Nieinwazyjne metody stymulacji mózgu w przewlekłym bólu
Appendices
Appendix 1. Main database search strategies for current update
CENTRAL (CRSO)
#1 MESH DESCRIPTOR pain EXPLODE ALL TREES 32731
#2 (((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*)):TI,AB,KY 15073
#3 ((sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*))):TI,AB,KY 6757
#4 #1 OR #2 OR #3 45871
#5 MESH DESCRIPTOR Transcranial Magnetic Stimulation 974
#6 MESH DESCRIPTOR Electronarcosis 33
#7 (((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*)):TI,AB,KY 4072
#8 (((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*))):TI,AB,KY 64
#9 (((non‐invasive or non*invasive) adj4 stimulat*)):TI,AB,KY 337
#10 ((theta burst stimulat* or iTBS or cTBS)):TI,AB,KY 150
#11 ((transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy)):TI,AB,KY 2912
#12 ((electrosleep or electronarco*)):TI,AB,KY 47
#13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 4355
#14 #4 AND #13 310
#15 31/07/2013 TO 30/09/2016:DL 264060
#16 #14 AND #15 176
MEDLINE (OVID)
1 exp Pain/ (283010)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (74023)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (28679)
4 or/1‐3 (325946)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (6328)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (25872)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (147)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (822)
9 (theta burst stimulat* or iTBS or cTBS).tw. (575)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7423)
11 (electrosleep or electronarco*).tw. (357)
12 or/5‐11 (28316)
13 randomized controlled trial.pt. (337806)
14 controlled clinical trial.pt. (84996)
15 randomized.ab. (241501)
16 placebo.ab. (134421)
17 drug therapy.fs. (1571905)
18 randomly.ab. (173459)
19 trial.ab. (248492)
20 groups.ab. (1134392)
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (2928552)
22 exp animals/ not humans.sh. (3751730)
23 21 not 22 (2487755)
24 4 and 12 and 23 (295)
25 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).ed. (2428299)
26 24 and 25 (112)
Embase (OVID)
1 exp Pain/ (1006798)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (158849)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (52041)
4 or/1‐3 (1044575)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (18453)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (50617)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (237)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (2843)
9 (theta burst stimulat* or iTBS or cTBS).tw. (1549)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (17745)
11 (electrosleep or electronarco*).tw. (383)
12 or/5‐11 (57298)
13 random$.tw. (1121981)
14 factorial$.tw. (28563)
15 crossover$.tw. (58949)
16 cross over$.tw. (26241)
17 cross‐over$.tw. (26241)
18 placebo$.tw. (244121)
19 (doubl$ adj blind$).tw. (172110)
20 (singl$ adj blind$).tw. (18218)
21 assign$.tw. (295873)
22 allocat$.tw. (107828)
23 volunteer$.tw. (211373)
24 Crossover Procedure/ (48595)
25 double‐blind procedure.tw. (236)
26 Randomized Controlled Trial/ (419274)
27 Single Blind Procedure/ (23071)
28 or/13‐27 (1749640)
29 (animal/ or nonhuman/) not human/ (5110486)
30 28 not 29 (1554658)
31 4 and 12 and 30 (1112)
32 (201307* or 201308* or 201309* or 201310* or 201311* or 201312* or 2014* or 2015* or 2016*).dd. (5443542)
33 31 and 32 (527)
34 limit 33 to embase (487)
PsycINFO (OVID)
1 exp Pain/ (48364)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (25922)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (4998)
4 or/1‐3 (56650)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (5956)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (17936)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (89)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (983)
9 (theta burst stimulat* or iTBS or cTBS).tw. (791)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7884)
11 (electrosleep or electronarco*).tw. (139)
12 or/5‐11 (18853)
13 clinical trials/ (9724)
14 (randomis* or randomiz*).tw. (62274)
15 (random$ adj3 (allocat$ or assign$)).tw. (35100)
16 ((clinic$ or control$) adj trial$).tw. (52603)
17 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (22429)
18 (crossover$ or "cross over$").tw. (8346)
19 random sampling/ (699)
20 Experiment Controls/ (856)
21 Placebo/ (4606)
22 placebo$.tw. (35030)
23 exp program evaluation/ (18184)
24 treatment effectiveness evaluation/ (20144)
25 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (70971)
26 or/13‐25 (221762)
27 4 and 12 and 26 (180)
28 limit 27 to yr="2013 ‐Current" (82)
CINAHL (EBSCO)
S26 S25 Limiters ‐ Published Date from: 20130701‐20160914
S25 S15 AND S24
S24 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 (allocat* random*)
S22 (MH "Quantitative Studies")
S21 (MH "Placebos")
S20 placebo*
S19 (random* allocat*)
S18 (MH "Random Assignment")
S17 (Randomi?ed control* trial*)
S16 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S15 S4 AND S14
S14 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 TI ( (electrosleep OR electronarco*) ) OR AB ( (electrosleep OR electronarco*) )
S12 TI ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") ) OR AB ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") )
S11 TI ( ("theta burst stimulat*" OR iTBS OR cTBS) ) OR AB ( ("theta burst stimulat*" OR iTBS OR cTBS) )
S10 TI ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) ) OR AB ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) )
S9 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S8 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S7 TI ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) ) OR AB ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) )
S6 (MH "Electric Stimulation")
S5 (MH "Electronarcosis")
S4 S1 OR S2 OR S3
S3 TI ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") ) OR AB ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") )
S2 TI ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*). ) OR AB ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*))
S1 (MH "Pain+")
LILACS
1. Pain$ or dolor$ or intractabl$ or neuropath$ or phantom or fantom or myofasc$ or temp$romandibular or sciatic$ or back‐ache or backache or ache or lumbago or fibromyalg$ or neuralg$ or dystroph$ or atroph$ or causalgi$ or whip‐lash or whiplash or polymyalg$ [Words]¬
2. ((Estimulaci$ or stimulat$) and (cerebra$ or brain$ or cortex or cortical or crania$ or transcranial$ or magneti$)) or electrostim$ or electrotherapy$ or electro‐therap$ or “theta burst stimul$” or iTBS or Ctbs or “transcrani$ magnet$ stimulat$” or rTMS or “transcrani$ direct current stimulat$” or tDCS or “cranial electrostimulat$” or “cranial electrotherapy$ or electrosleep or electronarco$ [Words]¬
3. ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
Appendix 2. Trials register search results for current update
| Register | Date of search | Search terms | Number of records |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 91 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 1 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 0 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 0 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain 01/01/2009 to 07/02/2013 adult | 60 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | |
| WHO ICTRP | 20/9/16 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 2 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
| Register | Date of search | Search terms | Number of records |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 6 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 3 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 3 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 0 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain 01/01/2009 to 07/02/2013 adult | 36 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 8 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 0 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 0 |
