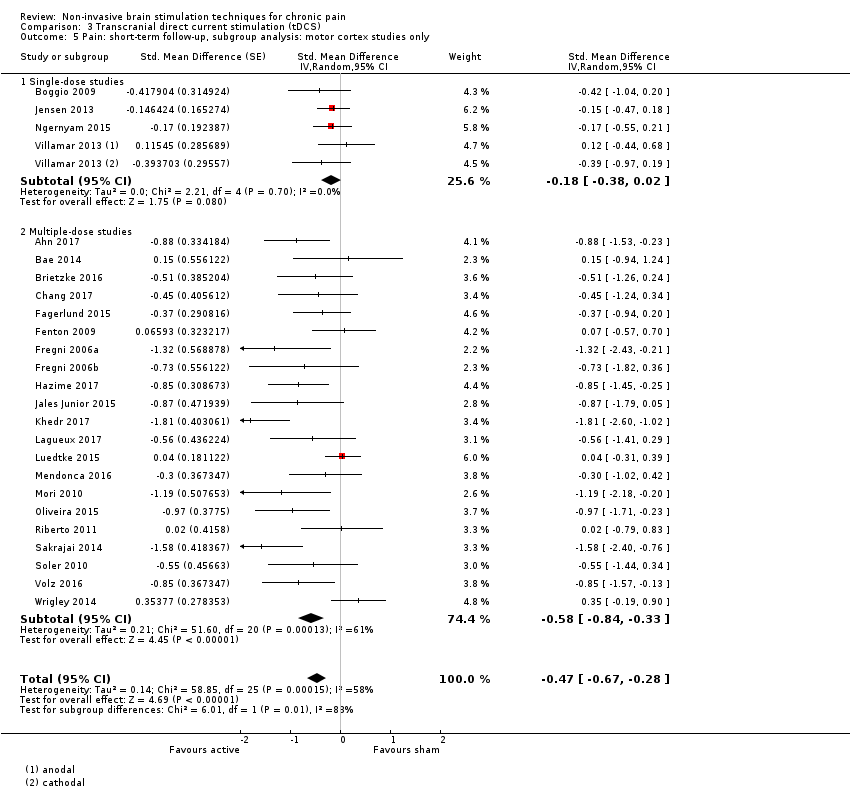 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only. | ||||
| 5.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 5.2 Multiple‐dose studies | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.58 [‐0.84, ‐0.33] | |
| 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.64, ‐0.26] | |
| Analysis 3.6  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased. | ||||
| 6.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.37, 0.01] | |
| 6.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.81, ‐0.30] | |
| 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] | |
| Analysis 3.7  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased. | ||||
| 7.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.03] | |
| 7.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] | |
| 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.56, ‐0.19] | |
| Analysis 3.8 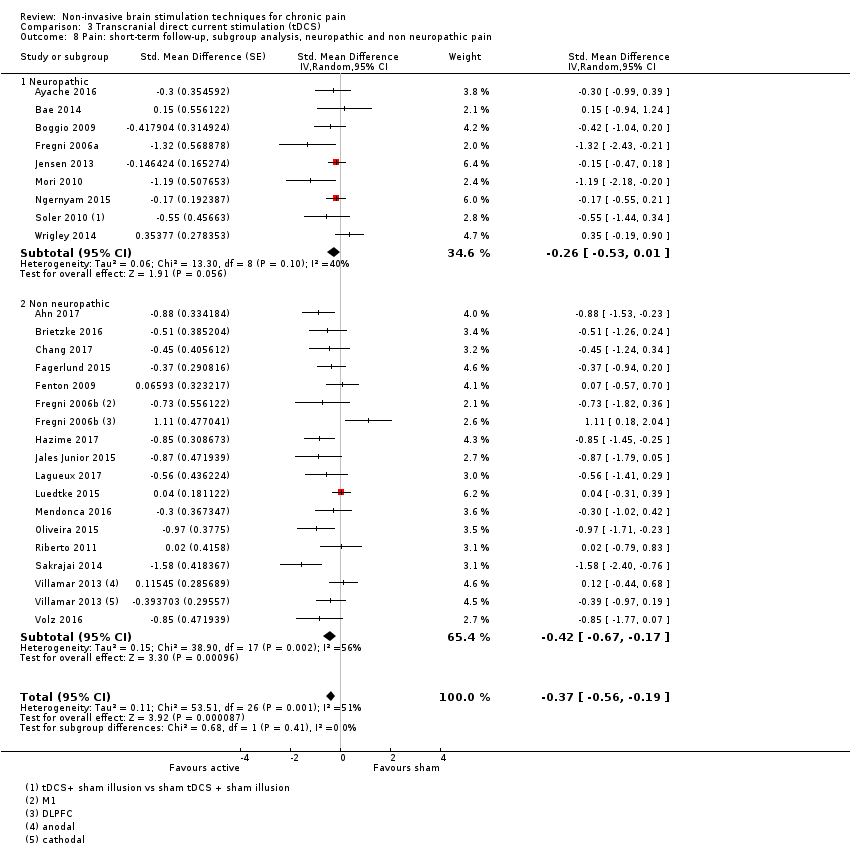 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain. | ||||
| 8.1 Neuropathic | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.53, 0.01] | |
| 8.2 Non neuropathic | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.67, ‐0.17] | |
| 9 Pain: short term follow‐up responder analysis 30% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.9  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 9 Pain: short term follow‐up responder analysis 30% pain reduction. | ||||
| 10 Pain: short term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.10 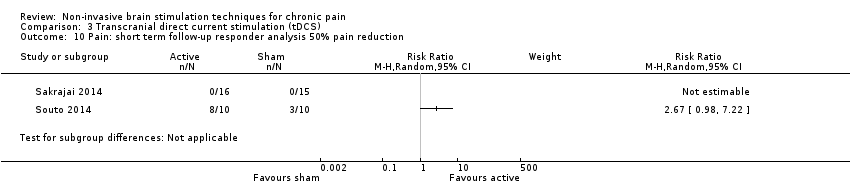 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 10 Pain: short term follow‐up responder analysis 50% pain reduction. | ||||
| 11 Pain: medium‐term follow‐up Show forest plot | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.13] | |
| Analysis 3.11  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 11 Pain: medium‐term follow‐up. | ||||
| 12 Pain: medium term follow‐up responder analysis 30% pain reduction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.12  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 12 Pain: medium term follow‐up responder analysis 30% pain reduction. | ||||
| 13 Pain: medium term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.13  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 13 Pain: medium term follow‐up responder analysis 50% pain reduction. | ||||
| 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.72, ‐0.18] | |
| Analysis 3.14 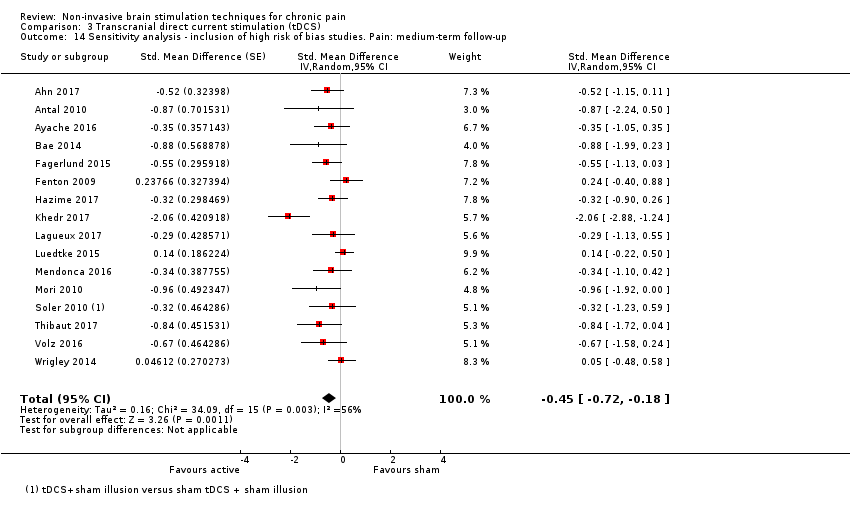 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up. | ||||
| 15 Pain: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.43, 0.41] | |
| Analysis 3.15  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 15 Pain: long‐term follow‐up. | ||||
| 16 Disability: short‐term follow‐up Show forest plot | 4 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.28, 0.26] |
| Analysis 3.16  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 16 Disability: short‐term follow‐up. | ||||
| 17 Disability: medium‐term follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.17 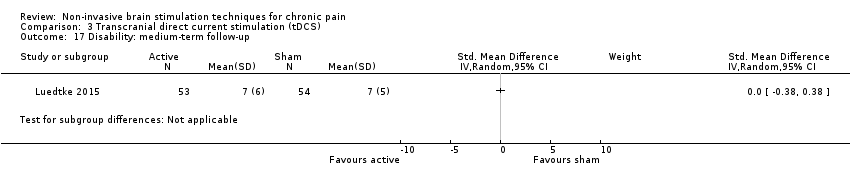 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 17 Disability: medium‐term follow‐up. | ||||
| 18 Quality of life: short‐term follow‐up Show forest plot | 4 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.21, 1.11] |
| Analysis 3.18  Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 18 Quality of life: short‐term follow‐up. | ||||
| 19 Quality of life: medium‐term follow‐up Show forest plot | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.09, 0.76] |
| Analysis 3.19 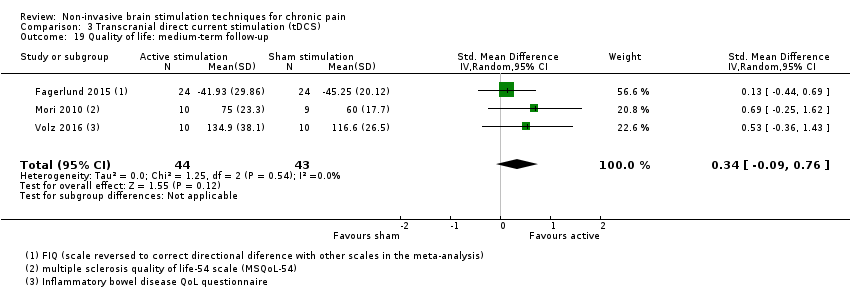 Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 19 Quality of life: medium‐term follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 1 Pain: short‐term follow‐up. | ||||
| 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.18] |
| Analysis 4.2  Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up. | ||||
| 3 Quality of Life: short term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3 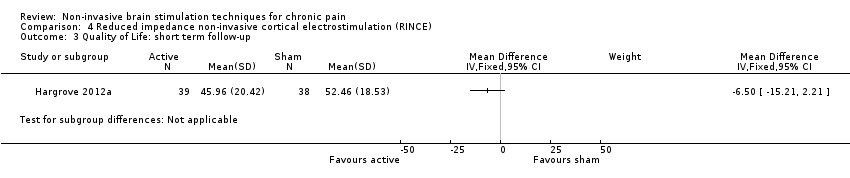 Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 3 Quality of Life: short term follow‐up. | ||||
| 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.91, 0.02] |
| Analysis 4.4  Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.64, 0.26] | |
| Analysis 5.1  Comparison 5 Transcranial random noise stimulation, Outcome 1 Pain. | ||||

Study flow diagram

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.1. Pain: short‐term follow‐up

Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.5. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only
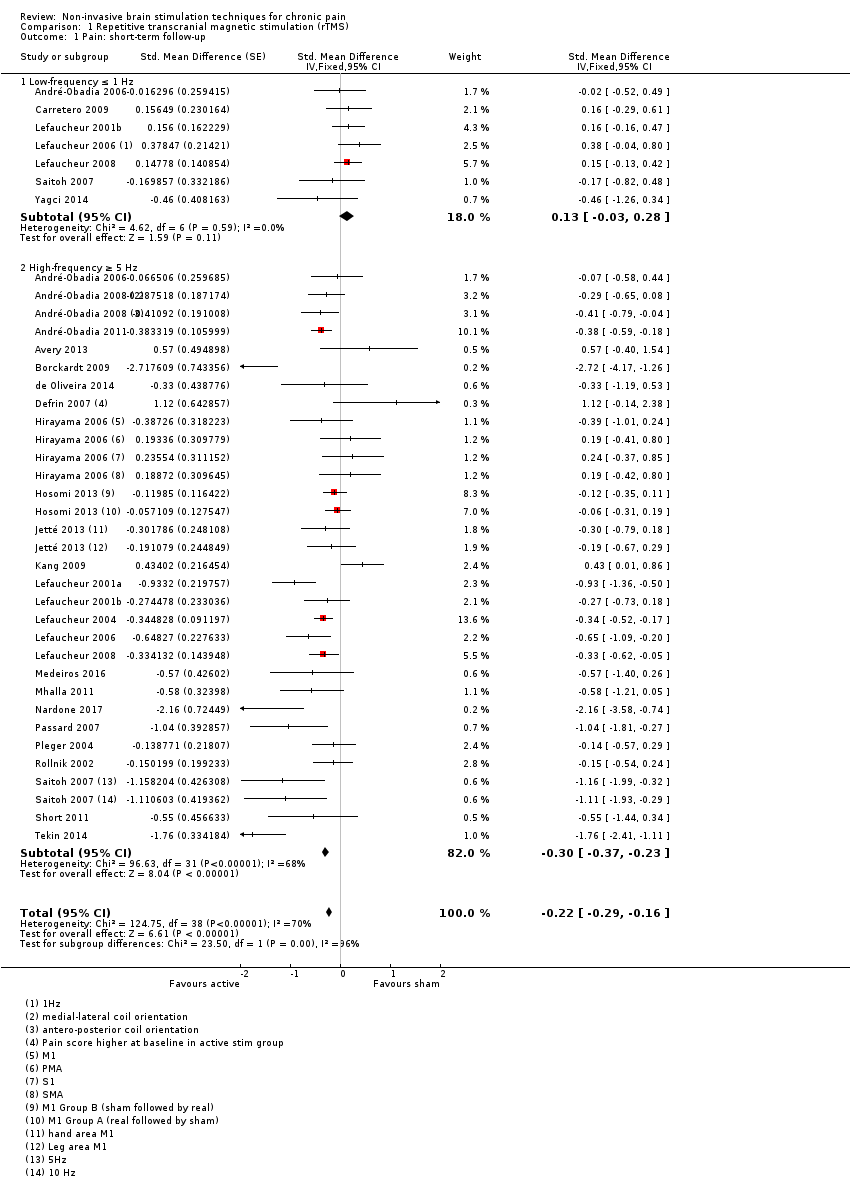
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 1 Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only.
