Вмешательства для использования тромбопрофилактики у госпитализированных пациентов с риском венозной тромбоэмболии
Appendices
Appendix 1. MEDLINE Ovid and Cochrane search strategy
1. exp Thrombosis/pc
2. exp Embolism/pc
3. (thrombosis or thrombotic or thrombus or thrombi or thromboembol*).tw.
4. (emboli* or embolus).tw.
6. clot?.tw.
7. (DVT or VTE or PE).tw.
8. or/1‐7
9. exp Anticoagulants/
10. anticoagulant*.tw.
11. (hydroxycoumarins or acenocoumarol or acenocoumar* or minisintrom or nicoumalone or s?nc?umar or sintrom or s?nthrom* or ancrod or ancrod or arvin or venacil or agkistrodon or arwinor or (blood adj3 coagulat* adj3 inhibit*) or "citric acid" or uralyt or dalteparin or tedelparin or fr‐860 or fr860 or dalteparin or kabi2165 or kabi‐2165 or fragmin* or "dermatan sulfate" or chondroitin or dextran or dextrans or hemodex or promit or macrodex or saviosol or rheodextran or polyglucin or hyskon or rheomacrodex or infukoll or rheopolyglucin or rheoisodex or rondex or dic?umarol or dicoumarin or bishydroxycoumarin or edetic or tetracemate or calcitetracemate or edta or ethylenedinitrilotetraacetic or edetate or (calcium adj3 tetacine) or versenate or coprin or edathamil or versene or dinitrilotetraacetate or "chelaton 3" or enoxaparin* or pk10169 or "pk 10169" or emt‐967 or emt96* or clexane or lovenox or emt‐966 or (ethyl adj3 biscoumacetate) or ethyldicoumarol or pelentan or tromexan or carbethoxydicoumarol or foy or gabexate or heparin* or at?eroid* or liquaemin or nadroparin* or fraxiparin* or cy‐216 or cy216 or "pentosan sulfuric polyester" or "pentosan sulphuric polyester" or ((polysulfate or polysulphate) adj sodium adj pentosan*) or ((sulfuric or sulphuric) adj polyester adj pentosan*) or fibrocid or ((hoe or bay or hoe‐bay) adj "946") or ((pentosan* or polypentose or xylan) adj (sulphate or sulfate or sp54 or sp‐54 or polysulfate* or polysulphate*)) or pz68 or pz‐68 or elmiron or hemoclar or phenindione or pindione or phenyline or fenilin or phenylindanedione or dindevan or phenprocoumon or falithrom or phenprogramma or phenprocoumalol or marcumar or phenylpropylhydroxycumarinum or phenprocoumarol or liquamar or marcoumar or "protein c" or "protein s" or warfarin marevan or coumadin* or warfant or aldocumar or tedicumar or "beta 2‐glycoprotein i" or apo‐h or anticardiolipin or "apoliprotein h" or ec‐vmfa or "endothelial cell viability maintaining factor" or "beta(2)gpi").tw.
12. exp Stockings, Compression/
13. exp Intermittent Pneumatic Compression Devices/
14. ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) adj3 (stocking* or hose or hosiery or device*)).tw.
15. (prophylaxis or prophylactic).tw.
16. pc.fs.
17. (prevent* or reduce or reduction or diminish or decrease* or inhibit*).tw.
18. or/9‐17
19. exp Medical Order Entry Systems/
20. exp Reminder Systems/
21. exp Drug Therapy, Computer‐Assisted/
22. (("computeri?ed physician" or system) adj5 "order entry").tw.
23. CPOE.tw.
24. ((computeri?ed or automat* or medicat* or electronic*) adj5 (alert* or reminder*)).tw.
25. sticker?.tw.
26. prescription aid?.tw.
27. exp Decision Support Systems, Clinical/
28. decision support.tw.
29. CDS.tw.
30. e‐iatrogenesis.tw.
31. alert fatigue.tw.
32. electronic tool?.tw.
33. exp Guideline/
34. exp Guidelines as Topic/
35. exp Guideline Adherence/
36. exp Clinical Protocols/
37. protocol*.tw.
38. guideline*.tw.
39. adhere*.tw.
40. (comply or compliance).tw.
41. or/19‐40
42. exp Inpatients/ or exp Hospitalization/ or exp Hospitals/
43. (inpatient* or "in?patient*").tw.
44. exp Adolescent, Hospitalized/ or exp Child, Hospitalized/
45. (hospitali?e* or hospitali?ation).tw.
46. (admitted adj3 (hospital or patient*)).tw.
47. ("high risk" or "at risk").tw.
48. or/42‐47
49. thromboprophyla*.mp.
50. 8 and 18 and 41 and 48
51. 48 and 49
52. 50 or 51
53. limit 52 to yr="1980 ‐Current"
Appendix 2. Embase Ovid search strategy
1. exp thrombosis prevention/
2. exp embolism prevention/
3. (thrombosis or thrombotic or thrombus or thrombi or thromboembol*).tw.
4. (emboli* or embolus).tw.
5. (phlebothrombo* or phlebitis).tw.
6. exp blood clotting/
7. clot.tw.
8. (DVT or VTE or PE).ti,ab.
9. or/1‐8
10. exp *anticoagulant agent/
11. anticoagulant*.tw.
12. (hydroxycoumarins or acenocoumarol or acenocoumar* or minisintrom or nicoumalone or s?nc?umar or sintrom or s?nthrom* or ancrod or ancrod or arvin or venacil or agkistrodon or arwinor or (blood adj3 coagulat* adj3 inhibit*) or "citric acid" or uralyt or dalteparin or tedelparin or fr‐860 or fr860 or dalteparin or kabi2165 or kabi‐2165 or fragmin* or "dermatan sulfate" or chondroitin or dextran or dextrans or hemodex or promit or macrodex or saviosol or rheodextran or polyglucin or hyskon or rheomacrodex or infukoll or rheopolyglucin or rheoisodex or rondex or dic?umarol or dicoumarin or bishydroxycoumarin or edetic or tetracemate or calcitetracemate or edta or ethylenedinitrilotetraacetic or edetate or (calcium adj3 tetacine) or versenate or coprin or edathamil or versene or dinitrilotetraacetate or "chelaton 3" or enoxaparin* or pk10169 or "pk 10169" or emt‐967 or emt96* or clexane or lovenox or emt‐966 or (ethyl adj3 biscoumacetate) or ethyldicoumarol or pelentan or tromexan or carbethoxydicoumarol or foy or gabexate or heparin* or at?eroid* or liquaemin or nadroparin* or fraxiparin* or cy‐216 or cy216 or "pentosan sulfuric polyester" or "pentosan sulphuric polyester" or ((polysulfate or polysulphate) adj sodium adj pentosan*) or ((sulfuric or sulphuric) adj polyester adj pentosan*) or fibrocid or ((hoe or bay or hoe‐bay) adj "946") or ((pentosan* or polypentose or xylan) adj (sulphate or sulfate or sp54 or sp‐54 or polysulfate* or polysulphate*)) or pz68 or pz‐68 or elmiron or hemoclar or phenindione or pindione or phenyline or fenilin or phenylindanedione or dindevan or phenprocoumon or falithrom or phenprogramma or phenprocoumalol or marcumar or phenylpropylhydroxycumarinum or phenprocoumarol or liquamar or marcoumar or "protein c" or "protein s" or warfarin marevan or coumadin* or warfant or aldocumar or tedicumar or "beta 2‐glycoprotein i" or apo‐h or anticardiolipin or "apoliprotein h" or ec‐vmfa or "endothelial cell viability maintaining factor" or "beta(2)gpi").tw.