Appendix 3. Search results summary table for current update
| Database searched | Date last searched | Number of results |
| CENTRAL (CRSO) 31/07/2013 TO 30/09/2016 | 11/10/17 | 243 |
| MEDLINE (OVID) July 2013 to Aug week 5 2016 | 11/10/17 | 217 |
| Embase (OVID) July 2013 to 2016 week 37 | 11/10/17 | 595 |
| PsycINFO (OVID) 2013 to July week 4 2016 | 11/10/17 | 117 |
| CINAHL (EBSCO) July 2013 to Sept 2016 | 11/10/17 | 42 |
| LILACS (Birme) 2013 to Sept 2016 | 11/10/17 | 42 |
| Total | 1256 | |
Appendix 4. Main database search strategies for 2014 update
CENTRAL (years 2009 to 2013 searched)
#1 MeSH descriptor: [Pain] explode all trees
#2 (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint" or "temperomandib* joint" or "tempromandib* joint" or central or (post next stroke) or complex or regional or "spinal cord") near/4 pain*:ti,ab,kw (Word variations have been searched)
#3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* near/2 neuralg*) or (herp* near/2 neuralg*) or (diabet* near/2 neuropath*) or (reflex near/4 dystroph*) or (sudeck* near/2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back near/4 surg*) or (failed back near/4 syndrome*)):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Transcranial Magnetic Stimulation] this term only
#6 MeSH descriptor: [Electronarcosis] explode all trees
#7 (brain* or cortex or cortical or transcranial* or cranial or magneti*) near/4 stimulat*:ti,ab,kw (Word variations have been searched)
#8 (transcrani* or crani* or brain*) near/4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*):ti,ab,kw (Word variations have been searched)
#9 (non‐invasive or non*invasive) near/4 stimulat*:ti,ab,kw (Word variations have been searched)
#10 "theta burst stimulat*" or iTBS or cTBS:ti,ab,kw (Word variations have been searched)
#11 "transcranial magnetic stimulation" or rTMS or "transcranial direct current stimulat*" or tDCS or "cranial electrostimulation" or "cranial electrotherap*":ti,ab,kw (Word variations have been searched)
#12 (electrosleep* or electronarco*):ti,ab,kw (Word variations have been searched)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 #4 and #13 from 2009 to 2013
MEDLINE and MEDLINE IN PROCESS (OVID)
1 exp Pain/ (283010)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (74023)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (28679)
4 or/1‐3 (325946)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (6328)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (25872)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (147)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (822)
9 (theta burst stimulat* or iTBS or cTBS).tw. (575)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7423)
11 (electrosleep or electronarco*).tw. (357)
12 or/5‐11 (28316)
13 randomized controlled trial.pt. (337806)
14 controlled clinical trial.pt. (84996)
15 randomized.ab. (241501)
16 placebo.ab. (134421)
17 drug therapy.fs. (1571905)
18 randomly.ab. (173459)
19 trial.ab. (248492)
20 groups.ab. (1134392)
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (2928552)
22 exp animals/ not humans.sh. (3751730)
23 21 not 22 (2487755)
24 4 and 12 and 23 (295)
25 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).ed. (2428299)
26 24 and 25 (112)
Embase (OVID)
1 exp Pain/ (729490)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (112128)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (41462)
4 or/1‐3 (759765)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (11875)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (35587)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (194)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (1314)
9 (theta burst stimulat* or iTBS or cTBS).tw. (770)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (10413)
11 (electrosleep or electronarco*).tw. (375)
12 or/5‐11 (39959)
13 4 and 12 (3078)
14 random$.tw. (793677)
15 factorial$.tw. (20700)
16 crossover$.tw. (46383)
17 cross over$.tw. (21096)
18 cross‐over$.tw. (21096)
19 placebo$.tw. (189884)
20 (doubl$ adj blind$).tw. (140353)
21 (singl$ adj blind$).tw. (13272)
22 assign$.tw. (220119)
23 allocat$.tw. (74677)
24 volunteer$.tw. (170305)
25 Crossover Procedure/ (36109)
26 double‐blind procedure.tw. (224)
27 Randomized Controlled Trial/ (338884)
28 Single Blind Procedure/ (16955)
29 or/14‐28 (1300700)
30 (animal/ or nonhuman/) not human/ (4566449)
31 29 not 30 (1146950)
32 13 and 31 (574)
33 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).dd. (4384183)
34 32 and 33 (303)
PsycINFO (OVID)
1 exp Pain/ (33859)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (17914)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (3654)
4 or/1‐3 (39372)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (3412)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (9508)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (55)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (401)
9 (theta burst stimulat* or iTBS or cTBS).tw. (441)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (4745)
11 (electrosleep or electronarco*).tw. (6)
12 or/5‐11 (9914)
13 4 and 12 (481)
14 clinical trials/ (6486)
15 (randomis* or randomiz*).tw. (39676)
16 (random$ adj3 (allocat$ or assign$)).tw. (22629)
17 ((clinic$ or control$) adj trial$).tw. (33763)
18 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (15332)
19 (crossover$ or "cross over$").tw. (5478)
20 random sampling/ (445)
21 Experiment Controls/ (435)
22 Placebo/ (2892)
23 placebo$.tw. (23869)
24 exp program evaluation/ (12521)
25 treatment effectiveness evaluation/ (11860)
26 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (45199)
27 or/14‐26 (142131)
28 13 and 27 (95)
29 limit 28 to yr="2009 ‐Current" (60)
CINAHL (EBSCO)
S26 S25 Limiters ‐ Published Date from: 20091101‐20130231
S25 S15 AND S24
S24 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 (allocat* random*)
S22 (MH "Quantitative Studies")
S21 (MH "Placebos")
S20 placebo*
S19 (random* allocat*)
S18 (MH "Random Assignment")
S17 (Randomi?ed control* trial*)
S16 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S15 S4 AND S14
S14 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 TI ( (electrosleep OR electronarco*) ) OR AB ( (electrosleep OR electronarco*) )
S12 TI ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") ) OR AB ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") )
S11 TI ( ("theta burst stimulat*" OR iTBS OR cTBS) ) OR AB ( ("theta burst stimulat*" OR iTBS OR cTBS) )
S10 TI ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) ) OR AB ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) )
S9 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S8 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S7 TI ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) ) OR AB ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) )
S6 (MH "Electric Stimulation")
S5 (MH "Electronarcosis")
S4 S1 OR S2 OR S3
S3 TI ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") ) OR AB ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") )
S2 TI ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*). ) OR AB ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*))
S1 (MH "Pain+")
LILACS (7 February 2013)
1. (chronic$ or back or musculoskel$ or intractabl$ or neuropath$ or phantom limb or fantom limb or neck or myofasc$ or temporomandib$ or temperomandib$ or tempromandib$ or central or (post stroke) or complex or regional or spinal cord sciatica or back‐ache or back ache or lumbago or fibromyalg$ or trigemin$ neuralg$ or herp$ neuralg$ or diabet$ neuropath$ or reflex dystroph$ or sudeck$ atrophy$ or causalg$ or whip‐lash or whip$lash or polymyalg$ or failed back) 69863
2. (brain$ or cortex or cortical or transcrani$ or cranial or magneti$ stimulat$ or electrostim$ or electro‐stim$ or electrotherapy$ or electro‐therap$ or non‐invasive or non invasive or stimul$ or theta burst stimulat$ or iTBS or cTBS or transcranial magnetic stimulat$ or rTMS or transcranial direct current stimulat$ or tDCS or cranial electrostimulation or cranial electrotherapy$ or electrosleep$ or electronarco$) 24787
3. 1&2 5559
4. (randomized controlled trial or controlled clinical trial or placebo or sham or randomly or trial or groups) 31227
5. 3&4 545
6. REMOVE ANY PRE 2009 (removed 292) 253
Appendix 5. Trials register search results for 2014 update
| Register | Date of search | Search terms | Number of records | Number of relevant records |
| NRR archive | 7 February 2013 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp*romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) al fields AND (2009 OR 2010 OR 2011 OR 2012 OR 2013) date started | 2 | 0 |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies
CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago
INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs
OUTCOME: pain 01/01/2009 to 07/02/2013 adult | 89 | 10 |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies
CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago
INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*
OUTCOME: pain | 20 | |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies
CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome
INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs
OUTCOME: pain
| 2 | |
| Clinical trials.gov | 7 February 2013 |
Field ‐ Interventional studies
CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome
INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*
OUTCOME: pain | 0 | |
| HSRProj | 11 February 2013 | ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*)) | 152 | 0 |
| Current controlled trials (excl clinicatrials.gov) | 11 February 2013 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (cranial electrotherapy OR electrosleep OR electronarco*) | 0 | 1 |
| Current controlled trials (excl clinicatrials.gov) | 11 February 2013 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation) | 0 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | TRANSCRANIAL and PAIN | 1 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | CRANIAL AND PAIN | 4 | |
| Current controlled trials (excl clinicatrials.gov) | 25/2/13 | STIMULATION AND PAIN | 75 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | (Cortex or cortical) and pain | 8 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | Brain and pain | 33 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | (Electro or electrical) and pain | 46 | |
| Total current controlled trials | 25 February 2013 |
| 167 | |
| Total relevant trial records, all databases | 11 | |||
Appendix 6. Search results summary table for 2014 update
| Database searched | Date searched | Number of results |
| CENTRAL Issue 6 of 12, 2013 (The Cochrane Library) | 24 July 2013 | 2 |
| MEDLINE (OVID) June 2013 to 19/7/2013 MEDLINE In Process (OVID) – current week | 24 July 2013 24 July 2013 | 5 19 |
| Embase (OVID) June 2013 to 2013 week 29 | 24 July 2013 | 8 |
| PsycINFO (OVID) June 2013 to July week 3 2013 | 24 July 2013 | 1 |
| CINAHL (EBSCO) June 2013 to July 2013 | 24 July 2013 | 4 |
| Total | 39 | |
| After de‐duplication | 35 | |
| After title abstract screening | 0 | |
| After expert checking | 2 | |
Appendix 7. Full list of searches and results for 2009 version of review
1. Cochrane PaPaS Group Specialised Register, saved search: 177 results
“electric* stimulat* therap*” or “brain* stimulat*” or “cort* stimulat*” or “transcranial* stimulat*” or “cranial stimulat*” or “magneti* stimulat*” or “direct current stimulat*” or “electric* stimulat*” or electrostim* or electrotherapy* or electro‐therap* or “theta burst stimulat*” or “transcran* magnet* stimulat*” or iTBS or cTBS or rTMS or “transcran* direct current stimulat*” or tDCS or electrosleep or electronarco*
2. CENTRAL in The Cochrane Library
| #1 | 25049 | |
| #2 | 7785 | |
| #3 | 3040 | |
| #4 | 30353 | |
| #5 | MeSH descriptor Transcranial Magnetic Stimulation explode all trees | 328 |
| #6 | 34 | |
| #7 | (brain* or cortex or cortical or transcranial* or cranial or magneti*) near/4 stimulat*:ti,ab,kw | 1388 |
| #8 | 45 | |
| #9 | 55 | |
| #10 | 9 | |
| #11 | 747 | |
| #12 | 45 | |
| #13 | 1505 | |
| #14 | 106 |
3a. MEDLINE
Database: Ovid MEDLINE(R) <1950 to November Week 3 2009>
1 exp Pain/ (252061)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (61945)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (25802)
4 1 or 3 or 2 (288507)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (4240)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (21248)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (116)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (526)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (359)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (5306)
11 (electrosleep or electronarco*).ab,ti. (357)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (23212)
13 4 and 12 (1069)
14 randomised controlled trial.pt. (291031)
15 controlled clinical trial.pt. (82962)
16 randomized.ab. (196258)
17 (placebo or sham).ab,ti. (164609)
18 drug therapy.fs. (1385685)
19 randomly.ab. (141449)
20 trial.ab. (203139)
21 groups.ab. (961704)
22 or/14‐21 (2562312)
23 exp animals/ not humans.sh. (3518581)
24 22 not 23 (2157467)
25 24 and 13 (219)
3b. Database: Ovid MEDLINE(R) In‐process & Other non‐indexed citations
<25 November 2009>
1 exp Pain/ (6)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (4772)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (1251)
4 1 or 3 or 2 (5661)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (0)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (1057)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (5)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (42)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (38)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (375)
11 (electrosleep or electronarco*).ab,ti. (0)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (1113)
13 4 and 12 (39)
4. Database: Embase
<1980 to 2009 Week 47>
1 exp Pain/ (394924)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (57196)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (21356)
4 1 or 3 or 2 (410258)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (5841)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (18227)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (74)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (498)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (330)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (5259)
11 (electrosleep or electronarco*).ab,ti. (20)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (19954)
13 4 and 12 (1331)
14 random*.ti,ab. (415216)
15 factorial*.ti,ab. (8708)
16 (crossover* or cross over* or cross‐over*).ti,ab. (40788)
17 placebo*.ti,ab. (114266)
18 (doubl* adj blind*).ti,ab. (87525)
19 (singl* adj blind*).ti,ab. (7775)
20 assign*.ti,ab. (113729)
21 allocat*.ti,ab. (36179)
22 volunteer*.ti,ab. (102464)
23 CROSSOVER PROCEDURE.sh. (21985)
24 DOUBLE‐BLIND PROCEDURE.sh. (74829)
25 RANDOMIZED CONTROLLED TRIAL.sh. (176320)
26 SINGLE BLIND PROCEDURE.sh. (8721)
27 or/14‐26 (691134)
28 ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ (3551150)
29 HUMAN/ (6702208)
30 28 and 29 (569432)
31 28 not 30 (2981718)
32 27 not 31 (601828)
33 32 and 13 (234)
5. Database: PsycINFO
<1806 to November Week 4 2009>
1 exp Pain/ (26560)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib* joint or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (14094)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (2649)
4 1 or 3 or 2 (30822)
5 Transcranial Magnetic Stimulation/ or Electrosleep treatment/ (1830)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (7832)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (47)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (144)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (259)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (2652)
11 (electrosleep or electronarco*).ab,ti. (140)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (8307)