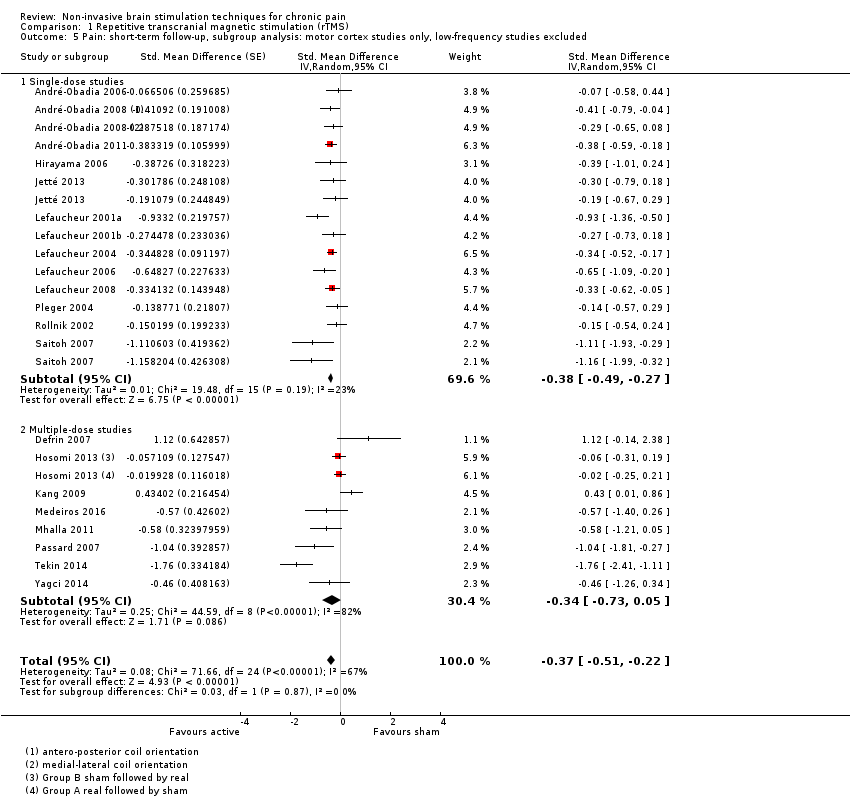
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
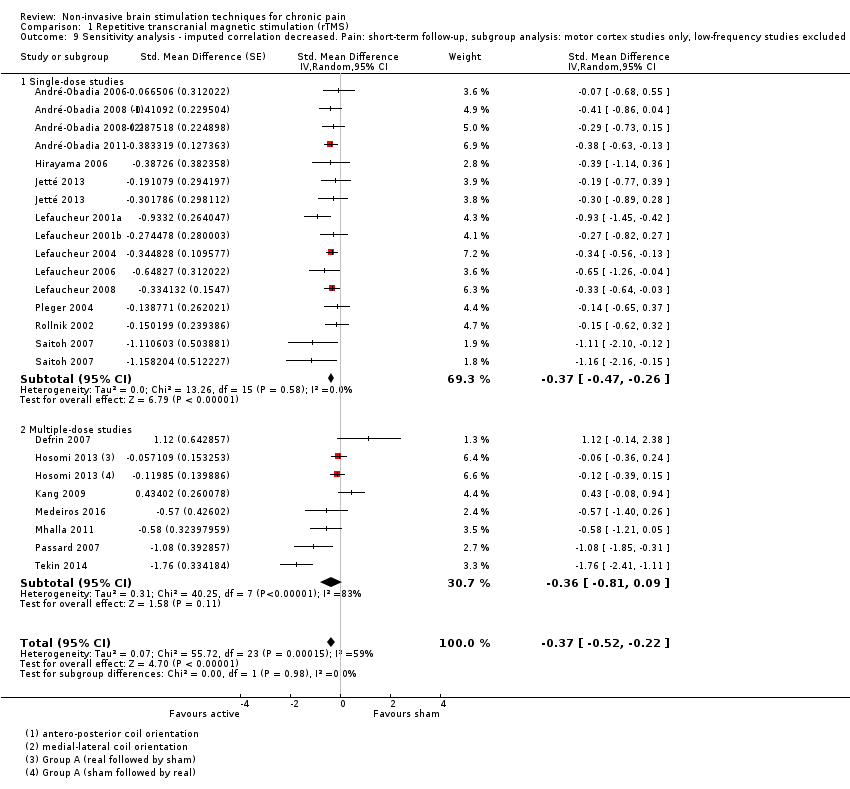
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
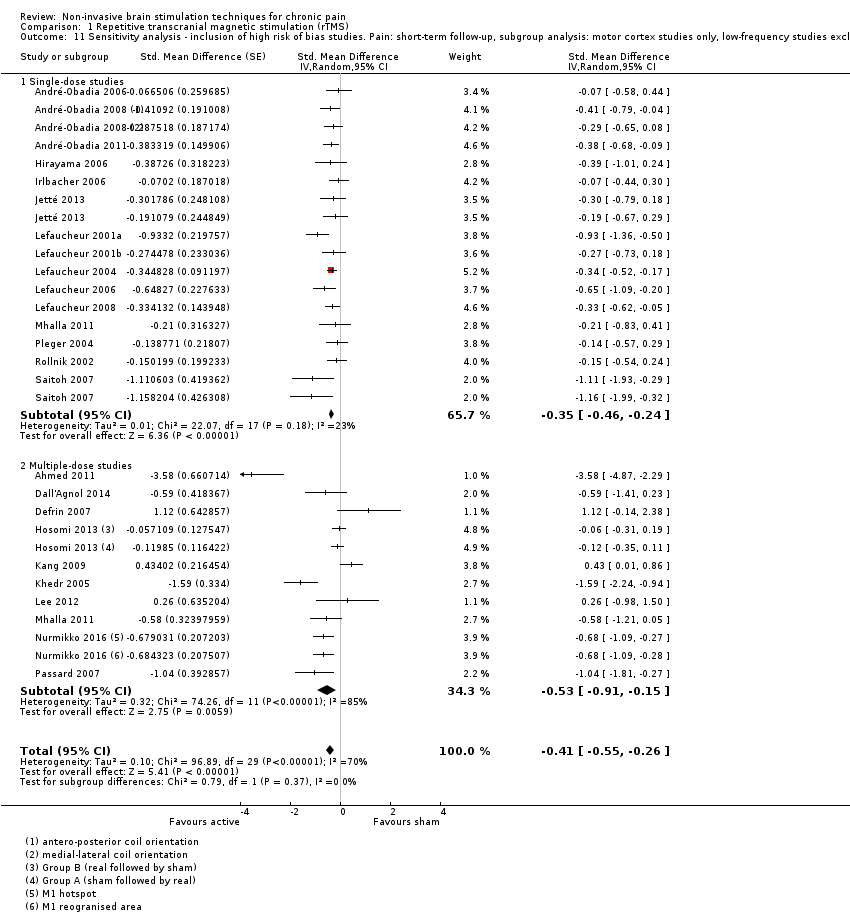
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 14 Pain: short term responder analysis 30% pain reduction.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 16 Pain: medium‐term follow‐up.
