皮质类固醇治疗普通感冒
摘要
研究背景
普通感冒是一种常见疾病,虽然是良性且自限性的,但会导致许多人去初级健康机构就诊,并导致大量缺课或缺勤。目前的对症治疗获益有限。皮质类固醇是治疗其他上呼吸道感染的有效方法,其抗炎作用也可能对普通感冒有益。本更新综述包括一项额外的研究。
研究目的
比较皮质类固醇与普通感冒的常规治疗对儿童和成人症状缓解和改善的影响。
检索策略
我们检索了Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)(2015年,第4期),其中包括急性呼吸道感染(Acute Respiratory Infections, ARI)组专业注册库、效果评价数据库(Database of Reviews of Effects, DARE)(2015年,第2期)、NHS健康经济数据库(2015年第2期)、MEDLINE(1948年至2015年5月第3周)和EMBASE(2010年1月至2015年5月)。
纳入排除标准
将皮质类固醇与安慰剂或标准临床治疗进行比较的随机、双盲、对照试验。
资料收集与分析
两名综述作者独立提取资料并评价试验质量。我们无法进行meta分析,而是对现有证据进行叙述性描述。
主要结果
我们纳入了三项试验,共353名受试者。两项试验将鼻内皮质类固醇与安慰剂进行比较,一项试验将鼻内皮质类固醇与常规治疗进行比较;没有试验研究口服皮质类固醇。在两项安慰剂对照试验中,没有证明鼻内皮质类固醇对症状的持续时间或严重程度有疗效。这两项试验的总体偏倚风险较低或不明确。在一项54名受试者参与的试验中,安慰剂组的平均出现症状天数为10.3天,而鼻内皮质类固醇组的平均出现症状天数为10.7天(P值=0.72)。对199名受试者进行的第二次试验报告称,症状持续时间没有显著差异。一项针对2至14岁儿童的单盲试验也接受口服抗生素治疗,但对症状缓解的结局指标报告不充分。该试验的总体偏倚风险很高。除口服阿莫西林外,接受鼻内类固醇治疗组的平均症状严重程度评分明显较低。一项安慰剂对照试验报告鼻吸出物中存在鼻病毒,但没有发现差异。三项试验中只有一项报告了不良事件;没有发现差异。两项试验报告了继发性细菌感染(一例鼻窦炎,一例急性中耳炎;均在皮质类固醇组中)。缺乏可比较的结局指标标准意味着我们无法合并数据。
作者结论
目前的证据不支持使用鼻内皮质类固醇来缓解普通感冒的症状。然而,只有三项试验,其中一项质量极低,总体统计学把握度有限。需要对成人和儿童进行进一步的大型、随机、双盲、安慰剂对照试验来回答这个问题。
PICO
简语概要
类固醇治疗普通感冒
研究问题
我们综述了使用类固醇药物改善普通感冒患者症状的证据。
研究背景
仅在美国,每年就有超过5亿患者患有普通感冒,并导致生产力显著下降。尽管有许多药物可以帮助改善普通感冒的症状,但没有一个有充分的证据表明其益处。类固醇(皮质类固醇)已被证明可以通过减少鼻子和喉咙内壁的炎症来帮助缓解其他类型的上呼吸道感染症状,这意味着它们也可以改善普通感冒的症状。
研究特征
证据截至2015年5月。我们总共发现了三个试验。两项试验从芬兰普通人群或医院工作人员中招募了成年人。这些试验(总共253名成年人)对鼻内类固醇喷雾剂(将类固醇喷入鼻孔)与仅含有安慰剂的喷雾剂进行了比较。我们发现了第三项试验,招募了100名转诊到伊朗儿科医院门诊的儿童。该试验将鼻内类固醇喷雾剂与不使用喷雾剂进行比较,并为所有受试者提供口服抗生素。
主要研究结果和证据质量
这两项比较成人类固醇喷雾剂和安慰剂喷雾剂的试验都没有显示出类固醇在一系列不同措施中的获益。对儿童进行类固醇喷雾与不使用喷雾比较的试验确实发现了一些获益的证据,但我们认为该试验的证据质量极低,结果尚不清楚。我们无法结合试验结果来进一步评估这个问题。没有关于不良事件的报告。
研究结论
现有证据表明,我们不应该使用鼻内类固醇来治疗普通感冒。然而,由于我们只发现了3项小型试验,因此如果不进行设计良好的大型试验,我们不能确定无效果。
Authors' conclusions
Background
Description of the condition
The common cold is the conventional term for upper respiratory tract viral infections that are benign and self limiting. Over 500 million patients develop colds in a year in the United States (Fendrick 2003), resulting in 22 million school days lost (Adams 1999), and an annual lost productivity of almost USD 25 billion (Bramley 2002).
The typical symptoms of a cold include nasal obstruction, rhinorrhoea, sneezing, sore throat and, on occasion, mild fever, headache and myalgia. The most common causative agent is the rhinovirus (Makela 1998), although several different viral families have been implicated and bacterial infection can give rise to the same symptoms (Kaiser 1996). Rhinoviral infection begins with deposition of virus on the nasal epithelium via airborne droplets or by hand from fomites (any inanimate object, e.g. kitchen sink, that can carry disease‐causing organisms). The inflammatory response of nasal mucosa to the viral infection involves vasodilation and increased vascular permeability, leading to the symptoms of sneezing, nasal congestion and rhinorrhoea.
Description of the intervention
Management options for common colds currently focus on symptom alleviation and include decongestants, where evidence has not recently been assessed, and antihistamines, for which there is no evidence of benefit (Wiest 2011). Whilst both of these therapies target the effects of the inflammatory response of the nasal mucosa to the virus, this inflammatory response could also be modulated by the use of corticosteroids, which inhibit the generation of pro‐inflammatory cytokines in nasal epithelium (Mygind 2001).
How the intervention might work
Corticosteroids have been demonstrated to increase the likelihood of resolution or improvement of symptoms in acute sinusitis (Zalmanovici 2013), as well as in viral croup (Russell 2011), and sore throats (Hayward 2009). Their anti‐inflammatory actions on the nasal mucosa may also reduce the symptoms and duration of the common cold.
Why it is important to do this review
The common cold results in significant morbidity and loss of productivity. Current treatment options have limited evidence of benefit. Corticosteroids may offer more effective symptom relief, given their actions in other infections of the upper respiratory tract, and it is important to examine the evidence for this. No previous systematic reviews have addressed this question.
Objectives
To compare corticosteroids versus usual care for the common cold on measures of symptom resolution and improvement in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing corticosteroids to placebo or to standard clinical management (for example, conservative measures such as pain relief) for the common cold.
Types of participants
Children and adults with the common cold, defined by clinical diagnosis. We excluded trials where a definitive diagnosis of another upper respiratory condition was present (for example, influenza or sinusitis). We also excluded trials where the common cold was experimentally induced if the intervention was initiated before the cold was induced. We did not impose any age limits.
Types of interventions
Oral or inhaled corticosteroids versus standard clinical care or placebo in the control group. We included trials reporting combined interventions if they allowed a direct comparison between corticosteroids and usual care for the common cold and were unconfounded. By unconfounded, we mean studies where the two groups were not treated differently, except for the provision of steroids to one group. Confounding can occur by the use of a different medication regime (for example, analgesics) for one of the two groups. We excluded them if the two groups were treated unequally apart from the corticosteroids.
Types of outcome measures
Primary outcomes
-
Proportion of participants with resolution or improvement of symptoms (individual and global) within one month.
-
Time lapse before resolution of symptoms.
Secondary outcomes
-
Adverse events necessitating discontinuation of treatment.
-
Relapse rates.
-
Microbiological consequences, for example, length of shedding of virus from nasopharyngeal secretions, bacterial culture from secretions.
-
Treatment for secondary infections.
-
Quality of life measures and economic costs.
Search methods for identification of studies
Electronic searches
For this 2015 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 4), which includes the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (May 2012 to May week 3, 2015) and EMBASE (May 2012 to May 2015). We also searched the Database of Reviews of Effects (DARE) (2015, Issue 2 of 4) and the NHS Health Economics Database (NHS EED) (2015, Issue 2 of 4) from The Cochrane Library.
Previously we searched CENTRAL (2012, Issue 5), the Database of Reviews of Effects (DARE) and the NHS Health Economics Database (searched 22 May 2012), MEDLINE (1948 to May week 2, 2012) and EMBASE (January 2010 to May 2012). We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version, Ovid format (Lefebvre 2011). See Appendix 1 for the MEDLINE and CENTRAL search strategy and Appendix 2 for the EMBASE search strategy.
