Kortikosteroidi za liječenje obične prehlade
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 200 "young adults" (59 males of mean age 24.0 years ± 2.7 and 141 females of mean age 24.1 years ± 3.6) with watery or purulent rhinitis and at least 1 of: cough, headache, hoarseness, myalgia, nasal congestion, oral temperature higher than 37.0°C or throat soreness were recruited. A total of 199 participants completed the study. Participants were recruited from the general population in Finland through advertisements and contact persons. Participants had to be healthy and without antibiotics for 4 weeks preceding entry into the study. Exclusion criteria ‐ allergic rhinitis, history of chronic or recurrent sinusitis or lower respiratory tract disease, major nasal septal deviation, nasal polyposis, pregnancy, lactation | |
| Interventions | Fluticasone propionate nasal spray daily dose 800 µg (administered as 2 puffs of 50 µg to each nostril 4 times a day at equal intervals during waking hours). Administration began 24 to 48 hours after onset of symptoms and continued for 6 days. Placebo spray was identical to the study drug without fluticasone propionate | |
| Outcomes | Symptom severity scores via diary card ‐ twice daily from days 1 to 6 then in the evening from days 7 to 20, assessing the severity of the symptoms of watery rhinitis, purulent rhinitis, nasal congestion, nasal irritation, nasal bleeding, blood in nasal mucous, cough, sputum, headache, fever, throat soreness, hoarseness, sweating, myalgia, lethargy. Oral temperature record on days 1 to 6 and then if participant felt feverish. Absence from study or work. Consumption of paracetamol tablets. Nasopharyngeal aspirate on days 1 and 7 for rhinovirus culture, rhinovirus PCR and bacterial culture | |
| Notes | Paracetamol was permitted in participants with fever or pain. However, drugs affecting nasal or lung function (including over‐the‐counter medications) were not allowed during the study Study drug and placebo were supplied by Glaxo Research and development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation is not described |
| Allocation concealment (selection bias) | Low risk | Study drug and placebo contained identical ingredients with the exception of fluticasone propionate. Steroid and placebo supplied by pharmaceutical company. Authors do not explicitly comment on the nature of the packaging |
| Blinding (performance bias and detection bias) | Unclear risk | Authors state that the study was double‐blind but do not give further detail regarding this |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 patient (0.5%) from the placebo group did not complete the study. They were excluded for improper use of study medication |
| Selective reporting (reporting bias) | Low risk | Reasonable reporting of outcomes, although often data were described in the text rather than presented and standard deviations were not mentioned. The use of study medication (i.e. compliance with study) was assessed but not reported |
| Other bias | High risk | Paracetamol use was recorded but not controlled: 141 tablets were used in the corticosteroid group and 170 in the placebo group. This difference may have affected symptom scores |
| Comparability of groups on different prognostic characteristics | Low risk | Reports "no differences in demographic characteristics" |
| Methods | Randomised, placebo‐controlled, double‐bind, parallel‐group design | |
| Participants | 54 patients (49 women, 5 men) over 18 years of age with symptoms of acute common cold having lasted from 1 to 3 days. Recruited from hospital staff in central Finland. Mean age 40.3, range 23 to 57 years. Exclusion criteria: chronic systemic diseases, ongoing treatment with corticosteroids, pregnancy | |
| Interventions | Beclomethasone dipropionate + lactose nasal spray 400 µg daily dose ‐ 2 puffs of 100 µg to each nostril once daily. Placebo spray lactose alone | |
| Outcomes | Symptom diaries ‐ recording severity of nasal blockage, rhinorrhoea, nasal itching, sneezing, cough, sore throat, hoarseness. Also, sum of symptom scores recorded. Rhinoscopic and ultrasonographic (of the maxillary sinuses) findings at days 1, 7 and 14 | |
| Notes | Orion Corporation Ltd supplied the study drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation code used ‐ no further details supplied |
| Allocation concealment (selection bias) | Low risk | Same inhaler used for both placebo and BDP administration. Non‐active ingredients the same |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind design stated. Data entry was blinded but no further details regarding this reported |
| Incomplete outcome data (attrition bias) | Low risk | 2/54 patients discontinued the study, 1 from each group |
| Selective reporting (reporting bias) | Low risk | All outcomes measured were reported in either text or data |
| Other bias | Unclear risk | A high percentage of patients had been treated for maxillary sinusitis previously: 19/26 in placebo and 14/28 in corticosteroid groups |
| Comparability of groups on different prognostic characteristics | Low risk | No statistically significant differences between groups at baseline on important prognostic characteristics, e.g. duration of cold symptoms before entry, symptom profile, rhinoscopy and ultrasonography appearances, patient characteristics. No baseline data were presented to support this |
| Methods | Single‐blind, randomised trial comparing intranasal steroids and oral amoxicillin to oral amoxicillin alone | |
| Participants | 100 children aged 2 to 14 with common colds lasting more than 10 days with nasal or postnasal discharge or common cold lasting less than 10 days with purulent nasal discharge and 3 to 4 days of rectally recorded fever greater than 39 °C. Exclusion criteria: allergic rhinitis, nasal obstruction due to deviated nasal septum, nasal polyps, lack of parental co‐operation, contraindications to use of the intervention medication, wound or lesion in the nasal mucosa. Children were recruited from outpatient clinics at the paediatric hospital in Iran | |
| Interventions | 50 µg of fluticasone propionate nasal spray (50 µg/puff, Flixonase, GSK) twice daily for 14 days Unclear which nostril was used Both groups received amoxicillin 80 to 100 mg/kg/day | |
| Outcomes | Severity of symptoms as documented by blinded healthcare workers by phone or face to face discussion on day 4 of the intervention and on days 10 to 14 Severity of symptoms was calculated for each symptom as 0 for not affected, 1 for very little problem, 2 for mild problem, 3 for moderately bad, 4 for bad and 5 for severe Total (mean) symptom severity score reported, however the authors do not describe how this is calculated. They also do not state how many days post‐intervention the individual symptom scores were reported ‐ this could be anywhere from 4 to 14 days after recruitment Complete recovery of symptoms ‐ based, according to personal communication with authors, on clinical assessment and patient self report, however, unclear method of calculation and time point of assessment Relative recovery of symptoms ‐ the authors state in direct communication that this was defined as recovery of associated symptoms such as cough, headache, malaise, facial pain, irritability but it remains unclear how this was calculated and the time point of assessment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with the authors: computer‐generated randomisation used |
| Allocation concealment (selection bias) | High risk | No control nasal spray used |
| Blinding (performance bias and detection bias) | High risk | Single‐blind study ‐ outcome assessors were blinded but the majority of the measures were based on patient self report |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were presented for all the children recruited in each arm The paper reports that patients were excluded if they showed no improvement by day 4 of the intervention Direct communication with authors revealed that no children were excluded for this reason |
| Selective reporting (reporting bias) | High risk | Very limited reporting of outcome measures |
| Other bias | High risk | If no improvement was seen in fever nasal congestion or cough, or if exacerbation of disease was evident, patients were reassessed and the antibiotics were changed if necessary. Following direct communication with the authors they stated that "As a whole, patients were assessed again and the antibiotics were changed if necessary at any time. In fact, most of the patient had received a different treatment, if they did not response to the first line antibiotic therapy after 3 days of the initial treatment." The type and duration of antibiotic usage if changed is not reported and so may have introduced performance bias The type and duration of antibiotics once changed is not reported and so may have introduced performance bias |
| Comparability of groups on different prognostic characteristics | Low risk | Symptom severity scores were 22.46 +/‐ 2.61 and 23.50 +/‐ 3.19 before treatment, however it is unclear how this was calculated The authors state in the text that "clinical features were almost similar at baseline of the study...and the differences between them are negligible" but the table they refer to in support of this statement does not offer any relevant data In personal communication the authors stated that there were no statistical differences in baseline prognostic characteristics |
BDP: beclomethasone dipropionate
PCR: polymerase chain reaction
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Defined non‐allergic rhinitis as those cases that were not infection | |
| Population was children with chronic nasal obstruction | |
| Experimentally induced rhinovirus infection. Steroid administered before inoculation | |
| Experimentally induced rhinovirus infection. Steroid administered before inoculation | |
| Symptoms lasted for longer than 10 days ‐ beyond the natural history of the common cold | |
| No relevant outcome measures reported. Same study population as Puhakka 1998 | |
| Review article focusing on perennial and allergic rhinitis (no abstract available initially and so we obtained full text) | |
| No direct comparison between steroid and placebo ‐ groups treated otherwise unequally in terms of type of vasoconstrictor and presence/absence of mucolytic | |
| Experimentally induced rhinovirus infection. Steroid administered before inoculation. Same patient population as Farr et al but examining biochemical markers rather than symptoms | |
| No direct comparison between steroid and placebo ‐ steroid group also received intranasal neomycin | |
| Symptoms lasted for longer than 10 days ‐ beyond the natural history of the common cold |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |
| Analysis 1.1  Comparison 1 Rhinovirus infection, Outcome 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment. | ||||

PRISMA flow chart.
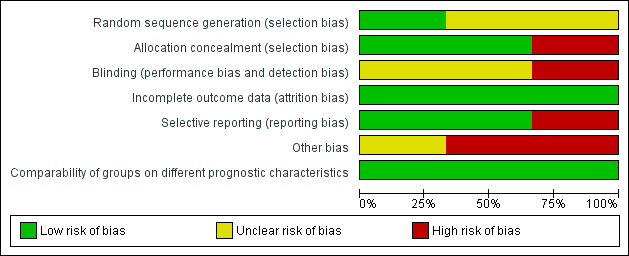
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
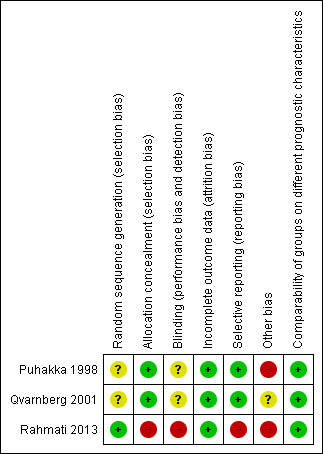
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
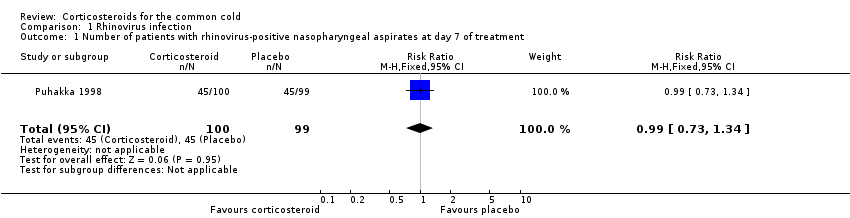
Comparison 1 Rhinovirus infection, Outcome 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |

