Daclizumab para la esclerosis múltiple recurrente remitente
Appendices
Appendix 1. Keywords
{daclizumab} OR {antigen} OR {zenapax} OR {dacliximab} OR {monoclonal antibody} OR {monoclonal antibodies} OR {antigens} AND {relapsing remitting} OR {relapsing‐remitting} OR {remitting‐relapsing} OR {remitting relapsing} OR {relapsing} OR {remitting} OR {relapsing AND remitting}

Study flow diagram.
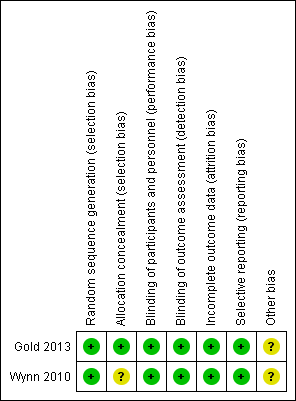
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
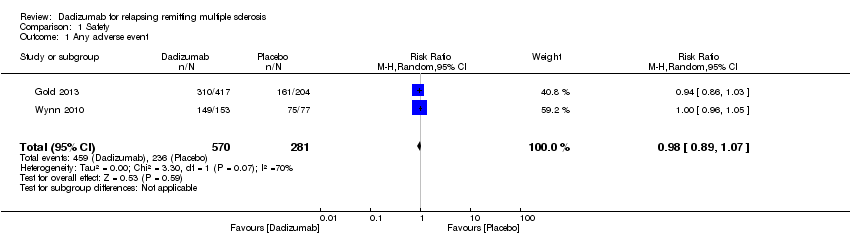
Comparison 1 Safety, Outcome 1 Any adverse event.
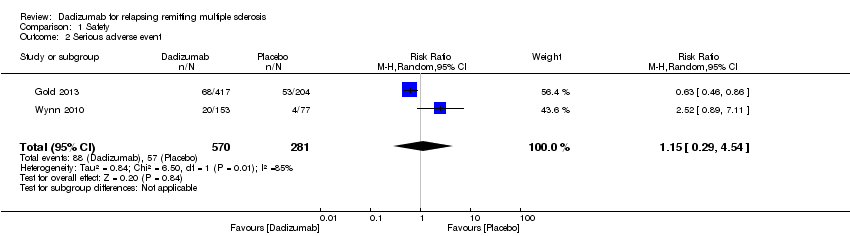
Comparison 1 Safety, Outcome 2 Serious adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.07] |
| 2 Serious adverse event Show forest plot | 2 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.29, 4.54] |