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov trials registries (latest search 19 May 2014). We searched the reference lists of all studies identified as relevant to increase the yield of relevant study references.
Data collection and analysis
Selection of studies
Two review authors (GH, CDM) independently reviewed the titles and abstracts of the electronic search results to select relevant articles. One review author (GH) obtained the full text of these articles. Two review authors (GH, CDM) independently reviewed full‐text articles for their inclusion in the review. A third review author (CH) resolved any disagreements by discussion. The review authors were not blinded to the journal of origin, the authors, the institutions or the magnitude of results.
Data extraction and management
Two review authors (GH, MT, CDM) independently extracted data from included trials, entering data into an extraction template and checking agreement. A third review author (CH) assisted with resolving any disagreements. A statistician (RP) independently reviewed all data extracted from original publications to verify the quality of methods and analysis used. We wrote to the trial authors for clarification of data where information was lacking.
Assessment of risk of bias in included studies
Two review authors (GH, MT) independently assessed the methodological quality of the included studies, with disagreements documented and resolved by discussion with a third review author (CH). The specific aspects of methodological quality assessed included random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), treatment adherence, percentage participation and comparability of groups on baseline characteristics. We used the Cochrane 'Risk of bias' tool to perform the assessment (Higgins 2011).
Measures of treatment effect
Symptom severity was reported as a mean symptom score in two trials (Qvarnberg 2001; Rahmati 2013). In neither trial was it clear how this was calculated. Duration of symptoms was reported as mean duration of symptoms in days in two trials. We were unable to combine data from individual trials. The number of patients who were rhinovirus‐positive at day seven is reported as a risk ratio (Puhakka 1998).
Unit of analysis issues
We did not encounter unit of analysis issues.
Dealing with missing data
Completion rates were very high for all of our included trials and so strategies for dealing with missing data were not required.
Assessment of heterogeneity
We did not assess heterogeneity.
Assessment of reporting biases
The small number of studies meant that the use of funnel plots was inappropriate. We attempted to contact trial authors to ask for unpublished results.
Data synthesis
As our data were not amenable to meta‐analysis, we addressed our primary outcomes using a narrative description of the available evidence.
Subgroup analysis and investigation of heterogeneity
We were unable to perform any subgroup analyses.
Sensitivity analysis
We were unable to perform sensitivity analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The initial search of the electronic databases retrieved 2947 records with duplicates removed. MEDLINE yielded 1492 records, CENTRAL 1577 and EMBASE 1211. We also searched HEED and DARE and these yielded 18 and 13 records, respectively. Of these records we identified 10 studies that were potentially eligible based on title and abstract. We obtained full‐text copies of all 10 articles. From these 10 we included two studies and excluded eight. In the updated search on 19 May 2015, MEDLINE yielded 166 records, EMBASE 1084, CENTRAL 504, NHS EED 10, HEED 0 and DARE 13 records. Once duplicates were removed the total number of new records was 1216 of which we identified four as potentially eligible based on title and abstract. We obtained full‐text copies and included one additional study (see Figure 1 for the PRISMA flow chart).
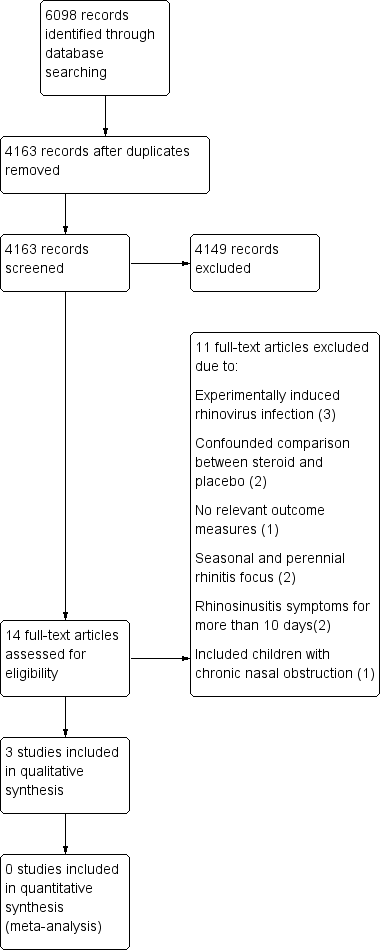
PRISMA flow chart.
Included studies
Two of our included studies involved 199 and 54 adult participants respectively, suffering from naturally developed colds (Puhakka 1998; Qvarnberg 2001). Both studies were performed in Finland and recruited from the general population (Puhakka 1998), or hospital staff (Qvarnberg 2001), and the majority were female (190/254). Participants received intranasal fluticasone propionate 200 μg four times daily (Puhakka 1998), or beclomethasone dipropionate 400 μg once daily (Qvarnberg 2001), for six or 14 days, respectively. The third included study involved 100 children aged two to 14 years attending the paediatric hospital in Bandar Abbas, Iran (Rahmati 2013). Participants received either amoxicillin 80 to 100 mg/kg alone for 14 days or amoxicillin and fluticasone nasal spray, one puff twice a day, for 14 days. This study aimed to recruit children with acute sinusitis. However, the eligibility criteria included children with symptoms of common cold for less than 10 days with purulent nasal discharge and three days of fever over 39 degrees celsius; criteria compatible with a diagnosis of common cold.
Excluded studies
We excluded 11 studies. Three studies involved experimentally induced rhinovirus infection and, in each case, the steroid intervention was started before inoculation of rhinovirus (Farr 1990; Gustafson 1996; Proud 1994). Two studies did not offer an unconfounded comparison between steroid and placebo, as their nasal sprays contained antibiotics or mucolytic/vasoconstrictor drugs, which were not also given to the placebo group (Peynegre 2005; Reinert 1991). One study used the same trial population as Puhakka et al to examine salivary constituents and reported no relevant outcome measures (Lenander‐Lumikari 1999). One study was a review focusing on seasonal and perennial rhinitis (Mygind 1977), and another excluded infection from its definition of non‐allergic rhinitis (Baccioglu Kavut 2013). Two studies assessed a population who presented with rhinosinusitis symptoms for more than 10 days, which we judged to be beyond the natural history of duration of the common cold (Keith 2012; Tugrul 2014), and the final study included children with chronic nasal obstruction (Bellodi 2006).
Risk of bias in included studies
Two of the studies were double‐blind trials comparing intranasal corticosteroid to placebo (Puhakka 1998; Qvarnberg 2001). The method of randomisation was not clearly reported in either study. We were unsuccessful in our attempt to elicit more information to support our assessment of risk of bias directly from the trial authors.
The third study was a single‐blind trial comparing intranasal corticosteroid and oral amoxicillin to amoxicillin alone (Rahmati 2013). We elicited further information directly from the authors and found that this study had a high risk of performance, selection and reporting bias.
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3. See Characteristics of included studies for further details of our risk of bias assessment.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
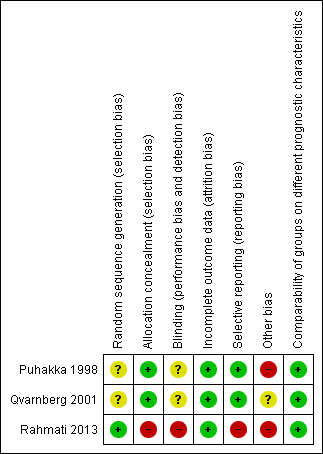
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies used placebo intranasal sprays with identical constituents to the intervention spray apart from the active corticosteroid (Puhakka 1998; Qvarnberg 2001). Qvarnberg 2001 used the 'Easyhaler' multidose powder inhaler designed for nasal application for both placebo and steroid. Puhakka 1998 did not directly describe the medication packaging used, although they do report that they received both placebo and corticosteroid sprays from the same pharmaceutical company.
Rahmati 2013 did not use a placebo comparison and therefore had no allocation concealment.
Blinding
Two studies were described as double‐blind (Puhakka 1998; Qvarnberg 2001). No further details regarding this were reported, although Qvarnberg 2001 reported that the randomisation code was only broken after data entry was complete. Rahmati 2013 stated that outcome assessors were blinded, yet a number of outcome measures required patient self report and patients were not blinded as no placebo was used.
Incomplete outcome data
Completion rates were high in all studies with only 3/353 participants failing to complete; two participants received placebo and one corticosteroid.
Selective reporting
In two studies, reporting of data was incomplete. Puhakka 1998 stated that data on usage of the trial medications were collected but they did not report these data; if participants in the steroid group had poor compliance with the trial this could reduce the likelihood of any positive effect. Rahmati 2013 displayed inadequate reporting of outcome measures in terms of both the time points of the assessment and the way in which the measures were assessed and calculated.
Other potential sources of bias
Puhakka 1998 reported that the placebo group as a whole consumed a greater quantity of paracetamol tablets than the steroid group (170 tablets compared to 141). This difference could influence the reporting of symptoms. The Puhakka 1998 trial was supported by GlaxoWellcome Ltd and one trial author was employed by GlaxoWellcome Ltd. This company also manufactured the steroid nasal spray used in the trial. No declarations of conflict of interest were made by the trial authors. The study drug for Qvarnberg 2001 was provided by Orion Pharma, and one of the trial authors worked for the company. No declarations of conflict of interest were made by the trial authors.
Rahmati 2013 stated that if no improvement was seen in fever, nasal congestion or cough, or if exacerbation of disease was evident, patients were reassessed and the antibiotics were changed if necessary. Following direct communication with the authors they stated that "As a whole, patients were assessed again and the antibiotics were changed if necessary at any time. In fact, most of the patients had received a different treatment, if they did not respond to the first line antibiotic therapy after 3 days of the initial treatment." The type and duration of antibiotics once changed is not reported and may have introduced performance bias.
Effects of interventions
The data extracted from the studies did not provide comparable outcome measures and we were unable to obtain further comparable data directly from the trial authors. Therefore, we have described the results of each trial according to our stated outcome measures.
Primary outcomes
1. Proportion of participants with resolution or improvement of symptoms (individual and global) within one month
Neither Puhakka 1998 nor Qvarnberg 2001 reported this outcome at any time point.
The outcomes of complete and relative resolution were reported as assessed by Rahmati 2013. However, despite direct communication with the authors, we were unable to establish the time point at which these outcomes were assessed or the criteria upon which they were based and therefore we do not feel the evidence is of sufficient quality to be included.
Rahmati 2013 reported a mean 'severity of symptoms' score. It was unclear how this was calculated in relation to the individual symptom scores they report. They state that the score refers to the end of treatment ‐ i.e. 14 days. They found that the score in those children receiving intranasal corticosteroid and amoxicillin reduced from 22.46 ± 2.61 to 11.68 ± 2.66. In those children just receiving amoxicillin the mean score reduced from 23.5 ± 3.19 to 14.84 ± 2.92. The final scores were significantly lower in the group receiving intranasal steroids. Qvarnberg 2001 reported that the sum of symptom severity scores over two weeks was similar in the two groups: 57.3 (maximum score 392) in the steroid group versus 51.6 in the placebo group (P value = 0.48). No clinically or statistically significant differences were shown in the summed severity (over two weeks follow‐up) of the seven individual symptom measures.
Rahmati 2013 also reported the percentage of patients scoring zero to five for individual symptoms (zero for not affected, one for very little problem, two for mild problem, three for moderately bad, four for bad and five for severe). We were unable to clarify the time point at which these scores were assessed. The paper includes a table, which suggests that scores are significantly lower for congestion, anterior discharge, posterior discharge, fullness, headache, cough and malodour, but that scores on exhaustion, fever and toothache were not significantly different. It is unclear how this statistical significance was calculated.
2. Time lapse before resolution of symptoms
Puhakka 1998 reported that the duration of the common cold symptoms of rhinorrhoea, nasal congestion and cough was equal in both groups (illustrated in figures in the original article). Mean duration of throat soreness was greater in the corticosteroid group than the placebo group: 5.3 days versus 3.7 days (P value < 0.001).
Qvarnberg 2001 reported the mean number of symptomatic days as 10.3 in the placebo group, compared to 10.7 in the corticosteroid group (P value = 0.72). Median time to recovery was 12 days in the steroid group and 11 days in the placebo group (log rank test P value = 0.81).
Rahmati 2013 did not report this outcome.
Secondary outcomes
1. Adverse events necessitating discontinuation of treatment
Only one study reported adverse events (Puhakka 1998). There were no adverse events necessitating discontinuation of treatment in either group.
2. Relapse rates
Puhakka 1998 reported that no participants had symptoms requiring additional follow‐up from 21 days after the start of the trial, suggesting a relapse rate of zero. Relapse rates were not assessed by Qvarnberg 2001 or Rahmati 2013.
3. Microbiological consequences
Puhakka 1998 was the only trial to assess the presence of rhinovirus by culture and polymerase chain reaction (PCR) of nasopharyngeal aspirates taken on day one and day seven (i.e. at the end of the course of treatment). There were no differences in the percentage of rhinovirus‐positive participants at baseline. When assessed by viral culture alone, there were significantly more rhinovirus‐positive participants at day seven in the corticosteroid group compared to the placebo group (36% versus 14%, P value < 0.001) (Analysis 1.1). However, when the total number of positive samples from day seven detected by PCR and culture were combined there were no significant differences between corticosteroid and placebo groups. Viral culture may offer a more accurate representation of presence of viable virus.
In an intention‐to‐treat‐infected (ITTI) population analysis of only those participants who were rhinovirus‐positive on day one, Puhakka 1998 reported no differences in the overall frequency of symptoms between steroid and placebo groups. The mean duration of cough was shorter (8.0 versus 10.8 days, P value < 0.05) and the severity of cough was lower on days three, four, seven, eight and nine in the corticosteroid group. Nasal congestion was less severe in the placebo group on days two and five.
No significant differences were seen between treatment groups in the number of positive bacterial cultures from nasopharyngeal aspirates. The effect on viral shedding was not assessed by any of our included studies.
4. Treatment for secondary infections
Puhakka 1998 reported that one out of 100 participants receiving corticosteroids and 0 out of 99 of participants receiving placebo required antibiotics for acute otitis media. Qvarnberg 2001 reported that one out of 28 participants in the corticosteroid group and 0 out of 26 in the placebo group developed maxillary sinusitis based on ultrasound. Rahmati 2013 treated all participants with antibiotics and offered a second course of alternative antibiotics if the child failed to improve after three days but did not supply data on the number of children for whom this was the case.
5. Quality of life measures and economic costs
No data were reported in relation to quality of life measures, economic costs or adverse events necessitating discontinuation of treatment.
Puhakka 1998 reported that the steroid group had no clinical changes and no symptoms classifiable as adverse events. Nasal irritation and bleeding did not occur significantly more often in the steroid group than the placebo group. Qvarnberg 2001 and Rahmati 2013 did not record or report upon adverse events.
Discussion
Summary of main results
This systematic review offers no evidence for benefit of intranasal corticosteroids for the common cold. The mean time to resolution of symptoms of the common cold was not significantly different in those participants using intranasal steroids compared to placebo in two of the studies included in this review. The symptom of sore throat had a longer duration in the corticosteroid group than the placebo group in one trial (Puhakka 1998), but this difference was not seen in the other trial (Qvarnberg 2001). The only trial to assess complete resolution of symptoms, Rahmati 2013, was of very poor quality and the outcome reporting was insufficient to allow us to report these data. Although they did demonstrate a significantly greater reduction in mean symptom severity score, this result must be interpreted in the context of a methodologically flawed trial performed in a population of patients that also included children with acute sinusitis.
In those participants shown to be rhinovirus‐positive, duration of cough was shorter in the group receiving intranasal corticosteroids but there was no difference when all participants were assessed; no differences were seen in the trial by Qvarnberg 2001. The use of corticosteroids did not result in any adverse consequences in terms of bacteriological growth and did not result in significantly greater requirement for secondary antibiotic therapy. However, there were too few events in the combined studies to reliably detect a potential difference.
A significantly higher percentage of participants in the corticosteroid group were found to be rhinovirus‐positive by viral culture in one trial (Puhakka 1998). This may imply that intranasal corticosteroids prolonged the duration of viable virus, which is of interest in the context of the known immunosuppressant actions of corticosteroids. However, prolonged presence of virus did not correlate with prolonged duration of symptoms.
Overall completeness and applicability of evidence
Only three trials of intranasal corticosteroids met the inclusion criteria for this review. One of these was a pilot study including only 54 participants (Qvarnberg 2001), and one was of very poor quality, with inadequate reporting of outcome measures (Rahmati 2013). This limits the conclusions that can be drawn. The data have limited applicability to older adults and there may be cultural differences that influence the predominantly self reported data from Finnish and Iranian patient groups. It is, of course, possible that a spray with inactive ingredients in itself is beneficial for the common cold. However, a recent systematic review found no convincing evidence of benefit of saline nasal spray for symptoms of upper respiratory tract infections (King 2015).