13 4 and 12 (277)
14 (random* or placebo* or sham or trial or groups).ti,ab. (391590)
15 13 and 14 (64)
6. CINAHL
<Search run 11 January 2010>
| 1 | exp PAIN/ | |
| 2 | ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*).ti,ab | |
| 3 | (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*").ti,ab | |
| 4 | 1 OR 2 OR 3 | |
| 5 | ELECTRONARCOSIS/ | |
| 6 | ELECTRIC STIMULATION/ | |
| 7 | ((brain* OR cortex OR cortical OR transcranial* OR cranial OR "magneti*) AND stimulat*).ti,ab | |
| 8 | ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)).ti,ab | |
| 9 | (("non‐invasive brain" OR "non*invasive brain") AND stimulat*).ti,ab | |
| 10 | ("theta burst stimulat*" OR iTBS OR cTBS).ti,ab | |
| 11 | ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy").ti,ab | |
| 12 | (electrosleep OR electronarco*).ti,ab | |
| 13 | 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 | |
| 14 | 4 AND 13 | |
| 15 | exp CLINICAL TRIALS/ | |
| 16 | (clinical AND trial*).af | |
| 17 | ((singl* OR doubl* OR trebl* OR tripl*) AND (blind* OR mask*)).ti,ab | |
| 18 | (Randomi?ed AND control* AND trial*).af | |
| 19 | RANDOM ASSIGNMENT/ | |
| 20 | (Random* AND allocat*).ti,ab | |
| 21 | placebo*.af | |
| 22 | PLACEBOS/ | |
| 23 | QUANTITATIVE STUDIES/ | |
| 24 | 15 OR 16 OR17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 | |
| 25 | 14 AND 24 |
7. SCOPUS
We did not search this database as it includes all of MEDLINE, all of Embase and some of CINAHL, which have been searched separately.
8. Search strategy for LILACS
http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/
1. Pain$ or dolor$ or intractabl$ or neuropath$ or phantom or fantom or myofasc$ or temp$romandibular or sciatic$ or back‐ache or backache or ache or lumbago or fibromyalg$ or neuralg$ or dystroph$ or atroph$ or causalgi$ or whip‐lash or whiplash or polymyalg$ [Words]
2. ((Estimulaci$ or stimulat$) and (cerebra$ or brain$ or cortex or cortical or crania$ or transcranial$ or magneti$)) or electrostim$ or electrotherapy$ or electro‐therap$ or “theta burst stimul$” or iTBS or Ctbs or “transcrani$ magnet$ stimulat$” or rTMS or “transcrani$ direct current stimulat$” or tDCS or “cranial electrostimulat$” or “cranial electrotherapy$ or electrosleep or electronarco$ [Words]
3. ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
4. 1 and 2 and 3 (68)
Appendix 8. Trials register search results for 2009 version of review
| Database | Date of search | Search strategy | No. hits | Agreed potential studies |
| National Research Register (NRR) Archive (NIHR) | 23 October 2009 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) IN “TITLE” Field | 366 | 2 |
| Clinicaltrials.gov | 23 October 2009 Search 1 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 62 |
|
| Clinicaltrials.gov | 23 October 2009 Search 2 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 8 (all also picked up in search 1) |
|
| Clinicaltrials.gov | 23 October 2009 Search 3 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain | 0 |
|
| Clinicaltrials.gov | 23 October 2009 Search 4 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain | 0 |
|
|
|
| TOTAL UNIQUE RESULTS FOR CLINICAL TRIALS.GOV | 62 | 7 |
| HSRProj (Health Services Research Projects in Progress) | 23 October 2009 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) | 77 | 0 |
| Current Controlled Trials | 23 October 2009 Search 1 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (cranial electrotherapy OR electrosleep OR electronarco*) | 0 |
|
| Current Controlled Trials | 23 October 2009 Search 2 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation) | 0 |
|
| Current Controlled Trials | 23 October 2009 Search 3 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 4 |
|
| Current Controlled Trials | 23 October 2009 Search 4 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC) | 13 |
|
| Current Controlled Trials | 23 October 2009 Search 5 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 |
|
| Current Controlled Trials | 23 October 2009 Search 6 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS ) | 9 |
|
| Current Controlled Trials | 3 November 2009 Search 7 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap*) | 36 |
|
| Current Controlled Trials | 23 October 2009 Search 8 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 53 |
|
| Current Controlled Trials | 3 November 2009 Search 9 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (cranial OR magneti* OR direct current OR DC) | 52 |
|
| Current Controlled Trials | 3 November 2009 Search 10 | (back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (brain* OR cortex OR cortical OR transcranial*) | 63 |
|
| Current Controlled Trials | 3 November 2009 Search 11 | (temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 |
|
| Current Controlled Trials | 3 November 2009 Search 12 | (temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (transcranial direct current stimulation OR tDCS) | 11 |
|
| Current Controlled Trials | 3 November 2009 Search 13 | (central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (iTBS OR cTBS OR transcranial magnetic stimulation OR rTMS) | 48 |
|
| Current Controlled Trials | 3 November 2009 Search 14 | (central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat*) | 199 |
|
| Current Controlled Trials | 3 November 2009 Search 15 | (central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR crani* OR electrostim*) | 1905 |
|
| Current Controlled Trials | 3 November 2009 Search 16 | (temp?romandib joint) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap*) | 0 |
|
| Current Controlled Trials | 3 November 2009 Search 17 | (temp?romandib joint) AND (iTBS OR cTBS OR transcranial magnetic stimulation OR rTMS) | 0 |
|
| Current Controlled Trials | 3 November 2009 Search 18 | (temp?romandib joint) AND (non‐invasive OR non*invasive OR theta burst stimulat*) | 0 |
|
| Current Controlled Trials | 3 November 2009 Search 19 | (chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 16 |
|
| Current Controlled Trials | 3 November 2009 Search 20 | (chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (Ctbs OR transcranial magnetic stimulation OR Rtms)
| 55 |
|
| Current Controlled Trials | 3 November 2009 Search 21 | (chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 557 |
|
| Current Controlled Trials | 3 November 2009 Search 22 | (chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC)
| 2385 |
|
| Current Controlled Trials | 3 November 2009 Search 23 | (temp*romandibular joint) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap*) | 8 |
|
| Current Controlled Trials | 3 November 2009 Search 24 | (temp*romandibular joint) AND (electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OR transcranial magnetic stimulation) | 1 |
|
| Current Controlled Trials | 3 November 2009 Search 25 | (temp*romandibular joint) AND (rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 |
|
|
|
| TOTAL RESULTS FOR CURRENT CONTROLLED TRIALS | 5415 | 14 |
|
|
| TOTAL RESULTS FROM ALL DATABASES |
| 23 |
|
|
| DUPLICATES BETWEEN DATABASES |
| 7 |
|
|
| FINAL TOTAL FROM TRIALS REGISTERS SEARCHES |
| 16 |

Study flow diagram

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.1. Pain: short‐term follow‐up

Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.5. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only
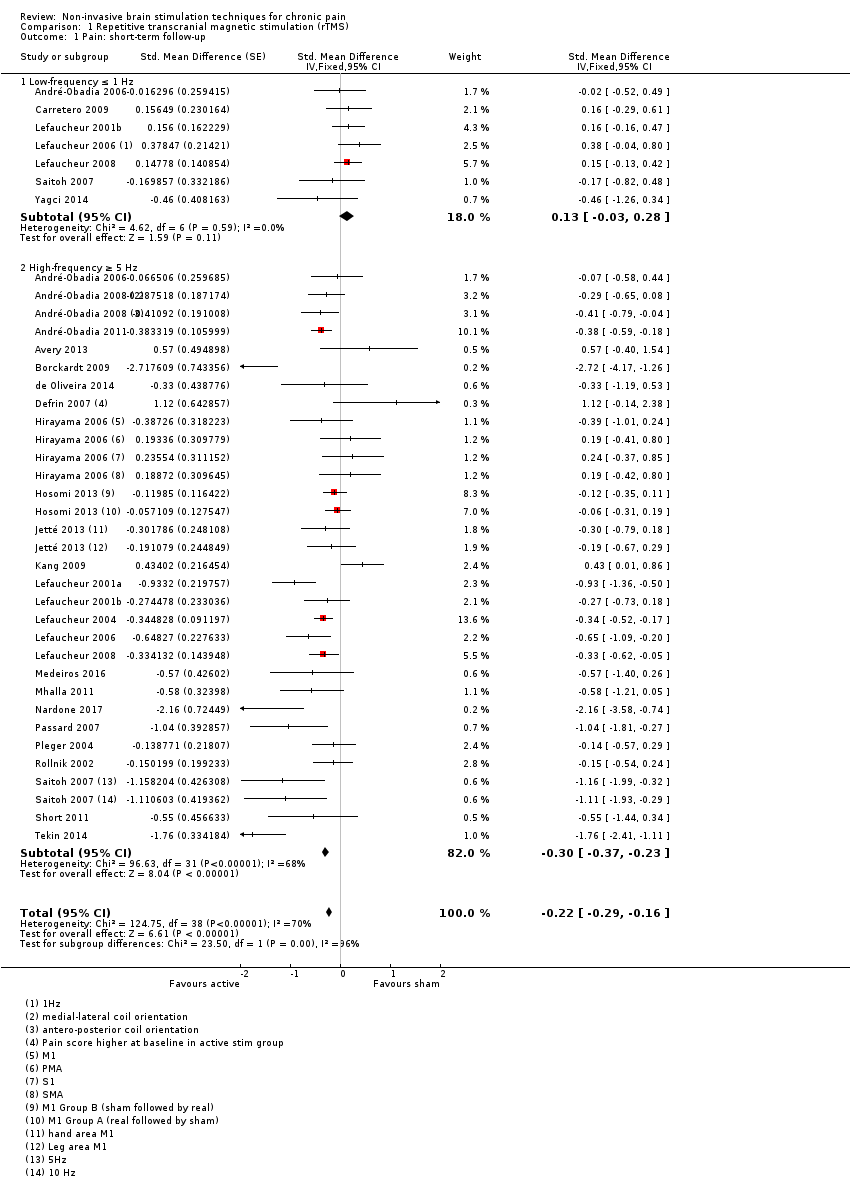
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 1 Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only.
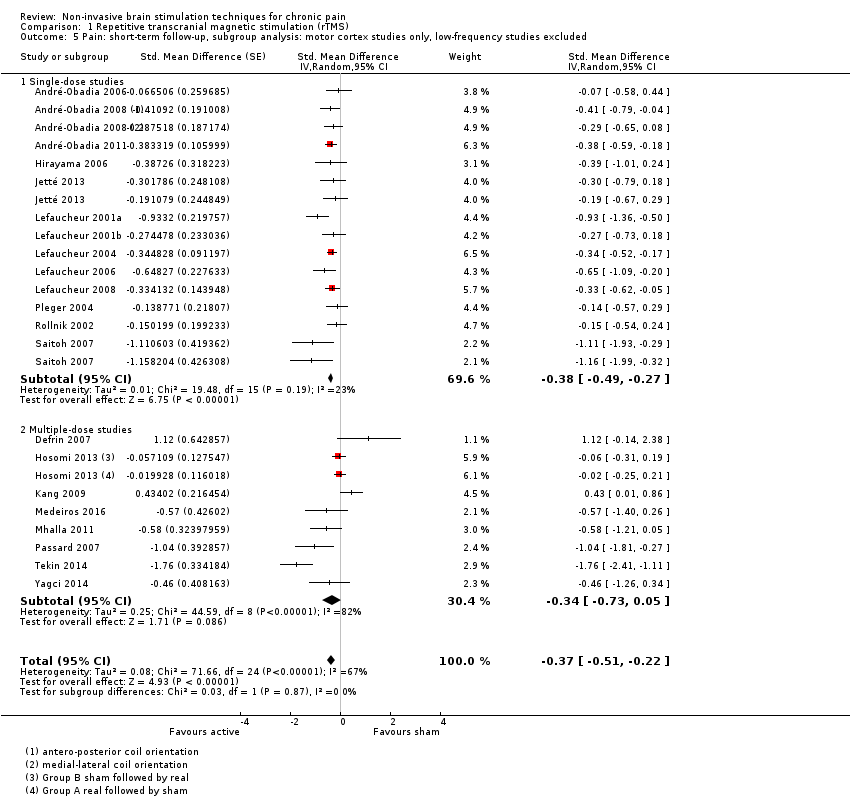
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
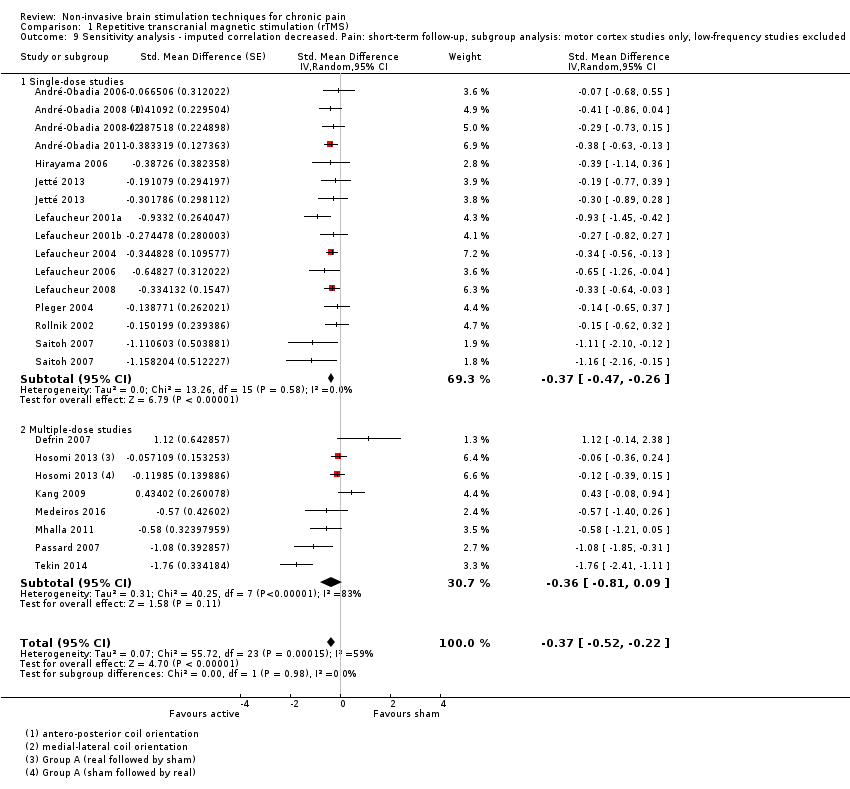
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
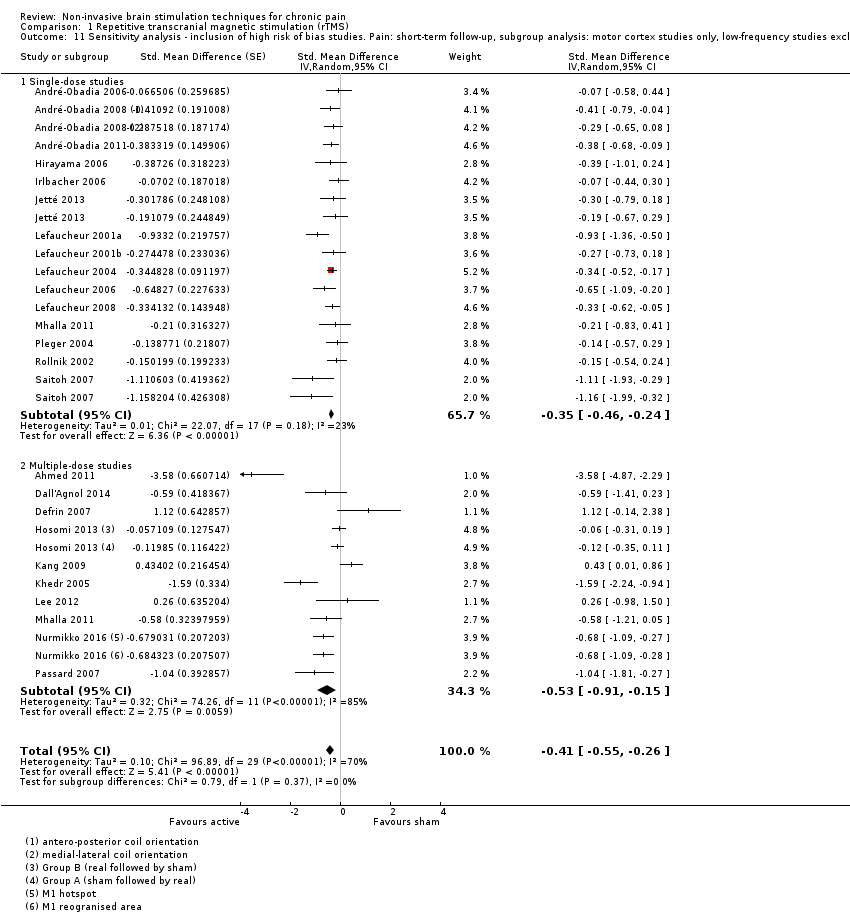
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 14 Pain: short term responder analysis 30% pain reduction.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 16 Pain: medium‐term follow‐up.
