Вмешательства, направленные на улучшение охвата иммунизацией детей в странах с низким и средним уровнем дохода
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cluster RCT in Pakistan | |
| Participants | Setting: Lasbela, 1 of the poorest districts in Balochistan Province in Pakistan. Aim: authors hypothesised that if the community accessed information on the cost‐benefits of immunisation, the uptake of vaccines would improve without requiring improvement in service delivery Participants: 180 community groups with each group having 8‐10 participants, both male and female. Outcome measured in children aged 12‐23 months; 911 children at pre‐intervention and 956 at post‐intervention | |
| Interventions | Intervention:evidence‐based discussion on immunisation in 18 clusters: trusted members of the committee were selected for a 3‐phased discussion. 9 field teams (facilitators) had discussion with 180 community groups of 8‐10 members each in 94 villages for the intervention group. 3 phases of discussion were held with the community groups. First phase the community groups discussed the situation of child immunisation in the union council, the smallest unit of the local government system. Facilitators discussed the risk of non‐vaccination for measles with the community groups. Second phase, discussed cost‐benefits of vaccination and treatment of measles. Third stage featured discussion on challenges of immunisation and identification of barriers and plans of action to increase access for immunisation services and means of spreading the discussion on vaccination Control: usual care in 14 clusters | |
| Outcomes | Proportion of 12‐23 month olds who had received measles vaccination Proportion of 12‐23 month olds who had received full course of DPT | |
| Duration of intervention | August 2006 to May 2007 (9 months) | |
| Notes | Follow‐up after 1 year (baseline conducted in spring 2005; follow‐up spring 2007) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator allocated baseline communities to 18 intervention enumeration areas and 14 control enumeration areas |
| Allocation concealment (selection bias) | Low risk | Sequence concealed and intervention assigned centrally |
| Blinding (performance bias and detection bias) | Low risk | Interviewers did not know which clusters had received the intervention, only the field co‐ordinator knew |
| Incomplete outcome data (attrition bias) | Low risk | Not applicable. Samples taken pre‐ and post‐intervention |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | High risk | "Although the facilitators discussed with participants their plans for disseminating the discussions within their communities, the intervention did not make special provision for the participants to 'take back' the discussion to others in the community, relying rather on endogenous networks for the information spill over." In addition, use of mothers' recall for immunisation uptake may under estimate vaccine coverage Unit of study was enumeration area, analysis done at participant level; no adjustment for cluster effect |
| Baseline outcome measurements similar? | Low risk | Yes |
| Baseline characteristics similar? | Low risk | Baseline characteristics similar except, "mothers willing to travel to vaccinate", which was higher in the intervention than the control group |
| Adequate protection against contamination? | Unclear risk | Measure to prevent contamination not stated |
| Methods | Cluster RCT in India | |
| Participants | Setting: disadvantaged rural community in Udiapur, India with 2% immunisation coverage Aim: to test the effect of reliable supply of free immunisation services and incentive to improve vaccine demand in a resource‐poor setting Participants: 1640 children aged 0‐6 months at baseline or 1‐3 years at the endpoint survey | |
| Interventions | Intervention A: once monthly reliable immunisation camp without incentive (379 children from 30 villages at endpoint). Intervention focused on establishing regular availability of immunisation services. Consisted of a mobile immunisation team, including a nurse and assistant, who conducted monthly immunisation camps in the villages. Camp held on a fixed date every month at a fixed time (11 am to 2 pm). Presence of nurse and assistant verified by requirement of timed and dated pictures of them in the villages and by regular monitoring Intervention B: once monthly reliable immunisation camp with small incentives consisting of raw lentils and metal plates for completion of schedule (382 children from 30 villages at endpoint). Intervention used the same infrastructure as intervention A but in addition offered parents 1 kg of raw lentils per immunisation administered and a set of "thalis" (metal plates used for meals) on completion of a child's full immunisation. Value of the lentils about USD1, equivalent to three‐quarters of 1 day's wage, and the value of the "thalis" about USD2 Control: no intervention (860 children in 74 villages at endpoint) | |
| Outcomes | Probability of receiving at least 1 immunisation (excluding OPV, which almost all children received) Presence of the BCG scar Number of immunisations received Probability of being fully immunised. A fully immunised child received all the vaccines in the EPI schedule (1 dose of BCG, 3 doses of DTP, 3 doses of OPV, and 1 dose of measles vaccine) by the age of 1 year | |
| Duration of intervention | 18 months | |
| Notes | Study conducted in rural state of Rajasthan, India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using the random number generator in the statistical package Stata (version 9), and after stratification by geographical block (the administrative unit above the village), one author (ED) randomly selected 30 of the 134 study villages to receive intervention A and 30 to receive intervention B. The 74 remaining villages were control villages and received no additional intervention" |
| Allocation concealment (selection bias) | Low risk | "Within each village, a household census was conducted, and 30 households containing children aged 0‐5 years were randomly selected with a random number generator to be part of the sample. The same households were surveyed again at the end point. The criterion for inclusion of a child in this study was to belong to a sampled household and to be aged 1‐3 at the end point of the study (main sample) or to have been aged 0‐6 months at baseline (baseline cohort)" |
| Blinding (performance bias and detection bias) | Low risk | "The allocation of villages to treatment or control was not blind... Surveys were undertaken in randomly selected households at baseline and about 18 months after the interventions started (end point)... Interviewers did not know which villages belonged to which intervention (or control) group" |
| Incomplete outcome data (attrition bias) | High risk | Households lost between baseline and endpoint: 16% (71/453) in intervention A group, 17% (72/481) in intervention B group, and 17% (210/1224) in control group; 17% (363/2158) overall |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | None |
| Baseline outcome measurements similar? | Low risk | Yes |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Low risk | "Villages from all three treatment groups were sufficiently far from each other (over 20 km) so we expected no contamination between the villages" |
| Methods | Cluster RCT in Mexico | |
| Participants | Setting: Nicaragua, Mexico with immunisation rate > 90% Participants: 506/50,000 eligible villages randomly chosen | |
| Interventions | Intervention: 2 cash transfers every 2 months; 1 general and 1 depending on school attendance
Control: Usual care | |
| Outcomes | Immunisation full coverage of children aged 12‐23 months with 3 doses of DPT, BCG, and measles vaccines | |
| Duration of intervention | 12‐35 months | |
| Notes | The controls should originally have acted as controls for 2 years, but for political reasons intervention in control communities occurred in late 1999 so only 18 months of comparison was possible and the control communities were, therefore, considered as cross‐over intervention communities after 1 year of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Study was not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Not applicable |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not seen |
| Other bias | Unclear risk | Not stated |
| Baseline outcome measurements similar? | High risk | Baseline level of vaccination rate lower in treatment group |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Unclear risk | Protection against contamination not stated. |
| Methods | RCT in Nepal | |
| Participants | Setting: main maternity hospital in Kathmandu, Nepal Aim: tested the effectiveness of 1‐to‐1 health education with perinatal mothers in a hospital setting in Nepal on infant care and family planning Participants: 540 post‐partum women | |
| Interventions | Intervention A: 20 minute, 1‐to‐1 health education immediately after birth and 3 months later Intervention B: 20 minute, 1‐to‐1 health education at birth only Intervention C: 20 minute, 1‐to‐1 health education at 3 months only Intervention D: control (no individual health education) | |
| Outcomes | Duration of exclusive breastfeeding Appropriate immunisation of infant Knowledge of oral rehydration solution and need to continue breastfeeding in diarrhoea Knowledge of infant signs suggesting pneumonia Uptake of postnatal family planning | |
| Duration of intervention | 20‐minute, individual health education at birth and 3 months later. Outcomes assessed at 3 and 6 months | |
| Notes | First education session conducted in quiet room before discharge from hospital. Second education session conducted in the mothers' home 3 months after delivery. Although the health education given at birth and 3 months covered broadly the same areas, more emphasis was placed on the importance of exclusive breastfeeding in the first session and on the need for family planning in the second session. Topics covered were infant feeding, treatment of diarrhoea, recognition of and response to symptoms suggesting acute respiratory infection in young infants, importance of immunisation, and importance of contraception after the puerperium. At the end of each session, health educator repeated the key messages covered and asked mother if she had any other questions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Restricted randomisation was used in blocks of 20, each block consisting of a random ordering of the numbers 019. Numbers 04, 59, 1014, and 1519 were assigned to groups A to D respectively" |
| Allocation concealment (selection bias) | Unclear risk | "Timing of assignment was when a mother was identified by the research team either in labour or shortly after delivery. The details of allocation to groups for consecutively recruited mothers were in sealed envelopes... The generator of the assignment was not involved in the execution of the allocation" |
| Blinding (performance bias and detection bias) | Low risk | "The mothers recruited and the health educators were not blind to the assignment of mothers to different groups. The outcome assessors were always blind to the assignment at both the 3 and 6 month follow up visits. Staff who were involved in data collection at the 3 month follow up were not involved in data collection at 6 months. The data analysts were not blind to the coding of the groups" |
| Incomplete outcome data (attrition bias) | High risk | Each of the 4 groups (A‐D) had 135 women. At 6 months, percentage of women lost‐to‐follow‐up was 29% in group A, 21% in B, 26% in C, and 24% in D |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | None |
| Baseline outcome measurements similar? | Low risk | Not applicable |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Low risk | Yes |
| Methods | Matched and cluster RCT in Ghana | |
| Participants | Setting: urban settings in Ghana with regular immunisation services Aim: addressing low immunisation coverage in spite of developed immunisation infrastructure Participants: children aged 12‐18 months. Included 200 mother‐and‐child pairs in the intervention group and 219 in the control group | |
| Interventions | Intervention:home visits in 30 clusters. During home visits, interviewers (university students) administered questionnaires to mothers or female caregivers and fathers or male caregivers of children aged 12‐18 months. Immunisations recorded from road‐to‐health card or clinic record (if card was missing) in a register. All respondents advised to bring identified children who had not completed immunisation schedule to the clinic for immunisation. A referral note was given to each child to bring to the clinic. Children who failed to complete immunisation were identified from the register and a maximum of 3 home visits made to each child within 6 months Control: standard care in 30 clusters | |
| Outcomes | Completion of polio1, OPV3, and measles Completion of schedule | |
| Duration of intervention | 6 months | |
| Notes | 6 months of follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Contiguous clusters were paired, as far as possible within enumeration areas, and one of each pair of clusters was randomly chosen for the survey..." |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | High risk | Neither the provider nor the child was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Lost to follow‐up not accounted for |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in protocol |
| Other bias | High risk | Children in registered and unregistered houses included in intervention group but only children in registered houses included in control group Analysis done at cluster level; also took matching into account at analysis |
| Baseline outcome measurements similar? | Low risk | Baseline immunisation coverage in the 2 groups were not statistically significant |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Unclear risk | Though "contiguous clusters were paired as far as possible within the enumeration area", it was unclear if they were protected from contamination |
| Methods | Cluster RCT in Mali | |
| Participants | Setting: Kolokani, a district in Mali hyperendemic for malaria and with immunisation level < 50% Participants: children aged 0‐23 months | |
| Interventions | Intervention: intermittent preventive treatment of malaria in infants (in 11 clusters), i.e. administration to infants of ½ tablet of sulphadoxine‐pyrimethamine along with EPI vaccines (DTP2, DTP3 and measles/yellow fever vaccine). Communities leaders were sensitised and health staff were trained. Supports for child health interventions were modified to allow the recording of the administration of the sulphadoxine‐pyrimethamine along with EPI vaccines and the health interventions Control: standard care in 11 clusters | |
| Outcomes | Proportion of 9‐23 months old children completely immunised with BCG, 3 doses of DTP, 1 dose of measles, and yellow fever vaccines | |
| Duration of intervention | 12 months | |
| Notes | Study conducted from December 2006 to December 2007. Sample size for the baseline survey estimated using the following assumptions. Based on a precision of 6% and alpha error of 5% and DTP3 coverage of two‐thirds (67%), a sample of 472 children was selected using a cluster effect of 2. This sample size was doubled to take into account analysis for specific age categories and increased by 10% to take into account missing information, making a total sample size of 1050 children aged 0‐23 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple balloting. "The health areas were numbered from 1 to 22 and each number was written on piece of paper that was folded. The 22 pieces of paper were then mixed and placed in box and 11 of them were randomly drawn to serve as intervention areas by one of the trainees in presence of the representatives of the 22 communities’ health centres" |
| Allocation concealment (selection bias) | High risk | "The study was an open cluster‐randomised trial... The health areas were numbered from 1 to 22 and each number was written on piece of paper that was folded. The 22 pieces of paper were then mixed and placed in box and 11 of them were randomly drawn to serve as intervention areas by one of the trainees in presence of the representatives of the 22 communities' health centres" |
| Blinding (performance bias and detection bias) | High risk | Study was open cluster‐randomised trial. 2 cross‐sectional surveys (using the WHO method of evaluation of vaccine coverage) performed, 1 at baseline and 1 after 1 year of the intervention. Did not state whether the people conducting the survey were aware of the treatment allocations or not |
| Incomplete outcome data (attrition bias) | Low risk | Not applicable; 2 independent samples taken pre‐ and post‐intervention |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Other bias | Low risk | None |
| Baseline outcome measurements similar? | High risk | No. Difference was statistically significant |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | High risk | Training of staff was carried out in both control and intervention communities, followed by public randomisation |
| Methods | Cluster RCT in Georgia | |
| Participants | Setting: low immunisation coverage despite healthcare reforms. Human resource management was weak with lack of knowledge and skills in management and supervision especially at the peripheral levels Participants: district immunisation managers, PHC providers. Number of health workers studied was 392 at pre‐intervention and 521 at post‐intervention. Apart from outcome measures from PHC workers, data were obtained on children's immunisation | |
| Interventions | Intervention:development of supportive supervision guidelines for district immunisation managers in 15 clusters: intervention consisted of development of supportive supervision guidelines and tools for district managers, training in continuous supportive supervision, monitoring, and evaluation of performance. Each district manager visited subordinated health facility at least once a month. On‐the‐job training was provided for immunisation managers to improve on supervision practices to help providers solve problems encountered in immunisation Control: no intervention in 15 control clusters | |
| Outcomes | DTP3, polio 3, and HBV3 coverage Difference in proportion of coverage from baseline | |
| Duration of intervention | 12 months | |
| Notes | Follow‐up study conducted after 1 year of intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified cluster randomisation was used to select the 30 cluster units out of the nation's 67 districts and allocate them into the two study groups (intervention and control), yielding two allocation sequences of 15 clusters each" |
| Allocation concealment (selection bias) | Unclear risk | "Given that immunization managers supervise health workers only within their districts, and similarly health workers provide immunization services to target population residing in communities within the same district, the risk of contamination of the control group with the intervention is negligible. Use of smaller units (e.g. village) would have posed a higher risk of contamination of intervention activities in control clusters" |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | Not applicable; 2 independent samples taken pre‐ and post‐intervention |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all the outcomes stated in the protocol were reported on |
| Other bias | High risk | During the course of intervention, the country improved healthcare financing for low‐income people and there was also improved country level economic growth thus improving access to health care. "It is possible that improved access to health care may have contributed to improved immunization coverage in Georgia" Unit of study was district, but unit of analysis was participant. No adjustment for clustering effect |
| Baseline outcome measurements similar? | Low risk | Yes |
| Baseline characteristics similar? | Low risk | Demographic and employment characteristics were similar among Center of Public Health staff respondents in the intervention and control groups, both at baseline and follow‐up except mean years of experience, which was more among the control group |
| Adequate protection against contamination? | Unclear risk | Protection against contamination unclear |
| Methods | Cluster RCT conducted in Nicaragua (Red de proteccion social) | |
| Participants | Setting: part of a social safety net programme targeted at poor households living in rural areas, but the pilot phase analysed in this study occurred in 2 departments (Madriz and Matagalpa) in the Northern part of the Central Region. This region is the only one in the country where poverty worsened during 1998 and 2001 These pilot sites were not representative of the country situation: within the 2 chosen departments, 6 municipalities were chosen (out of 20) because they had benefited from a previous programme that developed the capacity of the governing bodies to implement and monitor social projects: "it is possible that the selected municipalities had atypical capacities to run RPS" in the chosen municipalities, 78‐90% of the population was extremely poor/poor, compared to 21‐45% at national level. 42 eligible areas (the neediest) were chosen for the pilot programme based on wealth index Private providers were specifically trained to deliver the specific healthcare services required by the programme. Incentives were also given to teachers to compensate for the larger classes they had after the implementation of the programme. 10% of beneficiaries were penalised at least once during the first 2 years of the programme; 5% were expelled or left the programme. Some conditions (adequate weight gain) were dropped at the end of the pilot phase and others were not properly enforced (up‐to‐date vaccination while there were delays in the delivery of vaccines) Delays occurred in the implementation of the health component, which finally started in June 2001. Therefore, when the first follow‐up survey was realised in October 2001, the beneficiaries had been receiving the transfers for the education component for 13 months and those for the health and nutrition component for 5 months only Participants: All households except 169 (2.9% of households that lived in the intervention area) that owned either a vehicle (truck, pickup truck, or jeep) or land >14.1 hectares or both. | |
| Interventions | INTERVENTION: in 21 clusters Programme had 2 components:
Beneficiaries did not receive the food or education cash transfers if they failed to comply with any of the conditions CONTROL: no intervention in the 21 control clusters | |
| Outcomes | Immunisation coverage: reported up‐to‐date vaccination schedule (children aged 12‐23 months) Health services uptake: attendance of preventive care visits by children Anthropometric or nutritional outcomes: prevalence of stunting, wasting, and underweight (children aged < 5 years) Height for age Z‐score (children aged < 5 years) Prevalence of anaemia | |
| Duration of intervention | 5 years | |
| Notes | The "Red de Proteccion Social" project was financed by a loan from the Inter‐American Development Bank. The impact analysis of the pilot phase was done by the International Food Policy Research Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random selection by balloting within each stratum, randomisation was achieved by blindly drawing one of six coloured balls (three blue for intervention, three white for control) from a box after the name of each comarca [region] was called out" |
| Allocation concealment (selection bias) | High risk | Randomisation not concealed |
| Blinding (performance bias and detection bias) | High risk | Study not blinded |
| Incomplete outcome data (attrition bias) | High risk | Reasons for attrition not given |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all outcomes stated in the protocol were reported on |
| Other bias | High risk | "In October 2001, then, beneficiaries had been receiving transfers, and the educational components of the program had been monitored for 13 months, but they had only received five months of the health and nutrition services, including the health education workshops" "It is important to emphasize that for most of the indicators considered, the control group also showed large improvements over the period, although on a much smaller scale. A possible explanation for this increase is that other providers are bringing health services into the areas not covered by the program (program providers do not offer or deliver any services to non‐beneficiaries)" |
| Baseline outcome measurements similar? | Unclear risk | Baseline number of children aged 12‐23 months with updated immunisation similar between baseline and control |
| Baseline characteristics similar? | Unclear risk | Baseline characteristics on the intervention and control groups not stated. Author reported "few significant difference between households (or individuals) in intervention and control groups at baseline" but was unclear if the difference were related to outcomes of the review |
| Adequate protection against contamination? | High risk | "Control and intervention comarcas [regions] are at times adjacent to one another. A household may be a beneficiary while its neighbour is a nonbeneficiary, particularly in a few cases where boundaries such as roads divide two comarcas. Seeing the activity and the emphasis placed on the RPS objectives may lead non‐beneficiaries to undertake behavior they would not have otherwise. Reasons for such actions could be many ‐ including the possibility that the individuals thought this was a way to become eligible" |
| Methods | Cluster RCT in Honduras | |
| Participants | Participants: households in 70 clusters including pregnant women, new mothers, and children aged < 3 years. Outcome on immunisation was measured in 4359 children at pre‐intervention and 3876 at post‐intervention Aim: to drive demand, poor households benefited from cash transfer on the condition that they keep up‐to‐date with preventive healthcare services. | |
| Interventions | Intervention A:household monetary incentive in 20 clusters: consisted of distribution of vouchers worth GBP2.53 to mothers who were registered in 2000 census who were either pregnant or had a child < 3 years of age to a maximum of 2 children. In addition, mothers with children aged 6‐12 years enrolled in primary schools in grade 1‐4 given vouchers worth GBP3.69 per month. Beneficiaries lost aid if they were not up‐to‐date with routine antenatal care, and well‐child preventive health care and if child did not attend school regularly Intervention B:service‐level monetary incentive in 10 clusters: quality assurance teams set up at each health centre and trained on basic quality assurance methods. They developed work plans that included minor structural repairs; purchase of equipment, materials, and essential drugs; and money to pay lay assistants. Package included promotion of community‐based nutrition programme for children aged < 2 years Intervention C: combination of household and service‐level monetary incentives (i.e. Interventions A + B) in 20 clusters Control: standard (routine) care in 20 clusters | |
| Outcomes | Proportion of pregnant women immunised against tetanus Proportion of children aged 93 days to 3 years who received first dose of DTP or pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenzae type B, hepatitis B) at 42‐92 days of age Proportion of children aged 1 year old immunised against measles | |
| Duration of intervention | 1 year | |
| Notes | 2 years of follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children made to pick coloured balls from a box where aperture would not allow the children to see the ballot balls |
| Allocation concealment (selection bias) | High risk | "From the day of the randomisation onwards, there was no attempt to conceal the allocation, but it was not possible for a household to become eligible for the vouchers by moving into a beneficiary municipality. On the other hand, it was not possible to restrict usage of 'improved' health services to residents of the appropriate municipality" |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | "Loss to follow up did not exceed 5%" |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | High risk | Service package could not be provided according to the protocol and training on quality assurance was limited to only the introduction. Disbursement of funds for this was only 17% of the budget Unit of randomisation was municipalities. Analysis not adjusted for cluster effect |
| Baseline outcome measurements similar? | High risk | The coverage of DTP1 vaccine in the group receiving intervention C (intervention A + B) was lower than the other 3 groups |
| Baseline characteristics similar? | Low risk | Demographic and socioeconomic data of the 4 groups similar |
| Adequate protection against contamination? | High risk | It was possible for participants from other arms of study to attend services at improved centres. 14% of children aged < 3 years attended clinics in municipalities other than their municipality of residence 1 month prior to post‐intervention survey |
| Methods | RCT in Pakistan | |
| Participants | Setting: urban and semi‐urban communities with low literacy and low immunisation coverage Participants: 364 mother‐infant pairs, with infants aged ≤ 6 weeks. Excluded twin births, infants > 6 weeks of age, or infants born to mothers living outside the study surveillance areas. Cut‐off of 6 weeks used to ensure that the intervention was implemented before the first dose of DTP/hepatitis B became due | |
| Interventions | Intervention: 3 targeted pictorial messages regarding vaccines administered by trained CHWs. First key message highlighted how vaccines save children's lives. Second message provided logistic information about the address and location of the local vaccination centres. Third key message emphasised the significance of retaining immunisation cards, and role they could play at the time of the child's school admissions. Copy of these pictorial messages was left with the mother. Messages took about 5 minutes to impart Control: verbal general health promotion messages delivered by trained CHWs. Messages included information on hand washing, breastfeeding, clean water, benefits of using oral rehydration solutions during diarrhoea, bringing the infant to nearby health centre when there were symptoms of acute respiratory illnesses, importance of antenatal check‐ups for mothers, and some general information on vaccines. Length of each educational session was approximately 10‐15 minutes | |
| Outcomes | DTP/hepatitis B vaccine completion (3 doses) at 4 months after enrolment (4‐5 months of infant's age) | |
| Duration of intervention | 4 months | |
| Notes | Community‐based study conducted at 5 low‐income sites in Karachi, Pakistan. Participants were enrolled from August 2008 to November 2008 and followed up for assessment of outcome from December 2008 to March 2009, with each individual mother‐infant pair approached 4 months after the educational intervention session | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization lists, stratified for each of the five enrolment sites were generated by a computer and provided to the CHWs. Upon consent, mother‐infant pairs were assigned either to intervention or control arms through block randomisation (n = 4), according to the computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "As the intervention was educational, blinding of study staff and participants was not possible... Outcome assessment was done by an investigator ... blinded to the exposure status of participants" |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data not available for 2% (4/183) in the intervention group (0 deaths) and 3% (5/183) in the control group (3 deaths) |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | Low risk | None |
| Baseline outcome measurements similar? | Low risk | Yes |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Low risk | Yes |
| Methods | Cluster RCT in India | |
| Participants | Setting: community‐based trial Aim: tested the hypothesis that informing the community will enhance accountability of the health workers towards quantity and quality of services rendered. Resource poor rural populations were informed about entitled services Participants: households with at least 1 child going to public primary school in the village. Immunisation coverage targeted children aged 0‐35 months. 1025 children included | |
| Interventions | Intervention:information campaign in 11 clusters; 2 rounds of information campaigns consisting of 2 or 3 meetings and distribution of posters and leaflets. 15‐minute audiotaped message played twice at each meeting and 15 minutes given for questions. To ensure uniformity only questions for which answers were written in the leaflet were responded to Control: no intervention in 10 control clusters | |
| Outcomes | Received tetanus vaccination Received at least 1 vaccine | |
| Duration of intervention | Each of the 2 rounds of meetings lasted for 1 hour and each round was separated by 2 weeks | |
| Notes | Post‐intervention data collected 12 months after | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Low risk | Research assistants at post‐intervention had no knowledge of the intervention |
| Incomplete outcome data (attrition bias) | Low risk | 2.4% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | High risk | Proportion at campaign meetings 11‐14% and long recall period Unit of study was village; unit of analysis was household. No adjustment for clustering effect |
| Baseline outcome measurements similar? | Low risk | Difference between proportion of children immunised at baseline in the 2 groups was not statistically significant |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Unclear risk | "By randomly selecting only 5 village clusters of about 1000 in each district, we spread the selection of 105 village clusters over 21 districts to minimize any potential for contamination" |
| Methods | Matched, cluster RCT in 10 sites in Manicaland, Zimbabwe | |
| Participants | Aim: tested effect of conditional and unconditional cash transfer among poor and vulnerable populations in Zimbabwe Setting and participants: "We ranked households according to their index score and then divided them into quintiles in each study site, thus identifying the poorest 20% of households in each site... Eligible households contained children younger than 18 years and satisfied at least one other criteria: head of household was younger than 18 years; household cared for at least one orphan younger than 18 years, a disabled person, or an individual who was chronically ill; or household was in poorest wealth quintile. Households within the clusters were eligible for inclusion in the trial when they contained children younger than 18 years and satisfied at least one other criteria at baseline: the head of the household was younger than 18 years; the household cared for at least one orphan (a child younger than 18 years with one or more deceased parents), disabled person, or an individual who was chronically ill; or the household was in the poorest wealth quintile. Households in the richest wealth quintile and those already receiving cash transfers for orphans and vulnerable children were not eligible. We did a baseline survey of all households in the trial clusters between July, and September, 2009. We counted how many members made up each household and obtained information about trial endpoints and eligibility and exclusion criteria, including house hold asset data. We constructed a wealth index with a simple sum of reported household assets (appendix). We ranked households according to their index score and then divided them into quintiles in each study site, thus identifying the poorest 20% of households in each site. We obtained informed consent from the most senior member of the household available at time of interview" | |
| Interventions | Intervention: unconditional cash transfers in 1525 households, conditional cash transfers in 1319 households "Every household enrolled in the UCT [unconditional cash transfer] programme collected US$ [USD] 18 plus $4 per child in the household (up to a maximum of three children) from designated pay points every 2 months. Households in the CCT [conditional cash transfer] group could receive the same amount, but were monitored for compliance with several conditions: an application for a birth certificate had to be made within 3 months for all children younger than 18 years (including newborn babies) whose births had not been registered; children younger than 5 years had to be up‐to‐date with vaccinations and attend growth monitoring clinics twice a year; children aged 6–17 years had to attend school at least 90% of the time per month; and a representative from every household had to attend two‐thirds of local parenting skills classes. Compliance cards were issued to CCT households and were signed by service providers when beneficiaries accessed services. The signed cards were brought to the pay points every 2 months, along with other documents such as birth certificates, child health cards, and receipts for the payment of school fees. Community committees were familiar with most people living in the trial clusters. If a household provided a good reason for not meeting conditions (e.g. a child missing school because of illness), it was verified by the committee and judged on a case‐by‐case basis" Control: no intervention in 1199 households | |
| Outcomes | 3 domains of child well‐being (identity, health, and education) Proportion of children aged < 5 years with a birth certificate Proportion of children aged < 5 years with up‐to‐date vaccinations (measles, BCG, polio, and DTP) Proportion of children aged 6‐12 years attending school at least 80% of the time in the previous month | |
| Duration of intervention | 13 months | |
| Notes | "After the baseline survey, clusters were randomly assigned to UCT [unconditional cash transfer], CCT [conditional cash transfer], or control at public meetings that any community members could attend. In each site, one cluster was assigned to UCT, one to CCT, and one to control. Allocation was done by the drawing of lots from a hat. Participating households and individuals delivering the intervention were not masked to cluster assignment. At follow‐up, research assistants were not told the allocation of the household they were interviewing, but questions were included at the end of the questionnaire about whether households received transfers. LR was masked when doing the primary analysis" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation through balloting |
| Allocation concealment (selection bias) | High risk | Randomisation not concealed |
| Blinding (performance bias and detection bias) | Low risk | Study was single blinded. "LR was masked while doing the primary analysis" |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up accounted for and analysis was by intension to treat |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | Unclear risk | 2 villages randomised into the control group were mistakenly enrolled in the unconditional cash transfer group. Duration of study was shortened from 24 to 13 months due to lack of funds |
| Baseline outcome measurements similar? | Low risk | Yes |
| Baseline characteristics similar? | High risk | Some characteristics were dissimilar |
| Adequate protection against contamination? | High risk | Almost one‐third of those for UCT reported having to comply with conditions |
| Methods | RCT in Pakistan | |
| Participants | Setting: reminder intervention in an urban setting in Pakistan to reduce drop‐out rate in DTP3 Participants: 375 mothers visiting the EPI centre in each of 4 arms of study with 1125 children registering for DTP1 immunisation and residing in the study area for the past 6 months | |
| Interventions | Intervention A:redesigned ("reminder‐type") immunisation card; a larger card (15.5 cm by 11.5 cm when folded) that had only the date and day of next immunisation on both sides of the outer card printed with Microsoft Word font size 42 was designed as a reminder for mothers/carers for immunisation. Inner side of the card contained information about the child's complete immunization schedule dates and instructions for the mother/carer For those in the arm for redesigned card, the date and day for each DTP vaccination was written on the outer side of the card; dates of previous vaccinations were crossed out to avoid confusion. Mother was advised to place the card at a frequently visible place at home and to bring it to the clinic during immunisation visits Intervention B:centre‐base education; clinic‐based education that lasted 2‐3 minutes given to mothers at enrolment of their children in the EPI centre. The health education emphasised the importance of immunisation schedule completion Intervention C: intervention 1 + 2 Control: standard care | |
| Outcomes | Number of enrolled children with DTP3 completed within 90 days of duration of study | |
| Duration of intervention | 2‐3 minutes per session; follow‐up for 90 days | |
| Notes | Urban Pakistan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was by computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether allocation was concealed |
| Blinding (performance bias and detection bias) | High risk | Neither the participant nor the assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | Low risk | No other bias detected |
| Baseline outcome measurements similar? | Low risk | Not applicable |
| Baseline characteristics similar? | High risk | Most of the socioeconomic variables were similar but ownership of a television was more among group receiving education and a higher proportion of those receiving standard care lived close to the facility than those in the redesigned card group |
| Adequate protection against contamination? | Unclear risk | Unclear |
| Methods | RCT in Pakistan | |
| Participants | Setting: rural setting in Pakistan Aim: to test theory that reminder intervention can reduce drop‐out rate for DTP3 vaccination Participants: 1508 mother‐child pair visiting selected EPI centres for DTP1 who were resident in study area for at least 6 months. Criterion used to exclude 2 groups of temporary residents: women who temporarily relocated to their mothers' houses to deliver their children and internally displaced families who had migrated to the study area to avoid the aftermath of 2005 earthquake in the north of Pakistan | |
| Interventions | Intervention A:redesigned ("reminder‐type") immunisation card; a larger card than the existing EPI card (15.5 cm by 11.5 cm when folded), placed in a plastic jacket and provided with a hanging string. A "trained interviewer pasted the upcoming date and day of DTP2 immunization on both outer sides of the card and showed it to the mother. Mother was asked to hang the card in her home at a frequently visible place and requested that she bring the card along on her next immunization visit to the EPI centre. At DTP2 visit, the interviewer crossed out the date and day for DTP2 visit to avoid any confusion to the mothers, pasted the date and day for the upcoming DTP3 immunization visit on both sides of the card and showed the information to the mother." The inner side of the card contained information about the child's complete immunisation schedule dates and instructions for the mother Intervention B:centre‐base education; 2‐ to 3‐minute conversation between trained study interviewer and mother to convey the importance of completing the immunisation schedule and the potential adverse impact of incomplete immunisation on the child's health. Session was in simple vocabulary in the local language and deliberately kept short in prevision of potential large‐scale use by EPI staff in the future Intervention C: combination of redesigned card and centre‐based education Control: standard care i.e. routine EPI centre visit and neither intervention | |
| Outcomes | DTP3 coverage at the end of day 90 post‐enrolment. | |
| Duration of intervention | 2‐3 minutes per session; follow‐up at 90 days | |
| Notes | Rural areas around Karachi, Pakistan. Despite a small purchase volume, the cost of each card including the plastic jacket was USD0.05 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The lead investigator provided a computer‐generated randomisation list to each enrolment centre" |
| Allocation concealment (selection bias) | Unclear risk | "Each enrolled mother‐child pair received an identification number (ID) from the randomisation list and was assigned to the study group corresponding to the ID on the list" |
| Blinding (performance bias and detection bias) | High risk | "Because of the overt nature of interventions, neither the study participants nor the interviewers enrolling the study participants and recording the study outcome were blinded to the type of intervention received by the study participants" |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were stated in the protocol |
| Other bias | Low risk | No other bias detected |
| Baseline outcome measurements similar? | Low risk | Not applicable |
| Baseline characteristics similar? | Low risk | Yes |
| Adequate protection against contamination? | Low risk | "Interventions were provided in a private space to prevent contamination between study groups" |
BCG: Bacille Calmette‐Guérin; CHW: community health worker; DTP: diphtheria‐tetanus‐pertussis; EPI: Expanded Programme on Immunization; HBV3: three doses of hepatitis B virus; OPV: oral polio vaccine; PHC: primary healthcare; RCT: randomised controlled trial; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| Retrospective study | |
| Observational study | |
| Observational study | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| No relevant data on outcome | |
| Programme evaluation | |
| Observational study | |
| Programme evaluation | |
| No relevant data on outcome | |
| No relevant outcome. Reports on drop‐out rate | |
| Observational study | |
| Observational study | |
| No relevant data on outcome | |
| Observational study | |
| Retrospective study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| No relevant data on outcome | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| Observational study | |
| Observational study | |
| Observational study | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| Observational study | |
| Observational study | |
| Observational study | |
| Observational study | |
| Retrospective study | |
| Observational study | |
| Observational study | |
| Observational study | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| Observational study | |
| Observational study | |
| Data not summarised by the study groups | |
| Observational study | |
| Observational study | |
| Observational study | |
| A controlled before‐and‐after study with single unit for intervention and control arms | |
| Study had no control arm | |
| Observational study | |
| Observational study | |
| No relevant outcome for the review | |
| Observational study |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | A quasi‐experimental study in rural Pakistan |
| Participants | Household heads |
| Interventions | Community service in intervention clusters (government Basic Health unit) versus standard care in control clusters |
| Outcomes | Knowledge and practices regarding routine immunisation, Fully vaccinated children, partially vaccinated children, un‐vaccinated children. |
