Medición de la altura sínfisis‐fondo (ASF) durante el embarazo para la detección del crecimiento fetal anormal
Resumen
Antecedentes
La medición de la altura sínfisis‐fondo (ASF) es una práctica que se utiliza con frecuencia, principalmente para detectar el retraso del crecimiento intrauterino (RCIU) del feto. El RCIU no diagnosticado puede producir muerte fetal, así como aumentar la mortalidad y la morbilidad perinatales.
Objetivos
El objetivo de esta revisión es comparar la medición de la ASF con la medición por ecografía seriada de los parámetros fetales o la palpación clínica para detectar el crecimiento fetal anormal (RCIU y tamaño grande para la edad gestacional) y mejorar el resultado perinatal.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (14 de julio 2015) y en listas de referencias de los artículos recuperados.
Criterios de selección
Ensayos controlados aleatorizados que incluyen ensayos cuasialeatorizados y ensayos aleatorizados grupales que reclutaron a embarazadas con fetos únicos, con una gestación de 20 semanas y más, que compararon la medición con cinta de la ASF con la medición por ecografía seriada de los parámetros fetales o la palpación clínica mediante puntos de referencia anatómicos.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron los ensayos para la inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud.
Resultados principales
Se incluyó un ensayo con 1639 mujeres. Este ensayo comparó la medición de la ASF con la palpación abdominal clínica.
No hubo diferencias en los dos resultados primarios informados de la incidencia de «pequeño para la edad gestacional» (riesgos relativos (RR) 1,32; intervalo de confianza (IC) del 95%: 0,92 a 1,90, evidencia de baja calidad) o la muerte perinatal (RR 1,25; IC del 95%: 0,38 a 4,07; participantes = 1639, evidencia de baja calidad). No había datos sobre la detección neonatal de «grande para la edad gestacional» (definido de forma variada por los autores). No hubo diferencias en los resultados secundarios informados de hipoglucemia neonatal, ingreso en unidad de recién nacidos, ingreso en la sala de neonatos por RCIU (evidencia de baja calidad), inducción del trabajo de parto y cesárea (evidencia de muy baja calidad). El ensayo no abordó los demás resultados especificados en la tabla "Resumen de los hallazgos" (muerte intrauterina; resultado del neurodesarrollo en la infancia). Se utilizó el programa informático GRADEpro para evaluar la calidad de la evidencia, la disminución del grado de la evidencia se basó en la inclusión de un pequeño estudio único con un riesgo de sesgo poco claro y un amplio intervalo de confianza que cruza la línea de ningún efecto.
Conclusiones de los autores
No existe evidencia suficiente para determinar si la medición de la ASF es efectiva para detectar el RCIU. Por lo tanto, no se pueden recomendar cambios en la práctica actual. Se necesitan ensayos adicionales.
PICO
Resumen en términos sencillos
Medición de la altura del útero desde la sínfisis del pubis (ASF) en el embarazo para la detección de problemas en el crecimiento fetal
La monitorización del crecimiento del feto es importante durante el embarazo. Si el crecimiento es deficiente, se debe identificar cuanto antes porque el retraso de su detección puede dar lugar a la muerte del feto. La forma más simple de determinar el crecimiento es examinar el feto mediante la palpación abdominal de la madre y estimar el tamaño de su útero en comparación con un punto de referencia como el ombligo (umbilicus). Un método alternativo es utilizar una cinta métrica para tomar la medida, conocida como medición de la altura sínfisis‐fondo (ASF), desde el hueso púbico de la madre (sínfisis del pubis) hasta la parte superior del útero. La medición luego se aplica a la gestación con una regla general sencilla y se compara con el crecimiento normal.
Se quería saber cuál de estos dos métodos es más probable que detecte un crecimiento deficiente. La evaluación por ecografía también se puede utilizar para detectar un retraso del crecimiento, pero es costosa y no está siempre disponible y también existen inquietudes sobre su uso innecesario. Solo se encontró un ensayo aleatorizado (que incluyó a 1639 mujeres con una gestación de 20 semanas o más) que comparó medidas repetidas de la ASF con palpación abdominal. El ensayo no encontró diferencias entre los dos enfoques en la detección del crecimiento deficiente. Con tan poca evidencia, todavía no se sabe si un método es más efectivo que el otro, y cómo se comparan estos métodos con la medición por ecografía. Las principales conclusiones de esta revisión se evaluaron en cuanto a su calidad mediante un programa informático llamado GRADEpro. La evidencia en general era de baja/muy baja calidad.
Authors' conclusions
Summary of findings
| Tape measurement compared with clinical palpation for pregnancy for detecting abnormal fetal growth | ||||||
| Patient or population: Pregnant women with singleton fetuses who were of 20 weeks' gestation and above. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| clinical palpation | Tape measurement | |||||
| Neonatal detection of small‐for‐dates | Study population | RR 1.32 | 1639 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 58 per 1000 | 76 per 1000 | |||||
| Neonatal detection of large‐for‐gestational age | The study did not have data on this outcome. | |||||
| Perinatal mortality | Study population | RR 1.25 | 1639 | ⊕⊕⊝⊝ | ||
| 6 per 1000 | 7 per 1000 | |||||
| Moderate | ||||||
| 6 per 1000 | 8 per 1000 | |||||
| Intrauterine death | The study did not have data on this outcome. | |||||
| Caesarean section | Study population | RR 0.72 | 1639 | ⊕⊝⊝⊝ | ||
| 16 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 16 per 1000 | 11 per 1000 | |||||
| Neurodevelopmental outcome in childhood | The study did not have address this outcome. | |||||
| Admission to neonatal nursery | Study population | RR 1.06 | 1639 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 53 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 53 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study with unclear risk of bias 2Wide CI crossing the line of no effect 3One small study with few events and wide CI crossing the line of no effect 4 Only one small study | ||||||
Background
Description of the condition
Fetal growth assessment is an important part of antenatal care. Methods used in the past include clinical palpation of fundal height in relation to anatomical landmarks such as the umbilicus and xiphisternum, abdominal girth measurement and serial ultrasound measurement of the fetal parameters. Clinical palpation using anatomical landmarks is subjective and has a wide interobserver difference (Bais 2004), but is the only alternative in settings without ultrasound machines. Abdominal girth measurement rarely correlates with fetal outcomes (Rosenberg 1982). Serial ultrasound is thought to be an accurate tool to detect intrauterine growth restriction (IUGR) and macrosomia (large baby). The sensitivity for detection of IUGR is quoted as high as 93% and 90% for macrosomia (De Reu 2008).Though accurate, ultrasound is expensive when used as a screening tool for abnormal growth detection. The American College of Obstetricians and Gynecologists recommend symphysis fundal height (SFH) with ultrasound measurement where discrepancies of failure of fundal growth arise (ACOG 2000).
Description of the intervention
SFH measurement of the distance from the pubic symphysis to the uterine fundus is a simple, inexpensive and widely used method of detecting abnormal fetal growth. For fetuses after 24 weeks' gestation, the measurement is made by identifying the upper border of the symphysis pubis and the uterine fundus and measuring the distance between with a tape measure. The measurement in centimetres is then applied to the gestation by a simple rule of thumb (Belizan 1978). In a fetus which is growing normally, the SFH measurement in centimetres should correspond to the gestation (i.e., the SFH measurement should be 28 centimetres for a 28 weeks' gestation singleton pregnancy, with a allowance of +/‐ 2 centimetres difference).
How the intervention might work
SFH measurement is aimed at detecting small‐for‐dates fetuses but among these, the group that is important is those with IUGR. Many workers have found SFH measurement to be more scientific, objective, and reproducible to assess fetal growth (Belizan 1978; Challis 2002; Grover 1991; Lu 2003; Westin 1997). The primary and most important aim of the SFH measurement is the detection of fetuses that are poorly grown as delay in the diagnosis of this fetal condition may lead to intrauterine death (Challis 2002). It also has the potential to detect multiple pregnancies, large‐for‐gestational‐age fetuses, polyhydramnios and oligohydramnios. The assumption is that these conditions, if not picked up early enough during the course of routine antenatal care, will lead to an increased perinatal morbidity and perinatal mortality.
Use of SFH measurement reported detection rates of small‐for‐dates babies from observational studies of SFH, ranges between 56% (Rosenberg 1982) and 86% (Belizan 1978). Studies showing a reduced mortality have not been reported. SFH measurement appears to be in use in developing countries in most regions of the world (Goto 2013). .
In addition, there is disagreement in SFH measurement between observers regarding the ability to separate small fundal heights from those that are not small (Bailey 1989). This becomes an issue especially in a clinical setting where the pregnant woman sees more than one clinician during the course of her pregnancy. There is also the issue of clinicians being biased in the measurement of the SFH after knowing the gestational age (Jelks 2007). Despite this, SFH measurement continues to be used in many countries on a large scale simply because of its low cost, ease of use, and need for very little training. Another issue is the effect of body mass index on the accuracy of SFH measurement which is important in view of the emerging epidemic of obesity.
IUGR using ultrasound is detected by estimating fetal weight or fetal abdominal circumference that is less than the specified centile (usually 10th, 5th or 3rd) for gestation and sex, and detection of large‐for‐gestational age more than the specified centile (usually 90th, 95th, or 97th) fetuses is estimated by fetal weight or fetal abdominal circumference that is more than the 90th centile for its gestation and sex (Jelks 2007).
Why it is important to do this review
The evidence for the use of SFH measurement has great implications for low‐income countries with limited access to serial ultrasound assessment of the fetus. It is also important in high‐income countries as SFH measurement is still used as a screening tool to detect IUGR in many countries. Customised SFH measurement may also be used (seehttp://www.perinatal.nhs.uk).
Objectives
The objective of this review is to compare symphysis fundal height measurement with serial ultrasound measurement of fetal parameters or clinical palpation to detect abnormal fetal growth (intrauterine growth restriction and large‐for‐gestational age), and improving perinatal outcome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials including quasi‐randomised and cluster‐randomised trials. Trials using a cross‐over design were not eligible for inclusion.
Types of participants
Pregnant women with singleton fetuses who were of 20 weeks' gestation and above.
Types of interventions
Intervention
Tape measurement of symphysis fundal height.
Comparison
Serial ultrasound measurement of fetal parameters or clinical palpation using anatomical landmarks.
Types of outcome measures
Primary outcomes
-
Neonatal detection of small‐for‐dates (variously defined by authors).
-
Neonatal detection of large‐for‐gestational age (variously defined by authors).
-
Perinatal mortality (variously defined by authors).
Secondary outcomes
-
Complications associated with intrauterine growth restriction (IUGR) (fetal distress in labour, neonatal hypoglycaemia, admission to neonatal nursery because of IUGR).
-
Complications associated with large‐for‐gestational‐age fetuses (fetal macrosomia, shoulder dystocia, prolonged labour, fetal distress).
-
Intrauterine death.
-
Intrapartum asphyxia (however defined by trialists).
-
Detection of oligohydramnios (however detected by trialists).
-
Induction of labour.
-
Caesarean section and reasons for caesarean section.
-
Health service outcomes (admission to neonatal nursery, antenatal admission of women).
-
Detection of polyhydramnios (however detected by trialists).
-
Neurodevelopmental outcome in childhood.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (14 July 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved articles to look for further studies and any possible sources of unpublished data and contact known experts.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeRobert Peter 2012.
For this update, no new reports were identified as a result of the updated search.
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
Assessment of the quality of evidence
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons 'tape measurement versus clinical palpation':
-
Neonatal detection of small‐for‐dates (variously defined by authors).
-
Neonatal detection of large‐for‐gestational age (variously defined by authors).
-
Perinatal mortality (variously defined by authors)
-
Intrauterine death
-
Caesarean section and reasons for caesarean section.
-
Admission to neonatal nursery.
-
Neurodevelopmental outcome in childhood.
We used GRADE profiler (GRADEpro 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes has been produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
In future updates, if new reports are identified, we will use the methods described in Appendix 1.
Results
Description of studies
Results of the search
The original search of the Pregnancy and Childbirth Group's Trials Register found one report (Lindhard 1990). No new reports were identified for this update.
Included studies
The included study involved 1639 women (804 in the SFM group and 835 in the control group). The intervention group had serial measurements of symphysis fundal height (SFH) using a metric non‐elastic tape measure. The controls were assessed using abdominal palpation and were measured with an unmarked tape which was cut of and measured after the birth. The primary outcomes was detection of intrauterine growth restriction (IUGR). The characteristics of the study are included in the Characteristics of included studies.
Excluded studies
None identified.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of ’Risk of bias’ assessments.
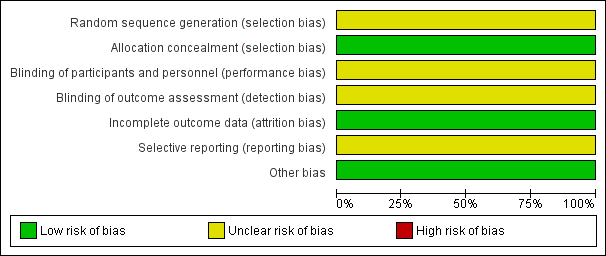
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
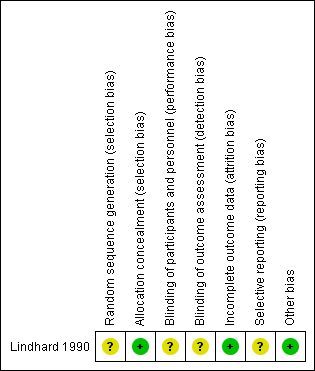
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the one included study (Lindhard 1990), the method of sequence generation was not described but the trialists used sealed opaque envelopes for group allocation.
Blinding
Blinding of participants and personnel was unlikely because of the nature of procedure. For the primary outcome there was no blinding of outcome assessment because the primary outcome (detection of small‐for‐gestational age (SGA)) in the SFM group was used to determine further management of the pregnancy. It is not mentioned whether the care‐giver carrying out the measurement was blinded for gestation prior to carrying out the measurement. For the neonatal outcome measures (perinatal death, neonatal hypoglycaemia, admission to the neonatal nursery), blinding of the outcome assessors would have been possible but it is not mentioned whether this was done.
Incomplete outcome data
All the patients who were randomised were accounted for and follow‐up was greater than 95%. It is not clear whether they used an intention‐to‐treat analysis.
Selective reporting
The protocol was not available. Methods section and the results were consistent.
Other potential sources of bias
None identified.
Effects of interventions
Tape measurement versus clinical palpation
There was only one included study (Lindhard 1990) involving 1639 women.
Primary outcomes
The incidence of small‐for‐gestational age (SGA) was not significantly different between the two groups; 7.6% in SFH group and 5.7% in control group (risk ratio (RR) 1.32, 95% confidence interval (CI) 0.92 to 1.90; participants = 1639;; Analysis 1.1). The numbers of perinatal deaths were not different between groups (RR 1.25, 95% CI 0.38 to 4.07; participants = 1639 ; Analysis 1.2). There were no data for the outcome neonatal detection of large‐for‐gestational age (LGA).
Secondary outcomes
The other reported outcomes were also not significant: neonatal hypoglycaemia (RR 1.10; 95% CI 0.47 to 2.58; Analysis 1.3), admission to neonatal nursery (RR 1.06; 95% CI 0.70 to 1.61; Analysis 1.4), admission to neonatal nursery for IUGR (RR 0.95; 95% CI 0.42 to 2.15; Analysis 1.5), induction of labour (RR 0.84; 95% CI 0.45 to 1.58; Analysis 1.6), caesarean section (RR 0.72; 95% CI 0.31 to 1.67; Analysis 1.7).
None of the other review prespecified secondary outcomes were mentioned in the study.
Discussion
Summary of main results
From the one included study we were unable to determine the effect of symphysis fundal height (SFH) measurement compared with abdominal palpation for detecting intrauterine growth restriction (IUGR). Neither were there any significant differences in fetal outcomes. The number of small‐for‐gestational age (SGA) infants, perinatal deaths and infants transferred to neonatal ward were similar in the two groups.The sample size was substantial compared with the calculated minimum sample required to answer this question. There were minor differences between the two groups in characteristics before pregnancy that would not have influenced the final outcome.
Overall completeness and applicability of evidence
This is an important question for women in most countries in the world and further trials should be performed. There is established technology for the detection of IUGR using ultrasound measurement of fetal parameters and Doppler velocimetry assists in clinical management. However, we could not identify any trials comparing SFH with serial ultrasound. It would be important to know how SFH measurement compares with that for the detection of IUGR as well as the palpation method.
Quality of the evidence
GRADEpro software was used to assess the quality of evidence for the seven outcomes stated above (summary of findings Table for the main comparison). We were able to provide quality assessment for four out of the seven prespecified outcomes. The evidence was of low quality for the outcome of neonatal detection of small‐for‐dates, perinatal mortality and admission to neonatal nursery, and of very low quality for the outcomes caesarean section and admission to neonatal nursery. Downgrading of evidence was based on the inclusion of only one study with unclear risk of bias and a wide confidence interval crossing the line of no effect. For the rare outcome, perinatal mortality, which we also judged to be less susceptible to the lack of blinding, we did not downgrade for imprecision.
Potential biases in the review process
The findings of this review are severely limited by the fact there is only one included study. Our search was very comprehensive and we do not have reasons to believe that we have missed studies due to publication bias. We attempted to minimise bias by having two review authors assessing studies and extracting data.
Agreements and disagreements with other studies or reviews
A systematic review examining interventions, including SFH measurement, for reducing IUGR‐related stillbirths (Imdad 2011) also found only one randomised controlled trial. However based on observational studies and a Delphi procedure performed among experts, the authors recommended its use for this purpose.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Tape measurement versus clinical palpation, Outcome 1 Neonatal detection of small‐for‐dates.

Comparison 1 Tape measurement versus clinical palpation, Outcome 2 Perinatal death.

Comparison 1 Tape measurement versus clinical palpation, Outcome 3 Neonatal hypoglycaemia.

Comparison 1 Tape measurement versus clinical palpation, Outcome 4 Admission to neonatal nursery.

Comparison 1 Tape measurement versus clinical palpation, Outcome 5 Admission to neonatal nursery because of intrauterine growth restriction.

Comparison 1 Tape measurement versus clinical palpation, Outcome 6 Induction of labour.

Comparison 1 Tape measurement versus clinical palpation, Outcome 7 Caesarean section.
| Tape measurement compared with clinical palpation for pregnancy for detecting abnormal fetal growth | ||||||
| Patient or population: Pregnant women with singleton fetuses who were of 20 weeks' gestation and above. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| clinical palpation | Tape measurement | |||||
| Neonatal detection of small‐for‐dates | Study population | RR 1.32 | 1639 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 58 per 1000 | 76 per 1000 | |||||
| Neonatal detection of large‐for‐gestational age | The study did not have data on this outcome. | |||||
| Perinatal mortality | Study population | RR 1.25 | 1639 | ⊕⊕⊝⊝ | ||
| 6 per 1000 | 7 per 1000 | |||||
| Moderate | ||||||
| 6 per 1000 | 8 per 1000 | |||||
| Intrauterine death | The study did not have data on this outcome. | |||||
| Caesarean section | Study population | RR 0.72 | 1639 | ⊕⊝⊝⊝ | ||
| 16 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 16 per 1000 | 11 per 1000 | |||||
| Neurodevelopmental outcome in childhood | The study did not have address this outcome. | |||||
| Admission to neonatal nursery | Study population | RR 1.06 | 1639 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 53 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 53 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study with unclear risk of bias 2Wide CI crossing the line of no effect 3One small study with few events and wide CI crossing the line of no effect 4 Only one small study | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal detection of small‐for‐dates Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.92, 1.90] |
| 2 Perinatal death Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.07] |
| 3 Neonatal hypoglycaemia Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.58] |
| 4 Admission to neonatal nursery Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.70, 1.61] |
| 5 Admission to neonatal nursery because of intrauterine growth restriction Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.42, 2.15] |
| 6 Induction of labour Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.45, 1.58] |
| 7 Caesarean section Show forest plot | 1 | 1639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.31, 1.67] |

