Enhanced care by generalists for functional somatic symptoms and disorders in primary care
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008142.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 octubre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos mentales comunes
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Marianne Rosendal and Chris Burton wrote the protocol draft, other authors contributed with critical feedback and discussions of methods. All authors accepted the final version of the protocol.
Chris Burton and Marianne Rosendal conducted the literature search, evaluated papers for inclusion and quality of studies, extracted and entered data, performed analyses and wrote the review draft. Annette H Blankenstein provided additional data from the original study in Amsterdam and extracted data. Michael Sharpe evaluated studies for inclusion when disagreements occurred. All authors contributed with critical feedback on the review and accepted the final version of the protocol.
Sources of support
Internal sources
-
Research Unit for General Practice, Århus, Denmark.
-
Community Health Sciences, General Practice Section, University of Edinburgh, UK.
-
Department of General Practice and Elderly Care Medicine, VU University Medical Center, Amsterdam, Netherlands.
-
The Research Clinic for Functional Disorders and Psychosomatics, Århus University Hospital, Denmark.
-
Department of Medicine and Regenstrief Institute, Indiana University, USA.
-
School of Molecular & Clinical Medicine, University of Edinburgh, UK.
-
Department of Biostatistics, Institute of Public Health, University of Århus, Denmark.
-
Department of Psychiatry, University of Nottingham, UK.
External sources
-
No sources of support supplied
Declarations of interest
Marianne Rosendal has been actively participating in the evaluation of RCTs cited in this review, and involved in the development of treatment guidelines for Danish primary care. The working hours spent on this Cochrane review have been part of her standard employment at the Research Unit for General Practice. No other conflicts of interest known.
Annette H Blankenstein has been the primary researcher in one of the RCTs on reattribution cited in this review. She has contributed to multidisciplinary and GP guidelines on MUS and somatoform disorders and she developed a training course for GPs on cognitive behavioural treatment for MUS. No other conflicts of interest known.
Richard Morriss has been the chief investigator of one of the RCTs cited in this review as well as a previous non‐randomised treatment trial. No other conflicts of interest known.
Per Fink has been involved in RCTs on treatment of medically unexplained symptoms in primary care cited in this review, and in the development of treatment guidelines for Danish primary care. No other conflicts of interest known.
Michael Sharpe: no conflicts of interest known.
Chris Burton: no conflicts of interest known.
Acknowledgements
We would like to thank Kurt Kroenke for his contributions to the protocol and feedback on the final review. Furthermore, we thank Morten Frydenberg for providing statistical advice on meta‐analyses and Winfried Rief, Lisbeth Frostholm and Eva Oernboel for providing additional data from the original studies in Marburg and Aarhus.
CRG funding acknowledgement
The UK National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Oct 18 | Enhanced care by generalists for functional somatic symptoms and disorders in primary care | Review | Marianne Rosendal, Annette H Blankenstein, Richard Morriss, Per Fink, Michael Sharpe, Christopher Burton | |
| 2009 Oct 07 | Enhanced care by generalists for functional somatic symptoms and disorders in primary care | Protocol | Marianne Rosendal, Chris Burton, Annette H Blankenstein, Per Fink, Kurt Kroenke, Michael Sharpe, Morten Frydenberg, Richard Morriss | |
Differences between protocol and review
The review has been performed in accordance with the protocol but with limitations as only few studies were included and original data could not be retrieved. Clarifications with regard to inclusion criteria and outcome measures have been made. Sensitivity analysis and subgroup analyses were planned but not performed as very few studies could be analysed. We originally planned to categorise outcomes as short (0 to 5 months), medium term (6 to 11) months, and longer term (12 or more months) but because of relatively small numbers of studies we merged the latter two. The protocol stated that meta‐analysis would use a fixed‐effect model and did not specify the circumstances in which meta‐analysis would be appropriate. In view of the variation in intervention intensity and assumed illness severity between studies, we chose to use a random‐effects model meta‐analysis with an exclusion threshold of heterogeneity of I2 > 50%. Furthermore, we changed our approach to dichotomous data for outcomes where continuous data were also available in order to combine them in analyses with continuous data. Finally, we supplemented the information in the section about measures of treatment effect to include specifications about the conducted calculations of standardised mean differences.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
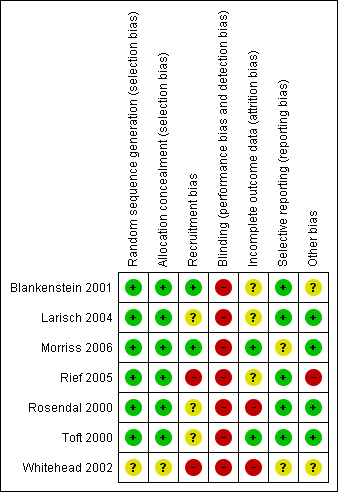
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
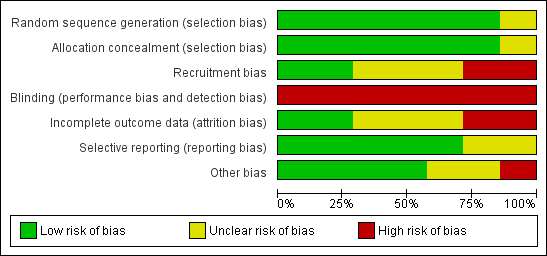
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Health related quality of life, Outcome 1 Change in physical health 6‐24 months.

Comparison 1 Health related quality of life, Outcome 2 Change in physical health 1‐3 months.

Comparison 1 Health related quality of life, Outcome 3 Change in mental health 6‐24 months.

Comparison 1 Health related quality of life, Outcome 4 Change in mental health 1‐3 months.
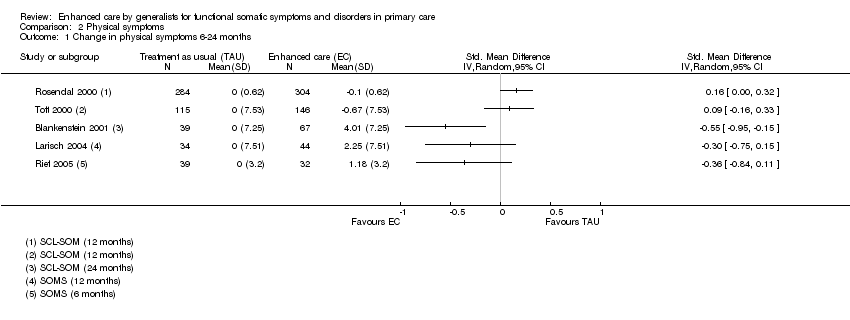
Comparison 2 Physical symptoms, Outcome 1 Change in physical symptoms 6‐24 months.
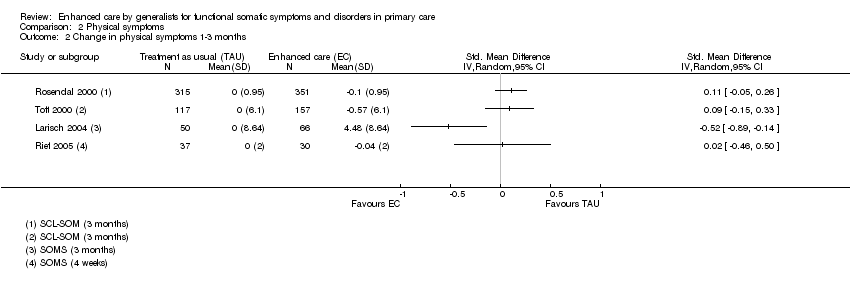
Comparison 2 Physical symptoms, Outcome 2 Change in physical symptoms 1‐3 months.
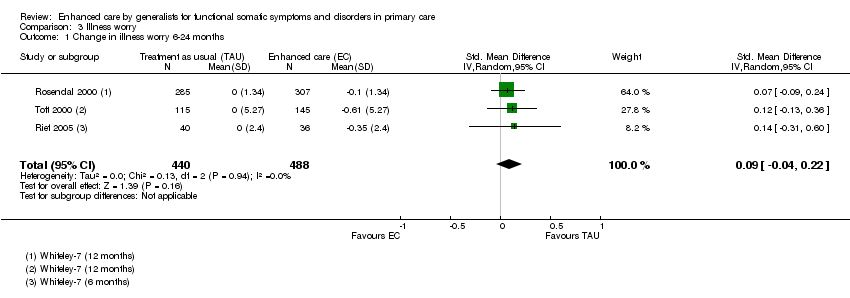
Comparison 3 Illness worry, Outcome 1 Change in illness worry 6‐24 months.

Comparison 3 Illness worry, Outcome 2 Change in illness worry 1‐3 months.
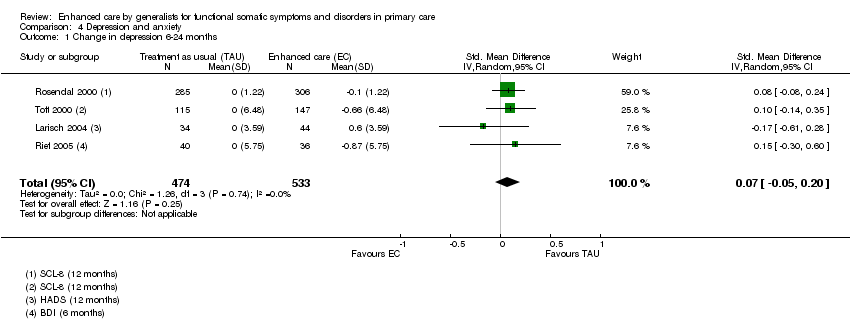
Comparison 4 Depression and anxiety, Outcome 1 Change in depression 6‐24 months.

Comparison 4 Depression and anxiety, Outcome 2 Change in depression 1‐3 months.
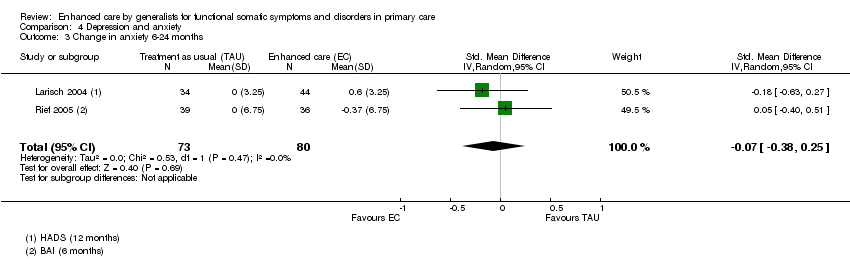
Comparison 4 Depression and anxiety, Outcome 3 Change in anxiety 6‐24 months.

Comparison 4 Depression and anxiety, Outcome 4 Change in anxiety 1‐3 months.
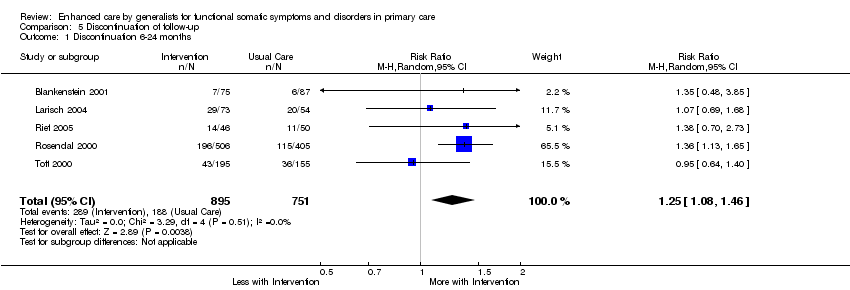
Comparison 5 Discontinuation of follow‐up, Outcome 1 Discontinuation 6‐24 months.
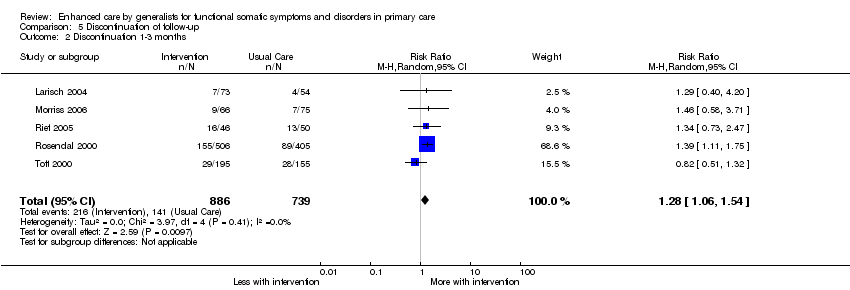
Comparison 5 Discontinuation of follow‐up, Outcome 2 Discontinuation 1‐3 months.
| Type of instrument | Name | references |
| Questionnaire | Abridged criteria/Escobar criteria/SOMS | |
|
| PHQ‐15 | |
|
| SCL‐12 (SCL‐90 somatization scale) | |
|
| Whiteley (7 or 14) | |
| GHQ | ||
| Interview | SCAN | |
|
| DIS | |
|
| CIDI | |
| Health care use | Frequent attendance | |
| The table may be supplemented by specific instruments for functional somatic syndromes. SOMS: Screening for somatoform Symptoms; PHQ: Patient Health Questionnaire; SCL: Symptom Check List; GHQ: General Health Questionnaire; SCAN: Schedules for Clinical Assessment in Neuropsychiatry; DIS: Diagnostic Interview Schedule; CIDI: Composite International Diagnostic Interview. | ||
| Rubric | Reviewer1 | Reviewer 2 | Conclusion | |
| Study ID, name of study | ||||
| Report ID | ||||
| Author name and year |
|
|
| |
| Title of paper |
|
|
| |
| Contact details |
|
|
| |
| Personal notes (e.g. name of disorder used) |
|
|
| |
| Assessment of eligibility | Intervention provided by generalist | |||
| Intervention deals with non‐biomedical aspects | ||||
| Patients have MUS or functional syndrome(s) | ||||
| RCT | ||||
| Personal notes | Reason for exclusion |
|
| |
| Study characteristics |
|
|
| |
| Methods | Study design including level of randomisation and measures of clustering |
|
|
|
| Study duration | ||||
| Sequence generation | ||||
| Allocation concealment | ||||
| Blinding (clinician, patient, researcher) | ||||
| Incomplete data (short term, longer term) | ||||
| Reporting (prespecified, incidental) | ||||
| Recruitment bias | ||||
|
| The risk of contamination (control patients receiving intervention unintentional or vice versa) |
|
|
|
| Other concerns about bias | ||||
| Participating GPs | Number of GPs/practices | |||
| Setting of the study (the primary care organisation and country) | ||||
| Doctor characteristics and sampling (previous training, years in practice, GP age) |
|
|
| |
| Participating patients | Number of patients in each group | |||
| Diagnostic criteria and instrument used | ||||
| Patient characteristics (inclusion, exclusion, ethnicity, diagnosis, symptom duration, psychiatric co‐morbidity) and sampling (population screening, waiting room screening, GP assessment, diagnostic instruments used) |
|
|
| |
| Date of study | ||||
| Intervention | Number of groups | |||
| Intervention* in active group and adherence |
|
|
| |
|
| Intervention in control group and adherence |
|
|
|
| Outcomes | Primary and secondary outcomes: time from randomisation, measure, tool used, definition, unit of measurement, definition of positive outcome |
|
|
|
|
| Length of maximum follow‐up |
|
|
|
| Analysis | Type (e.g. intention to treat) | |||
| Missing data (attempts to minimise, handling of) | ||||
| Covariate choice | ||||
| Power, anticipated effect and sample size adjustment | ||||
| Results | Number of participants in each group |
|
|
|
| Primary and secondary outcomes: sample size, missings, summary data for each group, estimate of effect, subgroup analyses | ||||
| Other | Funding, key conclusions by authors | |||
| Comments | ||||
| * Interventions
| ||||
| Study | Training intensity | Intervention | Delivery intensity | Manual and adherence | Proportion population eligible |
| Blankenstein | 20 hour programme, included discussion of audio taped consultations and booster sessions | Reattribution + (c) symptom diaries | “during usual care” at least two appointments. | Intervention registration forms filled in by GP during consultations with included patients. Adherence: intervention was applied to 51 of 75 patients (68%). Reattribution was achieved in 33 of 75 patients (31%) | Registered 33,000 (11,000 age 20‐45 ˜34% of population) Screened 900 (Frequent attenders: top 8%) Eligible 243 ( > = 5 on DSM3 symptoms checklist) (age 20‐45 only) 0.74% |
| Larisch | 12 hour programme, Teaching, pre‐recorded video, role playing | Training based on reattribution modified for German PHC setting: (a) emphasised examination (b) symptom diaries | 6x20 minute appointments every 2 weeks | Manual with therapy protocols for each of the 6 patient sessions. Adherence not reported. | Registered 34,000 (42 GPs x 800 patients / GP) Screened 847 (waiting room + GP opportunistic) Eligible 222 (4M/6F on SOMS + GHQ>2) 0.65% |
| Morriss | 3x2hours Teaching, pre‐recorded video, role playing | Reattribution: (a) feeling understood (c) making the link | Single clinic appointment | No manual but specific consultation model (reattribution) which was audio taped for evaluation. Adherence: Intervention GPs communicated reattribution in most of the consultation in 20 of 65 patients (31%) | Registered 102,000 (16 x 6400) Screened 4484 in 430 clinics Eligible 141 (current MUS >3 months) 0.14% |
| Rief | One day Teaching, pre‐recorded video, role playing (“if necessary”) | “Synthesis of” reattribution + additional information. (b) stopping medical investigations (c) handling organic health beliefs and need for reassurance (d) options for further treatment | Not specified. “encouraged to make regular appointments” | No manual or measurement of adherence. | Registered 20,800 (26 GPs x 800 patients / GP) Eligible 289 (96 in cohort 2) selected by GPs as 2 or more MUS (non‐randomised design, no record of individual consent / opt‐in or out??) 1.4% |
| Rosendal | 2 day residential + 3x2hour sessions Analysis of own video consultations + outreach visit after 6/12 | Extended reattribution and management (a) understanding – including brief focused exam (e) principles for management of chronic conditions | Not specified | No manual or measurement of adherence. | Population 66,500 (19% GPs serving 350,000 residents) 2880 screened Eligible 911 (either 4+ symptoms on SCL‐SOM or 2+ on Whitely‐7) 1.4% |
| Toft | 2 day residential + 3x2hour sessions Analysis of own video consultations + outreach visit after 6/12 | Extended reattribution and management (a) understanding – including brief focused exam (e) principles for management of chronic conditions | Not specified | No manual or measurement of adherence. | Population 54,000 (9%GPs serving 600,000 residents) 1785 screened (attenders 18‐65 consulting new health problem) Eligible 350 (Somatoform Disorder according to SCAN) 0.65% |
| Item | Description | Scoring: yes, unclear or no | |
| Selection bias | Random sequence generation | Was the allocation sequence adequately generated? | A method for random allocation at the level of the doctor or patient must have been applied and stated. |
| Allocation concealment | Was allocation adequately concealed? | Allocation should have been performed by an independent person without any influence on the allocation sequence or the decision about eligibility for inclusion. | |
| Recruitment bias | Were patients recruited before or after randomisation of doctors/practices? | If patients were recruited after randomisation at the level of the doctor/practice the recruitment could be affected by the practice awareness of allocation group to some degree (instruments for inclusion applied) or to a high degree (inclusion under direct influence of the doctor) | |
| Performance and detection bias | Blinding | Was knowledge of the allocated intervention prevented during the study at the level of the doctor, patient and assessor? | We defined: Low risk = attempt to conceal from doctor AND patients AND assessors blinded. Unclear risk = Unclear blinding of patient, doctor and/or assessor but none unblinded. High risk = either no attempt to conceal from doctor OR patients OR assessor not blinded |
| Attrition bias | Incomplete outcome data | Were loss to follow‐up acceptable and was attrition addressed? | Information about included patients lost to follow‐up. Attrition above 30% was rated as high risk. |
| Reporting bias | Selective reporting | Reporting of selective outcome? | Were primary outcomes stated and reported? Were other findings reported as such? |
| Other bias | Was the study free of other problems that could put it at a high risk of bias? | Specific attention paid to whether statistics were adjusted for the effect of clustering of patients with GPs or practices. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in physical health 6‐24 months Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Change in physical health 1‐3 months Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Change in mental health 6‐24 months Show forest plot | 3 | 795 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.18, 0.10] |
| 4 Change in mental health 1‐3 months Show forest plot | 3 | 904 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.13, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in physical symptoms 6‐24 months Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Change in physical symptoms 1‐3 months Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in illness worry 6‐24 months Show forest plot | 3 | 928 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.04, 0.22] |
| 2 Change in illness worry 1‐3 months Show forest plot | 4 | 1141 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in depression 6‐24 months Show forest plot | 4 | 1007 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.20] |
| 2 Change in depression 1‐3 months Show forest plot | 5 | 1259 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.16, 0.15] |
| 3 Change in anxiety 6‐24 months Show forest plot | 2 | 153 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.38, 0.25] |
| 4 Change in anxiety 1‐3 months Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Discontinuation 6‐24 months Show forest plot | 5 | 1646 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.08, 1.46] |
| 2 Discontinuation 1‐3 months Show forest plot | 5 | 1625 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.06, 1.54] |

