Ubat barisan pertama yang menghalang sistem renin angiotensin berbanding kelas ubat antihipertensi barisan pertama untuk hipertensi
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: computer‐generated randomization Blinding: double‐blinded, and blinded assessment Duration: 4.9 ± 1.4 years Funding: National Heart, Lung and Blood Institute and financial support from Pfizer | |
| Participants | Diagnosis: the risk factors included previous (> 6 months) MI or stroke, LVH demonstrated by ECG or echocardiography, history of T2DM, current cigarette smoking, HDL of < 35 mg/dL (< 0.91 mmol/L), or documentation of other atherosclerotic CVD N = 33357 Age: 55 years or older Sex: 47% women, 53% men History: not reported Inclusion criteria: stage 1 or 2 hypertension, 55 years or older, 1 additional risk factor for CHD events Excluded: individual with a history of hospitalized or treated symptomatic HF and/or left ventricular ejection fraction of < 35% | |
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine; thiazide: chlorthalidone Step 1: 12.5 mg/day, 12.5 mg/day (sham titration), 25 mg/day for chlorthalidone; 2.5 mg/day, 5 mg/day, 10 mg/day for amlodipine; 10 mg/day, 20 mg/day, 40 mg/day for lisinopril Step 2: add‐on, atenolol 25 mg/day‐100 mg/day; 0.05 mg/day‐0.2 mg/day of reserpine; clonidine 0.1 mg‐0.3 mg twice daily Step 3: 25 mg‐100 mg twice daily of hydralazine. Other drugs, including low doses of open‐label step 1 drug classes, were permitted if clinically indicated | |
| Outcomes | Primary outcomes: fatal CHD or non‐fatal MI combined Secondary outcomes:
Other secondary outcomes: cancer, incident ECG LVH, ESRF (dialysis, renal transplant, or death), slope of the reciprocal of longitudinal serum creatinine measurements | |
| Notes | Participants assigned to lisinopril were less likely to be black and more likely to be women, had untreated hypertension, evidence of CHD or atherosclerotic CVD, and a lower mean serum glucose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Clinical trials center was used |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical trials center judged by clinic investigator reports, copies of death certificates, and hospital discharge summaries |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Although the study was supported by the government and industry, insufficient information was found to evaluate the risk as high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: this study was supported by a grant from AstraZeneca | |
| Participants | Diagnosis: uncontrolled essential hypertension: 160/100 mmHg in untreated participants or 140/90 mmHg in treated participants plus evidence of target‐organ damage; accelerated hypertension: 220/120 mmHg N = 88 Median age (range): candesartan group 56 (37‐73) years; atenolol group 54 (39‐76) years Sex: 37.5% women, 62.5% men History: median duration of hypertension (range): candesartan 4 (1‐36) years; atenolol 3 (1‐36) years Inclusion criteria: uncontrolled essential hypertension Exclusion criteria: evidence of accelerated hypertension, MI or stroke within previous 6 months, DM, or any other condition that precluded participation | |
| Interventions | RAS inhibitor: candesartan; beta‐blocker: atenolol Candesartan 8 mg or 16 mg daily Atenolol 50 mg or 100 mg daily Add‐ons HCTZ, felodipine doxazosin | |
| Outcomes | SBP and DBP were measured in the right arm with an Omron HEM‐705‐CP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | There were no patient withdrawals |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 months Funding: supported in part by Abbott (Ludwigshafen, Germany) | |
| Participants | Diagnosis: arterial hypertension, defined as an untreated SBP ≥ 130 mmHg or a DBP ≥ 85 mmHg, or as the need for antihypertensive therapy to attain a SBP or DBP under these levels T2DM diagnosed according to the criteria of the WHO N = 604: trandolapril group 301; verapamil group 303 Age: trandolapril group 61.6 ± 8.1 years; verapamil group 62.5 ± 8.2 years Sex: 47% women, 53% men History: duration of diabetes (SD): trandolapril group mean 7.7 (6.7) years; verapamil group 8.2 (6.4) years Inclusion criteria: people aged 40 years or older with hypertension and a known history of T2DM not exceeding 25 years, a urinary albumin excretion rate > 20 μg/min in at least 2 of 3 consecutive, sterile, overnight samples, and a serum creatinine concentration of ≤ 1.5 mg/dL (133 μmol/L) Exclusion criteria: HbA1c > 11%, nondiabetic renal disease, and a specific indication for or contraindication to ACE‐inhibitor therapy or non‐dihydropyridine CCB therapy | |
| Interventions | RAS inhibitor: trandolapril; CCB: verapamil Verapamil 240 mg/day Trandolapril 2 mg/day Add‐ons step 1, HCTZ or furosemide; step 2, doxazosin, prazosin, clonidine, methyldopa, or beta‐blockers (allowed on the basis of specific indications, such as cardiac ischemic disease, but only if not contraindicated on the basis of ECG findings, such as bradyarrhythmias and delayed atrioventricular conduction); and step 3, minoxidil or long‐acting dihydropyridine CCBs. The use of potassium‐sparing diuretics, inhibitors of the renin–angiotensin system, and non‐dihydropyridine CCBs different from the study drugs was not allowed | |
| Outcomes | Trough SBP and DBP (Korotkoff phases 1 and 5, respectively) recorded as the mean of 3 morning measurements (to the nearest 2 mmHg) taken before the administration of a study drug CV death | |
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes in the Methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: this work was supported by grants from Institut de Recherches Internationales Servier and the Danish Heart Foundation. MJM had support from the Danish Medical Research Council | |
| Participants | Diagnosis: sitting DBP was 100 mmHg‐120 mmHg, measured 3 times with a mercury sphygmomanometer N = 30 Age: perindopril group 49 ± 2 years; atenolol group 51 ± 2 years Sex: 27% women, 73% men History: not reported Inclusion criteria: people with sitting DBP of 100mmHg‐120 mmHg. People suspected of secondary hypertension underwent isotope renography, spiral computed tomographic scan of the renal arteries, or urinary sampling of catecholamines, but none showed signs of secondary hypertension, and all were included Excluded: not reported | |
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg or 8 mg daily Atenolol 50 mg or 100 mg daily Add‐on, bendroflumethiazide | |
| Outcomes | HR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomization was balanced to ensure an equal gender and age distribution in the 2 groups Blinding: double‐blinded Duration: 1 year Funding: this work was supported in part by grants from Institute de Recherces Internationales Servier and the Danish Heart Foundation | |
| Participants | Diagnosis: not reported N = 31 Age: perindopril group 49 ± 7.7 years, atenolol group 51 ± 7.7 years Sex: 26% women, 74% men History: not reported Inclusion criteria: sitting DBP of 100 mmHg–120 mmHg Exclusion criteria: symptoms or signs of ischemic heart disease or secondary hypertension | |
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg or 8 mg daily Atenolol 50 mg or 100 mg daily Add‐ons, bendroflumethiazide | |
| Outcomes | BPs measured 3 times with a mercury sphygmomanometer, on 2 or 3 occasions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Low risk | Government funded trial |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: Gothenburg Medical Society and Merck Sharp and Dohme (Sweden) AB | |
| Participants | Diagnosis: non‐malignant essential hypertension: DBP > 95 mmHg at least 3 times on placebo N = 28 Age: 22‐64 years Sex: 100% men History: not reported Inclusion criterion: previously untreated men with non‐malignant essential hypertension Exclusion criteria: secondary hypertension or signs of CV end‐organ damage (except LVH and hypertensive retinopathy) | |
| Interventions | RAS inhibitor: enalapril; thiazide: HCTZ Enalapril average daily doses 34.9 mg HCTZ average daily doses 53.5 mg | |
| Outcomes | Supine BP: mercury sphygmomanometer, adequate cuff size, Korotkoff sounds 1 and 5 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "enalapril and hydrochlorothiazide were compared in a double‐blinded, randomised, parallel group design." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Quoted, "the groups were well balanced regarding demographic variables, cardiac hypertrophy and retinopathy." "All patients were still on randomised monotherapy after 6 months and were included in the analysis irrespective of blood pressure response." |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 36 weeks Funding: Merck Co Inc | |
| Participants | Diagnosis: not reported N = 210 Age: 21‐80 years Sex: 39% women, 61% men History: not reported Inclusion criteria: men and women, aged 21–80 years, with mild to moderate essential hypertension and ECG‐documented LVH assessed up to 30 days before enrolment. Eligible participants had trough sitting DBP of 95–115 mmHg or sitting SBP of 160 mmHg–200 mmHg, or both, while receiving placebo for 2–4 weeks, and a left ventricular mass index (LVMI) > 120 g/m² for men and > 105 g/m² for women Exclusion criteria: a LV end‐diastolic dimension > 60 mm, irrespective of the LVMI, systolic dysfunction or significant valvular disease | |
| Interventions | RAS inhibitor: losartan; beta‐blocker: atenolol Losartan 50 mg or 100 mg daily Atenolol 50 mg or 100 mg daily Add‐on, HCTZ | |
| Outcomes | Clinical DBP and SBP were measured at trough (22–26 hours after the previous study medication) with a standard mercury sphygmomanometer, with the participant in the sitting position after 5 min of rest, at every clinic visit (baseline and treatment). The trialists used means of 3 consecutive measurements at 2–3 min intervals | |
| Notes | The patient population included in this study differed from those included in the LIFE echocardiographic sub‐study, although the treatment regimens compared were the same | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: not reported | |
| Participants | Diagnosis: persistent microalbuminuria: AER 20 g/min‐200 g/min during the last 3 months; mild to moderate hypertension: mean DBP between 85 mmHg‐109 mmHg and SBP < 180 mmHg; T2DM: diagnosed according to the criteria of the WHO N = 180 Age: 40‐70 years Sex: 27% women, 73% men History: time since diabetes diagnosed (years): lercanidipine group 10 ± 6.4; atenolol group 11 ± 7.9 (means ± SD) Inclusion criteria: mild to moderate hypertensive people aged from 40‐70 years, affected by T2DM with the presence of persistent microalbuminuria Exclusion criteria: arterial hypertension outside the range specified above; secondary arterial hypertension; orthostatic hypotension (SBP decrease > 20 mmHg after standing for 2 min); AER < 20 μg/min, ≥ 20 μg/min not persistent, > 200 μg/min; HbA1c > 10%; cardiac insufficiency (classes NYHA III‐IV); arrhythmias; valvular disease; CHDs; unstable angina pectoris; complete left bundle branch block; HR < 50 or > 100 bpm; acute MI or cerebrovascular accident 3 months prior to recruitment; transaminases > 2 times the normal limit; serum creatinine > 141.4 μmol/L; anemia (hemoglobin <10 g/dL); hypertensive retinopathy grade III‐IV; obesity (body mass index > 35 kg/m2); known hypersensitivity to dihydropyridine derivates or to ACE‐inhibitors | |
| Interventions | RAS inhibitor: ramipril; CCB: lercanidipine Lercanidipine 10 mg or 20 mg/day Ramipril 5 mg or 10 mg/day Add‐ons HCTZ, atenolol | |
| Outcomes | BP measured using a mercury sphygmomanometer (Korotkoff phase 1 and 5) with participants in a sitting position after at least 10 min of rest. 2 blood pressure recordings, taken 3 min apart, were obtained. If the 2 DBP values differed by more than 5 mmHg, an additional measurement was taken and included in the calculated average HF Stroke | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomization accomplished by the drawing of envelopes containing randomization codes prepared by a statistician Blinding: double‐blinded. All study staff were blinded to treatment assignment Duration: 12 months Funding: not reported | |
| Participants | Diagnosis: nonsmoking people with T2DM for ≥ 2 years (HbA1c < 7.0%); mild hypertension (DBP 90mmHg‐99 mmHg) N = 116 Age: telmisartan group 52 ± 5 years, nifedipine group 53 ± 4 years Sex: 50% women, 50% men History: known DM for > 2 years Inclusion criteria: mild hypertension with T2DM Exclusion criteria: secondary hypertension; malignant hypertension; unstable angina; MI within the preceding 6 months; abnormalities of liver or renal function; or contraindications to or current use of ARBs or ACE inhibitors | |
| Interventions | RAS inhibitor: telmisartan; CCB: nifedipine Telmisartan 40 mg/day Nifedipine Gastro‐Intestinal Therapeutic System (GITS) 20 mg/day | |
| Outcomes | BP was measured using a standard mercury sphygmomanometer (Korotkoff 1 and 5) in the seated position | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was accomplished by the drawing of envelopes containing randomization codes prepared by a statistician." Whether the envelopes were opaque was not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "this 12‐months, randomised, double‐blind trial was conducted at the Department of Internal Medicine and Therapeutics of the University of Pavia in Italy." "All study staff were blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "a copy of the code was provided only to the individual responsible for performing the statistical analysis." Reviewer comment: possible high risk of bias because the statistical analysis was not blinded, but it would not result in detection bias; the method of blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | High risk | Quote: "at each clinical visit, heart rate was measured after the patient had been seated for >=10 minutes." Reviewer comment: high risk due to failure to report HR |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomization performed by the drawing of envelopes containing randomization codes prepared by a statistician Blinding: blinding of the investigators and participants was maintained by using identical numbered bottles prepared by the hospital pharmacy Duration: 12 months Funding: not reported | |
| Participants | Diagnosis: not reported N = 96 Age: doxazosin group 53 ± 9 years, irbesartan group 52 ± 10 years Sex: 51% women, 49% men History: not reported Inclusion criteria: T2DM; mild hypertension (DBP > 90 mmHg and < 105 mmHg) Exclusion criteria: secondary hypertension; malignant hypertension; unstable angina; MI; and/or liver/renal function abnormalities | |
| Interventions | RAS inhibitor: irbesartan; alpha‐blocker: doxazosin Doxazosin 4 mg daily Irbesartan 300 mg daily | |
| Outcomes | SBP (Korotkoff 1) and DBP (Korotkoff 4) measurements were obtained from participants in the seated position using a standard mercury sphygmomanometer (Erkameter 3000, ERKA, Bad Tolz, Germany) with a cuff of appropriate size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was performed by the drawing of envelopes containing randomization codes prepared by a statistician." Whether the envelopes were opaque was not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "ninety‐six patients with type 2 diabetes … were enrolled in this randomised, double‐blind trial." "Blinding of investigators and patients was maintained using identical numbered bottles prepared by the hospital pharmacy." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "… no subject experienced adverse effects serious enough to warrant discontinuing either drug … " Despite the absence of numbers for participants reported in the data tables, the statement above was sufficient |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blindness: double blind. Interventions were supplied as identical, opaque, white capsules in coded bottles to ensure the blind status of the study Duration: 24 months Funding: not reported | |
| Participants | Diagnosis: essential hypertension [DBP >90 and <110 mmHg and/or SBP>140 mmHg and <180 mmHg] N= 222 Age: < 65 years old Sex: 51.8% women, 48.2% men History: no reported Inclusion criteria: outpatients of both sex, aged < 65 years, with a first diagnosis of essential hypertension and naïve to antihypertensive treatment Excluded: secondary hypertension, severe hypertension (SBP >180 mmHg or DBP >110 mmHg), hypertrophic cardiomyopathies due to aetiologies other than hypertension, history of heart failure or a left ventricular ejection fraction (LVEF) ≤50%, history of angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery or myocardial infarction any time prior to visit 1, concurrent symptomatic arrhythmia, liver dysfunction (AST or ALT values exceeding 2‐fold the upper limit), creatinine >1.5 mg/dL and known hypersensitivity to the study drugs. Pregnant women as well as women of childbearing potential were excluded. Patients with endocrine, infective or inflammatory disorders were excluded, as well as were those taking anti‐inflammatory medications | |
| Interventions | RAS inhibitor: enalapril; CCB: lercanidipine Enalapril 20mg daily Lercanidipine 10mg daily | |
| Outcomes | Blood pressure measurements were obtained from each patient (using the right arm) in the seated position, using a standard mercury sphygmomanometer (Erkameter 3000; ERKA, Bad Tolz, Germany) (Korotkoff I and V) with a cuff of appropriate size. BP was measured by the same investigator at each visit, in the morning before daily drug intake and after the patient had rested for ≥10 min in a quiet room. Three successive BPreadings were obtained at 1‐min intervals, and the mean of the 3 readings was calculated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data was unlikely to have an impact on the results of the trial. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported. |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low. |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 weeks Funding: this study was supported by grant CDSP 964‐0A from Merck & Co, Whitehouse Station, NJ | |
| Participants | Diagnosis: not reported N = 303; enalapril group 148; nifedipine group 155 Age: nalapril group 3.5 ± 9.0 years; nifedipine group 63.0 ± 8.6 years Sex : 34.3% women, 65.7% men History: not reported Inclusion criteria: seated SBP of 140 mmHg and/or DBP of 90 mmHg (Korotkoff phase 5) for previous 4 weeks if taking antihypertensive medications or 150 mmHg and/or 90 mmHg, respectively, if unmedicated Exclusion criteria: people with left ventricular ejection fraction ,40%, severe valvular disease, or coexisting cardiomyopathy on screening ECG. Initially, people receiving treatment with ACE inhibitors or CCBs were excluded | |
| Interventions | RAS inhibitor: enalapril; CCB: nifedipine Enalapril 10 mg or 20 mg/day Nifedipine 30 mg or 60 mg/day Add‐ons HCTZ, atenolol | |
| Outcomes | Reduction of SBP, DBP, and HR | |
| Notes | When the frequent use of ACE inhibitors or CCBs by participants with LVH became evident, participants were enrolled with stratified randomization to assure balanced representation in both treatment arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: the randomization schedule was generated by a statistical analysis system Blinding: double‐blinded Duration: 3 years Funding: Pfizer Inc. The biostatistics department of Pfizer was responsible for entering data, quality controls, and blinded statistical analysis; Pfizer had no other role in the study performance, analysis, and reporting | |
| Participants | Diagnosis: malignant hypertension i.e. DBP > 120 mmHg; congestive heart disease according to New York Heart Association class II–IV N = 263 Age: 18‐80 years Sex: 40.7% women, 59.3% men History: not reported Inclusion criteria: nondiabetic adults, aged 18‐80 years, non‐nephrotic adult hypertensive patients with creatinine clearance of 20 mL‐ 60 mL/min·1.73 m² (Cockcroft‐Gault) Exclusion criteria: nephrotic proteinuria; secondary or malignant hypertension; a major CV event within previous 3 months; angina pectoris; CHD; uncontrolled arrhythmias; II–III degree atrioventricular block; need for steroids, nonsteroidal anti‐inflammatory or cytotoxic drugs; women of childbearing potential not using appropriate contraception; or any disease that could limit the ability of the patient to comply with the protocol requirements | |
| Interventions | RAS inhibitor: enalapril; CCB: amlodipine Amlodipine 5 mg or 10 mg/day Enalapril 5 mg or 10 mg/day Add‐ons: atenolol (50 mg/day‐100 mg/day), loop diuretics (furosemide, 20 mg/day‐500 mg/day or torsemide 5 mg/day‐200 mg/day), alpha‐blockers (prazosin, 2.5 mg/day‐5 mg/day or doxazosin 1 mg/day‐16 mg/day), and centrally acting drugs (rilmenidine, 1 mg/day‐2 mg/day or methyldopa 250 mg/day‐500 mg/day) | |
| Outcomes | All‐cause death, renal failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization schedule was generated by a statistical analysis system |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Although the role of the funding company was unlikely to have an impact on the study, no other information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomly assigned Blinding: double‐blinded Duration: 67 months Funding: Bayer Pharmaceutical Company, a grant (DK50298‐02) from the National Institute of Diabetes and Digestive and Kidney Diseases; Dr Hiatt was the recipient of an Academic Award in Vascular Disease from the National Institutes of Health | |
| Participants | Diagnosis: NIDDM, mean base‐line DBP ≥ 90 mmHg N = 470 (all in analysis) Age: 40‐74 years Sex: 32.6% women, 67.4% men History: not reported, probably outpatients Inclusion criteria: participants enrolled in the ABCD Trial were between the ages of 40 and 74 years at the time of recruitment and were identified according to diagnosis‐related groups from the pharmacy and billing lists of participating healthcare systems in the Denver metropolitan area. All participants in the ABCD Trial had NIDDM, diagnosed according to criteria based on those of the WHO report of 1985. All enrolled subjects had DBP of 80 mmHg or higher and were receiving no antihypertensive medications at the time of randomization Exclusion criteria: a known allergy to dihydropyridine CCBs or ACE inhibitors; MI or cerebrovascular accident within the previous 6 months; coronary‐artery bypass surgery within the previous 3 months; unstable angina pectoris within the previous 6 months; New York Heart Association class III or IV CHF; an absolute need for therapy with ACE inhibitors or CCBs; were receiving hemodialysis or peritoneal dialysis; or serum creatinine concentration > 3 mg/dL (265 μmol/L) | |
| Interventions | RAS inhibitor: enalapril; CCB: nisoldipine Nisoldipine 10 mg/day, with increases to 20 mg/day, 40 mg/day, and 60 mg/day, plus placebo in place of enalapril (Sular, Zeneca, Wilmington, Del) Enalapril 5 mg/day, with increases to 10 mg/day, 20 mg/day, and 40 mg/day, plus placebo in place of nisoldipine (Vasotec, Merck, Whitehouse Station, NJ) Open‐label, step‐wise, additional medication: metoprolol and HCTZ when participants did not achieve the target BP Notes: 99 participants in the enalapril group took a beta‐blocker, compared with 89 in the nisoldipine group (P value 0.035). 119 participants assigned to enalapril took a diuretic agent, as did 93 assigned to nisoldipine (P value 0.02) | |
| Outcomes | Binary data: fatal MI, non‐fatal MI, cerebrovascular accident, congestive HF, death from CV causes, death from any cause | |
| Notes | Significantly more participants discontinued nisoldipine than enalapril because of headaches (P value 0.009). Significantly more discontinued enalapril because of malaise or fatigue (P value 0.005) or uncontrolled hypertension (P value 0.04) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | The drugs and placebos were administered in a double‐blinded manner |
| Blinding of outcome assessment (detection bias) | Low risk | All CV events were reviewed by an independent endpoints committee whose members were blinded to the patients' assigned treatment groups |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | High risk | Add‐on medication was not balanced between groups. Quote: "Ninety‐nine patients in the enalapril group took a beta‐blocker, as compared with 89 patients in the nisoldipine group (P=0.035). One hundred nineteen patients assigned to enalapril took a diuretic agent, as compared to 93 assigned to nisoldipine (P=0.02)." |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: no funding source reported | |
| Participants | Diagnosis: stage I hypertension: SBP ≥ 140 mmHg and < 160 mmHg and/or DBP ≥ 90 mmHg and < 100 mmHg N = 378 Age: 68 ± 8 years old Sex: 54.8% women, 45.2% men History: 49.7% participants had enlarged atrial size Inclusion criteria: consecutive outpatients of either sex, age 40–80 years, with stage I hypertension; in sinus rhythm, but with ≥ 2 ECG‐documented episodes of symptomatic AF in the previous 6 months, each lasting > 60 min but < 7 days and terminating spontaneously Exclusion criteria: ECG evidence of (LVH; treatment with ARBs, ACE inhibitors, or antiarrhythmic agents; cardioversion within the previous 3 months; secondary hypertension; MI or stroke in the preceding 6 months; CHF; coronary heart disease; valvular disease; cardiac surgery during the previous 6 months; significant thyroid, pulmonary, renal, or hepatic disease;pregnancy or fertile woman; and known hypersensitivity or contraindications to the study medications | |
| Interventions | RAS inhibitor: telmisartan; CCB: amlodipine Telmisartan 80 mg per day for the previous 4 weeks, 120 mg per day for 5th to 8th week, 160 mg per day until the end of the study Amlodipine 5 mg per day for the previous 4 weeks, 7.5 mg per day for 5th to 8th week, 10 mg per day until the end of the study | |
| Outcomes | BP measured in the seated position using a standard mercury sphygmomanometer (Korotkoff I and V) with a cuff of appropriate size. Measurements always taken in the morning before daily drug intake (i.e. 24 hours after dosing, at trough) and after the subject had rested for 10 min in a quiet room. 3 successive BP readings taken at 1‐min intervals and averaged | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Statement as "randomised, controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Data flow was clearly stated, and missing data had little influence on results |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: Bayer | |
| Participants | Diagnosis: "The criteria for hypertension were a sitting DBP in the range 90–115 mmHg and a SBP < 200 mmHg, in patients who had not been administered blood pressure lowering drugs during the previous weeks." N = 80 Age: nitrendipine group 66.9 ± 6.2 years, enalapril group 58.8 ± 9.5 years Sex: 38.8% women, 61.2% men History: not reported Inclusion criteria: people with NIDDM and hypertension who were being treated by general practitioners in the Rotterdam area; DM diagnosed by general practitioner. Participants were being treated with diet or drugs (either an oral hypoglycemic or insulin); metabolic control had to be acceptable and was defined as an HbA1c level < 11.5% Exclusion criteria: class III or IV CHF, uncontrolled arrhythmias or severe or unstable angina pectoris; MI or stroke during the previous 3 months; history of other major illnesses or known intolerance to dihydropyridines or ACE inhibitors | |
| Interventions | RAS inhibitor: enalapril; CCB: nitrendipine Nitrendipine 20 mg twice a day for previous 4 weeks, 40 mg twice a day until the end of the study Enalapril 20 mg once a day for previous 4 weeks, 40 mg once a day until the end of the study Acebutolol added when needed. | |
| Outcomes | Changes in DBP, and SBP measured using an automated device (Dinamap, Arlington, Texas, USA) MI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: the Cooperative Studies Program of the Department of Veterans Affairs Research and Development Service | |
| Participants | Diagnosis: not reported N = 1105 Age: captopril group 57.4 ± 10 years, atenolol group 60.4 ± 9.4 years, diltiazem group 59.5 ± 9.2 years, prazosin group 60.1 ± 8.1 years, HCTZ group 58.1 ± 11.5 years, clonidine group 58.3 ± 9.8 years Sex: 100% men History: not reported Inclusion criteria: DBP 95 mmHg‐109 mmHg Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: captopril; CCB: diltiazem; thiazide: HCTZ; beta‐blocker: atenolol; alpha‐blocker: prazosin; CNS active drug: clonidine Atenolol 25 mg, 50 mg, 100 mg daily for 8‐week titration and 100mg daily for maintenance Captopril 12.5 mg, 25 mg, 50 mg twice daily for 8‐week titration and 50mg daily for maintenance Clonidine 0.1 mg, 0.2 mg, 0.3 mg twice daily for 8‐week titration and 0.3mg daily for maintenance Diltiazem‐SR 60 mg, 120 mg, 180 mg twice daily for 8‐week titration and 180mg daily for maintenance HCTZ 12.5 mg, 25 mg, 50 mg daily for 8‐week titration and 50mg daily for maintenance Prazosin 2 mg, 5 mg, 10 mg twice daily for 8‐week titration and 10mg daily for maintenance | |
| Outcomes | BP was measured with a cuff sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "… were randomly allocated to double‐blind treatment with 1 of 6 drugs." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | High risk | The number of participants accounted for in analysis of each group in Table 1 in the original article was far fewer than those included in the study |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 12 months Funding: NIA and NIH | |
| Participants | Diagnosis: hypertension i.e. SBP > 140 mmHg or DBP < 90 mmHg or receiving antihypertensive medications N = 47 Age: 60 years and above Sex: 57.4% women, 42.6% men History: Coronary artery disease: lisinopril group 35%; candesartan group 56%; HCTZ group 46% Hyperlipidemia: lisinopril group 35%; candesartan group 56%; HCTZ group 38% Inclusion criteria: 60 years or older, hypertension, executive dysfunction based on a score < 10 on the executive clock draw test (CLOX1) Exclusion criteria: individuals with possible dementia; intolerance to the study medications; SBP > 200 mmHg, DBP > 110 mmHg; serum creatinine > 2.0 mg/dL or serum potassium > 5.3 mEq/dL at baseline; receiving > 2 antihypertensive medications; presence of CHF, DM, or stroke; and inability to perform the study procedures or unwilling to stop currently used antihypertensive medications | |
| Interventions | RAS inhibitors: lisinopril, candesartan; thiazide: HCTZ Lisinopril: 10 mg increased to 20 mg then 40 mg if needed Candesartan: 8 mg increased to 16 mg then 32 mg if needed HCTZ: 12.5 mg increased to 25 mg if needed Long‐acting nifedipine (30 mg increased to 60 mg and 90 mg) was added, followed by long‐acting metoprolol (12.5 mg increased to 25 mg and 50 mg) if needed. | |
| Outcomes | BP: 2 seated blood pressure readings were performed and averaged at each visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a computer‐generated random allocation sequence occurred after baseline data collection |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | The drugs were administered in a double‐blinded manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Data flow was clearly stated |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded. All drugs were given as capsules of identical appearance Duration: 26 weeks Funding: not reported | |
| Participants | Diagnosis: not reported N = 220 Age: range:30‐77 years; mean age: carvedilol group 57 years, captopril group 58 years Sex: 40% women, 60% men History: not reported Inclusion criteria: essential hypertension with a DBP of 95 mmHg‐114 mmHg and dyslipidemia Exclusion criteria: secondary hypertension; unstable angina; gross hepatic or renal impairment; insulin‐dependent or unstable DM; or other major diseases | |
| Interventions | RAS inhibitor: captopril; beta‐blocker: carvedilol Carvedilol 25 mg‐50 mg daily Captopril 25 mg‐50 mg daily | |
| Outcomes | BP was measured with a calibrated mercury sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to fixed oral doses of …" Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was a multicenter, double‐blind, randomised (block size of 4), parallel group trial,…" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "233 were randomised to treatment (carvedilol 116, captopril 117)… 13 patents prematurely terminated the study after randomization, of whom 7 (carvedilol 1, captopril 6) were withdrawn because of protocol violation…The others who withdrew prematurely (carvedilol 5, captopril 1) were regarded as being eligible for the efficacy analysis until their last day in the trial." |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 42 weeks Funding: Novartis Pharmaceuticals | |
| Participants | Diagnosis: moderate hypertension i.e. SBP ≥ 140 mmHg, DBP < 110 mmHg, and pulse pressure ≥ 50 mmHg N = 109 Age: 50‐75 years Sex: 100% women History: duration of hypertension ± SD; taking valsartan for 6.8 ± 7 years; taking amlodipine for 8.3 ± 6.4 years Inclusion criteria: postmenopausal women; moderate hypertension Exclusion criteria: BP above the safety limit of SBP ≥ 180 mmHg and ⁄ or DBP ≥ 110 mmHg before or at any point during the study; people with a history of type 1 or T2DM; Raynaud disease; AF or other arrhythmia; evidence of secondary form of hypertension; cerebrovascular accidents; transient ischemic cerebral attack or MI; CHF; clinically significant valvular heart disease; history of malignancy including leukemia and lymphoma; life‐threatening disease; known hypersensitivity or contraindications to valsartan, other ARBs, thiazide diuretics, amlodipine or other CCBs, and glycerin trinitrite | |
| Interventions | RAS inhibitor: valsartan; CCB: amlodipine Valsartan 160 mg per day for previous 4 weeks, force‐titrated to 320 mg until the end of the study Amlodipine 5 mgper day for previous 4 weeks, force‐titrated to , 10 mg until the end of the study Open label HCTZ added if needed for week 12 onwards | |
| Outcomes | BP measured using a standard sphygmomanometer with the appropriate cuff size in accordance with the American Heart Association Committee Report on BP determination. All BPs were measured 3 times at 1‐min intervals while the participant was sitting for a minimum of 5 min | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "randomised, controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | High risk | The number of participants in each group in figure 1 and table 2 did not match |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: not reported | |
| Participants | Diagnosis: not reported N = 149 Age: cilazapril group 65 ± 6.9 years, atenolol group 67 ± 6.2 years Sex: 52.3% women, 47.7% men History: not reported Inclusion criteria: DBP 95 mmHg‐115 mmHg Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: cilazapril; beta‐blocker: atenolol Cilazapril 2.5 mg or 5 mg/day Atenolol 50 mg or 100 mg/day | |
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "a prospective, randomised, double blind trial …" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 52 weeks Funding: Pfizer International | |
| Participants | Diagnosis: hypertension defined as a sitting BP not taking drugs for hypertension > 140/90 mmHg. N = 25 Age: 24‐71 years Sex: 32% women, 68% men History: duration of hypertension: lisinopril group: 11 ± 3.60 years; amlodipine group: 54 ± 1.84 years Inclusion criteria: untreated hypertension (previously untreated or antihypertensive treatment discontinued for at least 1 year) Exclusion criteria: accelerated hypertension; secondary hypertension; DM; familial hypercholesterolemia; HF or any other significant concomitant disease | |
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine Amlodipine 5 mg‐10 mg daily Lisinopril 5 mg‐20 mg daily Open‐label add‐on medication, doxazosin and bendroflumethiazide | |
| Outcomes | Clinical SBP and DBP were measured in the right arm of the individual seated using a validated semiautomated sphygmomanometer (Sentron, Bard Biochemical, Illinois, USA) HR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: Bristol‐Myers Squibb Institute for Medical Research and Sanofi‐Synthelabo. Additionally, Dr Berl has received research grants from Pfizer | |
| Participants | Diagnosis: hypertension: SBP of > 135 mmHg while sitting, DBP of > 85 mmHg while sitting, or documented treatment with antihypertensive agents ESRF: as indicated by the initiation of dialysis, renal transplantation, or a serum creatinine concentration of at least 6.0 mg/dL (530 μmol/L) N = 1146 Age: irbesartan group 59.3 ± 7.1 years; amlodipine group 59.1 ± 7.9 years Sex: 35.7% women, 64.3% men History: CVD: irbesartan group 158 (27%); amlodipine group 171 (30%) Inclusion criteria: aged 30‐70 years, a documented diagnosis of T2DM, hypertension, and proteinuria, with urinary protein excretion of at least 900 mg/24 hours; serum creatinine concentration 1.0 mg/dL‐3.0 mg/dL (88 μmol/L and 265 μmol/L) in women and 1.2 mg/dL‐3.0 mg/dL (106 μmol/L and 265 μmol/L) in men Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: irbesartan; CCB: amlodipine Irbesartan 75 mg to 300 mg/day Amlodipine 2.5 mg to 10 mg/day Add‐ons, other antihypertensive agents except ACE inhibitors, ARBs, and CCB | |
| Outcomes | ESRF, death from any cause | |
| Notes | Randomization was performed by central office. However, generation of randomization sequence was not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | High risk | Many authors had received research grants form Bristol‐Myers Squibb |
| Methods | Allocation: used a computer‐generated allocation schedule Blinding: participants, clinicians, and assessment blinded. "LIFE is an investigator‐initiated, double‐masked, double‐dummy, randomised comparison", "An endpoint classification committee of two masked clinicians reviewed clinical records of all CV events reported by clinical centers to determine whether they met endpoint criteria." Duration: at least 4 years, mean 4.8 ± 0.9 years Funding: study data was in Merck database. Merck provided steering committee for this review free access to all data | |
| Participants | Diagnosis: not reported N = 9193 Age: 55‐80 years Sex: 54.0%, 46.0% men History: Any vascular disease: losartan group1203 (26%); atenolol group1104 (24%); all participants 2307 (25%) Coronary heart disease: losartan group 771 (17%); atenolol group 698 (15%); all participants 1469 (16%) Cerebrovascular disease: losartan group 369 (8%); atenolol group 359 (8%); all participants 728 (8%) Peripheral vascular disease: losartan group 276 (6%); atenolol group 244 (5%); all participants 520 (6%) AF: losartan group 150 (3%); atenolol group 174 (4%); all participants 324 (4%) Isolated systolic hypertension: losartan group 660 (14%); atenolol group 666 (15%); all participants 1326 (14%) DM: losartan group 586 (13%); atenolol group 609 (13%); all participants 1195 (13%) Inclusion criteria: previously treated or untreated hypertension and ECG signs of LVH Trough sitting SBP 160 mmHg–200 mmHg, DBP 95 mmHg‐115 mmHg, or both Exclusion criteria: secondary hypertension; MI or stroke within the previous 6 months; angina pectoris requiring treatment with beta‐blockers or calcium‐antagonists; HF or LV ejection fraction of ≤ 40%; or a disorder that, in the treating physician's opinion, required treatment with losartan or another angiotensin–II type 1‐receptor antagonist, atenolol or another beta‐blocker, HCTZ, or ACE inhibitors | |
| Interventions | RAS inhibitor: losartan; beta‐blocker: atenolol. Losartan: mean 82 ± 24 mg Atenolol: mean 79 ± 26 mg HCTZ added when needed | |
| Outcomes | Change in SBP, change in sitting SBP, sitting DBP, HR. Primary endpoint: CV morbidity, death and a composite endpoint (CV death, MI, stroke) An independent endpoint classification committee reviewed all the events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Masked endpoint classification committee was responsible |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the previous published paper were all reported |
| Other bias | High risk | Funded and conducted by Merck |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 48 weeks Funding: not reported | |
| Participants | Diagnosis: not reported N = 92 Age: irbesartan group 55 ± 9 years; atenolol group 54 ± 9 years Sex: 37.0% women, 63.0% men History: not reported Inclusion criteria: hypertensive people with ECG‐diagnosed LVH Exclusion criteria: known secondary hypertension; renal failure; LV dysfunction (ejection fraction 45%); coronary and valvular heart disease; stroke, and other serious concomitant diseases. No participant had a prior MI or AF | |
| Interventions | RAS inhibitor: irbesartan; beta‐blocker: atenolol Irbesartan 150 mg or 300 mg daily Atenolol 50 mg or 100 mg daily Add‐ons HCTZ, felodipine | |
| Outcomes | SBP and DBP at rest measured using a mercury sphygmomanometer after at least 10 min of rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported. |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low. |
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 12 months Funding: An unrestricted grant from the Institut de Recherches Internationales Servier | |
| Participants | Diagnosis: hypertensive Type 2 diabetic people with microalbuminuria N = 565 Age: Indapamide SR 60.7 ± 9.9 years, Enalapril 59.2 ± 10.0 years Sex: 37.5% women, 62.5% men History: Diabetes was required to be controlled by diet with or without 1 or more oral antidiabetic treatments, unchanged for at least 3 months Inclusion criteria: age 35 to 80 years, type 2 diabetes, essential hypertension (systolic BP 140 – 180 mmHg and diastolic BP < 110 mmHg), and persistent microalbuminuria (albumin excretion rate between 20 and 200 mg/min in 2 of 3 overnight urine samples collected during the placebo run–in period) Exclusion criteria: severe hypertension (systolic BP > 180 mmHg and/or diastolic BP > 110 mmHg), obesity (body mass index [BMI] > 40 kg/m2), hematuria or leucocyturia, and urinary tract infection | |
| Interventions | RAS inhibitor: Enalapril; thiazide: Indapamide SR Indapamide SR 1.5 mg daily n = 282 Enalapril 10 mg daily n = 283 from week 6, additional open–label antihypertensive treatment could be added in a stepwise manner to achieve target BP levels, with all steps separated by a 6–week interval Step 1: amlodipine 5 mg once daily Step 2: amlodipine 10 mg once daily Step 3: amlodipine 10 mg plus atenolol 50 mg once daily Step 4: amlodipine 10 mg plus atenolol 100 mg once daily | |
| Outcomes | 1. BMI: calculated as body weight (in kg) divided by body height (in meters) squared 2. Systolic and diastolic BP levels: with a mercury sphygmomanometer, in the morning before drug intake, after at least a 10–minute rest, in the supine position. 3 consecutive BP measurements were taken at 3–minute intervals, and averaged at W6, W12, W18, W24, W36, and W52 3. Medical history: reviewing the participants’ medical records 4. Fasting plasma sodium, potassium, creatinine, glucose, triglycerides, total cholesterol, high–density lipoprotein (HDL) cholesterol, low–density lipoprotein (LDL) cholesterol: using standard methods, in the central laboratory of each center, before randomization and at the end of the study 5. Creatinine clearance rate: calculated using the Modification of Diet in Renal Disease formula | |
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the prespecified outcomes were not available in the Methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: supported by the Parke Davis Company | |
| Participants | Diagnosis: hypertension i.e. supine BP 95 mmHg‐109 mmHg (Korotkoff phase 5) N = 60 Age: 35‐75 years old Sex: 38.3% women, 61.7% men History: Quinapril group median duration of DM 4.6 years; median duration of treated hypertension 11.7 years Metoprolol group median duration of DM 3.7 years; median duration of treated hypertension 8.8 years Inclusion criteria: NIDDM in stable blood glucose control, essential hypertension Exclusion criteria: CHF; MI; angina pectoris treated with drugs other than nitrates; hemodynamically serious valvular heart disease and secondary or malignant hypertension; treatment with thiazides or lipid‐lowering agents, or both, in the preceding 12 months; treatments with loop‐diuretics in the preceding 3 months; chronic therapy with non‐steroidal anti‐inflammatories. Serum levels of AST or ALT > 2 μkat/L(umol/(s*L); hyperlipoproteinemia; cholesterol > 8 mM; triglycerides > 4 mM; proteinuria (> 0.5 g/L) | |
| Interventions | RAS inhibitor: quinapril; beta‐blocker: metoprolol Quinapril 20 mg daily Metoprolol 100 mg daily Felodipine added when needed | |
| Outcomes | Supine BP measured by a sphygmomanometer | |
| Notes | "The doses were chosen to give equipotency in the antihypertensive effect." No differences between the reductions in standing SBP and DBP were found, however, standing SBP and DBP were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: double‐blinded randomization using a computer algorithm designed prior to commencement of the study Blinding: each participant was identified with an allocation number that was associated with treatment groups according to a computer‐generated allocation schedule; physicians were blinded to the treatment‐associated allocation number Duration: 12 months Funding: no funding sources reported | |
| Participants | Diagnosis: not reported N = 72 Age: range: 29‐63 years; mean age ± SD: 52 ± 12 years Sex: 44.4% women, 55.6% men History: not reported Inclusion criteria: a diagnosis of essential hypertension (ESH stage 1 or 2 hypertension) established by history and physical examination, together with the absence of clinical findings suggestive of a secondary hypertension, according to ESH guidelines Exclusion criteria: other CV diseases (defined as MI or angina pectoris, heart block, valvular disease, HF and claudication); concomitant LVH (defined according to ECG criteria); other target organ damage (including hypertensive retinopathy); micro‐ or macroalbuminuria or renal diseases; insulin‐dependent or NIDDM; electrolyte imbalances; alcoholism or psychiatric problems, or both; taking antihypertensive drugs; or contraindications to beta‐blockers | |
| Interventions | RAS inhibitor: losartan; beta‐blocker: bisoprolol Bisoprolol 5 mg daily Losartan 50 mg daily HCTZ was added when needed | |
| Outcomes | SBP and DBP measured in triplicate with a mercury sphygmomanometer after 5 min in a supine position. The Korotkoff phase V sound was used to determine DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blind randomization performed using a computer algorithm designed prior to commencement of the study |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Each participant was identified with an allocation number that was associated with treatment groups according to a computer‐generated allocation schedule; physicians were blinded to the treatment‐associated allocation number |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 24 months Funding: Danish Medical Research Council and Astra Cardiovascular, Denmark | |
| Participants | Diagnosis: not reported N = 14 Age: 25‐63 years Sex: 28.6% women, 71.4% men History: not reported Inclusion criteria: men and women aged 18‐65 years; chronic glomerulonephritis verified by renal biopsy; creatinine clearance 15 ml/min‐130 ml/min; and arterial hypertension with a DBP between 90 mmHg‐110 mmHg, calculated as the mean value of measurement on 3 different days after discontinuation of antihypertensive treatment for 2 weeks. Exclusion criteria: nephrotic syndrome; extracapillary glomerulonephritis; systemic disease with glomerulonephritis; liver disease; DM; HF; pregnancy; and unwillingness to participate Withdrawal criteria during the study were: development of exclusion criteria; progression to end‐stage renal disease; DBP > 110 mmHg at 3 consecutive visits in the outpatient clinic; and side effects | |
| Interventions | RAS inhibitor: ramipril; CCB: felodipine Three dose levels, low dose (LD), medium dose (MD), and high dose (HD) were used in each group. The dose of medicine was gradually increased(LD to MD to HD) in order to obtain a diastolic blood pressure of 90 mmHg or less: Ramipril 1.25 mg (LD), 2.5 mg (MD), 5.0 mg (HD) daily Felodipine 5 mg (LD), 10 mg (MD), 20 mg (HD) daily | |
| Outcomes | Blood pressures were means of 3 determinations measured after 1 hour's rest in the supine position with an interval of a few min between the determinations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was prospective, double‐blind and placebo‐controlled." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | High risk | 12 of 33 included subjects withdrew before the end of the study. The proportion of the participants dropping out of the trial was too much. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | High risk | Table 1 and Table 2 differ in the baseline BP data |
| Methods | Allocation: randomized: "The study was a randomised and double blind comparison" Blinding: double‐blinded Duration: 6 months before randomization, 21 months after randomization or until need of dialysis Funding: isradipine and spirapril were supplied by Novartis and the study was supported by Novartis. Statistical assistance was supported by a grant from the Danish Medical Research Council | |
| Participants | Diagnosis: chronic renal failure (serum creatinine between 150 μmol/L‐600 μmol/L) and hypertension (BP > 140/95 mmHg) N = 36 Age: 18‐75 years Sex: 36% women, 64% men History: previously treated and untreated people with hypertension Inclusion criteria: chronic, inactive renal disease and serum creatinine between 150 μmol/L‐600 μmol/L, (DBP > 95 mmHg, or SBP > 140 mmHg without treatment) Exclusion criteria: renal artery stenosis or severe CHF | |
| Interventions | RAS inhibitor: spirapril; CCB: isradipine Isradipine 5 mg daily Spirapril 6 mg daily Loop diuretics and labetolol were accepted add‐ons when target BP was not sufficient | |
| Outcomes | ESRF SBP, mercury sphygmomanometer and Korotkoff Phase 1, sitting DBP, mercury sphygmomanometer and Korotkoff Phase 5, sitting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Unclear risk | The data flow was not mentioned |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 6 months Funding: supported in part by a grant from Hoescht Marion Roussel, Inc | |
| Participants | Diagnosis: not reported N = 50 Age: ramipril group 52.7 ± 6.9 years; HCTZ group 50.1 ± 7.7 years Sex: 27% women; 73% men History: not reported Inclusion criteria: seated DBP of 95 mmHg‐114 mmHg Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: Ramipril; Thiazide: HCTZ Ramipril 5 mg, 10 mg, 20 mg daily HCTZ 12.5 mg, 25 mg, 50mg daily | |
| Outcomes | BP | |
| Notes | SD were not reported in the original article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation was not described. Some baseline characteristics (gender, height, body surface area, sleep blood pressure) differed between two active treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Fifty essential hypertensives participated in a double‐blind study for 6 months and were randomised to either ramipril or hydrochlorothiazide (HCTZ)." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Probably low, as other unrelated outcomes use blinded assessment |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | High risk | Some baseline characteristics (gender, height, body surface area, sleep blood pressure) differ between two active treatment groups |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: Hoffmann‐LaRoche Canada, Medical Research Council of Canada to the Multidisciplinary Research Group on Hypertension | |
| Participants | Diagnosis: hypertension, i.e. on more than 2 occasions recumbent SBP > 140 mmHg or DBP > 90 mmHg. The diagnosis of essential hypertension was established by absence of clinical evidence of secondary hypertension N = 17 Age: Cilazapril group 39.1 ± 2.3 years; Atenolol group 42.4 ± 1.6 years Sex: 100% men History: not reported Inclusion criteria: hypertensive men who were untreated or had not received antihypertensive medication for at least 6 months; 25‐50 years old Exclusion criteria: people who smoked > 5 cigarettes/day; abnormal fasting blood glucose level; serum creatinine concentration > 150 μmol/L; or any other systemic disease | |
| Interventions | RAS inhibitor: cilazapril; beta‐blocker: atenolol Atenolol identical 50 mg and 100 mg tablets Cilazapril 2.5 mg and 5 mg tablets Long‐acting nifedipine was added if needed | |
| Outcomes | SBP and DBP measured by standard mercury sphygmomanometer in sitting position after 15 min rest | |
| Notes | Men only included as participants due to the potential teratogenicity of nifedipine, which would be used if goal BP was not achieved with the drugs being studied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | ECG was read by a cardiologist unaware of the protocol |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized, "Randomization by center was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomization numbers. Randomization data were kept strictly confidential until the time of unblinding." Blinding: double‐blinded Duration: 1 year Funding: Novartis Pharmaceuticals Corporation, East Hanover, NJ | |
| Participants | Diagnosis: hypertension, mean sitting DBP > 90 mmHg and < 110 mmHg at the single‐blind placebo run‐in visit N = 962 Age: Aliskiren group 56.1 ± 10.9 years; HCTZ group 55.7 ± 10.9 years Sex: 36% women; 64% men History: mean duration of hypertension was 7.1 years. 35.2% of participants were classified as obese (body mass index 30 kg/m2), and 10.9% had DM (according to medical history) Inclusion criteria: outpatients aged 18 years or over with essential hypertension Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: aliskiren; thiazide: HCTZ Aliskiren 150 mg 300 mg daily HCTZ 12.5 mg 25 mg daily Amlodipine was added when needed | |
| Outcomes | Mean sitting DBP and SBP were measured by a mercury sphygmomanometer. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by center was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomization numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential until the time of unblinding." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 18 months Funding: sponsored in part by Bristol‐Myers Squibb and Sanofi Synthelabo, Germany | |
| Participants | Diagnosis: not reported N = 237 Age: irbesartan group 54.2 ± 8.0 years; atenolol group 55.5 ± 7.9 years Sex: 45% women; 55% men History: duration of hypertension: irbesartan group 5.3 ± 6.0 years; atenolol group 5.2 ± 6.7 years Inclusion criteria: men and women aged between 25‐65 years;SBP of 150 mmHg‐200 mmHg or a DBP of 95 mmHg‐115 mmHg and mild target organ damage defined as intima media thickness of the common carotid artery on the leading side ≥ 0.8 mm and ≤1.5 mm Exclusion criteria: known or suspected secondary hypertension; coronary heart disease; cerebrovascular disease; peripheral vascular disease; renovascular disease; insulin‐dependent DM; uncontrolled non‐insulin‐dependent DM; history of intolerance to atenolol, irbesartan, other angiotensin receptor blockers, HCTZ, or amlodipine; and pretreatment with an ACE inhibitor or an angiotensin receptor blocker within the last 6 months | |
| Interventions | RAS inhibitor: irbesartan; beta‐blocker: atenolol Irbesartan 150 mg/day or 300 mg/day Atenolol 50 mg/day or 100 mg/day Add‐on HCTZ, amlodipine | |
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | ECG measurement and assessment was blinded, but BP measurement was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 12 months Funding: "AstraZeneca provided funding for this clinical trial (to CDAS), but had no influence on the data analyses or manuscript preparation." | |
| Participants | Diagnosis: not reported N = 60 Age: Lisinopril group 62 ± 8 years; Candesartan group 60 ± 7 years; HCTZ group 63 ± 6 years Sex: 45% women; 55% men History: not reported Inclusion criteria: for the run‐in period were: T2DM ≥ 6 months (WHO criteria 1985); age 35‐70 years; "Caucasian ethnicity"; and urinary albumin excretion < 100 mg/24 hours. Patients with a sitting BP > 140/90 mmHg and < 190/120 mmHg after the run‐in period had an ECG. Participants were included if LVMI 490 g/m² in men or 470 g/m² in women Exclusion criteria: pregnancy or planning a pregnancy; a history of MI, angina pectoris, coronary artery bypass surgery, angioplasty, stroke, CHF, malignancy or other serious illnesses; serum creatinine > 140 mmol/L; body mass index 435 kg/m²; alcohol or drug abuse, or both; or participation in other clinical trials | |
| Interventions | RAS inhibitor: lisinopril, candesartan; thiazide: HCTZ HCTZ 12.5 mg daily Candesartan 8 mg daily Lisinopril 10 mg daily Add‐on: consecutively, 12.5 mg HCTZ, doubling study medication; 5 mg felodipine, 50 mg metoprolol, 2 mg doxazosin, 5mg felodipine; 50 mg metoprolol, 2 mg doxazosin, 5 mg felodipine, 100 mg metoprolol, and 4 mg doxazosin | |
| Outcomes | BP after 5 min of seated rest (mean of 3 consecutive measurements) | |
| Notes | Participants were limited to people of "Caucasian ethnicity". The reason was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Although the role of company was unlikely to have an impact on the study, no other information was found to evaluate the risk as either high or low |
| Methods | Allocation: alternative allocation Blinding: double‐blinded Duration: 1 year Funding: this study was supported by Institut de Recherches Internationales, (IRIS) France | |
| Participants | Diagnosis: DBP 95 mmHg‐115 mmHg. N = 100 Age: Perindopril group 54.3 ± 7.3 years; Atenolol group 56.5 ± 6.9 years Sex: 88% women; 12% men History: duration of hypertension was 8.2 ± 6.2 years and 99% of participants were on previous treatment.Duration of diabetes was 6.6 ± 5.4 years. Proteinuria was present in 40% of participants. Fundal changes consisting of hypertensive and diabetic retinopathy were present in 60% of participants Inclusion criteria: T2DM with hypertension and DBP 95 mmHg‐115 mmHg Exclusion criteria: albuminuria < 200 mg/min (300 mg in 24 hours) or macroalbuminuria > 3.5 g/24 hours; severe complications of hypertension such as stroke, HF, renal failure; severe diabetic retinopathy (neovascularization, vitreous hemorrhages or retinal detachment); contraindications to beta‐blockers or ACE inhibitors; people with poor metabolic control; and women with childbearing potential | |
| Interventions | RAS inhibitor: perindopril; beta‐blocker: atenolol Perindopril 4 mg, 8 mg daily Atenolol 50 mg, 100 mg daily HCTZ, nifedipine were added when needed | |
| Outcomes | Pulse rate and sitting and standing BP evaluated within 12 hours post administration at each review visit. BP determined by taking a mean of 3 readings with the Dinamap (Criticon, Johnson and Johnson) apparatus with the participant seated after 5 min rest | |
| Notes | There were no participants of European origin as the hospital serves only black and Indian people. Black people were excluded because they do not respond well to ACE inhibitors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | High risk | Alternative allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes in the methods were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 48 weeks Funding: Karolinska Institutet, Stockholm, Sweden, the Swedish Heart‐Lung Foundation,Stiftelsen Serafimerlasarettet, Stockholm, Sweden, Bristol‐Myers Squibb Pharmaceutical Research Institute, Princeton, NJ, USA, and Sanofi‐Synthelabo, Paris, France | |
| Participants | Diagnosis: Mild‐to‐moderate hypertension and left ventricular hypertrophy N = 101 Age: Irbesartan group 54 ± 8 years; Atenolol group 54 ± 10 years Sex: 32% women; 68% men History: Mild‐to‐moderate hypertension and LV hypertrophy Inclusion criteria: Women with mild‐to‐moderate hypertension and LV hypertrophy. All antihypertensive agents were withdrawn appropriately before the start of a 4‐ to 6‐week single‐blind placebo lead‐in period. At the end of the placebo period, participants were determined eligible for the double‐blind part of the study if the mean of 3 seated diastolic blood pressures (SeDBP) taken 1 min apart was 90 ‐ 115 mmHg on 2 consecutive visits, with values differing no more than 8 mmHg Exclusion criteria: If patients had an LV ejection fraction < 45%, any significant concomitant diseases, or were taking any other medications that might interfere with the efficacy assessments or that would present safety hazards | |
| Interventions | RAS inhibitor: Irbesartan; beta‐blocker: atenolol Irbesartan 150 mg/d Atenolol 50 mg/d If SeDBP was > 90 mmHg after 6 weeks,irbesartan 300 mg/d or atenolol 100 mg/d If SeDBP remained > 90 mmHg at week 12, open‐label hydrochlorothiazide (HCTZ) 12.5 mg/d (titrated to 25 mg if necessary) At week 24, open‐label felodipine 5 ‐ 10 mg/d if required At the end of the study, 40% of participants in the irbesartan group and 49% in the atenolol group remained on the monotherapy. | |
| Outcomes | 1. Blood pressure: At all clinic visits, trough (24 ± 3 h after the last dose) SeSBP and SeDBP were measured using a mercury sphygmomanometer. After resting for at least 10 mins in the seated position, blood pressure was determined as the average of 3 replicate measurements taken 1 min apart 2. Heart rate: Heart rate was then recorded in the seated position 3. Total peripheral resistance: Mean arterial pressure was calculated as SeDBP + (SeSBP ‐ SeDBP)/3. Total peripheral was calculated by dividing mean arterial pressure by cardiac output (i.e. stroke volume 3 heart rate), and expressed as peripheral resistance units (PRU) 4. Echocardiography (LVMI, left ventricular mass index; IVS, intraventricular septum; PWT, posterior wall thickness; LVEDD, left ventricular end‐diastolic diameter; RWT, relative wall thickness; EF, ejection fraction.): Echocardiography was performed with the woman in the left semilateral position. The ultrasound devices used were the Acuson 128 X P/10 (Mountain View, California, USA), Vingmed CFM 750 (Vingmed Sound,Horten, Norway) and HP SONOS 2500 (Andover,Massachusetts, USA). Measurements were performed on 3 ‐ 5 consecutive beats, from which the mean values were calculated. Basic measurements of LV dimensions in diastole (LVEDD) and systole (LVESD), and intra‐ventricular septum (IVS) thickness and posterior wall thickness in diastole (PWT) were made by M‐mode technique The ejection fraction was measured according to the recommendations of the American Society of Echocardiography | |
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's prespecified outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | High risk | Number of participants reported in different outcomes are not consistent |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: this study was supported by a grant from Bayer AG; lisinopril tablets were supplied by Zeneca | |
| Participants | Diagnosis: not reported N = 48; nisoldipine group 25, lisinopril group 23 Age: nisoldipine group 41 ± 9 years; lisinopril group 34 ± 7 years Sex: 33% women; 67% men History: duration of DM, nisoldipine group 25 ± 6 years; lisinopril group 24 ± 6 years (means ± SD) Inclusion criteria: type I DM with hypertension and diabetic nephropathy Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: lisinopril; CCB: nisoldipine Nisoldipine coat core 20 mg‐40 mg daily Lisinopril 10 mg‐20 mg daily | |
| Outcomes | BP measured with a standard clinical sphygmomanometer in the upper arm at heart level. DBP obtained as Korotkoff Phase 5 | |
| Notes | Coat core: a dosage form of Nisoldipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "We performed a 1‐year double‐blind, double‐dummy randomised controlled study …" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 1 year Funding: supported by Bayer AG; lisinipril was supplied by Zeneca | |
| Participants | Diagnosis: hypertension: DBP > 90 mmHg or ongoing treatment with antihypertensive medication N = 40 Age: Nisoldipine group 40 ± 9 years; Lisinopril group 34 ± 7 years Sex: 35% women; 65% men History: duration of DM: nisoldipine group 40 ± 9 years; lisinopril group 34 ± 7 years Inclusion criteria: hypertensive people between the ages of 18‐55 years with a GFR > 40 ml/min·1.73m², and had developed diabetes before the age of 41 years Exclusion criteria: not reported | |
| Interventions | RAS inhibitor: lisinopril; CCB: nisoldipine Nisoldipine coat core 20 mg or 40 mg daily Lisinopril 10 mg or 20 mg daily Add‐on, diuretic (mainly furosemide). 1 participant in the lisinopril group was prescribed a cardioselective beta‐blocker after 6 months | |
| Outcomes | BP was measured after 15 min rest in the supine position | |
| Notes | All patients were white, had been insulin‐dependent from the time of diagnosis, and received at least 2 daily injections of human insulin In 14 participants (6 in the nisoldipine group), diuretic treatment was continued because of edema. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | High risk | 10 of 50 withdrew before the end of the study |
| Selective reporting (reporting bias) | Low risk | Although the pre‐specified outcomes were not available in the methods, it is clear that all the expected outcomes were reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 26 months Funding: not reported | |
| Participants | Diagnosis: not reported N = 69 Age: 30‐73 years Sex: 48% women; 52% men History: not reported Inclusion criteria: DBP between 90 mmHg‐114 mmHg Exclusion criteria: recent MI or stroke; renal diseases; and CHF | |
| Interventions | RAS inhibitor: losartan; thiazide: HCTZ Losartan 50 mg daily HCTZ 25 mg daily | |
| Outcomes | Supine BP measurements using a mercury sphygmomanometer | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "After 2 weeks, in a double‐blind study, the subjects were randomly allocated to either treatment with…" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Questions in the Quality of life questionnaire were posed by a trained investigator blinded to clinical and active treatment The way in which BP assessments were made was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 2 years Funding: Pfizer, Netherlands | |
| Participants | Diagnosis: not reported N = 149 Age: amlodipine group 67 ± 4 years; lisinopril group 67 ± 4 years. Sex: 50% women, 50% men History: not reported Inclusion criteria: people with previously untreated mild to moderate hypertension Exclusion criteria: office BP > 220/115 mmHg; unstable BP after placebo treatment period, defined as the differences in DBP or SBP before placebo treatment of 10 mmHg or 20 mmHg, respectively; secondary hypertension of any etiology; angina pectoris; manifest coronary artery disease; current or recent history of CHF; hemodynamically significant valvular heart disease; cardiac arrhythmias; renal insufficiency; and insulin‐dependent DM | |
| Interventions | RAS inhibitor: lisinopril; CCB: amlodipine Amlodipine 5 mg, 10 mg Lisinopril 10 mg, 20 mg | |
| Outcomes | BP: Korotkoff phase 1 and 5, sitting position HR | |
| Notes | Participant numbers at 1 year and 2 year were not reported for BP; instead, "end of trial" was used in the tables of BP results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "we performed a double‐blind, randomised study in a Dutch rural population, …" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "ECG examinations were performed by the same observer, who was unaware of the identity of patients or BP measurements at baseline and after 1 and 2 years of active treatment." Statistical analysis was performed by an independent agency |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | High risk | HR was listed in the "Methods", but no detailed data were reported in "Results", though statements like "heart rate did not significantly change during treatment, …" were evident Participant numbers in Table 2 do not match those stated in the article |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 4.4 years Funding: this study was supported by grant NIH‐R01‐HL34767 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md; and Pfizer Inc, New York, NY, and Merck Sharp & Dohme Research Laboratories, Rahway, NJ | |
| Participants | Diagnosis: not reported N = 597 Age: 45‐69 years Sex: 62% women, 38% men History: not reported Inclusion criteria: men and women aged 45–69 years, DBP 90 mmHg‐99 mmHg at both of the first 2 eligibility visits and averaged 90 mmHg‐99 mmHg over the 3 eligibility visits Exclusion criteria: use of > 1 type of antihypertensive drug; inability to obtain a technically satisfactory baseline ECG; angina; at least 50% of meals eaten away from home; unwillingness or inability to make nutritional changes; CV disease; life threatening illness; LVH | |
| Interventions | RAS inhibitor: enalapril; CCB: amlodipine; thiazide: chlorthalidone; beta‐blocker: acebutolol; alpha‐blocker: doxazosin Nutritional‐hygienic intervention plus one of the following 6 treatments:
| |
| Outcomes | BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
| Methods | Allocation: randomized. Computer‐generated random sequence centrally prepared by sponsor Blinding: double‐blinded (patients and clinicians) Duration: 4 ‐ 6 years, 4.2 ± 1.2 years (means ± SD) Funding: Novartis for design (interactively), data management, data analysis | |
| Participants | Diagnosis: hypertension defined as a mean sitting SBP between 160 mmHg ‐ 210 mmHg (inclusive), and a mean sitting DBP of < 115 mmHg N = 15,245: valsartan group 7649; amlodipine group 7596 Age: 50 years or older Sex: 42% women, 58% men Antihypertensive medication taken at time of randomization: Previously treated for hypertension: valsartan group 7088 (92.7%); amlodipine group 6989 (92.0%) ACE inhibitor: valsartan group 3148 (41.3%); amlodipine group 3135 (41.4%) Angiotensin‐receptor blocker: valsartan group 812 (10.7%); amlodipine group 800 (10.6%) Alpha‐blockers: valsartan group 540 (7.1%); amlodipine group 495 (6.5%) Beta‐blockers: valsartan group 2496 (32.7%); amlodipine group 2551 (33.7%) Calcium‐channel antagonist: valsartan group 3181 (41.7%); amlodipine group 3048 (40.2%) Diuretics as monotherapy: valsartan group 2047 (26.9%); amlodipine group 2020 (26.7%) Fixed‐dose diuretic combinations: valsartan group 686 (9.0%); amlodipine group 634 (8.4%) Qualifying disease factors: Coronary heart disease: valsartan group 3490 (45.6%); amlodipine group 3491 (46.0%) Peripheral arterial disease: valsartan group 1052 (13.8%); amlodipine group 1062 (14.0%) Stroke or TIA: valsartan group 1513 (19.8%); amlodipine group 1501 (19.8%) LVH with strain pattern: valsartan group 454 (5.9%); amlodipine group 462 (6.1%) Inclusion criteria: men or women of any racial background, 50 years of age and older, with CV risk factors or disease according to an algorithm based on age and sex Exclusion criteria: renal artery stenosis; pregnancy; acute MI; percutaneous trans luminal coronary angioplasty or coronary artery bypass graft within the past 3 months; clinically relevant valvular disease; cerebrovascular accident in the past 3 months; severe hepatic disease; severe chronic renal failure; CHF requiring ACE inhibitor therapy; monotherapy with beta‐blockers for both coronary artery disease and hypertension | |
| Interventions | RAS inhibitor: valsartan; CCB: amlodipine Valsartan 80 mg, median 151.7 mg, range 83.2 mg‐158.5 mg Amlodipine 5 mg, median 8.5 mg, range 5.0 mg‐9.9 mg | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random sequence centrally prepared by sponsor |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quoted: "A statistician on the executive committee independently analyzed data to validate and further explore the analyses done by statisticians employed by the sponsor." And "An endpoint committee, blinded to therapy allocation, reviewed the clinical records of all CV events reported by clinical centers and adjudicated according to the protocol criteria." |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | High risk | Quoted: "The proportion of patients receiving valsartan monotherapy as the last recorded study medication was significantly smaller than that of patients receiving amlodipine monotherapy, and a larger proportion of patients in the valsartan group received the highest dose of study drug plus hydrochlorothiazide or plus other antihypertensive drugs than in the amlodipine group." Reviewer comment: the proportion of monotherapy and highest dose (include HCTZ and other non‐study add‐on drugs) was not balanced between the 2 groups |
| Methods | Allocation: randomized Blindness: double‐blinded Duration: 36 months Funding: Clinical project of Third Military Medical University (2010XLC04) and 3 grants from the Natural Science Foundation of China (Nos 81172773, 81202286 and 81473068) | |
| Participants | Diagnosis: essential hypertension combined with i‐IFG N = 227 Age: mean 45.02 in Losartan potassium group, 46.59 in Levamlodipine besylate Sex: 46% women, 54% men History: patients with EH combined with i‐IFG Inclusion criteria: (1) age between 18 and 70 years (2) i‐IFG criteria: participants received at least 2 fasting glucose (FG) examinations on different days in the Clinical Laboratory of the Southwest Hospital, and the results showed 5.6 mmol l‐1 < FPG < 7 mmol l‐1 and postprandial 2‐hr plasma glucose (2hPG) < 7.8 mmol l‐1 (3) hypertension criteria: the BP was the average of 3 measurements of BP in the right arm after sitting still for 5 minutes using a cuff sphygmomanometer, which conformed to the standards formulated in the 2010 Chinese guidelines for the management of hypertension: systolic BP (SBP) ≥140 mmHg and diastolic BP (DBP) ≥ 90 mmHg (4) participants had not used antihypertensive drugs within the previous 2 weeks (5) participants who would like to come back for follow‐up in the next 3 years Exclusion criteria: (1) women who were incapable or unwilling to provide written informed consent (2) evidence of liver disease (alanine aminotransferase or aspartate aminotransferase greater than twice the normal upper limit) or kidney disease (serum creatinine > 95 μmol l‐1) (3) secondary hypertension, urinary tract infection, renal artery stenosis, hyperkalemia, pregnancy, lactation, recent cerebral hemorrhage or cerebral infarction, or severe heart failure (4) use of hypoglycemic medication or insulin in the previous 5 years (5) allergy to the drugs in this study (6) participants who refused to come back to the hospital for follow‐up | |
| Interventions | RAS inhibitor: losartan; CCB: levamlodipine Losartan potassium at 50 or 100 mg Levamlodipine besylate at 2.5 or 5 mg | |
| Outcomes | SBP, DBP: monitored in the long term and re‐examined every 12 months in the Southwest Hospital Fasting insulin (FINS): tested in the Department of Nuclear Medicine in Southwest Hospital by radioimmunoassay Insulin sensitivity index (ISI): ISI = In (1/(FPG× FINS)) FPG: tested in Clinical Laboratory in Southwest Hospital by the glucose oxidase method 2‐hr insulin (2hINS): tested in the Department of Nuclear Medicine in Southwest Hospital by radioimmunoassay 2Hpg: tested in Clinical Laboratory in Southwest Hospital by the glucose oxidase method Glycohemoglobin (HbA1C): tested in the Clinical Laboratory in Southwest Hospital by enzymatic methods Body mass index (BMI) : BMI ≥ 24 kg m2 was overweight and BMI ≥ 28 kg m2 was obesity Total cholesterol, total triglycerides (TGs), low‐density lipoprotein cholesterol(LDL‐C), high‐density lipoprotein cholesterol (HDL‐C): tested in the Clinical Laboratory in Southwest Hospital by enzymatic methods Dyslipidemia: diagnosed according to the dyslipidemia indicators in the diagnostic standards of metabolic syndrome proposed by the International Diabetes Federation in 2005: TG ≥ 1.7 mmol l1 and HDL‐C < 1.29 mmol l−1 (females) | |
| Notes | New for 2018 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the Methods were all reported |
| Other bias | Unclear risk | Insufficient information found to evaluate the risk as either high or low |
| Methods | Allocation: randomized Blinding: double‐blinded Duration: 3 years Funding: Astra‐Zeneca provided the study medication | |
| Participants | Diagnosis: autosomal dominant polycystic kidney disease (ADPKD) defined by ultrasonographic criteria as described by Ravine et al (Ravine 1994) and a positive family history Hypertension: casual BP 140/90 mmHg or presence of an antihypertensive medication, or both N = 37; ramipril group 17; metoprolol group 20 Age: 18 ‐ 65 years Sex: 54% women, 46% men (only per‐protocol subjects available) Inclusion criteria: confirmed diagnosis of ADPKD; aged 18–65 years; evidence for hypertension; serum creatinine ≤ 4.0 mg/dL. Exclusion criteria: serum creatinine > 4.0 mg/dL; MI or cerebrovascular accident in the past 12 months; known intolerance to study medication; pregnancy or women not using contraception; evidence for severe hepatic disease; use of immunosuppressants or non‐steroidal anti‐inflammatory drugs; CHF; alcohol abuse or consumption of narcotics; the presence of a malignant disease or non‐compliance of the participants | |
| Interventions | RAS inhibitor: ramipril; beta‐blocker: metoprolol Ramipril 2.5 mg or 5 mg daily Metoprolol 50 mg or 100 mg daily Add‐on medication, open‐label, felodipine, doxazosin, furosemide | |
| Outcomes | Casual SBP and DBP measured with a standard mercury sphygmomanometer at each clinical visit. BP readings were taken with the participant seated after 5 min of rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method of blinding was not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were unlikely to have an impact on the results of the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the methods were all reported |
| Other bias | Unclear risk | Insufficient information was found to evaluate the risk as either high or low |
Abbreviations
ACE: angiotensin converting enzyme
ADPKD: autosomal dominant polycystic kidney disease
AER: albumin excretion rate
AF: atrial fibrillation
ALT: alanine transaminase
ARB: angiotensin II receptor agonist
AST: aspartate transaminase
bpm: beats per minute
BP: blood pressure
CCB: calcium channel blocker
CHD: coronary heart disease
CHF: congestive heart failure
CV: cardiovascular
CVD: cardiovascular disease(s)
DBP: diastolic blood pressure
DM: diabetes mellitus
ECG: electrocardiograph
ESH: European Society of Hypertension
ESRF: end stage renal failure
GFR: glomerular filtration rate
HbA1c: glycosylated hemoglobin
HCTZ: hydrochlorothiazide
HDL: high‐density lipoprotein
HF: heart failure
HR: heart rate
LV: left ventricular
LVH: left ventricular hypertrophy
LVMI: left ventricular mass index
MI: myocardial infarction
min: minute(s)
NIA: national institutes of aging
NIDDM: non‐insulin‐dependent diabetes mellitus
NIH: national institute on health
SBP: systolic blood pressure
SD: standard deviation
SEM: standard error of mean
SR: slow release
TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Mortality and morbidity data were not reported in a form that could be extracted and entered | |
| Not double‐blinded trial; study used PROBE (prospective, randomized, open‐label design, with blinded assessments of end points) design | |
| The outcome of this study was BP control rate, so we could not extract relevant data | |
| Outcomes were grouped and analyzed according to blood potassium. We have no available data to extract associated with different medications | |
| This study included participants with high risk of hypertension but excluded people diagnosed as hypertensive | |
| The study focused on choice of an initial antihypertensive agent by using renin profile methods versus age‐race methods. There were no available data for this review |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 5 | 35226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| Analysis 1.1  Comparison 1 RAS inhibitors vs CCBs, Outcome 1 All‐cause death. | ||||
| 2 Total CV events Show forest plot | 6 | 35223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.02] |
| Analysis 1.2  Comparison 1 RAS inhibitors vs CCBs, Outcome 2 Total CV events. | ||||
| 3 Total HF Show forest plot | 5 | 35143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| Analysis 1.3  Comparison 1 RAS inhibitors vs CCBs, Outcome 3 Total HF. | ||||
| 4 Total MI Show forest plot | 5 | 35043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| Analysis 1.4  Comparison 1 RAS inhibitors vs CCBs, Outcome 4 Total MI. | ||||
| 5 Total stroke Show forest plot | 4 | 34673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.32] |
| Analysis 1.5  Comparison 1 RAS inhibitors vs CCBs, Outcome 5 Total stroke. | ||||
| 6 ESRF Show forest plot | 4 | 19551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| Analysis 1.6  Comparison 1 RAS inhibitors vs CCBs, Outcome 6 ESRF. | ||||
| 7 SBP Show forest plot | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [0.90, 1.56] |
| Analysis 1.7  Comparison 1 RAS inhibitors vs CCBs, Outcome 7 SBP. | ||||
| 8 DBP Show forest plot | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [0.79, 1.18] |
| Analysis 1.8  Comparison 1 RAS inhibitors vs CCBs, Outcome 8 DBP. | ||||
| 9 HR Show forest plot | 5 | 540 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.63, 2.22] |
| Analysis 1.9  Comparison 1 RAS inhibitors vs CCBs, Outcome 9 HR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| Analysis 2.1  Comparison 2 RAS inhibitors vs thiazides, Outcome 1 All‐cause death. | ||||
| 2 Total CV events Show forest plot | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.00, 1.11] |
| Analysis 2.2  Comparison 2 RAS inhibitors vs thiazides, Outcome 2 Total CV events. | ||||
| 3 Total HF Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.07, 1.31] |
| Analysis 2.3  Comparison 2 RAS inhibitors vs thiazides, Outcome 3 Total HF. | ||||
| 4 Total MI Show forest plot | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| Analysis 2.4  Comparison 2 RAS inhibitors vs thiazides, Outcome 4 Total MI. | ||||
| 5 Total stroke Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| Analysis 2.5  Comparison 2 RAS inhibitors vs thiazides, Outcome 5 Total stroke. | ||||
| 6 ESRF Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.37] |
| Analysis 2.6  Comparison 2 RAS inhibitors vs thiazides, Outcome 6 ESRF. | ||||
| 7 SBP Show forest plot | 10 | 26382 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.20, 1.99] |
| Analysis 2.7  Comparison 2 RAS inhibitors vs thiazides, Outcome 7 SBP. | ||||
| 8 DBP Show forest plot | 9 | 26335 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.13] |
| Analysis 2.8  Comparison 2 RAS inhibitors vs thiazides, Outcome 8 DBP. | ||||
| 9 HR Show forest plot | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐2.87, 4.19] |
| Analysis 2.9  Comparison 2 RAS inhibitors vs thiazides, Outcome 9 HR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| Analysis 3.1  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 1 All‐cause death. | ||||
| 2 Total CV events Show forest plot | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| Analysis 3.2  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 2 Total CV events. | ||||
| 3 Total HF Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| Analysis 3.3  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 3 Total HF. | ||||
| 4 Total MI Show forest plot | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.27] |
| Analysis 3.4  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 4 Total MI. | ||||
| 5 Total stroke Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.63, 0.88] |
| Analysis 3.5  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 5 Total stroke. | ||||
| 6 ESRF Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| Analysis 3.6  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 6 ESRF. | ||||
| 7 SBP Show forest plot | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐1.22, 0.11] |
| Analysis 3.7  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 7 SBP. | ||||
| 8 DBP Show forest plot | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.14, 0.83] |
| Analysis 3.8  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 8 DBP. | ||||
| 9 HR Show forest plot | 10 | 9979 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [5.59, 6.50] |
| Analysis 3.9  Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 9 HR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐3.98, ‐0.78] |
| Analysis 4.1  Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 3 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.09, 0.85] |
| Analysis 4.2  Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 2 DBP. | ||||
| 3 HR Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.1 [‐2.41, 8.61] |
| Analysis 4.3  Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 3 HR. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐6.01, 8.61] |
| Analysis 5.1  Comparison 5 RAS inhibitors vs CNS active drug, Outcome 1 SBP. | ||||
| 2 DBP Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| Analysis 5.2  Comparison 5 RAS inhibitors vs CNS active drug, Outcome 2 DBP. | ||||
| 3 HR Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐4.13, 7.13] |
| Analysis 5.3  Comparison 5 RAS inhibitors vs CNS active drug, Outcome 3 HR. | ||||
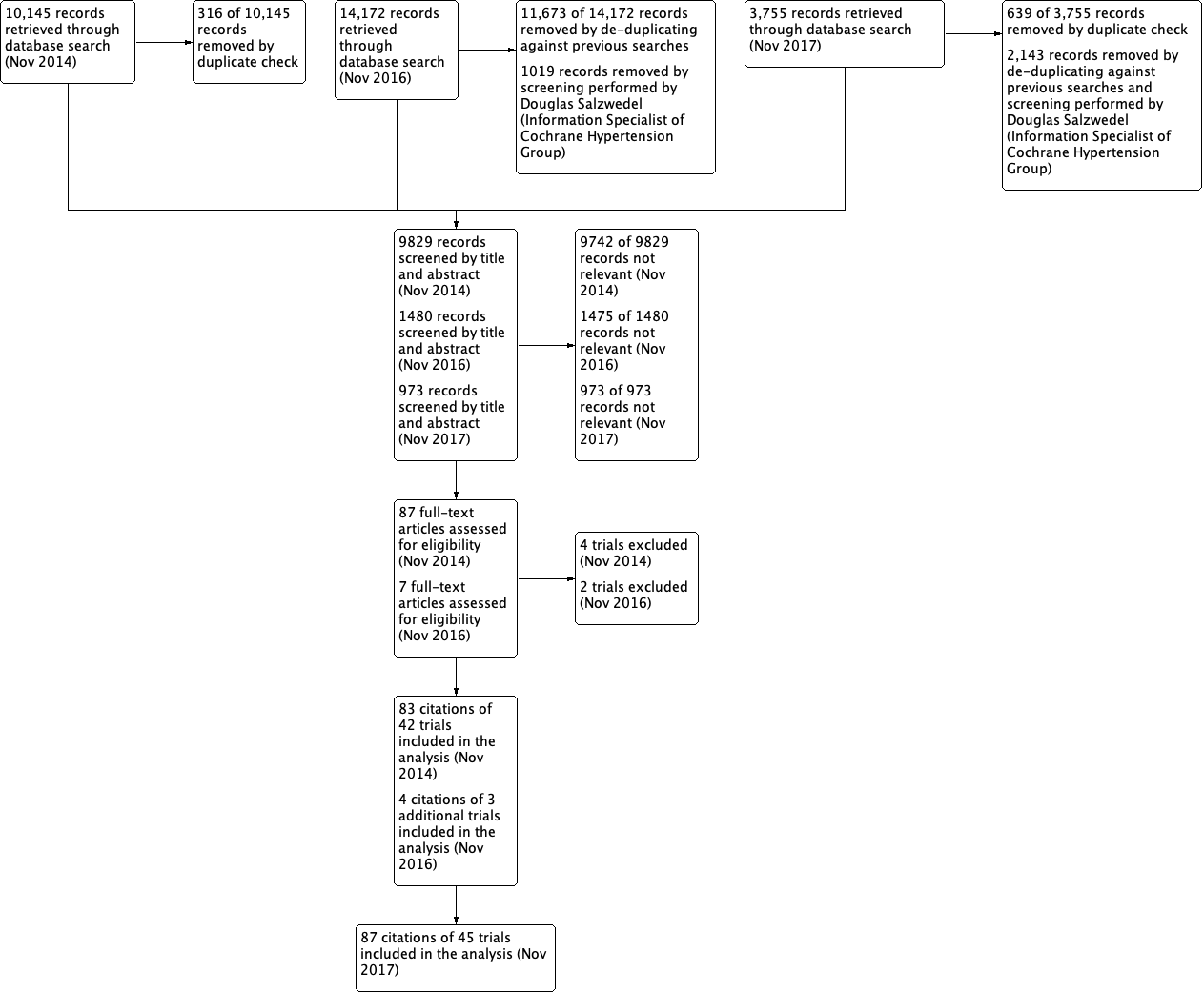
PRISMA Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included citations