| Notes |
| Methods | Randomised controlled trial in Kadoma City, Zimbabwe |
| Participants | Women at delivery |
| Interventions | SMS reminders versus standard care |
| Outcomes | Immunisation coverage, timely vaccinations |
| Notes |
| Methods | Cluster RCT in Rwanda |
| Participants | Healthcare providers |
| Interventions | Performance‐based payment of healthcare providers (payment for performance; P4P) versus traditional input‐based funding |
| Outcomes | Immunisation, prenatal care visits and institutional deliveries, quality of prenatal care, and child preventive care visits |
| Notes |
| Methods | controlled before‐after study in rural Kenya |
| Participants | Caregivers of children aged 2‐13 months |
| Interventions | Free hygiene kits and education about water treatment and hand hygiene |
| Outcomes | Fully vaccinated children |
| Notes |
| Methods | Cluster RCT in Ibadan, Nigeria |
| Participants | Children aged 0‐12 weeks |
| Interventions | Mobile phone reminders and recall versus Primary Health Care immunisation providers' training versus combined Mobile phone reminders and recall versus Primary Health Care immunisation providers' training versus standard care |
| Outcomes | Children fully vaccinated at 12 months of age |
| Notes |
| Methods | A field experiment in rural Guatemala |
| Participants | Families whose children were due for a vaccine |
| Interventions | Personal reminders versus standard care |
| Outcomes | Fully vaccinated children |
| Notes |
| Methods | RCT in Guatemala City |
| Participants | Caregivers of infants aged 8‐14 weeks presenting for first dose of primary immunisation series |
| Interventions | Mobile phone short message service versus standard care |
| Outcomes | Fully vaccinated infants |
| Notes |
| Methods | Random allocation of paraprofessionals and Midwives to "visiting area" in Istanbul, Turkey |
| Participants | Midwives and lady home visitors (paraprofessionals) and children aged < 5 years |
| Interventions | Use of lay home visitors vs. midwives for home visit |
| Outcomes | Infants fully vaccinated, children aged < 5 fully vaccinated. |
| Notes |
| Methods | Random allocation of three facilities in three districts in Kenya to two interventions and control |
| Participants | children less than 12 months |
| Interventions | Reminder text message vs reminder sticker |
| Outcomes | Receipt of DTP 2 and DTP 3 at 10 and 14 weeks; dropout rate |
| Notes |
| Methods | Cluster RCT in rural Uttar Pradesh, India |
| Participants | Mothers of children 0‐23 months of age were eligible |
| Interventions | Home visits by volunteers plus community mobilisation to promote immunisation versus community mobilisation to promote nutrition |
| Outcomes | Primary outcomes were feasibility of recruitment, randomisation and retention of participants |
| Notes |
| Methods | Randomised controlled trial |
| Participants | Children aged < 2 years, had telephone numbers listed in pre‐exiting computerised database, and were due or late for immunisation(s) during the 4‐month enrolment period |
| Interventions | Household of children were randomised to receive or not receive a general or vaccine‐specific computer generated telephone reminder message 1 day before the child was due, or immediately after randomisation if the child was late |
| Outcomes | The rate of immunisation visits in the 30‐day follow‐up period |
| Notes |
| Methods | Non randomised trial in urban and rural Bangladesh |
| Participants | Families of children in need of vaccination |
| Interventions | Mobile phone short message service versus standard care |
| Outcomes | Fully vaccinated children |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Measles vaccine Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 1.1 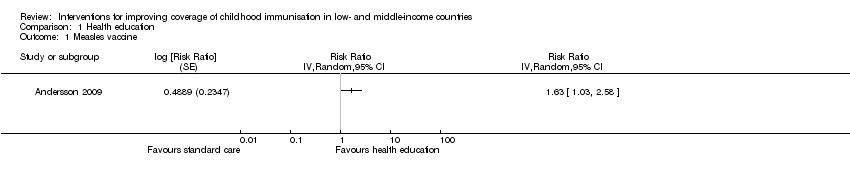 Comparison 1 Health education, Outcome 1 Measles vaccine. | ||||
| 2 DTP3 Show forest plot | 5 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Health education, Outcome 2 DTP3. | ||||
| 2.1 Community‐based education | 2 | Risk Ratio (Random, 95% CI) | 1.68 [1.09, 2.59] | |
| 2.2 Facility‐based education | 3 | Risk Ratio (Random, 95% CI) | 1.20 [0.97, 1.48] | |
| 3 Received at least 1 vaccine Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 1.3 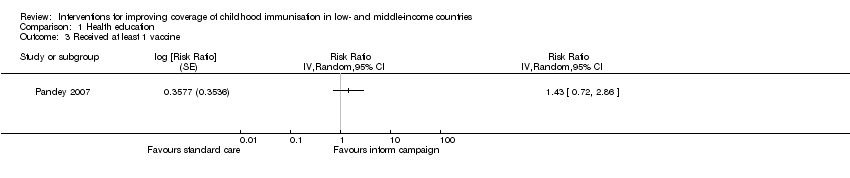 Comparison 1 Health education, Outcome 3 Received at least 1 vaccine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DTP3 Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 1.50 [1.21, 1.87] | |
| Analysis 2.1  Comparison 2 Health education plus redesigned reminder card, Outcome 1 DTP3. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 3.1 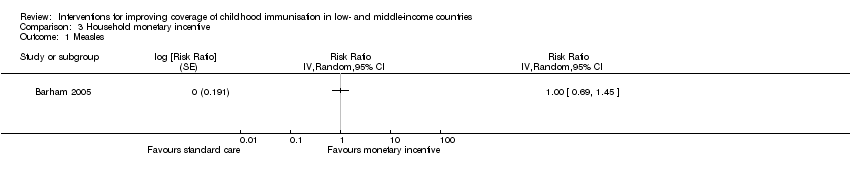 Comparison 3 Household monetary incentive, Outcome 1 Measles. | ||||
| 2 Fully immunised children Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] | |
| Analysis 3.2  Comparison 3 Household monetary incentive, Outcome 2 Fully immunised children. | ||||
| 3 BCG Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 3.3 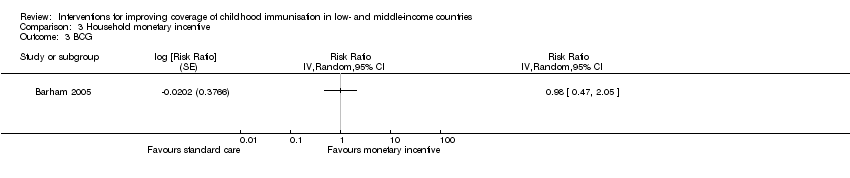 Comparison 3 Household monetary incentive, Outcome 3 BCG. | ||||
| 4 MMR Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Household monetary incentive, Outcome 4 MMR. | ||||
| 4.1 Household monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Household + service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 DTP1 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 3.5 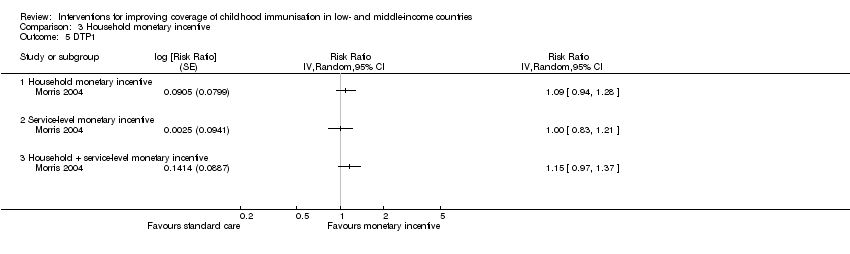 Comparison 3 Household monetary incentive, Outcome 5 DTP1. | ||||
| 5.1 Household monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Household + service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OPV3 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 4.1 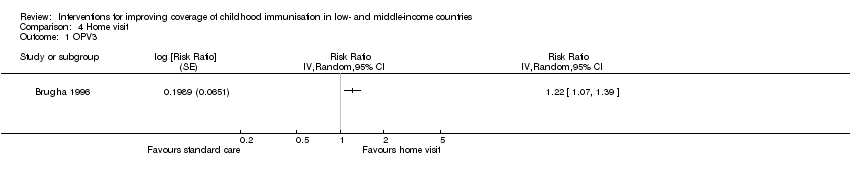 Comparison 4 Home visit, Outcome 1 OPV3. | ||||
| 2 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Home visit, Outcome 2 Measles. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fully immunised children Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Regular immunisation outreach, Outcome 1 Fully immunised children. | ||||
| 1.1 Regular immunisation outreach only | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Regular immunisation outreach + incentive | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BCG Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 6.1 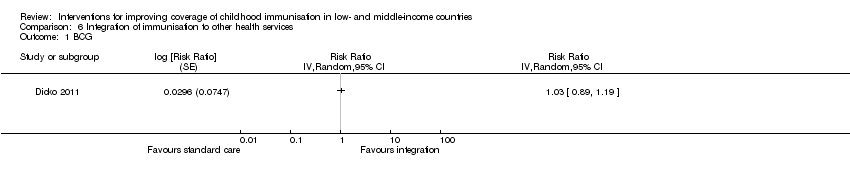 Comparison 6 Integration of immunisation to other health services, Outcome 1 BCG. | ||||
| 2 DTP3 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Integration of immunisation to other health services, Outcome 2 DTP3. | ||||
| 3 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Integration of immunisation to other health services, Outcome 3 Measles. | ||||
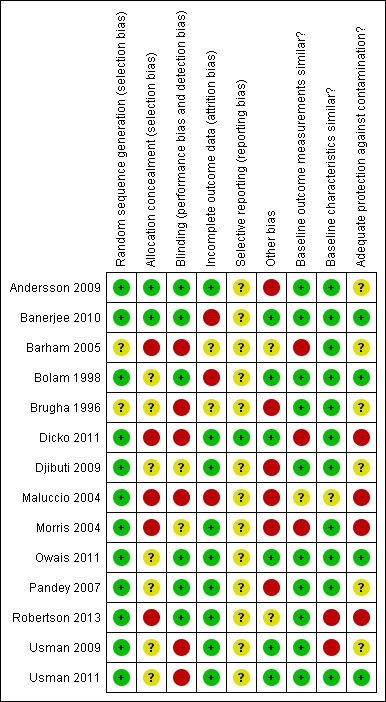
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
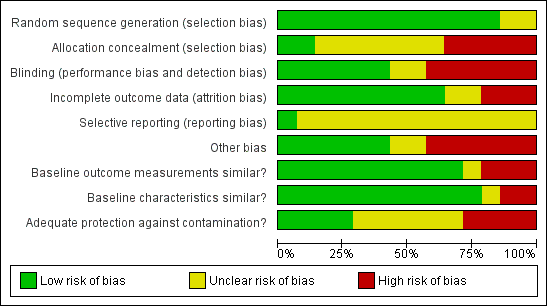
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Study flow diagram.
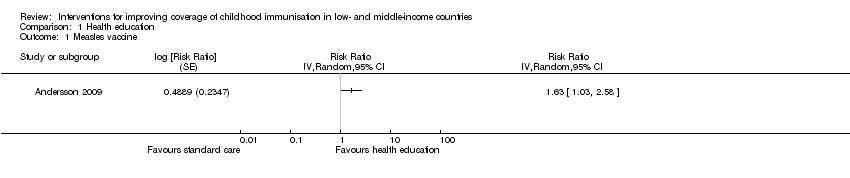
Comparison 1 Health education, Outcome 1 Measles vaccine.

Comparison 1 Health education, Outcome 2 DTP3.

Comparison 1 Health education, Outcome 3 Received at least 1 vaccine.
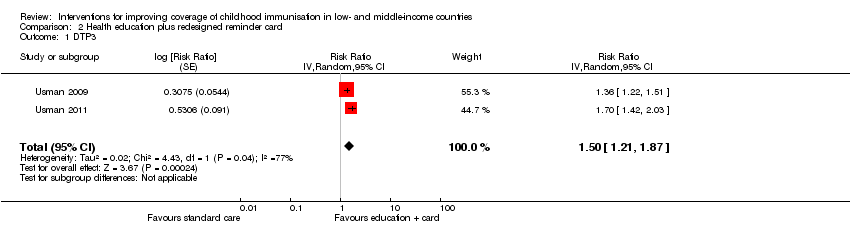
Comparison 2 Health education plus redesigned reminder card, Outcome 1 DTP3.

Comparison 3 Household monetary incentive, Outcome 1 Measles.

Comparison 3 Household monetary incentive, Outcome 2 Fully immunised children.

Comparison 3 Household monetary incentive, Outcome 3 BCG.

Comparison 3 Household monetary incentive, Outcome 4 MMR.
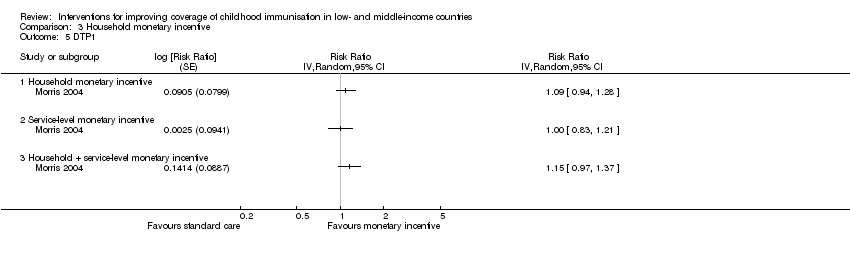
Comparison 3 Household monetary incentive, Outcome 5 DTP1.
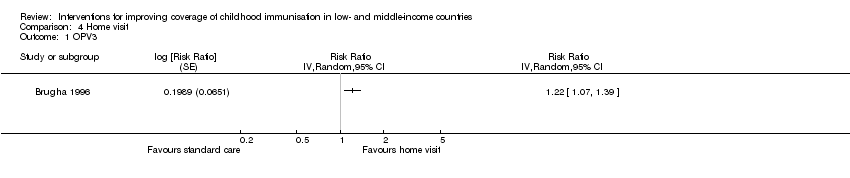
Comparison 4 Home visit, Outcome 1 OPV3.
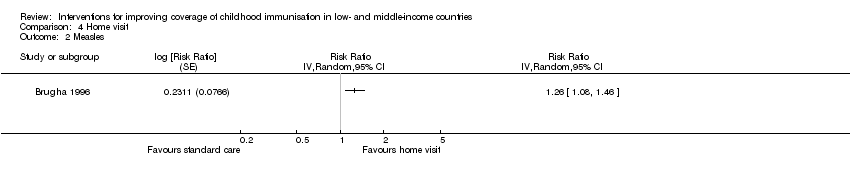
Comparison 4 Home visit, Outcome 2 Measles.

Comparison 5 Regular immunisation outreach, Outcome 1 Fully immunised children.