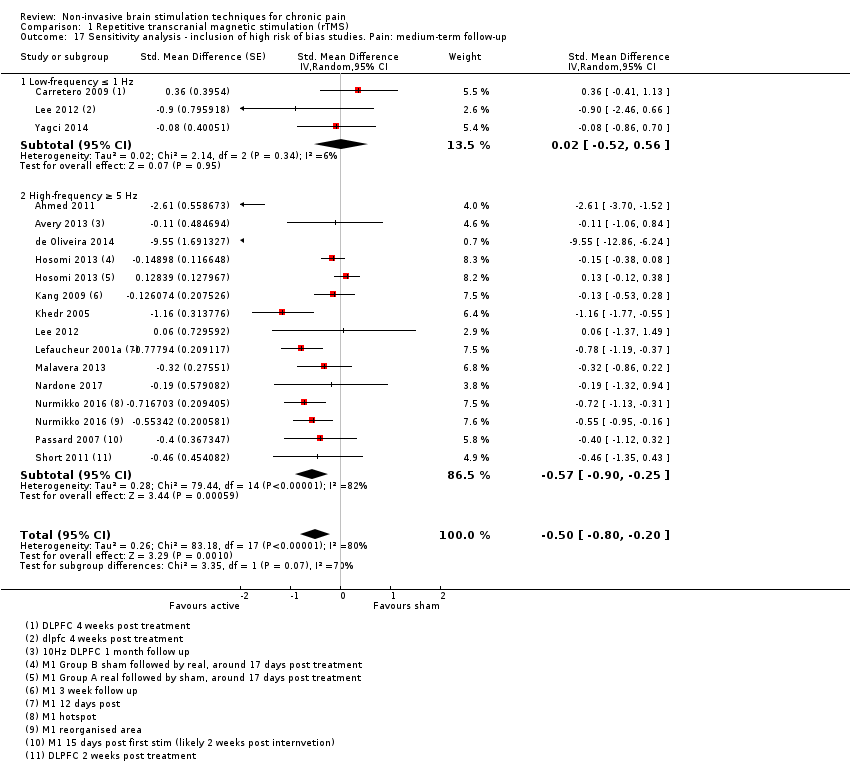
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only.
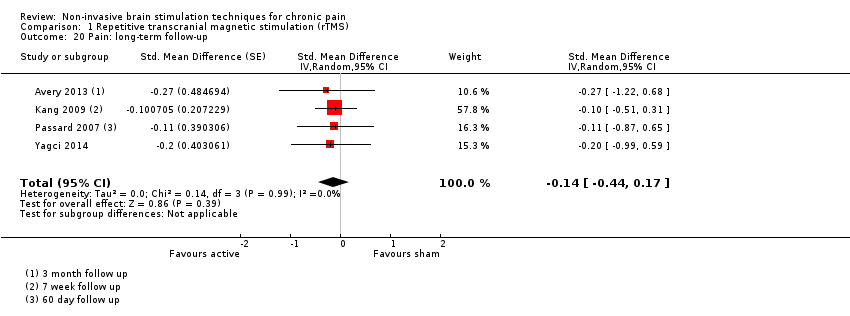
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 20 Pain: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 22 Disability: short‐term follow‐up.
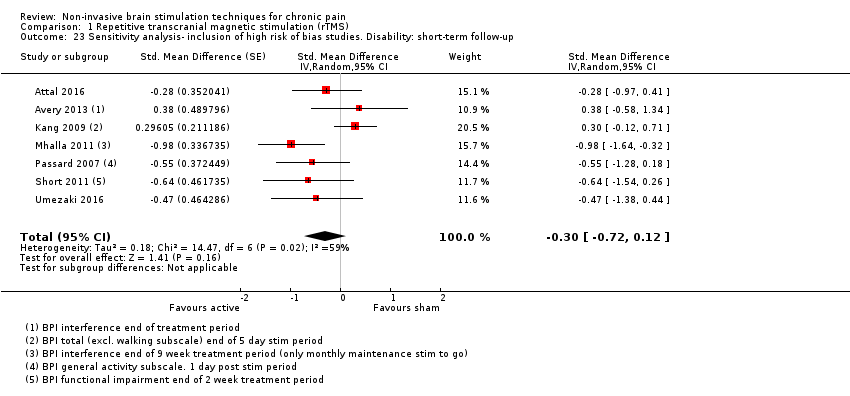
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 24 Disability: medium‐term follow‐up.
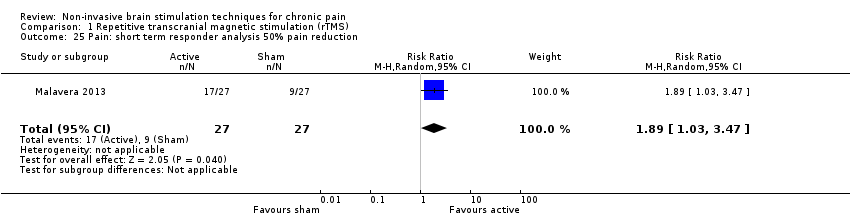
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 25 Pain: short term responder analysis 50% pain reduction.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 26 Disability: long‐term follow‐up.
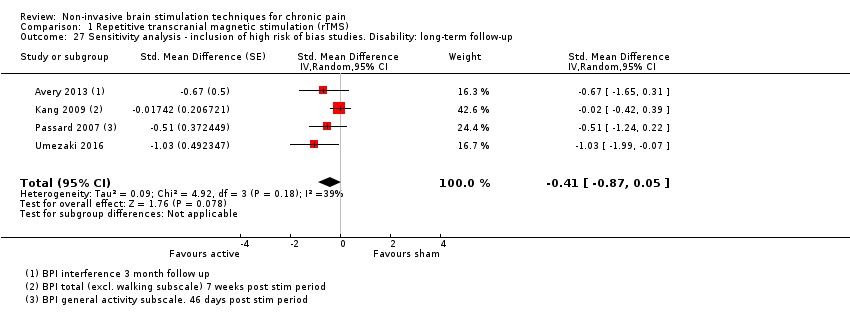
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 31 Quality of life: long‐term follow‐up.
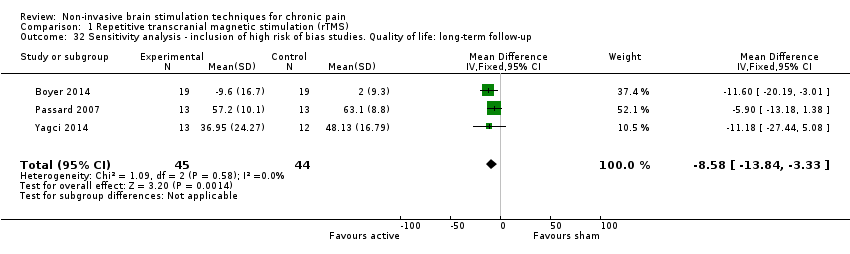
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up.