13. exp compression stocking/
14. ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) adj3 (stocking* or hose or hosiery)).tw.
15. (prophylaxis or prophylactic).tw.
16. pc.fs.
17. (prevent* or reduce or reduction or diminish or decrease* or inhibit*).tw.
18. or/10‐17
19. exp hospital information system/
20. exp reminder system/
21. exp computer assisted drug therapy/
22. (("computeri?ed physician" or system) adj5 "order entry").tw.
23. CPOE.tw.
24. ((computeri?ed or automat* or medicat* or electronic*) adj5 (alert* or reminder*)).tw.
25. sticker*.tw.
26. prescription aid*.tw.
27. exp decision support system/
28. "decision support".tw.
29. CDS.tw.
30. e‐iatrogenesis.tw.
31. alert fatigue.tw.
32. electronic tool*.tw.
33. exp practice guideline/
34. exp clinical protocol/
35. (protocol* or guideline* or adhere*).tw.
36. (comply or compliance).tw.
37. or/19‐36
38. exp hospital patient/ or exp hospitalization/ or (*exp * hospital/ and exp patient/)
39. (inpatient* or "in?patient").tw.
40. (hospitali?e* or hospitali?ation).tw.
41. (admitted adj3 (hospital or patient*)).tw.
42. ("high risk" or "at risk").tw.
43. or/38‐42
44. thromboprophyla*.mp.
45. 9 and 18 and 37 and 43
46. 43 and 44
47. 45 or 46
48. limit 47 to yr="1980 ‐Current"
Appendix 3. BIOSIS previews Ovid search strategy
1. (thrombosis or thrombotic or thrombus or thrombi or thromboembol*).mp.
2. (emboli* or embolus).mp.
3. (phlebothrombo* or phlebitis).mp.
4. clot*.mp.
5. (DVT or VTE or PE).tw.
6. or/1‐5
7. anticoagulant*.mp.
8. (hydroxycoumarins or acenocoumarol or acenocoumar* or minisintrom or nicoumalone or s?nc?umar or sintrom or s?nthrom* or ancrod or ancrod or arvin or venacil or agkistrodon or arwinor or (blood adj3 coagulat* adj3 inhibit*) or "citric acid" or uralyt or dalteparin or tedelparin or fr‐860 or fr860 or dalteparin or kabi2165 or kabi‐2165 or fragmin* or "dermatan sulfate" or chondroitin or dextran or dextrans or hemodex or promit or macrodex or saviosol or rheodextran or polyglucin or hyskon or rheomacrodex or infukoll or rheopolyglucin or rheoisodex or rondex or dic?umarol or dicoumarin or bishydroxycoumarin or edetic or tetracemate or calcitetracemate or edta or ethylenedinitrilotetraacetic or edetate or (calcium adj3 tetacine) or versenate or coprin or edathamil or versene or dinitrilotetraacetate or "chelaton 3" or enoxaparin* or pk10169 or "pk 10169" or emt‐967 or emt96* or clexane or lovenox or emt‐966 or (ethyl adj3 biscoumacetate) or ethyldicoumarol or pelentan or tromexan or carbethoxydicoumarol or foy or gabexate or heparin* or at?eroid* or liquaemin or nadroparin* or fraxiparin* or cy‐216 or cy216 or "pentosan sulfuric polyester" or "pentosan sulphuric polyester" or ((polysulfate or polysulphate) adj sodium adj pentosan*) or ((sulfuric or sulphuric) adj polyester adj pentosan*) or fibrocid or ((hoe or bay or hoe‐bay) adj "946") or ((pentosan* or polypentose or xylan) adj (sulphate or sulfate or sp54 or sp‐54 or polysulfate* or polysulphate*)) or pz68 or pz‐68 or elmiron or hemoclar or phenindione or pindione or phenyline or fenilin or phenylindanedione or dindevan or phenprocoumon or falithrom or phenprogramma or phenprocoumalol or marcumar or phenylpropylhydroxycumarinum or phenprocoumarol or liquamar or marcoumar or "protein c" or "protein s" or warfarin marevan or coumadin* or warfant or aldocumar or tedicumar or "beta 2‐glycoprotein i" or apo‐h or anticardiolipin or "apoliprotein h" or ec‐vmfa or "endothelial cell viability maintaining factor" or "beta(2)gpi").tw.
9. ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) adj3 (stocking* or hose or hosiery)).mp.
10. (prophylaxis or prophylactic).mp.
11. (prevent* or reduce or reduction or diminish or decrease* or inhibit*).mp.
12. or/7‐11
13. (("computeri?ed physician" or system) adj5 "order entry").tw.
14. CPOE.tw.
15. ((computeri?ed or automat* or medicat* or electronic*) adj5 (alert* or reminder*)).tw.
16. sticker*.tw.
17. prescription aid*.tw.
18. "decision support".tw.
19. CDS.tw.
20. e‐iatrogenesis.tw.
21. alert fatigue.tw.
22. electronic tool*.tw.
23. (guideline* or protocol* or adhere*).tw.
24. (comply or compliance).tw.
25. or/13‐24
26. (inpatient* or "in?patient").tw.
27. (hospitali?e* or hospitali?ation).tw.
28. (admit* adj3 (hospital or patient*)).tw.
29. ("high risk" or "at risk").tw.
30. or/26‐29
31. thromboprophyla*.mp.
32. 6 and 12 and 25 and 30
33. 30 and 31
34. 32 or 33
Appendix 4. CINAHL search strategy
S46 S44 OR S45
S45 S42 AND S43
S44 S8 AND S15 AND S32 AND S42
S43TI thromboprophyla* OR AB thromboprophyla*
S42 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41
S41TI ("high risk" OR "at risk") OR AB ("high risk" OR "at risk")
S40TI (admitted N3 (hospital or patient*)) OR AB (admitted N3 (hospital or patient*))
S39TI (hospitali?e* OR hospitali?ation) OR AB (hospitali?e* OR hospitali?ation)
S38(MH "Child, Hospitalized")
S37(MH "Adolescent, Hospitalized")
S36TI (inpatient* OR in?patient*) OR AB (inpatient* OR in?patient*)
S35(MH "Hospitals+")
S34(MH "Hospitalization+")
S33(MH "Inpatients")
S32 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S31TI (protocol* or guideline* OR adhere*) OR AB (protocol* or guideline* OR adhere*)
S30(MH "Practice Guidelines")
S29TI electronic tool* OR AB electronic tool*
S28TI alert fatigue OR AB alert fatigue
S27TI e‐iatrogenesis OR AB e‐iatrogenesis
S26TI CDS OR AB CDS
S25TI decision support* OR AB decision support*
S24(MH "Decision Support Systems, Clinical")
S23TI prescription aid* OR AB prescription aid*
S22TI sticker* OR AB sticker*
S21TI ((computeri?ed or automat* or medicat* or electronic*) N5 (alert* or reminder*)) OR AB ((computeri?ed or automat* or medicat* or electronic*) N5 (alert* or reminder*))
S20TI CPOE OR AB CPOE
S19TI (("computeri?ed physician" or system) N5 "order entry") OR AB (("computeri?ed physician" or system) N5 "order entry")
S18(MH "Drug Therapy, Computer Assisted")
S17(MH "Reminder Systems")
S16(MH "Electronic Order Entry")
S15 S9 OR S10 OR S11 OR S12 OR S13 OR S14
S14TI (prevent* or reduce or reduction or diminish or decrease* or inhibit*) OR AB (prevent* or reduce or reduction or diminish or decrease* or inhibit*)
S13TI (prophylaxis or prophylactic) OR AB (prophylaxis or prophylactic)
S12TI ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) N3 (stocking* or hose or hosiery or device*)) OR AB ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) N3 (stocking* or hose or hosiery or device*))
S11(MH "Compression Garments")
S10TI anticoagulant* OR AB anticoagulant*
S9(MH "Anticoagulants+")
S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7
S7TX (DVT OR VTE OR PE) OR AB (DVT OR VTE OR PE)
S6TX (clot or clots) OR AB (clot or clots)
S5TX (phlebothrombo* or phlebitis) OR AB (phlebothrombo* or phlebitis)
S4TX (emboli* OR embolus) OR AB (emboli* or embolus)
S3TX (thrombosis or thrombotic or thrombus or thrombi or thromboembol*) OR AB (thrombosis or thrombotic or thrombus or thrombi or thromboembol*)
S2(MH "Embolism+/PC")
S1(MH "Thrombosis+/PC")
Appendix 5. WEB OF SCIENCE search strategy
#1 TS=(thrombosis or thrombotic or thrombus or thrombi or thromboembol* OR emboli* OR embolus OR phlebothrombo* or phlebitis OR clot OR DVT OR VTE OR PE)
#2 TS=(anticoagulant* OR hydroxycoumarins or acenocoumarol or acenocoumar* or minisintrom or nicoumalone or s?nc?umar or sintrom or s?nthrom* or ancrod or ancrod or arvin or venacil or agkistrodon or arwinor or (blood NEAR/3 coagulat* NEAR/3 inhibit*) or "citric acid" or uralyt or dalteparin or tedelparin or fr‐860 or fr860 or dalteparin or kabi2165 or kabi‐2165 or fragmin* or "dermatan sulfate" or chondroitin or dextran or dextrans or hemodex or promit or macrodex or saviosol or rheodextran or polyglucin or hyskon or rheomacrodex or infukoll or rheopolyglucin or rheoisodex or rondex or dic?umarol or dicoumarin or bishydroxycoumarin or edetic or tetracemate or calcitetracemate or edta or ethylenedinitrilotetraacetic or edetate or (calcium NEAR/3 tetacine) or versenate or coprin or edathamil or versene or dinitrilotetraacetate or "chelaton 3" or enoxaparin* or pk10169 or "pk 10169" or emt‐967 or emt96* or clexane or lovenox or emt‐966 or (ethyl NEAR/3 biscoumacetate) or ethyldicoumarol or pelentan or tromexan or carbethoxydicoumarol or foy or gabexate or heparin* or at?eroid* or liquaemin or nadroparin* or fraxiparin* or cy‐216 or cy216 or "pentosan sulfuric polyester" or "pentosan sulphuric polyester" or ((polysulfate or polysulphate) NEAR/1 sodium NEAR/1 pentosan*) or ((sulfuric or sulphuric) NEAR/1 polyester NEAR/1 pentosan*) or fibrocid or ((hoe or bay or hoe‐bay) NEAR/1 "946") or ((pentosan* or polypentose or xylan) NEAR/1 (sulphate or sulfate or sp54 or sp‐54 or polysulfate* or polysulphate*)) or pz68 or pz‐68 or elmiron or hemoclar or phenindione or pindione or phenyline or fenilin or phenylindanedione or dindevan or phenprocoumon or falithrom or phenprogramma or phenprocoumalol or marcumar or phenylpropylhydroxycumarinum or phenprocoumarol or liquamar or marcoumar or "protein c" or "protein s" or warfarin marevan or coumadin* or warfant or aldocumar or tedicumar or "beta 2‐glycoprotein i" or apo‐h or anticardiolipin or "apoliprotein h" or ec‐vmfa or "endothelial cell viability maintaining factor" or "beta(2)gpi" OR ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) NEAR/3 (stocking* or hose or hosiery)) OR prophylaxis or prophylactic or prevent* or reduce or reduction or diminish or decrease* or inhibit*)
#3 TS=((("computeri?ed physician" or system) NEAR/5 "order entry") OR CPOE OR ((computeri?ed or automat* or medicat* or electronic*) NEAR/5 (alert* or reminder*)) or sticker* OR "prescription aid*" OR "decision support" OR CDS OR e‐iatrogenesis OR "alert fatigue" OR "electronic tool*" OR guideline* or protocol* OR adhere* OR comply or compliance)
#4 TS=(inpatient* OR "in‐patient*" or hospitali?e* or hospitali?ation or (admitted NEAR/3 (hospital* or patient*)) OR "high risk" or "at risk")
#5 TS=(thromboprophyla*)
#6 #4 AND #3 AND #2 AND #1
#7 #5 AND #4
#8 #7 OR #6
Appendix 6. LILACS Search Strategy
((thrombosis or thrombotic or thrombus or thrombi or thromboembol* or phlebothrombo* or phlebitis or clot* or DVT or VTE) AND (prophylaxis or prophylactic or prevent* or reduce or reduction or diminish or decrease* or inhibit*)) OR thromboprophyla*
Appendix 7. PubMed search strategy
#65,"Search #64 NOT medline[sb]"
#64,"Search #62 OR #63"
#63,"Search #60 AND #61"
#62,"Search #15 AND #27 AND #52 AND #60"
#61,"Search thromboprophyla*[tw]"
#60,"Search #52 OR #53 OR #54 OR #55 OR #56 OR #58 OR #59"
#59,"Search high risk[tw] or at risk[tw]"
#58,"Search admitted[tw] AND (hospital[tw] or patient[tw] or patients[tw])"
#56,"Search hospitalise*[tw] or hospitalisation[tw] or hospitalize*[tw] or hospitalization[tw]"
#55,"Search Adolescent, Hospitalized[Mesh] or Child, Hospitalized[Mesh]"
#54,"Search inpatient[tw] or inpatients[tw] or in‐patient[tw] or in‐patients[tw]"
#53,"Search Inpatients[Mesh] or Hospitalization[Mesh] or Hospitals[Mesh]"
#52,"Search #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51"
#51,"Search comply[tw] or compliance[tw]"
#50,"Search adhere*[tw]"
#49,"Search guideline*[tw]"
#48,"Search protocol*[tw]"
#47,"Search Clinical Protocols[Mesh]"
#46,"Search Guideline Adherence[Mesh]"
#45,"Search Guidelines as Topic[Mesh]"
#44,"Search Guideine[Mesh] Schema: all"
#43,"Search Guideine[Mesh]"
#42,"Search electronic tool*[tw]"
#41,"Search alert fatigue[tw]"
#40,"Search e‐iatrogenesis[tw]"
#39,"Search CDS[tw]"
#38,"Search decision support[tw]"
#37,"Search ""Decision Support Systems, Clinical""[Mesh]"
#36,"Search prescription aid*[tw]"
#35,"Search sticker*[tw]"
#34,"Search ((computerised or computerized or automat* or medicat* or electronic*) AND (alert* or reminder*))[tw]"
#33,"Search CPOE[tw]"
#32,"Search ((""computerised physician"" or ""computerized physician"" or system) AND ""order entry"")[tw]"
#31,"Search ""Drug Therapy, Computer‐Assisted""[Mesh]"
#30,"Search ""Reminder Systems""[Mesh]"
#29,"Search ""Medical Order Entry Systems""[Mesh]"
#27,"Search #16 OR #17 OR #19 OR #21 OR #23 OR #24 OR #25 OR #26"
#26,"Search prevent*[tw] or reduce[tw] or reduction[tw] or diminish[tw] or decrease*[tw] or inhibit*[tw]"
#25,"Search prophylaxis[tw] or prophylactic[tw]"
#24,"Search ((compression* or thromboembolism‐deterrent or anti‐embolism or TED) AND (stocking* or hose or hosiery or device*))[tw]"
#23,"Search ""Intermittent Pneumatic Compression Devices""[Mesh]"
#21,"Search ""Stockings, Compression""[Mesh]"
#19,"Search hydroxycoumarins[tw] or acenocoumarol[tw] or acenocoumar*[tw] or minisintrom[tw] or nicoumalone[tw] or syncumar[tw] or sintrom[tw] or sinthrom*[tw] or synthrom*[tw] or ancrod[tw] or arvin[tw] or venacil[tw] or agkistrodon[tw] or arwinor[tw] or blood coagulation inhibitor[tw] or blood coagulation inhibitors[tw] or citric acid[tw] or uralyt[tw] or dalteparin[tw] or tedelparin[tw] or fr‐860[tw] or fr860[tw] or dalteparin[tw] or kabi2165[tw] or kabi‐2165[tw] or fragmin*[tw] or ""dermatan sulfate""[tw] or chondroitin[tw] or dextran[tw] or dextrans[tw] or hemodex[tw] or promit[tw] or macrodex[tw] or saviosol[tw] or rheodextran[tw] or polyglucin[tw] or hyskon[tw] or rheomacrodex[tw] or infukoll[tw] or rheopolyglucin[tw] or rheoisodex[tw] or rondex[tw] or dicumarol[tw] or dicoumarol[tw] or dicoumarin[tw] or bishydroxycoumarin[tw] or edetic[tw] or tetracemate[tw] or calcitetracemate[tw] or edta[tw] or ethylenedinitrilotetraacetic[tw] or edetate[tw] or (calcium AND tetacine)[tw] or versenate[tw] or coprin[tw] or edathamil[tw] or versene[tw] or dinitrilotetraacetate[tw] or ""chelaton 3""[tw] or enoxaparin*[tw] or pk10169[tw] or ""pk 10169""[tw] or emt‐967[tw] or emt96*[tw] or clexane[tw] or lovenox[tw] or emt‐966[tw] or ""ethyl biscoumacetate""[tw] or ethyldicoumarol[tw] or pelentan[tw] or tromexan[tw] or carbethoxydicoumarol[tw] or foy[tw] or gabexate[tw] or heparin*[tw] or ateroid*[tw] or atheroid*[tw] or liquaemin[tw] or nadroparin*[tw] or fraxiparin*[tw] or cy‐216[tw] or cy216[tw] or ""pentosan sulfuric polyester""[tw] or ""pentosan sulphuric polyester""[tw] or ((polysulfate or polysulphate) AND sodium AND pentosan*)[tw] or ((sulfuric or sulphuric) AND polyester AND pentosan*)[tw] or fibrocid[tw] or ((hoe or bay or hoe‐bay) AND ""946"")[tw] or ((pentosan* or polypentose or xylan)[tw] AND (sulphate or sulfate or sp54 or sp‐54 or polysulfate* or polysulphate*))[tw] or pz68[tw] or pz‐68[tw] or elmiron[tw] or hemoclar[tw] or phenindione[tw] or pindione[tw] or phenyline[tw] or fenilin[tw] or phenylindanedione[tw] or dindevan[tw] or phenprocoumon[tw] or falithrom[tw] or phenprogramma[tw] or phenprocoumalol[tw] or marcumar[tw] or phenylpropylhydroxycumarinum[tw] or phenprocoumarol[tw] or liquamar[tw] or marcoumar[tw] or ""protein c""[tw] or ""protein s""[tw] or ""warfarin marevan""[tw] or coumadin*[tw] or warfant[tw] or aldocumar[tw] or tedicumar[tw] or ""beta 2‐glycoprotein i""[tw] or apo‐h[tw] or anticardiolipin[tw] or ""apoliprotein h""[tw] or ec‐vmfa[tw] or ""endothelial cell viability maintaining factor""[tw] or ""beta(2)gpi""[tw]"
#17,"Search anticoagulant*[tw]"
#16,"Search ""Anticoagulants""[Mesh]"
#15,"Search #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #14"
#14,"Search DVT[tiab] OR VTE[tiab] OR PE[tiab]"
#12,"Search clot[tw]
#11,"Search phlebothrombo*[tw] or phlebitis[tw]"
#10,"Search emboli[tw] or embolus[tw]"
#9,"Search thrombosis[tw] or thrombotic[tw] or thrombus[tw] or thrombi[tw] or thromboembol*[tw]"
#8,"Search ""Embolism/prevention and control""[Mesh]"
#7,"Search ""Thrombosis/prevention and control""[Mesh]"
Appendix 8. Clinicaltrials.gov search strategy
thrombosis or thrombotic or thrombus or thrombi or thromboembol* or emboli* or embolus or phlebothrombo* or phlebitis or clot or clots or DVT or VTE or PE
Appendix 9. Funnel Plots
Appendix 10. Influence analysis
| Influence analyses | |||
| Alerts ‐ Received prophylaxis | |||
| Study omitted | RD estimate without study | 95% Confidence interval | |
| 0.21 | 0.06 | 0.36 | |
| 0.22 | 0.16 | 0.28 | |
| 0.19 | 0.16 | 0.22 | |
| Combined | 0.21 | 0.15 | 0.27 |
| Alerts ‐ Received appropriate prophylaxis | |||
| Study omitted | RD estimate without study | 95% Confidence interval | |
| 0.16 | 0.12 | 0.21 | |
| 0.14 | 0.06 | 0.22 | |
| 0.16 | 0.12 | 0.20 | |
| Combined | 0.16 | 0.12 | 0.20 |
| Alerts ‐ Symptomatic VTE | |||
| Study omitted | RR estimate without study | 95% Confidence interval | |
| 0.65 | 0.50 | 0.85 | |
| 0.57 | 0.18 | 1.86 | |
| 0.56 | 0.34 | 0.92 | |
| Combined | 0.64 | 0.47 | 0.86 |
| Multifaceted interventions ‐ Received prophylaxis (adjusted) | |||
| Study omitted | RD estimate without study | 95% Confidence interval | |
| 0.04 | 0.01 | 0.06 | |
| 0.04 | 0.02 | 0.06 | |
| 0.03 | 0.01 | 0.06 | |
| 0.04 | 0.02 | 0.06 | |
| 0.02 | ‐0.02 | 0.06 | |
| Combined | 0.04 | 0.02 | 0.06 |
VTE: venous thromboembolism
Appendix 11. Abbreviations
CDSR: Cochrane Database of Systematic Rviews
CENTRAL: Cochrane Central Register of Controlled Trials
CI: confidence interval
CINAHL: Cumulative Index to Nursing and Allied Health Literature
CME: continuous medical education
Cochrane EPOC: Cochrane Effective Practice and Organisation of Care
CRT: cluster randomized controlled trial
DARE: Database of Abstracts of Reviews of Effects
DVT: deep vein thrombosis
GRADE: Grading of Recommendations Assessment, Development and Evaluation
I²: statistical index of heterogeneity
ICC: intraclass correlation
LILACS: Latin American and Caribbean health sciences LIterature
MeSH: Medical Subject Headings
NHS EED: NHS Economic Evaluation Database
PE: pulmonary embolism
PICOS: patients, intervention, comparator, outcome, setting
QA: quality assurance
QRT: quasi‐randomized controlled trial
RAP: received appropriate prophylaxis
RCT: randomized controlled trial
RD: risk difference
ROB: risk of bias
RP: received prophylaxis
RR: risk ratio
VTE: venous thromboembolism
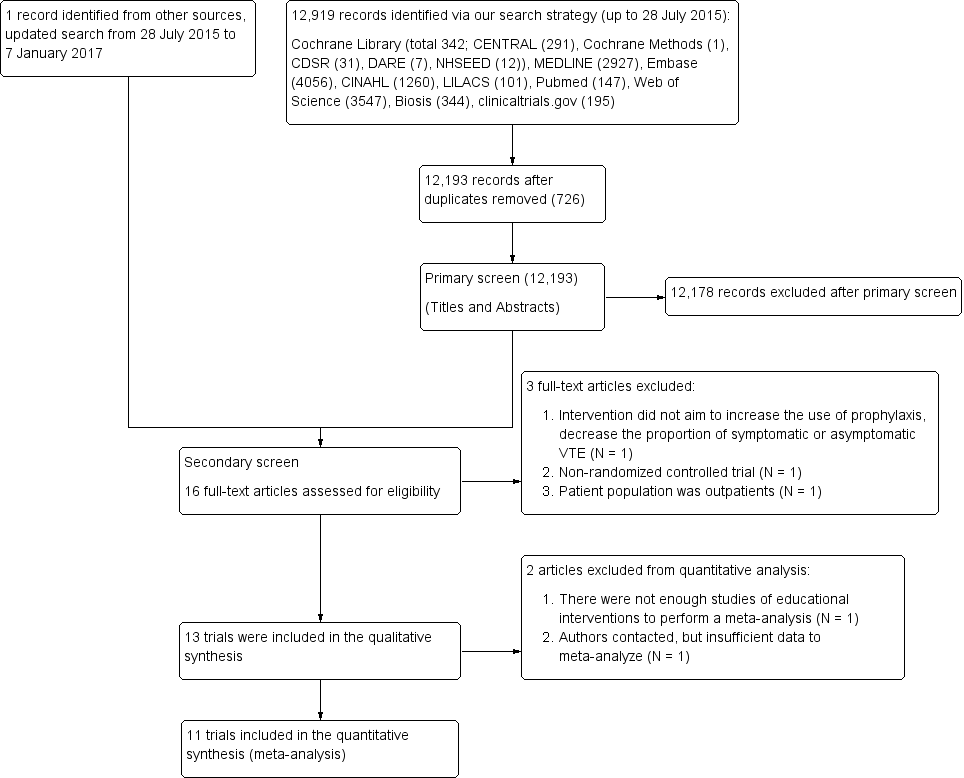
Study flow diagram.
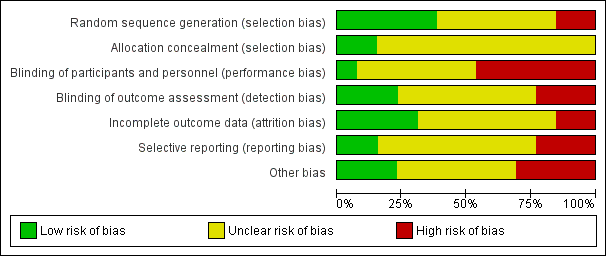
Summary of risk of bias: review authors’ judgements about each 'Risk of bias' item presented as percentages across all included studies
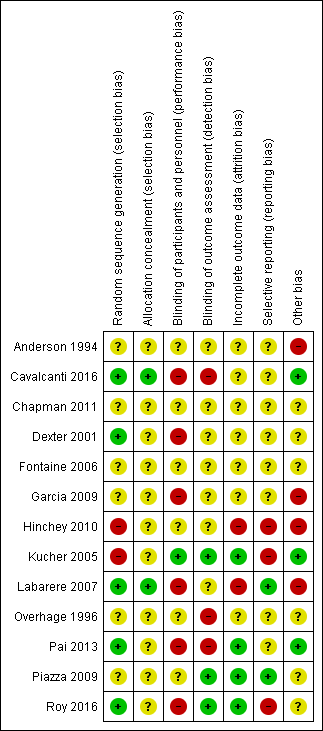
Summary of risk of bias: review authors’ judgements about each 'Risk of bias' item for each included study

Funnel plot of comparison: 1 Alerts versus standard care, outcome: 1.1 Received prophylaxis.
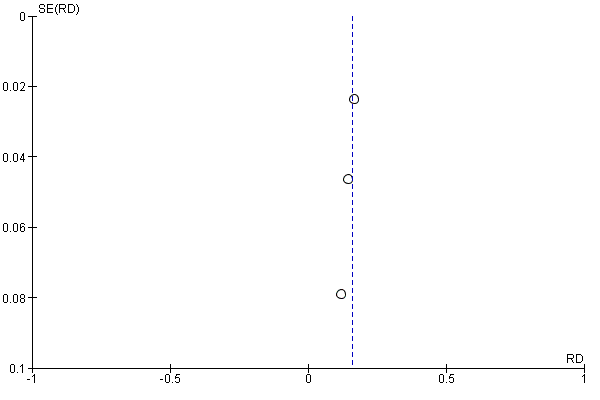
Funnel plot of comparison: 1 Alerts versus standard care, outcome: 1.2 Received appropriate prophylaxis.