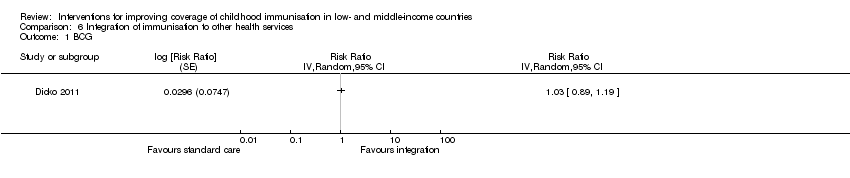
Comparison 6 Integration of immunisation to other health services, Outcome 1 BCG.

Comparison 6 Integration of immunisation to other health services, Outcome 2 DTP3.

Comparison 6 Integration of immunisation to other health services, Outcome 3 Measles.
| Population: children aged < 24 months | |||||
| Outcomes | Anticipated absolute effects (95% CI)* | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Health education | ||||
| DTP3 (Follow‐up: 4‐9 months) | 577 per 1000 | 969 per 1000 | RR 1.68 | 1692 | ⊕⊕⊕⊝ |
| *The effect in the 'health education' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;DTP3: 3 doses of diphtheria‐tetanus‐pertussis containing vaccines; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. 'Substantially different' implies a large enough difference that it might affect a decision. | |||||
| 1 We rated down by 1 level because we judged the included studies at high risk of bias. 2 We rated down by 1 level because of unexplained heterogeneity of effects across studies, P value < 0.00001, I2 = 68%. | |||||
| Population: children aged 6 weeks Setting: Pakistan | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Health education plus redesigned card | ||||
| DTP3 (Follow‐up: 90 days) | 470 per 1000 | 705 per 1000 | RR 1.50 | 1502 | ⊕⊕⊝⊝ |
| *The effect in the 'health education + redesigned card' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;DTP3: 3 doses of diphtheria‐tetanus‐pertussis containing vaccines; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. 'Substantially different' implies a large enough difference that it might affect a decision. | |||||
| 1 We rated down by 1 level because of unexplained heterogeneity of effects across studies; P value = 0.04; I2 = 77%. 2 We rated down by 1 level because we judged the 2 included studies at unclear risk of selection bias and at high risk of performance and detection bias. 3 Usman 2009; Usman 2011. | |||||
| Population: children aged < 5 years | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Monetary incentive | ||||
| Fully immunised children (Follow‐up: 13 months to 5 years) | 701 per 1000 | 736 per 1000 | RR 1.05 | 1000 | ⊕⊕⊝⊝ |
| *The effect in the 'monetary incentive' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. 'Substantially different' implies a large enough difference that it might affect a decision. | |||||
| 1 We rated down by 2 levels because we judged the 2 included studies at high risk of bias. | |||||
| Population: children aged 12‐18 months Setting: Ghana | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Home visits | ||||
| OPV3 | 73 per 100 | 89 per 100 | RR 1.22 | 419 | ⊕⊕⊝⊝ |
| *The effect in the 'home visits' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. | |||||
| 1 We rated down by 2 levels because the 1 included study was judged to be at high risk of bias. | |||||
| Population: children aged 0‐6 months | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Immunisation outreach | ||||
| Fully immunised ‐ regular immunisation outreach only (Follow‐up: 18 months) | 58 per 1000 | 180 per 1000 | RR 3.09 | 1239 | ⊕⊕⊝⊝ |
| Fully immunised ‐ regular immunisation outreach + non‐monetary incentive (Follow‐up: 18 months) | 58 per 1000 | 387 per 1000 | RR 6.66 | 1242 | ⊕⊕⊝⊝ |
| *The effect in the 'immunisation outreach' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. 'Substantially different' implies a large enough difference that it might affect a decision. | |||||
| 1 We rated down by 2 levels because we judged the 1 included study at high risk of bias. | |||||
| Population: children aged 0‐23 months | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Standard care | Integration | ||||
| DTP3 | 602 per 1000 | 1000 per 1000 | RR 1.92 | 1481 | ⊕⊕⊝⊝ |
| *The effect in the 'integration' group (and its 95% CI) was based on the assumed risk in the 'standard care' group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high. 'Substantially different' implies a large enough difference that it might affect a decision. | |||||
| 1 We rated down by 2 levels because we judged the 1 included study at high risk of bias. 2 Dicko 2011. | |||||
| Target | Interventions | Purpose of the interventions |
| Recipients | Communication interventions to inform and educate targeting individuals, groups, communities or providers, or a combination of these through face‐to‐face interaction, use of mass media, printed material, etc | To improve understanding on vaccination; its relevance; benefits and risks of vaccination; where, when, and how to receive vaccine services; and who should receive vaccine services (Willis 2013) |
| Communication interventions to recall or remind using face‐to‐face interaction, telephone, mail, etc | To remind those who are overdue for vaccination in order to reduce drop‐out rate (Willis 2013) | |
| Communication interventions to teach skills, e.g. parenting skills | To provide people with the ability to operationalise knowledge through the adoption of practical skills (Willis 2013) | |
| Communication interventions to provide support | To provide assistance or advice for consumers (Willis 2013) | |
| Interventions to facilitate decision‐making, e.g. decision aids on vaccination for parents | To assist carers in participating in decision making (Dubé 2013) | |
| Interventions to enable communication through traditional media, internet, etc | To make communication possible (Dubé 2013) | |
| Interventions, including communication, to enhance community ownership, e.g. community dialogues involving traditional and religious rulers | To increase demand for vaccination To ensure sustainability To build trust in vaccination and vaccination services To drive demand for vaccination | |
| Incentives | To reward service uptake; to cover out‐of‐pocket cost | |
| Providers | Training | To improve knowledge on vaccination, to improve skills, to improve attitudes to clients, to reduce missed opportunities for vaccination |
| Audit and feedback | To ensure quality and client satisfaction with services | |
| Supportive supervision | To ensure quality and maintain standards, to reduce missed opportunities for vaccination | |
| Incentives | To boost morale and enhance performance | |
| Health system | Infrastructural development, e.g. provision of health facilities, provision of road to improve access to health facilities | To ensure access to services |
| Logistic support | To improve service quality service and so improve utilisation to ensure availability of services | |
| Service delivery, e.g. outreach; home visits; integration of vaccination with other services; guidelines/protocol for vaccination; increased resources | Outreach to improve access to services Home visits to remind parents about vaccination and identify unimmunised children for immunisation Integration to encourage vaccine uptake Guidelines and protocols to ensure quality of services Improved resources to ensure availability of services | |
| Policy makers | Advocacy for: development of supporting policies, increased funding of health services | To promote the development of policies to support vaccine uptake To increase funding to the health sector |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Measles vaccine Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2 DTP3 Show forest plot | 5 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Community‐based education | 2 | Risk Ratio (Random, 95% CI) | 1.68 [1.09, 2.59] | |
| 2.2 Facility‐based education | 3 | Risk Ratio (Random, 95% CI) | 1.20 [0.97, 1.48] | |
| 3 Received at least 1 vaccine Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DTP3 Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 1.50 [1.21, 1.87] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2 Fully immunised children Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] | |
| 3 BCG Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 4 MMR Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 4.1 Household monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Household + service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 DTP1 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 5.1 Household monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Household + service‐level monetary incentive | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OPV3 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fully immunised children Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Regular immunisation outreach only | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Regular immunisation outreach + incentive | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BCG Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2 DTP3 Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 3 Measles Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Totals not selected | |