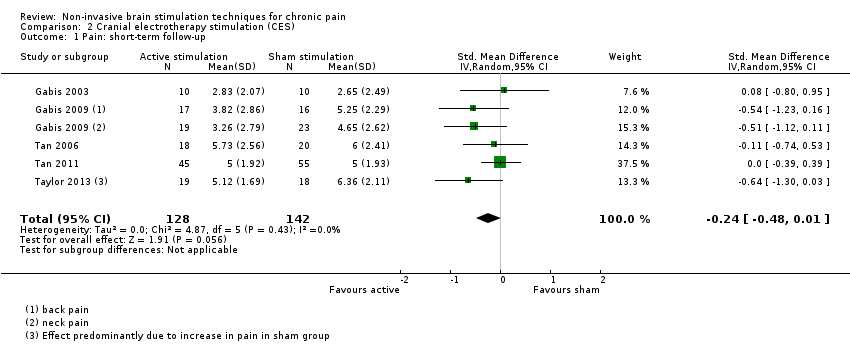
Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 1 Pain: short‐term follow‐up.

Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 2 Quality of life: short term follow up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 1 Pain: short‐term follow‐up.
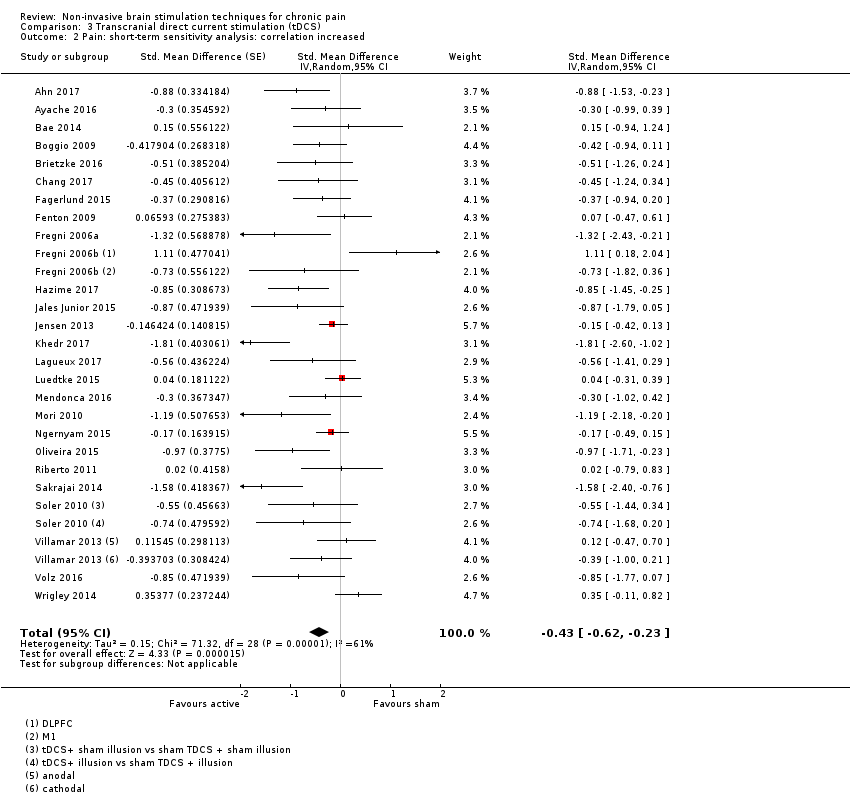
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 2 Pain: short‐term sensitivity analysis: correlation increased.
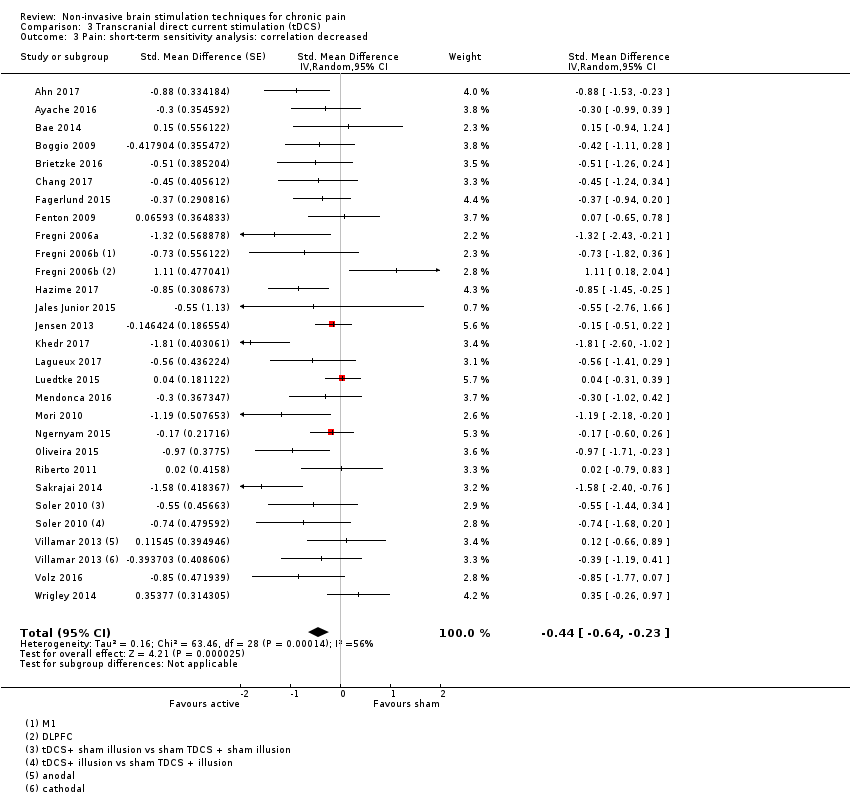
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 3 Pain: short‐term sensitivity analysis: correlation decreased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 9 Pain: short term follow‐up responder analysis 30% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 10 Pain: short term follow‐up responder analysis 50% pain reduction.
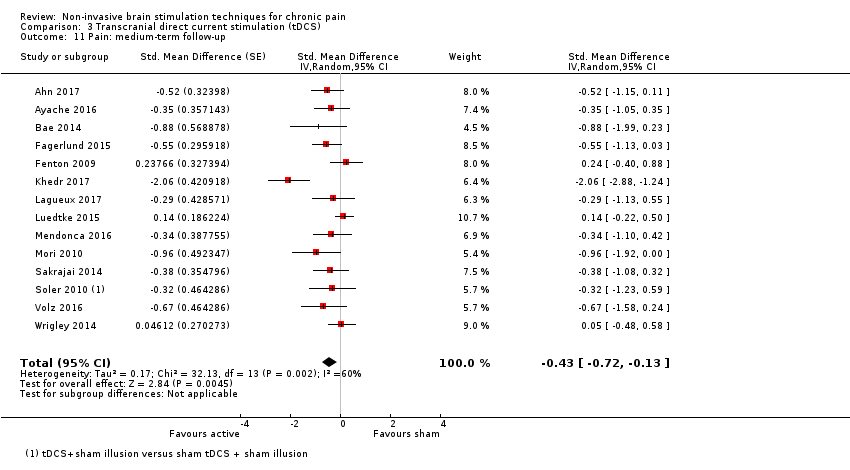
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 11 Pain: medium‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 12 Pain: medium term follow‐up responder analysis 30% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 13 Pain: medium term follow‐up responder analysis 50% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 15 Pain: long‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 16 Disability: short‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 17 Disability: medium‐term follow‐up.