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included citations

Comparison 1 RAS inhibitors vs CCBs, Outcome 1 All‐cause death.
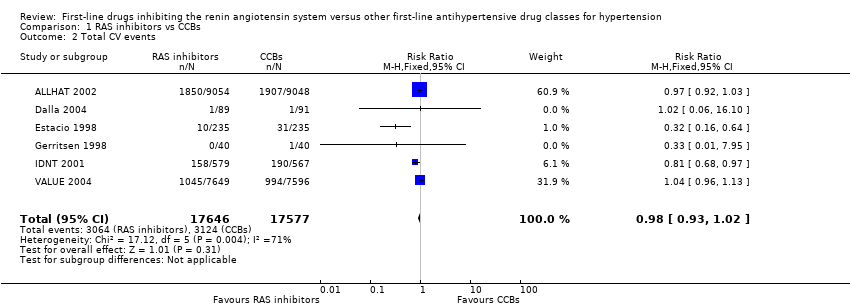
Comparison 1 RAS inhibitors vs CCBs, Outcome 2 Total CV events.
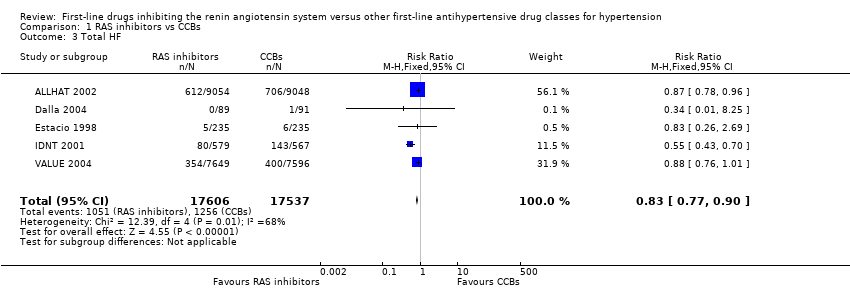
Comparison 1 RAS inhibitors vs CCBs, Outcome 3 Total HF.

Comparison 1 RAS inhibitors vs CCBs, Outcome 4 Total MI.

Comparison 1 RAS inhibitors vs CCBs, Outcome 5 Total stroke.

Comparison 1 RAS inhibitors vs CCBs, Outcome 6 ESRF.

Comparison 1 RAS inhibitors vs CCBs, Outcome 7 SBP.

Comparison 1 RAS inhibitors vs CCBs, Outcome 8 DBP.
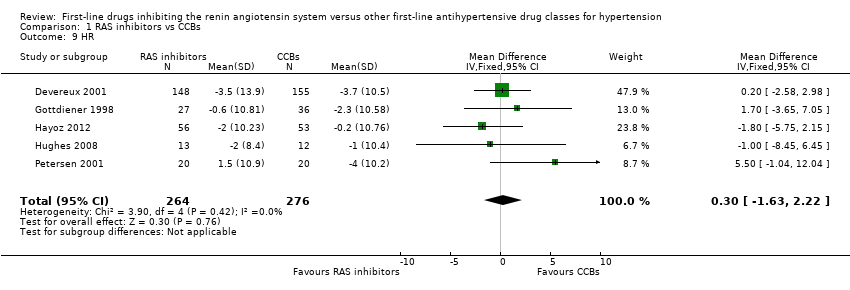
Comparison 1 RAS inhibitors vs CCBs, Outcome 9 HR.

Comparison 2 RAS inhibitors vs thiazides, Outcome 1 All‐cause death.

Comparison 2 RAS inhibitors vs thiazides, Outcome 2 Total CV events.
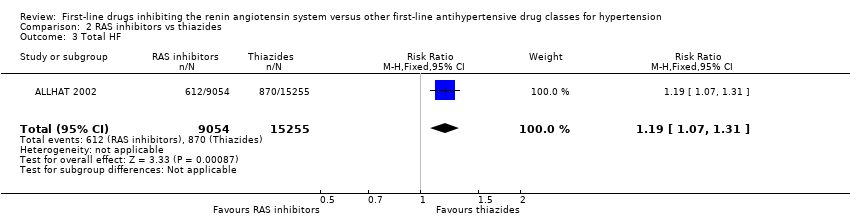
Comparison 2 RAS inhibitors vs thiazides, Outcome 3 Total HF.
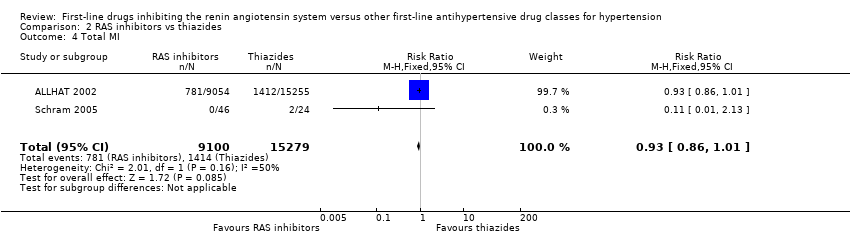
Comparison 2 RAS inhibitors vs thiazides, Outcome 4 Total MI.

Comparison 2 RAS inhibitors vs thiazides, Outcome 5 Total stroke.

Comparison 2 RAS inhibitors vs thiazides, Outcome 6 ESRF.

Comparison 2 RAS inhibitors vs thiazides, Outcome 7 SBP.

Comparison 2 RAS inhibitors vs thiazides, Outcome 8 DBP.

Comparison 2 RAS inhibitors vs thiazides, Outcome 9 HR.
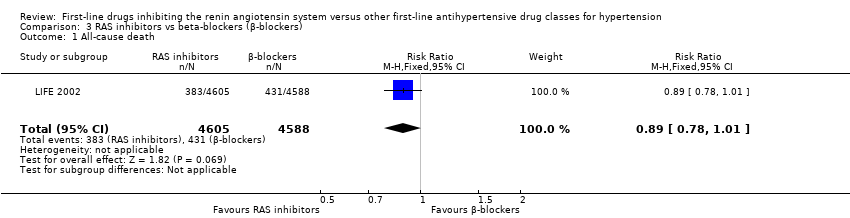
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 1 All‐cause death.

Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 2 Total CV events.
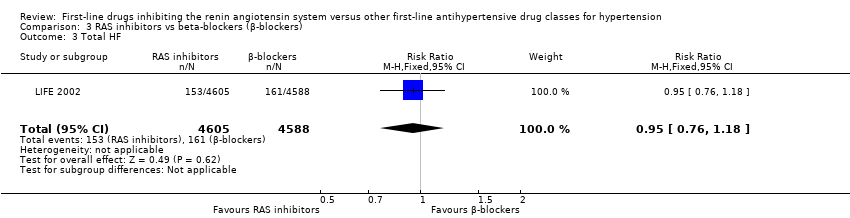
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 3 Total HF.
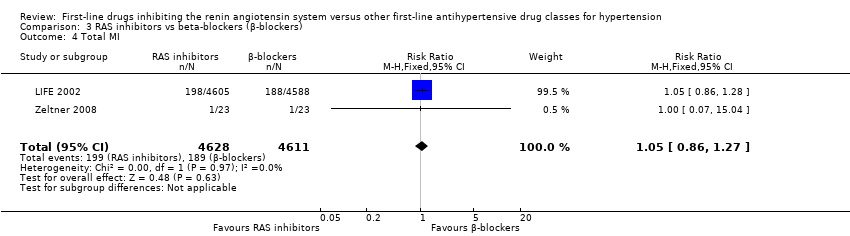
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 4 Total MI.

Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 5 Total stroke.

Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 6 ESRF.
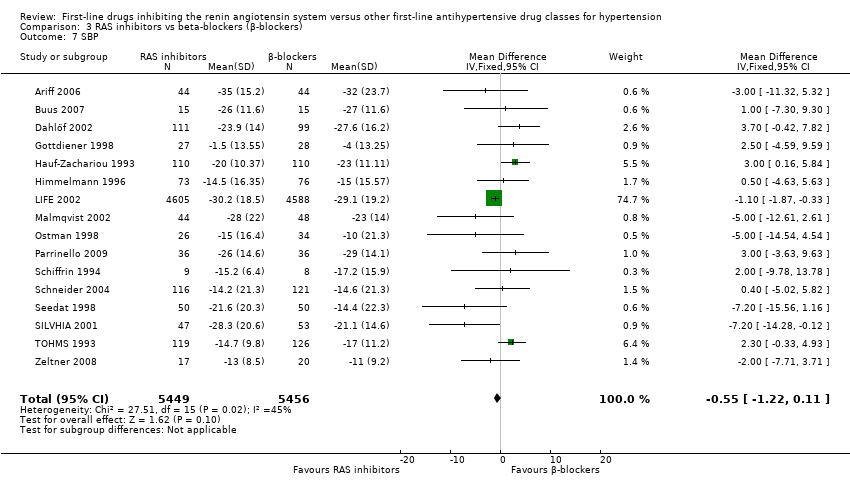
Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 7 SBP.

Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 8 DBP.

Comparison 3 RAS inhibitors vs beta‐blockers (β‐blockers), Outcome 9 HR.
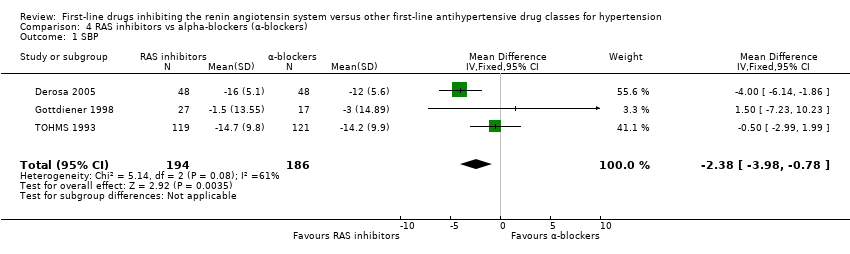
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 1 SBP.
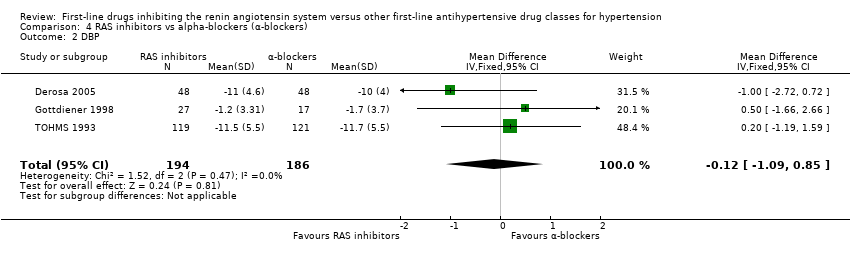
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 2 DBP.
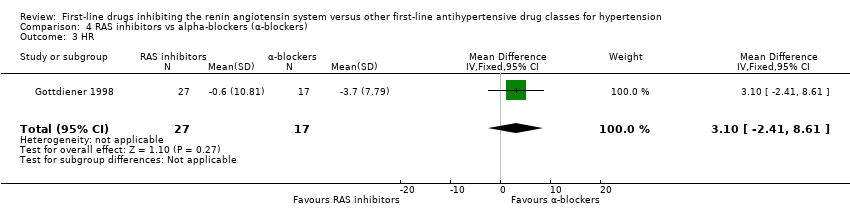
Comparison 4 RAS inhibitors vs alpha‐blockers (α‐blockers), Outcome 3 HR.

Comparison 5 RAS inhibitors vs CNS active drug, Outcome 1 SBP.
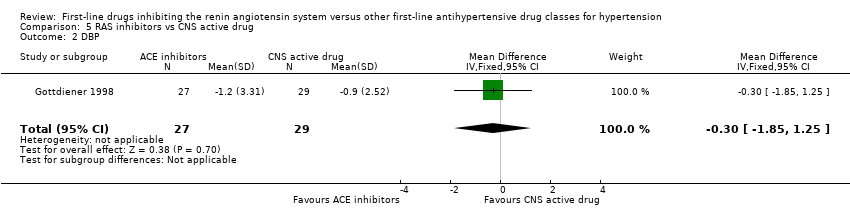
Comparison 5 RAS inhibitors vs CNS active drug, Outcome 2 DBP.

Comparison 5 RAS inhibitors vs CNS active drug, Outcome 3 HR.
| First‐line RAS inhibitors compared to first‐line CCBs for hypertension | |||||||
| Patient or population: people with hypertension | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| CCBs | RAS inhibitors | ||||||
| All‐cause death | 124 per 1000 | 127 per 1000 | RR 1.03 | 35,226 | ⊕⊕⊕⊝ | ||
| Total cardiovascular events | 178 per 1000 | 174 per 1000 | RR 0.98 | 35,223 | ⊕⊕⊕⊝ | ||
| Death or hospitalization for heart failure | 72 per 1000 | 60 per 1000 | RR 0.83 | 35,143 | ⊕⊕⊕⊝ | ARR = 1.2% NNTB = 83 | |
| Total myocardial infarction | 68 per 1000 | 69 per 1000 | RR 1.01 | 35,043 | ⊕⊕⊕⊝ | ||
| Total stroke | 39 per 1000 | 46 per 1000 | RR 1.19 | 34,673 | ⊕⊕⊕⊝ | ARI = 0.7% NNTH = 143 | |
| End stage renal failure | 25 per 1000 | 22 per 1000 | RR 0.88 | 19,551 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded because we judged some of the included trials to be at high risk of bias. | |||||||
| First‐line RAS inhibitors compared to first‐line thiazides for hypertension | ||||||
| Patient or population: people with hypertension Comparison: First‐line thiazides | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Thiazides | RAS inhibitors | |||||
| All‐cause death | 144 per 1000 | 144 per 1000 (135 to 154) | RR 1.00 (0.94 to 1.07) | 24,309 (1) | ⊕⊕⊕⊝ | |
| Total cardiovascular events | 194 per 1000 | 204 per 1000 | RR 1.05 | 24,379 | ⊕⊕⊕⊝ | |
| Death or hospitalization for heart failure | 57 per 1000 | 68 per 1000 (61 to 75) | RR 1.19 (1.07 to 1.31) | 24,309 (1) | ⊕⊕⊕⊝ | ARI = 1.1% NNTH = 91 |
| Total myocardial infarction | 93 per 1000 | 86 per 1000 | RR 0.93 | 24,379 | ⊕⊕⊕⊝ | |
| Total stroke | 44 per 1000 | 50 per 1000 (45 to 56) | RR 1.14 (1.02 to 1.28) | 24,309 (1) | ⊕⊕⊕⊝ | ARI = 0.6% NNTH = 167 |
| End stage renal failure Follow‐up: mean 4.9 years | 13 per 1000 | 14 per 1000 (11 to 18) | RR 1.10 (0.88 to 1.37) | 24,309 (1) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on one large trial (ALLHAT 2002). | ||||||
| First‐line RAS inhibitors compared to first‐line beta‐blockers for hypertension | ||||||
| Patient or population: people with hypertension | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Β‐blockers | RAS inhibitors | |||||
| All‐cause death | 94 per 1000 | 84 per 1000 | RR 0.89 (0.78 to 1.01) | 9193 (1) | ⊕⊕⊝⊝ | |
| Total cardiovascular events | 143 per 1000 | 126 per 1000 | RR 0.88 | 9239 | ⊕⊕⊝⊝ | ARR = 1.7% NNTB = 59 |
| Total heart failure | 35 per 1000 | 33 per 1000 (27 to 41) | RR 0.95 (0.76 to 1.18) | 9193 (1) | ⊕⊕⊝⊝ | |
| Total myocardial infarction | 41 per 1000 | 43 per 1000 | RR 1.05 | 9239 | ⊕⊕⊝⊝ | |
| Total stroke | 67 per 1000 | 50 per 1000 (42 to 59) | RR 0.75 (0.63 to 0.88) | 9193 (1) | ⊕⊕⊝⊝ | ARR = 1.7% NNTB = 59 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based primarily on one moderate‐sized trial (LIFE 2002). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 5 | 35226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| 2 Total CV events Show forest plot | 6 | 35223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.02] |
| 3 Total HF Show forest plot | 5 | 35143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 4 Total MI Show forest plot | 5 | 35043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 5 Total stroke Show forest plot | 4 | 34673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.32] |
| 6 ESRF Show forest plot | 4 | 19551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| 7 SBP Show forest plot | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [0.90, 1.56] |
| 8 DBP Show forest plot | 20 | 36437 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [0.79, 1.18] |
| 9 HR Show forest plot | 5 | 540 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.63, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 2 Total CV events Show forest plot | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.00, 1.11] |
| 3 Total HF Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.07, 1.31] |
| 4 Total MI Show forest plot | 2 | 24379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 5 Total stroke Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 6 ESRF Show forest plot | 1 | 24309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.37] |
| 7 SBP Show forest plot | 10 | 26382 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.20, 1.99] |
| 8 DBP Show forest plot | 9 | 26335 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.13] |
| 9 HR Show forest plot | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐2.87, 4.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 2 Total CV events Show forest plot | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| 3 Total HF Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 4 Total MI Show forest plot | 2 | 9239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.27] |
| 5 Total stroke Show forest plot | 1 | 9193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.63, 0.88] |
| 6 ESRF Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 7 SBP Show forest plot | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐1.22, 0.11] |
| 8 DBP Show forest plot | 16 | 10905 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.14, 0.83] |
| 9 HR Show forest plot | 10 | 9979 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [5.59, 6.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 3 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐3.98, ‐0.78] |
| 2 DBP Show forest plot | 3 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.09, 0.85] |
| 3 HR Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.1 [‐2.41, 8.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SBP Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐6.01, 8.61] |
| 2 DBP Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.85, 1.25] |
| 3 HR Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐4.13, 7.13] |