Funnel plot of comparison: 1 Alerts versus standard care, outcome: 1.3 Symptomatic VTE.

Funnel plot of comparison: 2 Multifaceted interventions versus standard care or another intervention, outcome: 2.1 Received prophylaxis (unadjusted).
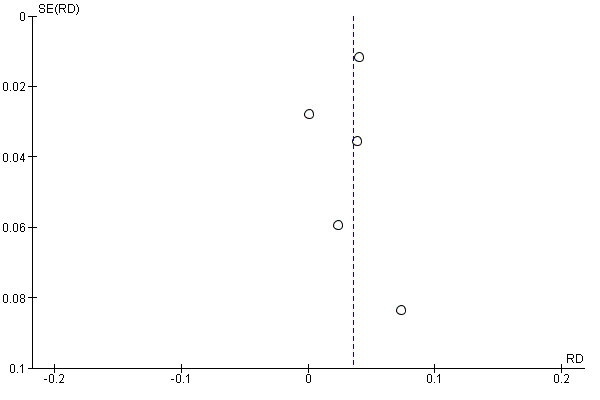
Funnel plot of comparison: 2 Multifaceted interventions versus standard care or another intervention, outcome: 2.2 Received prophylaxis (adjusted).

Comparison 1 Alerts versus standard care, Outcome 1 Received prophylaxis.

Comparison 1 Alerts versus standard care, Outcome 2 Received appropriate prophylaxis.

Comparison 1 Alerts versus standard care, Outcome 3 Symptomatic VTE.

Comparison 2 Multifaceted interventions versus standard care or another intervention, Outcome 1 Received prophylaxis (unadjusted).
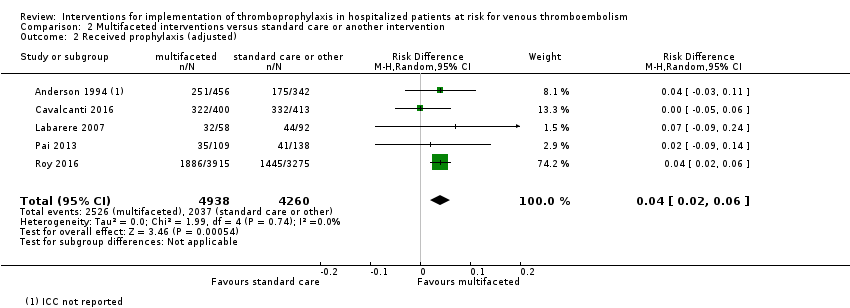
Comparison 2 Multifaceted interventions versus standard care or another intervention, Outcome 2 Received prophylaxis (adjusted).
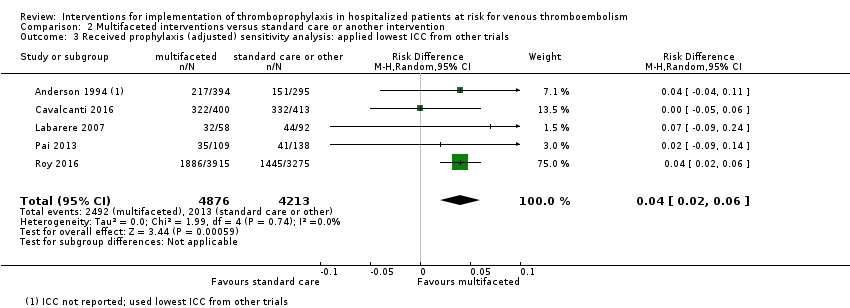
Comparison 2 Multifaceted interventions versus standard care or another intervention, Outcome 3 Received prophylaxis (adjusted) sensitivity analysis: applied lowest ICC from other trials.

Comparison 2 Multifaceted interventions versus standard care or another intervention, Outcome 4 Received prophylaxis (adjusted) sensitivity analysis: applied mean ICC from other trials.
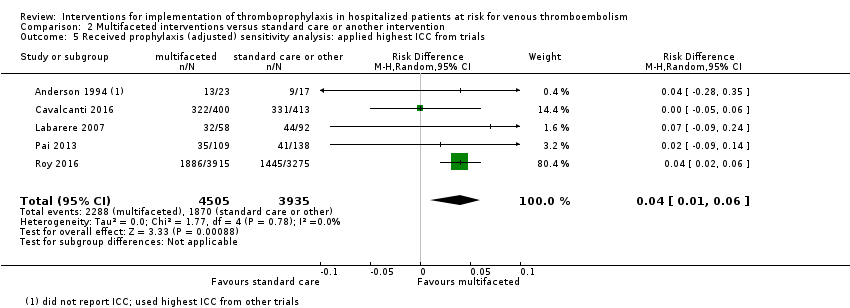
Comparison 2 Multifaceted interventions versus standard care or another intervention, Outcome 5 Received prophylaxis (adjusted) sensitivity analysis: applied highest ICC from trials.
| Computer or human alerts compared with standard care for VTE prophylaxis. | ||||||
| Patient or population: adult medical and surgical patients at risk for VTE Settings: hospital Intervention: automatic reminder systems, such as computer alerts or human alerts, designed to increase the implementation of thromboprophylaxis and/or decrease the incidence of symptomatic or asymptomatic VTE Comparison: standard care (no intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Measures of effect (RD, RR) (95% CI; I²) | No of Participants | Quality of the evidence | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Control group | Intervention group | |||||
| Received prophylaxis** (Follow‐up: 3 months) | Study population | RD 0.21 (0.15 to 0.27; 75%) | 5057 | ⊕⊕⊝⊝ | ||
| 178 per 1000 | 390 per 1000 | |||||
| Low risk population | ||||||
| 145 per 1000 | 318 per 1000 | |||||
| High risk population | ||||||
| 357 per 1000 | 782 per 1000 | |||||
| Received appropriate prophylaxis** (Follow‐up: 36 hours | Study population | RD 0.16 (0.12 to 0.20; 0%) | 1820 | ⊕⊕⊕⊝ | ||
| 305 per 1000 | 460 per 1000 | |||||
| Low risk population | ||||||
| 175 per 1000 | 249 per 1000 | |||||
| High risk population | ||||||
| 663 per 1000 | 941 per 1000 | |||||
| Symptomatic VTE (Follow‐up: 3 months) | Study population | RR 0.64 (0.47 to 0.86; 15%) | 5353 | ⊕⊕⊝⊝ | ||
| 56 per 1000 | 36 per 1000 | |||||
| Low risk population | ||||||
| 29 per 1000 | 19 per 1000 | |||||
| High risk population | ||||||
| 82 per 1000 | 52 per 1000 | |||||
| * Control risk was used as assumed risk (baseline risk), due to lack of well‐designed observational studies that measure this in detail to be presented as baseline risk for the population. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Clustered trials did not provide sufficient data (intraclass correlation (ICC) or adjusted confidence intervals) for us to pool cluster adjusted estimates. CI: confidence interval; I²: statistical index of heterogeneity; RD: risk difference; RR: risk ratio; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the level of certainty of evidence from high to low based on the following reasons: serious study limitations (quasi‐random sequence generation in 1/3 RCTs, no blinding of outcome assessment in 1/3 RCTs, selective reporting of safety outcomes in 1/3 RCTs. Random sequence generation, allocation concealment, blinding of participants and personnel, and other potential biases were unclear in most studies). No indirectness of evidence; some inconsistency of pooled results; no imprecision of pooled results; and undetected publication bias. 2 We downgraded the level of certainty of evidence from high to moderate based on the following reasons: serious study limitations (no blinding of participants and personnel in 2/3 RCTs, incorrect analysis that did not account for the clustered nature of the data in 1/3 RCTs. Random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases were unclear in most studies). No indirectness of evidence; no inconsistency and imprecision of pooled RD results; and undetected publication bias. 3 We downgraded the level of certainty of evidence from high to low based on the following reasons: serious study limitations (quasi‐random sequence generation in 1/3 RCTs, selective reporting of safety outcomes in 1/3 RCTs. Random sequence generation, allocation concealment, blinding of participants and personnel, and other potential biases were unclear in most studies). No indirectness of evidence, no inconsistency of pooled RR results, some imprecision of pooled results related to the small number of events, and undetected publication bias. | ||||||
| Multifaceted interventions compared with standard care or another type of intervention for VTE prophylaxis. | ||||||
| Patient or population: adult medical and surgical patients at risk for VTE Settings: hospital Intervention: multifaceted interventions (combination of interventions that may include education, audit and feedback, and alert), designed to trigger need for thromboprophylaxis Comparison: standard care (no intervention) or another type of intervention* | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Absolute effect (RD) (95% CI; I²) | No of Participants | Quality of the evidence | Comments | |
| Assumed risk** | Corresponding risk | |||||
| Control group | Intervention group | |||||
| Received prophylaxis (Unadjusted; Follow‐up: 2 to 4 months) | Study population | RD 0.03 (0.00 to 0.05; 64%) | 26,330 | ⊕⊕⊕⊝ | Clustered trials did not provide sufficient data (intraclass correlation (ICC) or adjusted confidence intervals) for us to pool cluster‐adjusted estimates Length of follow‐up was not specified in one study (Labarere 2007) | |
| 526 per 1000 | 558 per 1000 | |||||
| Low risk population | ||||||
| 299 per 1000 | 317 per 1000 | |||||
| High risk population | ||||||
| 803 per 1000 | 851 per 1000 | |||||
| Received prophylaxis (Adjusted; Follow‐up: 2 to 4 months) | Study population | RD 0.04 (0.02 to 0.06; 0%) | 9198 | ⊕⊕⊕⊝ | ICCs were available for 4/5 (Cavalcanti 2016 = 0.13, Labarere 2007 = 0.24, Pai 2013 = 0.022, Roy 2016 = 0.002) trials included in this meta‐analysis. ICC's were not available for Anderson 1994. Adjustment for the cluster design effect was performed via reported ICCs, no ICC was applied to the one trial that did not report an ICC (Anderson 1994) Total patients are lower because cluster design effect applied to the numbers of events and participants. Length of follow‐up was not specified in one study (Labarere 2007) | |
| 478 per 1000 | 507 per 1000 | |||||
| Low risk population | ||||||
| 297 per 1000 | 315 per 1000 | |||||
| High risk population | ||||||
| 804 per 1000 | 852 per 1000 | |||||
| * 'another type of intervention' was a multifaceted intervention targeted at different types of healthcare professionals (intervention targeted physicians and nurses; control targeted physicians only). ** Control risk was used as assumed risk (baseline risk), due to lack of well‐designed observational studies that measure this in detail to be presented as baseline risk for the population.The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; I²: Statistical index of heterogeneity; ICC: intraclass correlation; RCT: randomized controlled trial; RD: risk difference; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded the level of certainty of evidence from high to moderate based on the following reasons: serious study limitations (no blinding of participants and personnel in 4/5 RCTs, no blinding of outcome assessment in 2/5 RCTs, incomplete outcome data in 1/5 RCTs, selective reporting in 1/5 RCTs, baseline imbalances and incorrect analysis in 1/5 RCTs, and loss of clusters in 1/5 RCTs. Allocation concealment and selective reporting were unclear in most studies. No indirectness of evidence; no inconsistency and imprecision of pooled results; and undetected publication bias. | ||||||
| Quantitative risk of bias score for sensitivity analysis | ||
| Trial | Summary ROB Score | Overall ROB |
| ‐1 | Unclear | |
| +1 | Unclear | |
| 0 | Unclear | |
| 0 | Unclear | |
| 0 | Unclear | |
| ‐2 | High | |
| ‐4 | High | |
| +2 | Low | |
| 0 | Unclear | |
| ‐1 | Unclear | |
| +1 | Unclear | |
| +3 | Low | |
| +1 | Unclear | |
| ROB: risk of bias | ||
| Intervention | Outcome | Risk Difference (RD) (95% CI) | I² Statistic for RD | Relative Risk (RR) (95% CI) |
| Multifaceted unadjusted | Received prophylaxis | 0.03 (0.00 to 0.05) | 64% | 1.07 (1.00 to 1.14) |
| Multifaceted adjusted | Received prophylaxis | 0.04 (0.02 to 0.06) | 0% | 1.06 (1.02 to 1.11) |
| Multifaceted lowest ICC | Received prophylaxis | 0.04 (0.02 to 0.06) | 0% | 1.06 (1.02 to 1.11) |
| Multifaceted mean ICC | Received prophylaxis | 0.04 (0.01 to 0.06) | 0% | 1.06 (1.01 to 1.11) |
| Multifaceted highest ICC | Received prophylaxis | 0.04 (0.01 to 0.06) | 0% | 1.06 (1.01 to 1.11) |
| ICCs were available for 4/5 (Cavalcanti 2016 = 0.13, Labarere 2007 = 0.24, Pai 2013 = 0.022, Roy 2016 = 0.002) trials included in this meta‐analysis. ICC's were not available for Anderson 1994. In this table adjustment for the cluster design effect was performed via reported ICCs. No ICC was applied to the one trial that did not report an ICC (Anderson 1994). We performed a sensitivity analysis using the lowest reported ICC (0.002), the mean reported ICC (0.0985), and the highest reported ICC (0.24) for the trial that did not report an ICC (Anderson 1994). All trials in the meta‐analysis were clustered designs. | ||||
| ICC: intracluster correlation Anderson 1994; Cavalcanti 2016; Labarere 2007; Pai 2013; Roy 2016 | ||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 3 | 0.21 | (0.15 to 0.27) | 1003/2523 | 451/2534 |
| Received appropriate prophylaxis | 3 | 0.16 | (0.12 to 0.20) | 419/906 | 279/914 |
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | 3 | 0.64 | (0.47 to 0.86) | 94/2675 | 149/2678 |
| Symptomatic DVT | 2 | 0.43a | (0.23 to 0.78) | 15/1255 | 35/1251 |
| 0.80b | (0.44 to 1.46) | 19/1238 | 24/1255 | ||
| Symptomatic PE | 2 | 0.40a | (0.22 to 0.74) | 14/1255 | 35/1251 |
| 0.63b | (0.21 to 1.93) | 5/1238 | 8/1255 | ||
| Asymptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | 2 | 1.01a | (0.87 to 1.17) | 282/1255 | 279/1251 |
| 1.04b | (0.88 to 1.24) | 215/1238 | 209/1255 | ||
| Sudden death | ‐ | ‐ | ‐ | ‐ | ‐ |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | 2 | 1.00a | (0.53 to 1.87) | 19/1255 | 19/1251 |
| 0.91b | (0.53 to 1.54) | 25/1238 | 28/1255 | ||
| Minor bleeding | 1 | 0.92a | (0.69 to 1.23) | 81/1255 | 88/1251 |
| Thrombocytopenia | ‐ | ‐ | ‐ | ‐ | ‐ |
| DVT: deep vein thrombosis Chapman 2011; Dexter 2001; Garcia 2009; Kucher 2005; Piazza 2009; Overhage 1996 | |||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 2 | 0.19a | (0.16 to 0.22) | 421/1255 | 182/1251 |
| 0.08b | (‐0.17 to 0.33) | 13/30 | 10/28 | ||
| Received appropriate prophylaxis | 1 | 0.17c | (0.12 to 0.21) | 228/664 | 116/662 |
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | 1 | 0.59a | (0.43 to 0.80) | 61/1255 | 103/1251 |
| Symptomatic DVT | 1 | 0.43a | (0.23 to 0.78) | 15/1255 | 35/1251 |
| Symptomatic PE | 1 | 0.40a | (0.22 to 0.74) | 14/1255 | 35/1251 |
| Asymptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | 1 | 1.01a | (0.87 to 1.17) | 282/1255 | 279/1251 |
| Sudden death | ‐ | ‐ | ‐ | ‐ | ‐ |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | 1 | 1.00a | (0.53 to 1.87) | 19/1255 | 19/1251 |
| Minor bleeding | 1 | 0.92a | (0.69 to 1.23) | 81/1255 | 88/1251 |
| Thrombocytopenia | ‐ | ‐ | ‐ | ‐ | ‐ |
| DVT: deep vein thrombosis | |||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 1 | 0.25a | (0.22 to 0.29) | 569/1238 | 259/1255 |
| Received appropriate prophylaxis | 2 | 0.14b | (0.05 to 0.24) | 147/182 | 114/172 |
| 0.12c | (‐0.03 to 0.28) | 44/60 | 49/80 | ||
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | 2 | 0.79a | (0.50 to 1.25) | 32/1238 | 41/1255 |
| 0.19b | (0.02 to 1.60) | 1/182 | 5/172 | ||
| Symptomatic DVT | 1 | 0.80a | (0.44 to 1.46) | 19/1238 | 24/1255 |
| Symptomatic PE | 1 | 0.63a | (0.21 to 1.93) | 5/1238 | 8/1255 |
| Asymptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | 1 | 1.04a | (0.88 to 1.24) | 215/1238 | 209/1255 |
| Sudden death | ‐ | ‐ | ‐ | ‐ | ‐ |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | 1 | 0.91a | (0.53 to 1.54) | 25/1238 | 28/1255 |
| Minor bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thrombocytopenia | ‐ | ‐ | ‐ | ‐ | ‐ |
| DVT: deep vein thrombosis | |||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 5 | 0.03 | (0.00 to 0.05) | 7306/13611 | 6509/12722 |
| Received appropriate prophylaxis | 2 | 0.03a | (‐0.00 to 0.06) | 263/1154 | 290/1457 |
| 0.02b | (0.01 to 0.03) | 1474/8359 | 1094/6992 | ||
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | 1 | 0.97b | (0.77 to 1.23) | 150/8068 | 128/6692 |
| Symptomatic DVT | 1 | 1.17b | (0.76 to 1.81) | 48/8068 | 34/6692 |
| Symptomatic PE | 1 | 0.71b | (0.44 to 1.15) | 31/8068 | 36/6692 |
| Asymptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | 1 | 1.21c | (0.86 to 1.70) | 49/315 | 64/497 |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | 2 | 1.02b | (0.93 to 1.12) | 940/8298 | 764/6884 |
| 0.95d | (0.88 to 1.01) | 1096/3327 | 1196/3434 | ||
| Sudden death | 1 | 1.01b | (0.72 to 1.43) | 72/8298 | 59/6884 |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | 1 | 0.96b | (0.72 to 1.28) | 100/8068 | 86/6692 |
| Minor bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thrombocytopenia | 1 | 0.53c | (0.02 to 12.86) | 0/315 | 1/497 |
| DVT: deep vein thrombosis Anderson 1994 CME + QA Group; Cavalcanti 2016; Hinchey 2010; Labarere 2007; Pai 2013; Roy 2016 | |||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 1 | ‐0.02 | (‐0.09 to 0.05) | 252/513 | 175/342 |
| Received appropriate prophylaxis | ‐ | ‐ | ‐ | ‐ | ‐ |
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Symptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Symptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sudden death | ‐ | ‐ | ‐ | ‐ | ‐ |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thrombocytopenia | ‐ | ‐ | ‐ | ‐ | ‐ |
| Anderson 1994 CME group | |||||
| DVT: deep vein thrombosis | |||||
| Outcomes | Number of studies | Risk Difference (RD) | 95% Confidence interval | Events, intervention | Events, control |
| Received prophylaxis | 1 | ‐0.05 | (‐0.12 to 0.02) | 115/360 | 133/359 |
| Received appropriate prophylaxis | ‐ | ‐ | ‐ | ‐ | ‐ |
| Venous thromboembolism outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Symptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Symptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Symptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic VTE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic DVT | ‐ | ‐ | ‐ | ‐ | ‐ |
| Asymptomatic PE | ‐ | ‐ | ‐ | ‐ | ‐ |
| Mortality | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sudden death | ‐ | ‐ | ‐ | ‐ | ‐ |
| Safety outcomes | Number of studies | Risk Ratio (RR) | 95% Confidence interval | Events, intervention | Events, control |
| Major bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thrombocytopenia | ‐ | ‐ | ‐ | ‐ | ‐ |
| DVT: deep vein thrombosis | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Received prophylaxis Show forest plot | 3 | 5057 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.15, 0.27] |
| 2 Received appropriate prophylaxis Show forest plot | 3 | 1820 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [0.12, 0.20] |
| 3 Symptomatic VTE Show forest plot | 3 | 5353 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Received prophylaxis (unadjusted) Show forest plot | 5 | 26330 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.05] |
| 2 Received prophylaxis (adjusted) Show forest plot | 5 | 9198 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.02, 0.06] |
| 3 Received prophylaxis (adjusted) sensitivity analysis: applied lowest ICC from other trials Show forest plot | 5 | 9089 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.02, 0.06] |
| 4 Received prophylaxis (adjusted) sensitivity analysis: applied mean ICC from other trials Show forest plot | 5 | 8491 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 5 Received prophylaxis (adjusted) sensitivity analysis: applied highest ICC from trials Show forest plot | 5 | 8440 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |