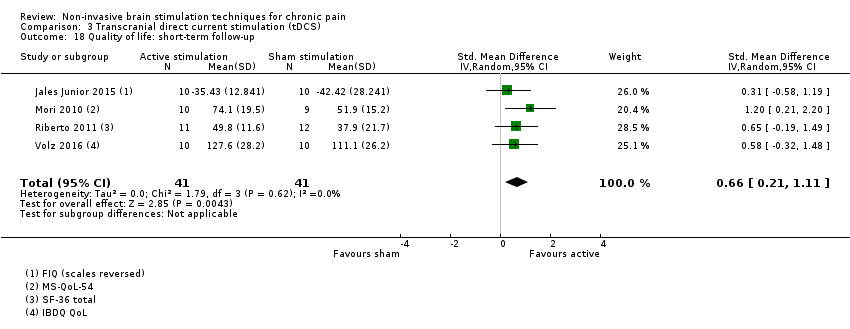
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 18 Quality of life: short‐term follow‐up.
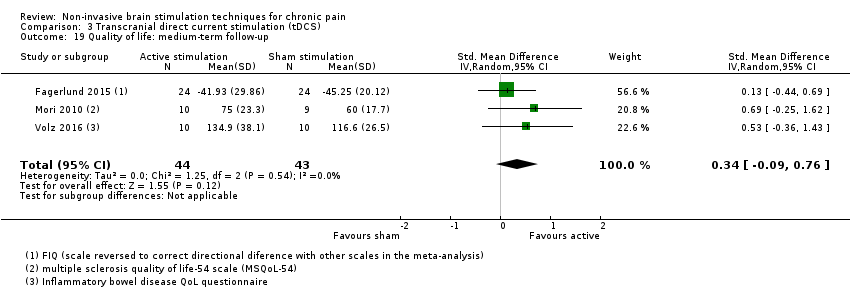
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 19 Quality of life: medium‐term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 1 Pain: short‐term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
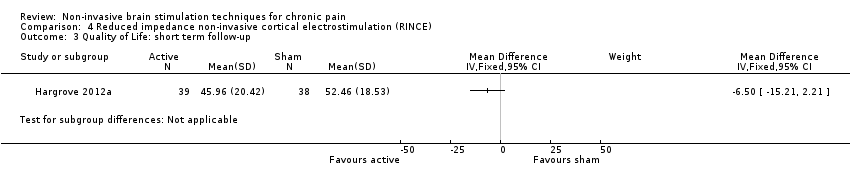
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 3 Quality of Life: short term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up.

Comparison 5 Transcranial random noise stimulation, Outcome 1 Pain.
| rTMS compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active rTMS Comparison: sham rTMS | ||||
| Outcomes | Effect size | Relative and absolute effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.22 (‐0.29 to ‐0.16) | This equates to a 7% (95% CI 5% to 9%) reduction in pain intensity, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale. | 655 (27) | ⊕⊕⊝⊝ low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | SMD ‐0.29, 95% CI ‐0.87 to 0.29 | ‐ | 119 (5) | ⊕⊝⊝⊝ very low2 |
| Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire | MD ‐10.80, 95% CI ‐15.04 to ‐6.55 | ‐ | 105 (4) | ⊕⊕⊝⊝ low3 |
| CI: confidence interval; MD: mean difference; rTMS: repetitive transcranial magnetic stimulation; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias and once for inconsistency due to heterogeneity. | ||||
| CES compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active CES Comparison: sham CES | ||||
| Outcomes | Effect size | Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.24 (‐0.48 to 0.01) | ‐ | 270 (5) | ⊕⊕⊝⊝ low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | No data available | No data available | No data available | No data available |
| Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire | MD ‐25.05 (‐37.82 to ‐12.28) | ‐ | 36 (1) | ⊕⊝⊝⊝ very low2 |
| CI: confidence interval; CES: cranial electrotherapy stimulation; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias and once for imprecision due to low participant numbers. | ||||
| tDCS compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active tDCS Comparison: sham tDCS | ||||
| Outcomes | Effect size | Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.43 (‐0.63 to ‐0.22) | This equates to a 17% (95% CI 9% to 25%) reduction in pain intensity or a 0.82 (95% CI 0.42 to 1.2) point reduction on a 0 to 10 pain intensity scale. | 747 (27) | ⊕⊝⊝⊝ very low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | SMD ‐0.01, (95% CI ‐0.28 to 0.26) | ‐ | 212 (4) | ⊕⊕⊝⊝ low2 |
| Quality of life (0 to < 1 week postintervention) measured using different scales across studies | SMD 0.66, 95% CI 0.21 to 1.11 | ‐ | 82 (4) | ⊕⊕⊝⊝ low2 |
| CI: confidence interval; MD: mean difference; SMD: standardised mean difference; tDCS: transcranial direct current stimulation | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias, once for inconsistency due to heterogeneity and once for evidence of possible publication bias. | ||||
| Study | Location of stimulation | Coil orientation | Frequency (Hz) | Intensity (% RMT) | Number of trains | Duration of trains | Inter‐train intervals (sec) | Number of pulses per session | Treatment sessions per group |
| M1 stump region | 45° angle from sagittal line | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily | |
| M1 contralateral to painful side | Anteroposterior induced current | 10 | 80 | 30 | 10 | 20 | 3000 | 3, x1 daily | |
| M1 contralateral to painful side | Posteroanterior | 20, 1 | 90 | 20 Hz: 20 1 Hz: 1 | 20 Hz: 4 sec 1 Hz: 26 min | 20 Hz: 84 | 1600 | 1 | |
| M1 contralateral to painful side | Posteroanterior Medial‐lateral | 20 | 90 | 20 | 4 sec | 84 | 1600 | 1 | |
| M1 hand area, not clearly reported but likely contralateral to painful side | Not specified | 20 | 90 | 20 | 4 sec | 84 | 1600 | 1 | |
| Left DLPFC | Not specified | 10 | 120 | 75 | 4 | 26 | 3000 | 15 | |
| Left PFC | Not specified | 10 | 100 | 40 | 10 sec | 20 | 4000 | 3 over a 5‐day period | |
| Left M1 | anteroposterior | 10 | 90 | 20 | 10 | 50 | 2000 | 14, 10 sessions in 2 weeks followed by maintenance phase of 1 session at weeks 4, 6, 8, and 10 | |
| Right DLPFC | Not specified | 1 | 110 | 20 | 60 sec | 45 | 1200 | Up to 20 on consecutive working days | |
| Left M1 | 45° angle from sagittal line | 10 | 80 | 16 | 10 | 26 | 1600 | 10, timescale not specified | |
| M1 midline | Not specified | 5 | 115 | 500 | 10 sec | 30 | ? 500* | 10, x 1 daily | |
| Left DLPFC/premotor | not specified | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x 1 daily (working days) for 2 weeks | |
| Left and right SII | Not specified | 1 or 20 | 90 | Not specified | Not specified | Not specified | 1600 | 1 | |
| Right SII | Not specified | 1 | 70% maximum stimulator output intensity (not RMT) | 1 | Not specified | Not specified | 1600 | 10, x 1 daily (weekdays only) | |
| M1, S1, PMA, SMA | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 1 | |
| M1 corresponding to painful region | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 10, x 1 daily (weekdays only) | |
| M1 contralateral to painful side | Not specified | 5, 1 | 95 | Not specified | Not specified | Not specified | 500 | 1 | |
| M1 hand or leg area with neuro navigation | 45º postero‐lateral | 10 | 90 | 40 | 5 | 25 | 2000 | 1, per stimulation condition | |
| Right M1 | 45º postero‐lateral | 10 | 80 | 20 | 5 sec | 55 | 1000 | 5, x 1 daily | |
| M1 contralateral to painful side | Not specified | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily | |
| Right DLPFC (low‐frequency) Left M1 (high‐frequency) | Not specified | 10, 1 | 10 Hz: 80 1 Hz: 110 | 10 Hz: 25 1 Hz: 2 | 10 Hz: 8 sec 1 Hz: 800 sec | 10 Hz: 10 1 Hz: 60 | 10 Hz: 2000 1 Hz: 1600 | 10, x 1 daily (weekdays only) | |
| M1 contralateral to painful side | Not specified | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 0.5 | 80 | 10 Hz: 20 0.5 Hz: 1 | 10 Hz: 5 sec 0.5 Hz: 20 min | 10 Hz: 55 | 10 Hz: 1000 0.5 Hz: 600 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 | 10 Hz: 6 sec 1 Hz: 20 min | 10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 | 10 Hz: 6 sec 1 Hz: 20 min | 10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 | 1 | |
| M1 contralateral to painful side | 45° angle from sagittal line | 10 | 90 | 20 | 6 | 54 | 1200 | 10, x 1 daily (weekdays only) | |
| Left M1 | 45° angle from sagittal line | 10 | 80 | not reported | not reported | not reported | 1600 | 10, x 1 daily | |
| Left M1 | Posteroanterior | 10 | 80 | 15 | 10 sec | 50 | 1500 | 14, 5 x 1 daily (working days), then 3 x 1 weekly, then 3 x 1 fortnightly, then 3 x 1 monthly | |
| Left PFC | Posteroanterior | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x5 per week for 2 weeks | |
| M1 hotspot contralateral to pain M1 in reorganised area contralateral to pain | Posteroanterior | 10 | 90 | 20 | 10 sec | 60 | 2000 | 5, x 3‐5 times per week | |
| M1 deep central sulcus | H‐coil | 20 | 100 | 30 | 2.5 sec | 30 | 1500 | 5, x 1 daily on consecutive days | |
| M1 contralateral to painful side | Posteroanterior | 10 | 80 | 25 | 8 sec | 52 | 2000 | 10, x 1 daily (working days) | |
| M1 contralateral to painful side | Posteroanterior | 10 | 100 | 25 | 10 sec | 60 | 2500 | 10, x 1 daily (working days) | |
| M1 hand area | Not specified | 10 | 110 | 10 | 1.2 sec | 10 | 120 | 1 | |
| M1 midline | Not specified | 20 | 80 | 20 | 2 sec | Not specified | 800 | 1 | |
| M1 over motor representation of painful area | Not specified | 10, 5, 1 | 90 | 10 Hz; 5 5 Hz: 10 1 Hz: 1 | 10 Hz: 10 sec 5 Hz: 10 sec 1 Hz: 500 sec | 10 Hz: 50 5 Hz: 50 | 500 | 1 | |
| Left DLPFC | Parasagittal | 10 | 120 | 80 | 5 sec | 10 sec | 4000 | 10, x 1 daily (working days) for 2 weeks | |
| M1 midline | 45° angle from sagittal line | 10 | 100 | 30 | 5 | 12 | 1500 | 10, x 1 daily (not clear if only work days) | |
| Targeted to ACC | 4‐coil configuration | 1 Hz (10 Hz data excluded as not randomised) | 110 | Not reported | Not reported | Not reported | 1800 | 20, x 1 daily (working days) | |
| Left DLPFC | Not specified | 10 | 100 | 10 | 5 | 10 | 3000 | 10, x1 daily (working days) | |
| Left M1 | Not specified | 1 | 90 | 20 | 60 | 45 | 1200 | 10, x1 daily (working days) | |
| M1 midline | Handle pointing posteriorly | 10 | 10 | 30 | 5 | 25 | 1500 | 10, x1 daily (working days) | |
| ACC: anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; M1: primary motor cortex; PFC: prefrontal cortex; PMA: pre‐motor area; RMT: resting motor threshold; dS1: primary somatosensory cortex; SII: secondary somatosensory cortex; SMA: supplementary motor area *Inconsistency between stimulation parameters and reported total number of pulses in study report. See Included studies section for mored detail. | |||||||||
| Study | Electrode placement | Frequency (Hz) | Pulse width (ms) | Waveform shape | Intensity | Duration (min) | Treatment sessions per group |
| Ear clip electrodes | 10 | 2 | Not specified | 12 μA | 53 | x 2 daily for 4 days | |
| Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | ? daily for 3 weeks | |
| Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days | |
| Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days | |
| Mastoid processes and forehead | Not specified | Not specified | 2 conditions: symmetric, asymmetric | 11 to 15 mA | 40 | x 1 daily for 5 days | |
| Ear clip electrodes | 0.5 | Not specified | Biphasic square wave | 100 μA | 60 | x 1 daily for 30 days | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 40 | x 1 daily for 6 weeks | |
| Ear clip electrodes | 0.5 | Not specified | Not specified | 10 to 600 μA | 20 | 12 (timing not specified) | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 to 500 μA | 60 | x 1 daily for 21 days | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 60 | x 1 daily for 21 days | |
| Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | x 1 daily for 8 weeks |
| Study | Location of stimulation (Anode) | Electrode pad size | Intensity (mA) | Anodal or cathodal? | Stimulus duration (min) | Treatment sessions per group |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 left hand area | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily | |
| Left DLPFC | 25 cm2 | 2mA | Anodal | 20 | 3, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 3 per week for 3 weeks | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 30 | 1 | |
| Left M1 | 25‐35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 16, x 2 weekly for 8 weeks | |
| M1 contralateral to painful side | HD‐tDCS | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1, side not specified | 35 cm2 | 2mA | Anodal | 20 | 5, x 1 daily | |
| M1 dominant hemisphere | 35 cm2 | 1 mA | Anodal | 20 | 2 | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 40 cm2 | 1mA | Anodal | 20 | Daily, self‐administered for 14 days | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 12, x 3 per week for 4 weeks | |
| Left M1 | 15 cm2 | 1mA | Anodal | 20 | x 1 weekly for 10 weeks | |
| M1 left | 35cm2 | 2 mA | Anodal | 20 | 1 | |
| M1 contralateral to painful side | 24 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily, 5 days per week for 2 weeks | |
| M1, side not specified DLPFC | 25 cm2 | 2mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 14, x 5 weekly for 2 weeks, x 1 weekly for 4 weeks | |
| M1 left side not specified | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| Group 1: anodal left M1 Group 2: cathodal left M1 Group 3: anodal supraorbital Group 4: cathodal supraorbital Group 5: sham | 35 cm2 | 2 mA | Anodal or cathodal | 20 | 1 | |
| Left M1 | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 1 | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily, then x 2 weekly for 3 weeks, up to 10 sessions | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 1 per condition | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 weekly | |
| M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily (weekdays only) | |
| Left M1 | 25 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 left | HD‐tDCS 4 x 1‐ring montage | 2 mA | Anodal or cathodal | 20 | x 1 per condition | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| DLPFC: dorsolateral prefrontal cortex; HD‐tDCS: high definition tDCS; M1: primary motor cortex | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 27 | Std. Mean Difference (Fixed, 95% CI) | ‐0.22 [‐0.29, ‐0.16] | |
| 1.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | 0.13 [‐0.03, 0.28] | |
| 1.2 High‐frequency ≥ 5 Hz | 25 | Std. Mean Difference (Fixed, 95% CI) | ‐0.30 [‐0.37, ‐0.23] | |
| 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies Show forest plot | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 2.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.10] | |
| 2.2 Multiple‐dose studies | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.76, ‐0.05] | |
| 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only Show forest plot | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.13] | |
| 3.1 Low‐frequency ≤ 1 Hz | 5 | Std. Mean Difference (Fixed, 95% CI) | 0.15 [‐0.02, 0.32] | |
| 3.2 High‐frequency ≥ 5 Hz | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.28 [‐0.36, ‐0.20] | |
| 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only Show forest plot | 8 | Std. Mean Difference (Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.17] | |
| 4.1 Low‐frequency ≤ 1 Hz | 1 | Std. Mean Difference (Fixed, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 4.2 High‐frequency ≥ 5 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | ‐0.56 [‐0.81, ‐0.31] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.51, ‐0.22] | |
| 5.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.49, ‐0.27] | |
| 5.2 Multiple‐dose studies | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.73, 0.05] | |
| 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up Show forest plot | 29 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.40, ‐0.14] | |
| 6.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.15 [0.01, 0.29] | |
| 6.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.49, ‐0.22] | |
