Corticosteroides para el resfriado común
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008116.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 octubre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Gail Hayward wrote the review. The manuscript was revised by all review authors.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
British Society for Antimicrobial Chemotherapy, UK.
Funding for this work was provided in part by a Systematic Review Grant (GA722SRG) from the British Society for Antimicrobial Chemotherapy
Declarations of interest
Gail Hayward: none known
Matthew J Thompson: none known
Rafael Perera: none known
Chris B Del Mar: none known
Paul P Glasziou: none known
Carl J Heneghan: none known
Acknowledgements
We would like to thank the British Society for Antimicrobial Chemotherapy for a seed grant to assess treatment of common upper respiratory tract infections with corticosteroids.
The University of Oxford Nuffield Department of Primary Care Health Sciences is part of the National Institute of Health Research School of Primary Care Research, which provides financial support for senior investigators who contributed to this article. The opinions expressed are those of the review authors and not of the Department of Health.
The review authors wish to thank the following people for commenting on the draft protocol: Morio Aihara, Jean‐Michel Klossek, Nicola Principi, Sree Nair and Anca Zalmanovici. We thank the following people for commenting on the draft review: Amanda Young, Harri Hemilä, Rashmi Das, Sree Nair and Anca Zalmanovici; and we thank the following people for commenting on the draft update of this review: Jenny Negus, Amanda Roberts, Ravishankar and Anca Zalmanovici Trestioreanu.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Oct 13 | Corticosteroids for the common cold | Review | Gail Hayward, Matthew J Thompson, Rafael Perera, Chris B Del Mar, Paul P Glasziou, Carl J Heneghan | |
| 2012 Aug 15 | Corticosteroids for the common cold | Review | Gail Hayward, Matthew J Thompson, Rafael Perera, Chris B Del Mar, Paul P Glasziou, Carl J Heneghan | |
| 2009 Oct 07 | Corticosteroids for the common cold | Protocol | Gail Hayward, Matthew J Thompson, Carl J Heneghan, Rafael Perera, Chris B Del Mar, Paul P Glasziou | |
Differences between protocol and review
We have added an additional exclusion criterion as follows: "We also excluded trials where the common cold was experimentally induced if the intervention was initiated before the cold was induced." We made this decision once the range of eligible papers was established as we had not anticipated trials using experimentally induced infections.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Adult; Child; Child, Preschool; Female; Humans; Male;
PICO

PRISMA flow chart.
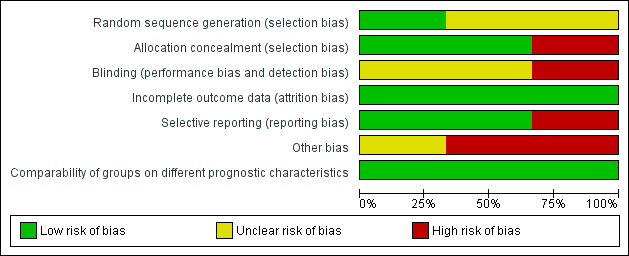
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
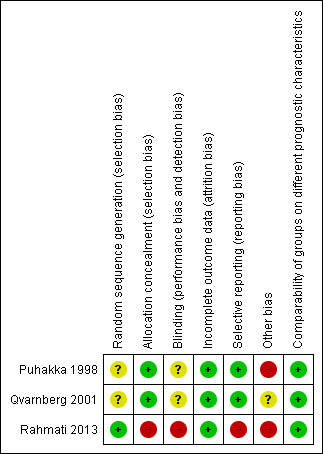
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
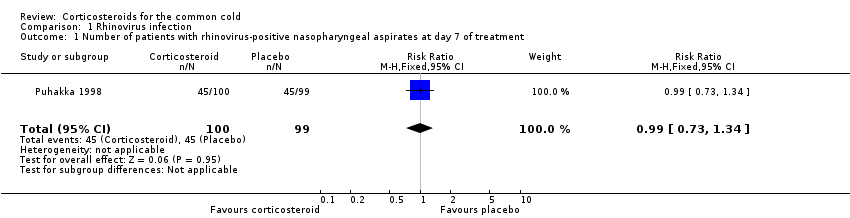
Comparison 1 Rhinovirus infection, Outcome 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |