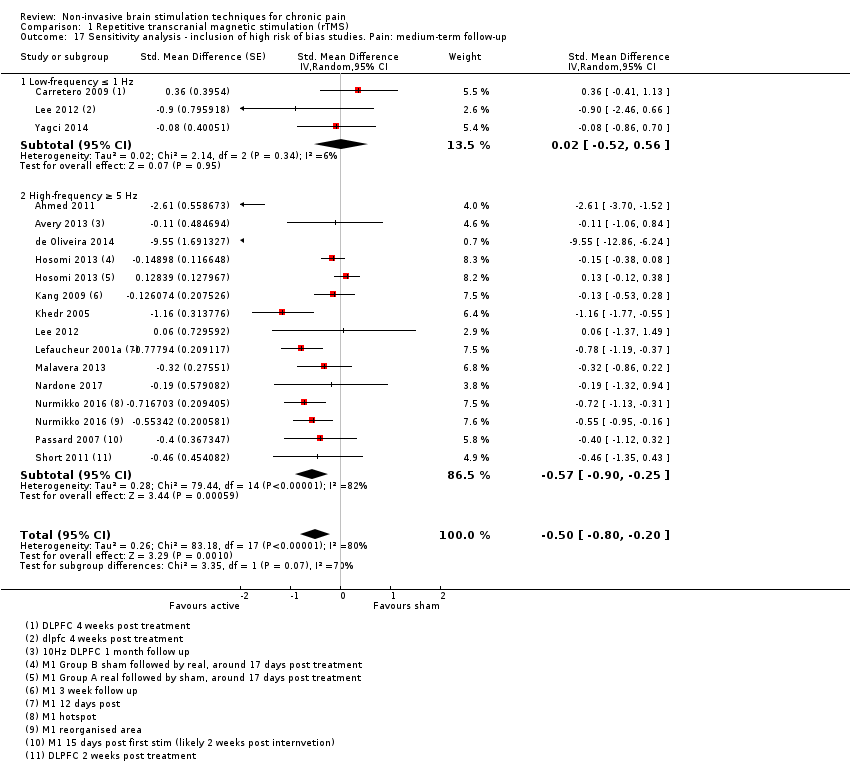
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only.
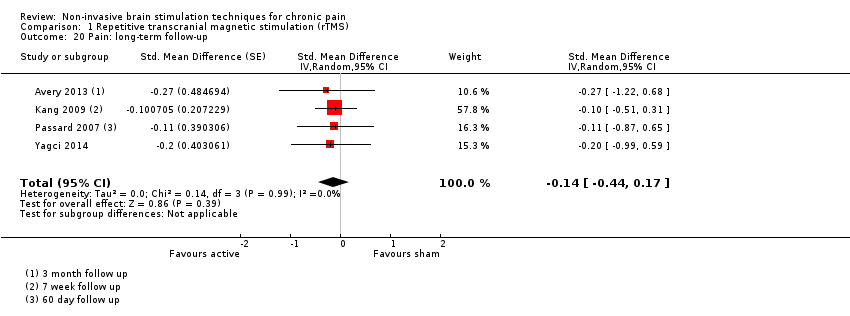
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 20 Pain: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 22 Disability: short‐term follow‐up.
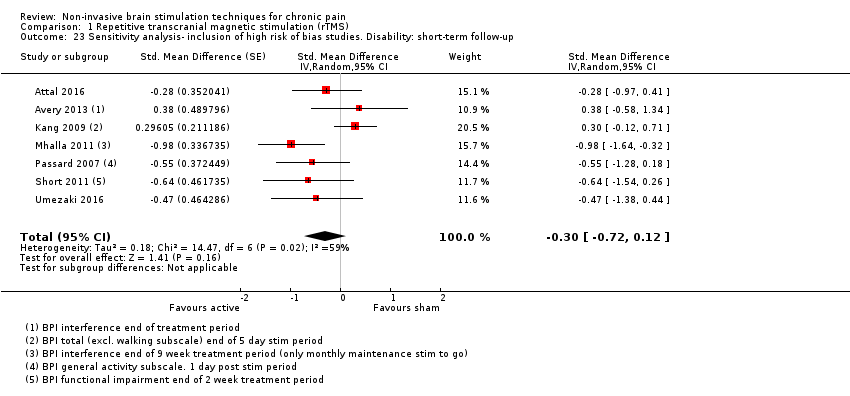
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 24 Disability: medium‐term follow‐up.
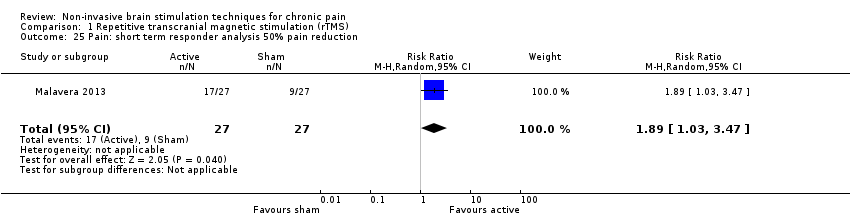
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 25 Pain: short term responder analysis 50% pain reduction.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 26 Disability: long‐term follow‐up.
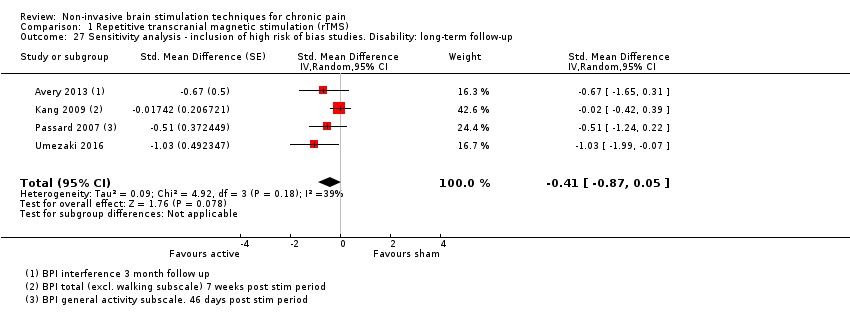
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up.

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).

Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 31 Quality of life: long‐term follow‐up.
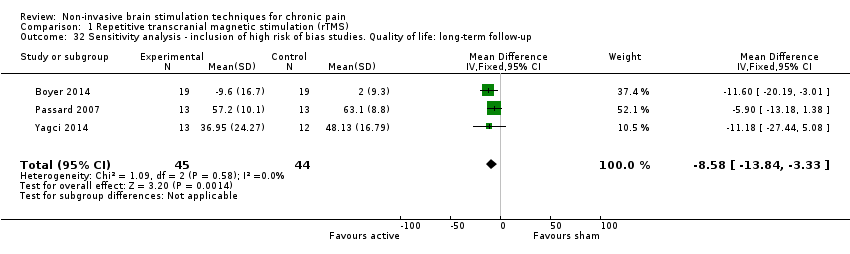
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up.
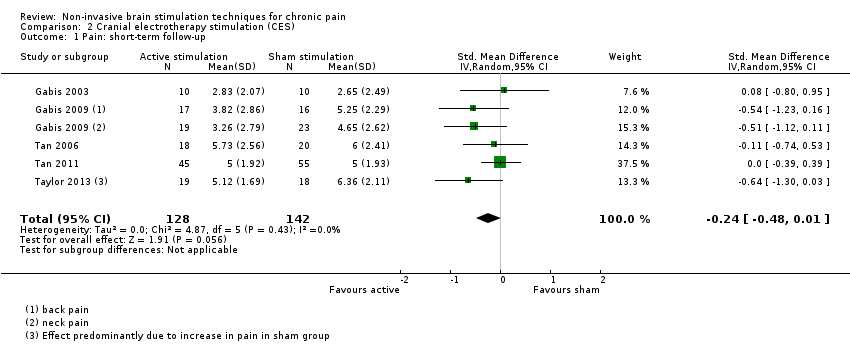
Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 1 Pain: short‐term follow‐up.

Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 2 Quality of life: short term follow up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 1 Pain: short‐term follow‐up.
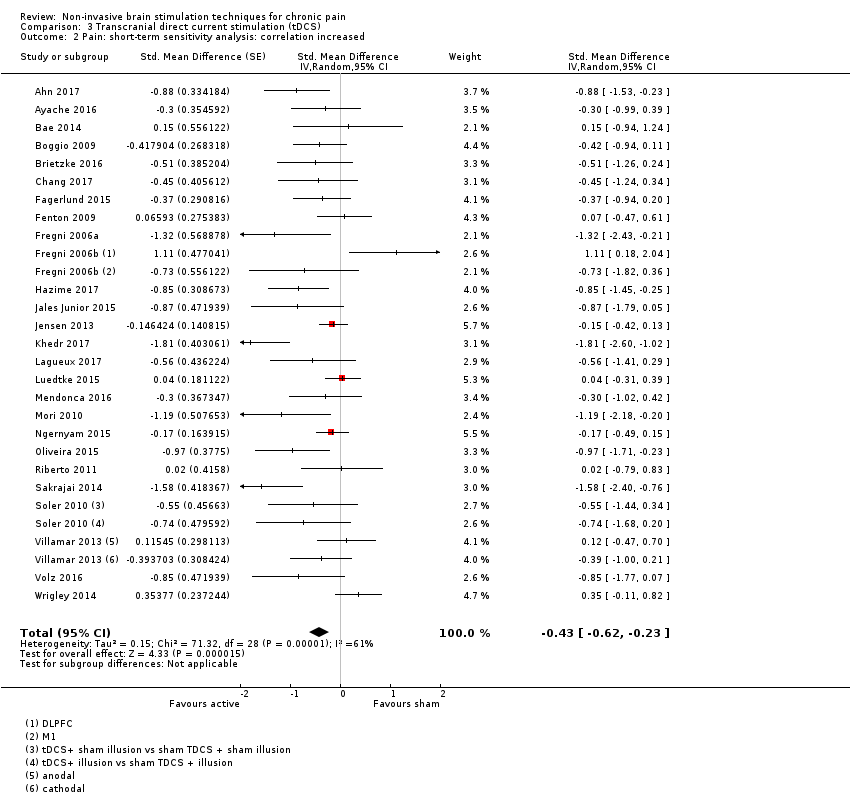
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 2 Pain: short‐term sensitivity analysis: correlation increased.
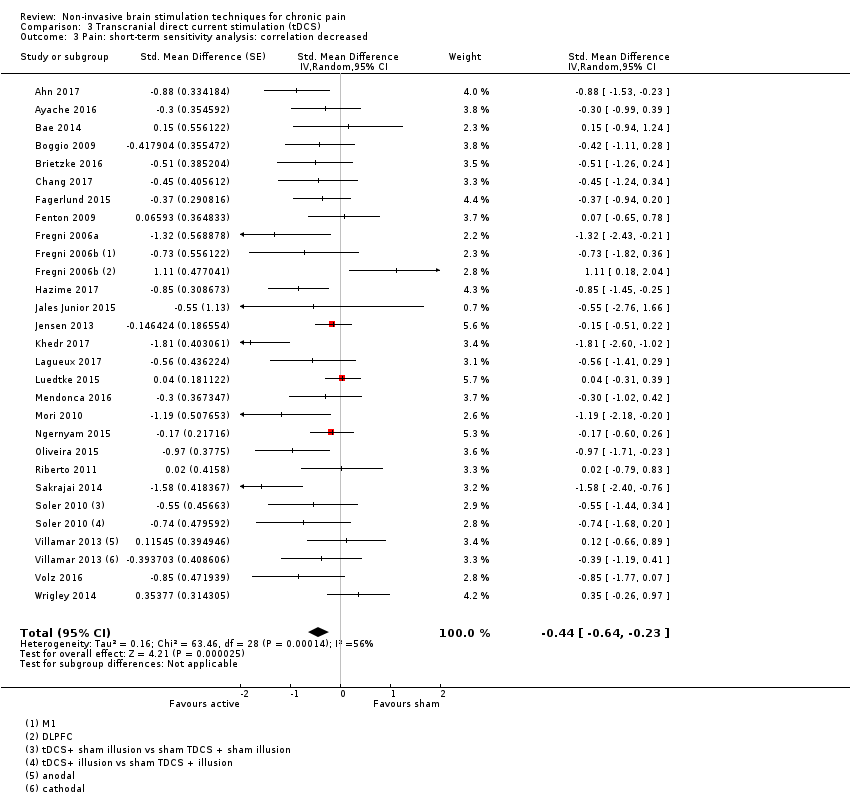
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 3 Pain: short‐term sensitivity analysis: correlation decreased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 9 Pain: short term follow‐up responder analysis 30% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 10 Pain: short term follow‐up responder analysis 50% pain reduction.
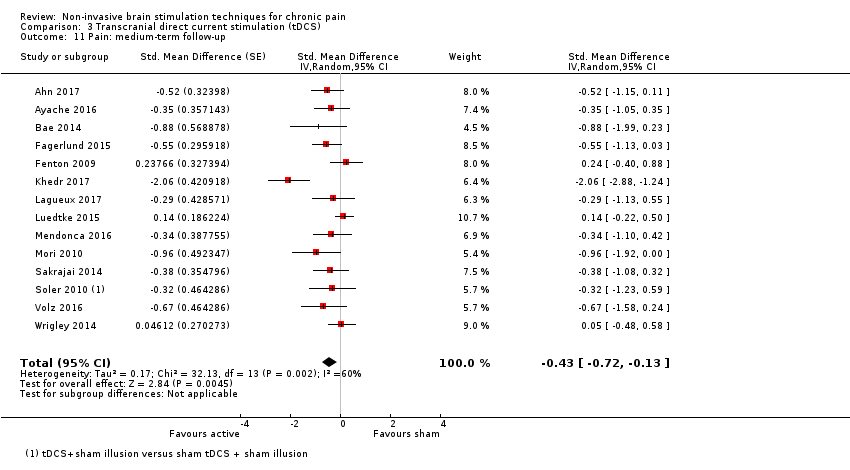
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 11 Pain: medium‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 12 Pain: medium term follow‐up responder analysis 30% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 13 Pain: medium term follow‐up responder analysis 50% pain reduction.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 15 Pain: long‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 16 Disability: short‐term follow‐up.

Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 17 Disability: medium‐term follow‐up.
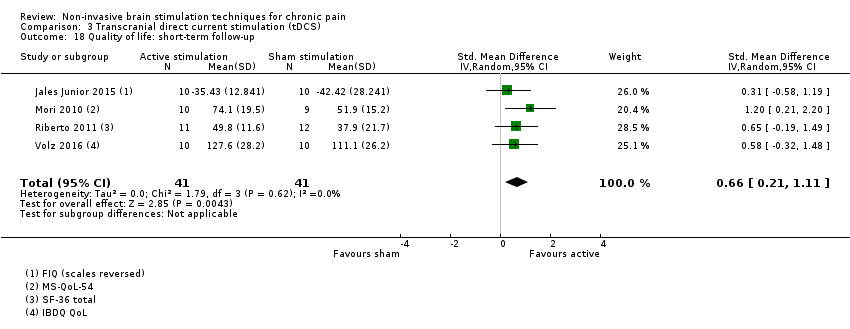
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 18 Quality of life: short‐term follow‐up.
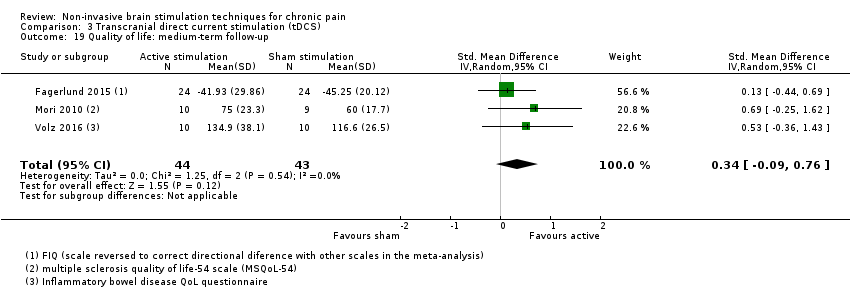
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 19 Quality of life: medium‐term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 1 Pain: short‐term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
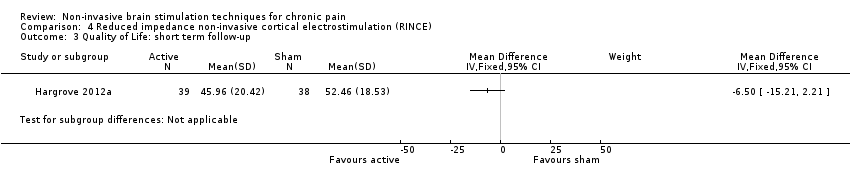
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 3 Quality of Life: short term follow‐up.

Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up.

Comparison 5 Transcranial random noise stimulation, Outcome 1 Pain.
| rTMS compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active rTMS Comparison: sham rTMS | ||||
| Outcomes | Effect size | Relative and absolute effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.22 (‐0.29 to ‐0.16) | This equates to a 7% (95% CI 5% to 9%) reduction in pain intensity, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale. | 655 (27) | ⊕⊕⊝⊝ low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | SMD ‐0.29, 95% CI ‐0.87 to 0.29 | ‐ | 119 (5) | ⊕⊝⊝⊝ very low2 |
| Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire | MD ‐10.80, 95% CI ‐15.04 to ‐6.55 | ‐ | 105 (4) | ⊕⊕⊝⊝ low3 |
| CI: confidence interval; MD: mean difference; rTMS: repetitive transcranial magnetic stimulation; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias and once for inconsistency due to heterogeneity. | ||||
| CES compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active CES Comparison: sham CES | ||||
| Outcomes | Effect size | Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.24 (‐0.48 to 0.01) | ‐ | 270 (5) | ⊕⊕⊝⊝ low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | No data available | No data available | No data available | No data available |
| Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire | MD ‐25.05 (‐37.82 to ‐12.28) | ‐ | 36 (1) | ⊕⊝⊝⊝ very low2 |
| CI: confidence interval; CES: cranial electrotherapy stimulation; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias and once for imprecision due to low participant numbers. | ||||
| tDCS compared with sham for chronic pain | ||||
| Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active tDCS Comparison: sham tDCS | ||||
| Outcomes | Effect size | Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. | No of participants | Quality of the evidence |
| Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales | SMD ‐0.43 (‐0.63 to ‐0.22) | This equates to a 17% (95% CI 9% to 25%) reduction in pain intensity or a 0.82 (95% CI 0.42 to 1.2) point reduction on a 0 to 10 pain intensity scale. | 747 (27) | ⊕⊝⊝⊝ very low1 |
| Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales | SMD ‐0.01, (95% CI ‐0.28 to 0.26) | ‐ | 212 (4) | ⊕⊕⊝⊝ low2 |
| Quality of life (0 to < 1 week postintervention) measured using different scales across studies | SMD 0.66, 95% CI 0.21 to 1.11 | ‐ | 82 (4) | ⊕⊕⊝⊝ low2 |
| CI: confidence interval; MD: mean difference; SMD: standardised mean difference; tDCS: transcranial direct current stimulation | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
| 1Downgraded once for study limitations due to high or unclear risk of bias, once for inconsistency due to heterogeneity and once for evidence of possible publication bias. | ||||
| Study | Location of stimulation | Coil orientation | Frequency (Hz) | Intensity (% RMT) | Number of trains | Duration of trains | Inter‐train intervals (sec) | Number of pulses per session | Treatment sessions per group |
| M1 stump region | 45° angle from sagittal line | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily | |
| M1 contralateral to painful side | Anteroposterior induced current | 10 | 80 | 30 | 10 | 20 | 3000 | 3, x1 daily | |
| M1 contralateral to painful side | Posteroanterior | 20, 1 | 90 | 20 Hz: 20 1 Hz: 1 | 20 Hz: 4 sec 1 Hz: 26 min | 20 Hz: 84 | 1600 | 1 | |
| M1 contralateral to painful side | Posteroanterior Medial‐lateral | 20 | 90 | 20 | 4 sec | 84 | 1600 | 1 | |
| M1 hand area, not clearly reported but likely contralateral to painful side | Not specified | 20 | 90 | 20 | 4 sec | 84 | 1600 | 1 | |
| Left DLPFC | Not specified | 10 | 120 | 75 | 4 | 26 | 3000 | 15 | |
| Left PFC | Not specified | 10 | 100 | 40 | 10 sec | 20 | 4000 | 3 over a 5‐day period | |
| Left M1 | anteroposterior | 10 | 90 | 20 | 10 | 50 | 2000 | 14, 10 sessions in 2 weeks followed by maintenance phase of 1 session at weeks 4, 6, 8, and 10 | |
| Right DLPFC | Not specified | 1 | 110 | 20 | 60 sec | 45 | 1200 | Up to 20 on consecutive working days | |
| Left M1 | 45° angle from sagittal line | 10 | 80 | 16 | 10 | 26 | 1600 | 10, timescale not specified | |
| M1 midline | Not specified | 5 | 115 | 500 | 10 sec | 30 | ? 500* | 10, x 1 daily | |
| Left DLPFC/premotor | not specified | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x 1 daily (working days) for 2 weeks | |
| Left and right SII | Not specified | 1 or 20 | 90 | Not specified | Not specified | Not specified | 1600 | 1 | |
| Right SII | Not specified | 1 | 70% maximum stimulator output intensity (not RMT) | 1 | Not specified | Not specified | 1600 | 10, x 1 daily (weekdays only) | |
| M1, S1, PMA, SMA | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 1 | |
| M1 corresponding to painful region | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 10, x 1 daily (weekdays only) | |
| M1 contralateral to painful side | Not specified | 5, 1 | 95 | Not specified | Not specified | Not specified | 500 | 1 | |
| M1 hand or leg area with neuro navigation | 45º postero‐lateral | 10 | 90 | 40 | 5 | 25 | 2000 | 1, per stimulation condition | |
| Right M1 | 45º postero‐lateral | 10 | 80 | 20 | 5 sec | 55 | 1000 | 5, x 1 daily | |
| M1 contralateral to painful side | Not specified | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily | |
| Right DLPFC (low‐frequency) Left M1 (high‐frequency) | Not specified | 10, 1 | 10 Hz: 80 1 Hz: 110 | 10 Hz: 25 1 Hz: 2 | 10 Hz: 8 sec 1 Hz: 800 sec | 10 Hz: 10 1 Hz: 60 | 10 Hz: 2000 1 Hz: 1600 | 10, x 1 daily (weekdays only) | |
| M1 contralateral to painful side | Not specified | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 0.5 | 80 | 10 Hz: 20 0.5 Hz: 1 | 10 Hz: 5 sec 0.5 Hz: 20 min | 10 Hz: 55 | 10 Hz: 1000 0.5 Hz: 600 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 | 10 Hz: 6 sec 1 Hz: 20 min | 10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 | 1 | |
| M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 | 10 Hz: 6 sec 1 Hz: 20 min | 10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 | 1 | |
| M1 contralateral to painful side | 45° angle from sagittal line | 10 | 90 | 20 | 6 | 54 | 1200 | 10, x 1 daily (weekdays only) | |
| Left M1 | 45° angle from sagittal line | 10 | 80 | not reported | not reported | not reported | 1600 | 10, x 1 daily | |
| Left M1 | Posteroanterior | 10 | 80 | 15 | 10 sec | 50 | 1500 | 14, 5 x 1 daily (working days), then 3 x 1 weekly, then 3 x 1 fortnightly, then 3 x 1 monthly | |
| Left PFC | Posteroanterior | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x5 per week for 2 weeks | |
| M1 hotspot contralateral to pain M1 in reorganised area contralateral to pain | Posteroanterior | 10 | 90 | 20 | 10 sec | 60 | 2000 | 5, x 3‐5 times per week | |
| M1 deep central sulcus | H‐coil | 20 | 100 | 30 | 2.5 sec | 30 | 1500 | 5, x 1 daily on consecutive days | |
| M1 contralateral to painful side | Posteroanterior | 10 | 80 | 25 | 8 sec | 52 | 2000 | 10, x 1 daily (working days) | |
| M1 contralateral to painful side | Posteroanterior | 10 | 100 | 25 | 10 sec | 60 | 2500 | 10, x 1 daily (working days) | |
| M1 hand area | Not specified | 10 | 110 | 10 | 1.2 sec | 10 | 120 | 1 | |
| M1 midline | Not specified | 20 | 80 | 20 | 2 sec | Not specified | 800 | 1 | |
| M1 over motor representation of painful area | Not specified | 10, 5, 1 | 90 | 10 Hz; 5 5 Hz: 10 1 Hz: 1 | 10 Hz: 10 sec 5 Hz: 10 sec 1 Hz: 500 sec | 10 Hz: 50 5 Hz: 50 | 500 | 1 | |
| Left DLPFC | Parasagittal | 10 | 120 | 80 | 5 sec | 10 sec | 4000 | 10, x 1 daily (working days) for 2 weeks | |
| M1 midline | 45° angle from sagittal line | 10 | 100 | 30 | 5 | 12 | 1500 | 10, x 1 daily (not clear if only work days) | |
| Targeted to ACC | 4‐coil configuration | 1 Hz (10 Hz data excluded as not randomised) | 110 | Not reported | Not reported | Not reported | 1800 | 20, x 1 daily (working days) | |
| Left DLPFC | Not specified | 10 | 100 | 10 | 5 | 10 | 3000 | 10, x1 daily (working days) | |
| Left M1 | Not specified | 1 | 90 | 20 | 60 | 45 | 1200 | 10, x1 daily (working days) | |
| M1 midline | Handle pointing posteriorly | 10 | 10 | 30 | 5 | 25 | 1500 | 10, x1 daily (working days) | |
| ACC: anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; M1: primary motor cortex; PFC: prefrontal cortex; PMA: pre‐motor area; RMT: resting motor threshold; dS1: primary somatosensory cortex; SII: secondary somatosensory cortex; SMA: supplementary motor area *Inconsistency between stimulation parameters and reported total number of pulses in study report. See Included studies section for mored detail. | |||||||||
| Study | Electrode placement | Frequency (Hz) | Pulse width (ms) | Waveform shape | Intensity | Duration (min) | Treatment sessions per group |
| Ear clip electrodes | 10 | 2 | Not specified | 12 μA | 53 | x 2 daily for 4 days | |
| Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | ? daily for 3 weeks | |
| Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days | |
| Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days | |
| Mastoid processes and forehead | Not specified | Not specified | 2 conditions: symmetric, asymmetric | 11 to 15 mA | 40 | x 1 daily for 5 days | |
| Ear clip electrodes | 0.5 | Not specified | Biphasic square wave | 100 μA | 60 | x 1 daily for 30 days | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 40 | x 1 daily for 6 weeks | |
| Ear clip electrodes | 0.5 | Not specified | Not specified | 10 to 600 μA | 20 | 12 (timing not specified) | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 to 500 μA | 60 | x 1 daily for 21 days | |
| Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 60 | x 1 daily for 21 days | |
| Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | x 1 daily for 8 weeks |
| Study | Location of stimulation (Anode) | Electrode pad size | Intensity (mA) | Anodal or cathodal? | Stimulus duration (min) | Treatment sessions per group |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 left hand area | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily | |
| Left DLPFC | 25 cm2 | 2mA | Anodal | 20 | 3, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 3 per week for 3 weeks | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 30 | 1 | |
| Left M1 | 25‐35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 16, x 2 weekly for 8 weeks | |
| M1 contralateral to painful side | HD‐tDCS | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1, side not specified | 35 cm2 | 2mA | Anodal | 20 | 5, x 1 daily | |
| M1 dominant hemisphere | 35 cm2 | 1 mA | Anodal | 20 | 2 | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 40 cm2 | 1mA | Anodal | 20 | Daily, self‐administered for 14 days | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 12, x 3 per week for 4 weeks | |
| Left M1 | 15 cm2 | 1mA | Anodal | 20 | x 1 weekly for 10 weeks | |
| M1 left | 35cm2 | 2 mA | Anodal | 20 | 1 | |
| M1 contralateral to painful side | 24 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily, 5 days per week for 2 weeks | |
| M1, side not specified DLPFC | 25 cm2 | 2mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 14, x 5 weekly for 2 weeks, x 1 weekly for 4 weeks | |
| M1 left side not specified | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| Group 1: anodal left M1 Group 2: cathodal left M1 Group 3: anodal supraorbital Group 4: cathodal supraorbital Group 5: sham | 35 cm2 | 2 mA | Anodal or cathodal | 20 | 1 | |
| Left M1 | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 1 | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily, then x 2 weekly for 3 weeks, up to 10 sessions | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 1 per condition | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 weekly | |
| M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily (weekdays only) | |
| Left M1 | 25 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 left | HD‐tDCS 4 x 1‐ring montage | 2 mA | Anodal or cathodal | 20 | x 1 per condition | |
| M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily | |
| DLPFC: dorsolateral prefrontal cortex; HD‐tDCS: high definition tDCS; M1: primary motor cortex | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 27 | Std. Mean Difference (Fixed, 95% CI) | ‐0.22 [‐0.29, ‐0.16] | |
| 1.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | 0.13 [‐0.03, 0.28] | |
| 1.2 High‐frequency ≥ 5 Hz | 25 | Std. Mean Difference (Fixed, 95% CI) | ‐0.30 [‐0.37, ‐0.23] | |
| 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies Show forest plot | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 2.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.10] | |
| 2.2 Multiple‐dose studies | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.76, ‐0.05] | |
| 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only Show forest plot | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.13] | |
| 3.1 Low‐frequency ≤ 1 Hz | 5 | Std. Mean Difference (Fixed, 95% CI) | 0.15 [‐0.02, 0.32] | |
| 3.2 High‐frequency ≥ 5 Hz | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.28 [‐0.36, ‐0.20] | |
| 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only Show forest plot | 8 | Std. Mean Difference (Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.17] | |
| 4.1 Low‐frequency ≤ 1 Hz | 1 | Std. Mean Difference (Fixed, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 4.2 High‐frequency ≥ 5 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | ‐0.56 [‐0.81, ‐0.31] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.51, ‐0.22] | |
| 5.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.49, ‐0.27] | |
| 5.2 Multiple‐dose studies | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.73, 0.05] | |
| 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up Show forest plot | 29 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.40, ‐0.14] | |
| 6.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.15 [0.01, 0.29] | |
| 6.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.49, ‐0.22] | |
| 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up Show forest plot | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 7.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 7.2 High‐frequency ≥ 5 Hz | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.49, ‐0.19] | |
| 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] | |
| 8.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.50, ‐0.28] | |
| 8.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.71, 0.04] | |
| 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.52, ‐0.22] | |
| 9.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.47, ‐0.26] | |
| 9.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.81, 0.09] | |
| 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 31 | Std. Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.34, ‐0.20] | |
| 10.1 Low‐frequency ≤ 1 Hz | 10 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.07, 0.22] | |
| 10.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Fixed, 95% CI) | ‐0.36 [‐0.44, ‐0.29] | |
| 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded Show forest plot | 24 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.55, ‐0.26] | |
| 11.1 Single‐dose studies | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.46, ‐0.24] | |
| 11.2 Multiple‐dose studies | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐0.91, ‐0.15] | |
| 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.48, 0.15] | |
| 12.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 12.2 High frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.92 [‐1.95, 0.12] | |
| 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 13.1 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 14 Pain: short term responder analysis 30% pain reduction Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.17, 3.80] |
| 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐1.01, 0.17] | |
| 16 Pain: medium‐term follow‐up Show forest plot | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.61, 0.05] | |
| 16.1 Low‐frequency ≤ 1 Hz | 2 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.41, 0.69] | |
| 16.2 High‐frequency ≥ 5 Hz | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.73, 0.00] | |
| 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] | |
| 17.1 Low‐frequency ≤ 1 Hz | 3 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.52, 0.56] | |
| 17.2 High‐frequency ≥ 5 Hz | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐0.90, ‐0.25] | |
| 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.46, 0.02] | |
| 18.1 Low frequency ≤ 1Hz | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.86, 0.70] | |
| 18.2 High‐frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.49, 0.03] | |
| 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐1.08 [‐2.49, 0.32] | |
| 19.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.36 [‐0.41, 1.13] | |
| 19.2 High‐frequency ≥ 5 Hz | 4 | Std. Mean Difference (Random, 95% CI) | ‐1.74 [‐3.66, 0.19] | |
| 20 Pain: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.44, 0.17] | |
| 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.89, 0.10] | |
| 22 Disability: short‐term follow‐up Show forest plot | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.87, 0.29] | |
| 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.72, 0.12] | |
| 24 Disability: medium‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐1.07, 0.33] | |
| 25 Pain: short term responder analysis 50% pain reduction Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.03, 3.47] |
| 26 Disability: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.62, 0.16] | |
| 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up Show forest plot | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.87, 0.05] | |
| 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐15.04, ‐6.55] |
| 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 4 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐11.49 [‐16.73, ‐6.25] |
| 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) Show forest plot | 5 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐8.93 [‐13.49, ‐4.37] |
| 31 Quality of life: long‐term follow‐up Show forest plot | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐6.78 [‐13.43, ‐0.14] |
| 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up Show forest plot | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐13.84, ‐3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.48, 0.01] |
| 2 Quality of life: short term follow up Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.63, ‐0.22] | |
| 1.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 1.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.77, ‐0.25] | |
| 2 Pain: short‐term sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.62, ‐0.23] | |
| 3 Pain: short‐term sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.64, ‐0.23] | |
| 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies Show forest plot | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.67, ‐0.29] | |
| 4.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 4.2 Multiple‐dose studies | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.79, ‐0.32] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.67, ‐0.28] | |
| 5.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 5.2 Multiple‐dose studies | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.58 [‐0.84, ‐0.33] | |
| 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.64, ‐0.26] | |
| 6.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.37, 0.01] | |
| 6.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.81, ‐0.30] | |
| 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased Show forest plot | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] | |
| 7.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.03] | |
| 7.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] | |
| 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain Show forest plot | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.56, ‐0.19] | |
| 8.1 Neuropathic | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.53, 0.01] | |
| 8.2 Non neuropathic | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.67, ‐0.17] | |
| 9 Pain: short term follow‐up responder analysis 30% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10 Pain: short term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11 Pain: medium‐term follow‐up Show forest plot | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.13] | |
| 12 Pain: medium term follow‐up responder analysis 30% pain reduction Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Pain: medium term follow‐up responder analysis 50% pain reduction Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up Show forest plot | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.72, ‐0.18] | |
| 15 Pain: long‐term follow‐up Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.43, 0.41] | |
| 16 Disability: short‐term follow‐up Show forest plot | 4 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.28, 0.26] |
| 17 Disability: medium‐term follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18 Quality of life: short‐term follow‐up Show forest plot | 4 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.21, 1.11] |
| 19 Quality of life: medium‐term follow‐up Show forest plot | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.09, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: short‐term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.18] |
| 3 Quality of Life: short term follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up Show forest plot | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.91, 0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.64, 0.26] | |