| 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up Show forest plot | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 7.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 7.2 High‐frequency ≥ 5 Hz | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.49, ‐0.19] | |
| 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] | |
| 8.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.50, ‐0.28] | |
| 8.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.71, 0.04] | |
| 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.52, ‐0.22] | |
| 9.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.47, ‐0.26] | |
| 9.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.81, 0.09] | |
| 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 31 | Std. Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.34, ‐0.20] | |
| 10.1 Low‐frequency ≤ 1 Hz | 10 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.07, 0.22] | |
| 10.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Fixed, 95% CI) | ‐0.36 [‐0.44, ‐0.29] | |
| 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 24 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.55, ‐0.26] | |
| 11.1 Single‐dose studies | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.46, ‐0.24] | |
| 11.2 Multiple‐dose studies | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐0.91, ‐0.15] | |
| 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.48, 0.15] | |
| 12.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 12.2 High frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.92 [‐1.95, 0.12] | |
| 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 13.1 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 14 Pain: short term responder analysis 30% pain reduction Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.17, 3.80] |
| 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐1.01, 0.17] | |
| 16 Pain: medium‐term follow‐up Show forest plot | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.61, 0.05] | |
| 16.1 Low‐frequency ≤ 1 Hz | 2 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.41, 0.69] | |
| 16.2 High‐frequency ≥ 5 Hz | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.73, 0.00] | |
| 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] | |
| 17.1 Low‐frequency ≤ 1 Hz | 3 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.52, 0.56] | |
| 17.2 High‐frequency ≥ 5 Hz | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐0.90, ‐0.25] | |
| 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.46, 0.02] | |
| 18.1 Low frequency ≤ 1Hz | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.86, 0.70] | |
| 18.2 High‐frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.49, 0.03] | |
| 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐1.08 [‐2.49, 0.32] | |
| 19.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.36 [‐0.41, 1.13] | |
| 19.2 High‐frequency ≥ 5 Hz | 4 | Std. Mean Difference (Random, 95% CI) | ‐1.74 [‐3.66, 0.19] | |
| 20 Pain: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.44, 0.17] | |
| 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.89, 0.10] | |
| 22 Disability: short‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.87, 0.29] | |
| 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.72, 0.12] | |
| 24 Disability: medium‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐1.07, 0.33] | |
| 25 Pain: short term responder analysis 50% pain reduction Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.03, 3.47] |
| 26 Disability: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.62, 0.16] | |
| 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.87, 0.05] | |
| 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐15.04, ‐6.55] |
| 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐11.49 [‐16.73, ‐6.25] |
| 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 5 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐8.93 [‐13.49, ‐4.37] |
| 31 Quality of life: long‐term follow‐up Show forest plot | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐6.78 [‐13.43, ‐0.14] |
| 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up Show forest plot | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐13.84, ‐3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.48, 0.01] |
| 2 Quality of life: short term follow up Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.63, ‐0.22] | |
| 1.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 1.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.77, ‐0.25] | |
| 2 Pain: short‐term sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.62, ‐0.23] | |
| 3 Pain: short‐term sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.64, ‐0.23] | |
| 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies Show forest plot | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.67, ‐0.29] | |
| 4.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 4.2 Multiple‐dose studies | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.79, ‐0.32] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.67, ‐0.28] | |
| 5.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 5.2 Multiple‐dose studies | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.58 [‐0.84, ‐0.33] | |
| 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.64, ‐0.26] | |
| 6.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.37, 0.01] | |
| 6.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.81, ‐0.30] | |
| 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] | |
| 7.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.03] | |
| 7.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] | |
| 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.56, ‐0.19] | |
| 8.1 Neuropathic | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.53, 0.01] | |
| 8.2 Non neuropathic | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.67, ‐0.17] | |
| 9 Pain: short term follow‐up responder analysis 30% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10 Pain: short term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11 Pain: medium‐term follow‐up Show forest plot | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.13] | |
| 12 Pain: medium term follow‐up responder analysis 30% pain reduction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Pain: medium term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.72, ‐0.18] | |
| 15 Pain: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.43, 0.41] | |
| 16 Disability: short‐term follow‐up Show forest plot | 4 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.28, 0.26] |
| 17 Disability: medium‐term follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18 Quality of life: short‐term follow‐up Show forest plot | 4 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.21, 1.11] |
| 19 Quality of life: medium‐term follow‐up Show forest plot | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.09, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.18] |
| 3 Quality of Life: short term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.91, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.64, 0.26] | |