Quality of the evidence
The two double‐blind trials included in this review failed to describe in detail the procedures followed for randomisation and blinding (Puhakka 1998; Qvarnberg 2001). However, both trials reported almost complete outcome data, were at low risk of reporting bias and described procedures for allocation concealment. Although no conflict of interest was reported, another potential source of bias may have been the sponsorship of one of the trials by the pharmaceutical company manufacturing the steroid spray and the inclusion of one of its employees on the authorship of the paper. The single‐blind trial retrieved from our update of this review was at high risk of selection, performance and reporting bias, and results were not clearly presented (Rahmati 2013).
Potential biases in the review process
No potential biases are expected in this review process.
Agreements and disagreements with other studies or reviews
We excluded two trials that evaluated the clinical effectiveness of corticosteroids commenced in advance of inoculation with rhinovirus. Although the results of these studies have very limited applicability to clinical practice, the results are interesting in the context of the findings of our review. There were two trials involving 91 participants, of whom 75 became infected by rhinovirus. In Farr 1990, the active treatment group of 19 participants received a 10‐day course of intranasal steroid beginning four days before inoculation and a three‐day course of twice daily 30 mg prednisolone beginning one day before inoculation. A significantly lower proportion of the corticosteroid group met the criteria for a cold and also believed that they had a cold. The reported severity of the cold was also lower on days one, two and five after inoculation. However, there was no difference in individual symptom score totals and summed symptom scores between corticosteroid and placebo groups, nor in total mean mucus weights and tissue use.
Gustafson 1996 examined the effect of 20 mg prednisolone three times daily starting 11 hours before inoculation for five days in an active treatment group of 21 participants. In contrast to Farr 1990, they found no difference in the number of participants who met the criteria for a cold. There were no differences in total symptom scores, mucus production and tissue use between corticosteroid and placebo groups. They reported increased mean viral titres in the corticosteroid group but no difference in the frequency or duration of viral shedding. In summary, trials using inoculation of rhinovirus do not provide any consistent evidence of symptomatic benefit of corticosteroids in the common cold.

PRISMA flow chart.
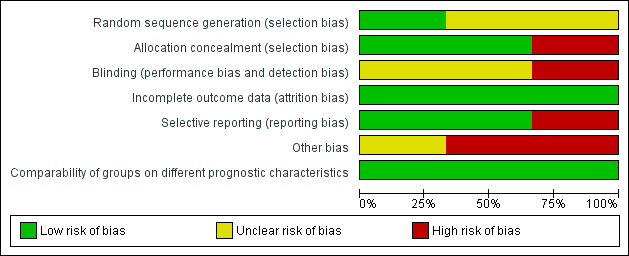
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
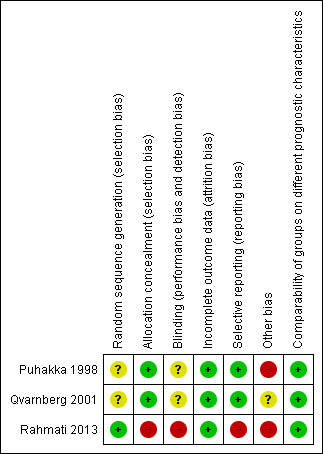
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
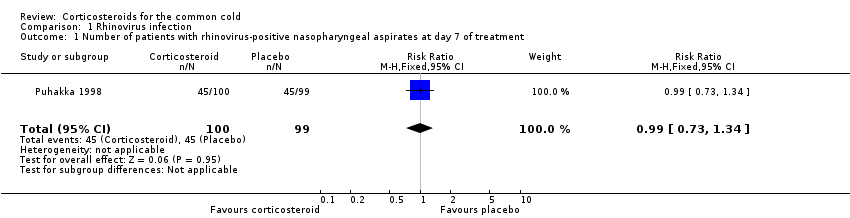
Comparison 1 Rhinovirus infection, Outcome 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with rhinovirus‐positive nasopharyngeal aspirates at day 7 of treatment Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.73, 1.34] |

