用于预防心源性猝死的胺碘酮与其他药物干预的比较
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with stable heart failure due to ischemic or nonischemic causes and a left ventricular ejection fraction of no more than 35 percent Country: United States & Canada | |
| Participants | N = 1692 (845 amiodarone, 847 placebo) Sex: 76‐77% male Age: median 60 years Inclusion: heart failure with LVEF < 35% (primary prevention) | |
| Interventions | Group 1: ICD (not analysed) Group 2: amiodarone (up to 800 mg/day initially, 300 mg/d on average at the end of the study) Group 3: placebo Duration: 2 to 5 years | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD, quality of life | |
| Notes | The outcome 'quality of life was published in a separate report by Marks et al. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Placebo and amiodarone were administered in a double‐blind fashion with the use of identical appearing 200‐mg tablets. . ." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Placebo and amiodarone were administered in a double‐blind fashion with the use of identical appearing 200‐mg tablets. . ." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Pairwise comparisons of amiodarone with placebo and ICD with placebo were performed according to the intention‐to‐treat principle." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of 2521 patients who underwent randomisation, 2479 (98%) completed quality‐of‐life questionnaires at baseline. . . Overall, from a total of 9171 expected contacts with patients, 8747 quality‐of‐life questionnaires (95%) were collected. Only 1.2% of patients declined to complete the questionnaires, and only 1.4% of forms were judged to be incomplete." |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Patients with heart failure with low ejection fraction and ventricular ectopies Country: India | |
| Participants | N = 90 (46 amiodarone, 44 placebo) Sex: 67‐69% male Age: male 56‐58 years Inclusion: CHF with LVEF < 35% (primary prevention) | |
| Interventions | Group 1: amiodarone (200 mg/day final dose) Group 2: placebo Duration: 1 year | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The medication was given in a single‐blind fashion." We are certain that the patients received placebo, but we don't know whether the physicians were blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description of the methods used to assess the outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Forty‐six participants were randomly selected to receive low‐dose amiodarone and 44 patients received placebo. . ." In all the tables and figures, the numbers are 36 for amiodarone and 40 for placebo, with no explanation for the discrepancy. Furthermore, 5 participants from the amiodarone arm were excluded for showing "proarrhythmia on second Holter monitoring". |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients at after myocardial infarction who persisted with complex ventricular ectopic activity up to hospital discharge were entered into the study Country: Switzerland | |
| Participants | N = 312 (98 amiodarone, 100 individualised antiarrhythmic drug, 114 no treatment) Sex: 86% male Age: mean 61 years Inclusion: 7 to 28 days post‐AMI (primary prevention) | |
| Interventions | Group 1: amiodarone 1000 mg/d first week, then 200 mg/d (final dose) Group 2: individualized antiarrhythmic drug Group 3: no antiarrhythmic therapy Duration: 1 year In the 'individualised antiarrhythmic drug' arm the participants used as first line quinidine or mexiletine. If the first drug did not achieve this goal, the second drug was tested. In case of failure of both drugs, clinicians tried other antiarrhythmic drugs (ajmaline, disopyramide, flecainide, propafenone or sotalol). If none of these drug regimens was effective, amiodarone was given. | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Other measured outcomes were effectiveness of the different regimens in suppressing ventricular ectopic activity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were then randomised to one of the three treatment groups on the basis of their hospital entry number and the randomisation list." |
| Allocation concealment (selection bias) | Unclear risk | The trial was performed at three hospitals, but there is no mention of the allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the 'no therapy' arm didn't use placebo. The participants included in the individualised antiarrhythmic drug therapy used different schemes. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Deaths were assessed without knowledge of the treatment group assignment; evaluation was based on death certificates, physician and hospital records, autopsy records and information obtained from relatives and witnesses." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Each death was assigned to the treatment group according to the intention to treat principle." Quote: "Patients no longer willing to adhere to the treatment regimen or to show up for follow‐up evaluation were contacted by telephone at the end of the year to obtain information about survival." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with recent AMI (on their 7th day at least) and a mean of at least 10 Ventricular Premature Depolarizations/hr or at least one run of VT in a 24hr electrocardiographic monitoring Country: Canada | |
| Participants | N = 77 (48 amiodarone, 29 placebo) Sex: 73‐79% male Age: mean 64‐66 years Inclusion: ≥ 7 days postacute myocardial infarction (primary prevention) | |
| Interventions | Group 1: amiodarone 10 mg/kg/d the first 2 weeks then 300‐400 mg/d (final dose) Group 2: placebo Duration: 2 years | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Quote: "The principal outcomes were the composite of arrhythmic death or resuscitated VF, arrhythmic death, other cardiac death, noncardiac vascular death, and nonvascular death." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Eligible patients who gave informed consent were randomly allocated in a double‐blind fashion in a 2:1 ratio to amiodarone or an identical‐appearing placebo in 200‐mg tablets." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "When a patient died or experienced cardiac arrest, details of the event were gathered from the hospital, ambulance, or emergency department records and from interviews with family, treating physicians, and nurses. The narrative summary was then reviewed independently and without knowledge of treatment allocation by two of the principal investigators (J.A.C. and S.J.C.)." |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Survivors of myocardial infarction with frequent or repetitive Ventricular Premature Depolarizations Country: Canada | |
| Participants | N = 1202 (606 amiodarone, 596 placebo) Sex: 82% male Age: mean 64 years Inclusion: ≥ 7 days post acute MI (primary prevention) | |
| Interventions | Group 1: amiodarone 10 mg/kg/d the first 2 weeks then 200‐400 mg/d (final dose) Group 2: placebo Duration: 2 years | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ". . . according to the computer generated randomisation code (stratified by centre in blocks of four) prepared by the External Safety and Efficacy Monitoring Committee. . ." |
| Allocation concealment (selection bias) | Low risk | Quote: "The complete randomisation code was available only to the chair of the External Safety and Efficacy Monitoring Committee. Thus, standard masked conditions were extended to include the Steering Committee, the External Safety and Efficacy Monitoring Committee, the Coordinating and Methods Centre, and Sanofi Winthrop, all of whom were unaware of treatment allocation." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "An independent company was contracted to pack the active drug (amiodarone 200 mg tablets) and matching placebo tablets." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The outcome events reported by the clinical investigators were all reviewed by the External Validation Committee under masked conditions. This committee had final responsibility for the verification of resuscitated ventricular fibrillation and the classification of deaths." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[A]ll outcomes were also analysed by the intention‐to‐treat principle, in which all patients were judged to be at risk from the time of enrollment until the predefined completion of follow‐up, irrespective of whether the study drug was discontinued." Quote: ". . . intention‐to‐treat analyses included all randomised patients. . ." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients who survived the early phase of myocardial infarction and who were considered unsuitable to receive beta‐blockers because of contraindications. Country: Poland | |
| Participants | N = 613 (305 amiodarone, 308 to placebo) Sex: 68‐71% male Age: mean 58‐59 years Inclusion: 5‐7 days post acute MI (primary prevention) | |
| Interventions | Group 1: amiodarone (400 mg/d final dose) Group 2: placebo Duration: 1 year | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[P]atients were randomised between the 5th and 7th days after admission, separately in each centre according to random numbers in sealed envelopes prepared by the independent statistical unit." |
| Allocation concealment (selection bias) | Unclear risk | The authors used sealed envelopes for patient allocation, but it is not stated whether these envelopes were opaque. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: ". . . allocated to treatment in double‐blind fashion. . ." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Independent consultants who were unaware of treatment allocation verified classification of each event." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients who were randomised were included in the analysis whether or not the allocated regimen had been discontinued. Follow‐up for clinical events was 100% complete." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with spontaneous or inducible VT or VF, receiving a dual chamber ICD Countries: Canada, Germany, United States, England, Sweden, and Austria | |
| Participants | N = 278 (140 amiodarone + beta blocker (BB), 138 to BB alone) Sex: 78‐83% male Age: mean 63‐65 years Inclusion: participants with ICD (secondary prevention) | |
| Interventions | Group 1: amiodarone 800 mg/d for 6 weeks then 200 mg/d (final dose) + BB Group 2: beta‐blocker alone Group 3: sotalol alone (not analyzed) Duration: 1 year | |
| Outcomes | All‐cause mortality, SCD and ICD shocks | |
| Notes | Primary outcome was the delivery of ICD shocks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ". . . predetermined random sequence incorporating random block sizes of 3 and 6, with stratification for center and the rate of the slowest documented VT (150/min)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible consenting patients were registered and allocated to open‐study treatment via a call to an automatic computer‐based system at the study's Coordinating and Methods Center. . ." |
| Blinding of participants and personnel (performance bias) | High risk | There was no matching placebo in the BB alone arm. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: ". . . adjudicated by a committee blinded to treatment allocation to determine the underlying heart rhythm before the event and the appropriateness of the delivered therapy". There is no mention to the assessment of the clinical outcomes, but it should not be different from what is stated above. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: ". . . with the analysis based on intention to treat (patients were included in the analysis even if they never took or stopped the assigned therapy)". Loss to follow‐up was less than 5%. |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with severe Heart failure adequately treated Country: Argentina | |
| Participants | N = 516 (260 amiodarone, 256 standard treatment) Sex: 79‐82% male Age: mean 58‐60 years Inclusion: CHF with LVEF < 35% (primary prevention) | |
| Interventions | Group 1: amiodarone (600 mg/day for 2 weeks, then 300 mg/day) Group 2: standard treatment Duration: 2 years | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out with a computer allocation schedule in 10‐patient blocks, stratified. . ." |
| Allocation concealment (selection bias) | Low risk | Quote: " [H]istory. . . of eligible patients w[as] submitted to and analysed by the coordinating centre" |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the control arm didn't use matching placebo |
| Blinding of outcome assessment (detection bias) | Low risk | The information was reclassified at the coordinating centre, blinded to the assigned group. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "These patients remained in the assigned group according to the intention to treat." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | High risk | The trial was stopped early for benefit. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: patients admitted within 24 h of the onset of symptoms of an acute myocardial infarction and heart failure Country: Argentina | |
| Participants | N= 1073 (542 amiodarone, 531 to placebo) Sex: 75‐80% male Age: mean 60 years Inclusion: ≥ 24 h postacute MI (primary prevention) | |
| Interventions | Group 1: amiodarone Group 2: placebo Original protocol: "The overall dose of intravenous amiodarone or placebo was 2700 mg in the first 48 hours. Meanwhile, oral amiodarone/placebo was started at the same time as the intravenous infusion, at a dose of 600 mg every 12 h during the first 4 days. From day 5 to day 90 the oral dose consisted of a single daily dose of 400 mg of amiodarone/placebo. Afterwards and until completion of the study (180 days) the patients received 200 mg/day orally." Amended protocol: ". . . first and second day: 600 mg intravenous/day plus oral amiodarone/placebo 800 mg/ day in one intake; from day 3 to day 90, 400 mg/day and from day 91 to day 180, 200 mg/day. This amendment of the protocol meant a 52% reduction in the amiodarone loading dose in the initial 96 h of treatment for those persons assigned to the amiodarone group." Duration: 6 months | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Quote: "the second interim analysis [516 participants] showed higher mortality among patients receiving amiodarone. This difference in mortality was suspected to be related to arterial hypotension with or without myocardial ischaemia during intravenous and/or oral administration of the drug. Consequently, the Safety and Monitoring Board suggested to the Steering Committee the need to change the amiodarone/placebo doses. . ." Quote: "This amendment of the protocol meant a 52% reduction in the amiodarone loading dose in the initial 96 h of treatment for those persons assigned to the amiodarone group." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to receive either amiodarone or placebo in a double‐blind manner. The complete randomisation list was made before the beginning of the study. . . Randomisation was carried out in balanced blocks of four patients (two for each treatment) in such a way that each centre incorporated an equal number of patients for each treatment. The actual treatment composition was not identifiable unless the corresponding codes were opened." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were. . . centrally assigned to treatment in a random fashion." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were randomly assigned. . . in a double‐blind manner." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "On discharge, case report forms. . . were submitted by the researcher to the coordinating centre." We think that because it is not explicitly stated, and there's no mention about the analysis of the causes of death, the risk of bias is unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All the randomised patients were included in the analysis of results, whether having completed the protocol or not." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | High risk | There are small and unexplained differences between the sample size reported in the English and Spanish (Carbajales 1997) published data. This trial was stopped early for lack of benefit for the primary outcome. |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Efficacy in the treatment of ventricular arrhythmias during the post‐AMI period Country: France | |
| Participants | N = 97 (48 amiodarone, 49 propanolol) Sex: 83‐85% male Age: mean 53.9‐56.4 years Inclusion: ≥ 9 days postacute MI (primary prevention) | |
| Interventions | Group 1: amiodarone 600 mg/d for 8 days then 400 mg/d for 5 days a week (final dose) Group 2: propanolol 60 mg/d initially which then was increased to 160 mg/d over a few days Duration: 6 months | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Electrocardiographic alterations were the main outcome for the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in propanolol arm used a different scheme than participants included in the amiodarone arm. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated for the outcome of death. For the electrocardiographical outcomes, there is a quote: "The investigators who read the LEM, although this remark can apply to them, did not know for certain which drug the patients were receiving." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[T]he analysis should also take into account patients who stopped treatment because of side effects and patients lost to follow‐up who could have stopped treatment and follow‐up for the same reason. All these patients were regarded as failures. This approach, thus, took account of all patients in the study." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with heart failure and asymptomatic complex ventricular arrhythmias Country: Argentina | |
| Participants | N = 127 (66 amiodarone, 61 no treatment) Sex: 73‐82% male Age: mean 60‐62 years Inclusion: CHF with LVEF < 35% (primary prevention) | |
| Interventions | Group 1: amiodarone (800 mg/day for 2 weeks, and 400 mg/d as the final dose) Group 2: no treatment Duration: 1 year | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Low risk | Quote: "[T]he researchers telephoned the corresponding provincial coordinator for treatment assignment. Only the provincial coordinator and the directors of the study knew about the randomisation procedures." |
| Blinding of participants and personnel (performance bias) | High risk | In this trial the participants included in the control arm didn't use placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is no mention in the report regarding the outcome assessment. |
| Incomplete outcome data (attrition bias) | High risk | 21 participants were lost of follow‐up (9 in amiodarone arm, 12 in non‐treatment arm). |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: PAtients who had been resuscitated from an episode of out‐of‐hospital VF without a privary reversible cause and in whom a myocardial infarction did not occur at the time of the episode of VF. Country: United States | |
| Participants | N = 228 (113 amiodarone, 115 conventional therapy) Sex: 89% male Age: mean 62 years Inclusion: resuscitated SCD (secondary prevention) | |
| Interventions | Group 1: amiodarone 1200 mg/d for 10 days, then 200‐800 mg/d for up to 2 months and finally 100‐400 mg/d Group 2: other antiarrhythmic drugs (conventional therapy) guided by EP testing Duration: 2 years The participants included in the 'conventional therapy' arm used procainamide, quinidine, disopyramide, tocainide, mexiletine, encainide, flecainide, propafenone, or combination therapy | |
| Outcomes | All‐cause mortality (primary outcome), cardiac death, SCD and ICD shocks | |
| Notes | 50% of participants had an ICD installed during the trial. The implantation rate increased from 11% in 1984 to 73% in 1990. We were not able to obtain the individual data for the participants with or without ICD. Many data were retrieved from the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[T]he study is not blinded with respect to the drug assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | There's no mention to the outcome assessment except for the cardiac mortality, which is stated to be "an end point difficult to misclassify and includes sudden arrhythmic cardiac deaths, documented resuscitated out‐of‐hospital VF, and nonarrhythmic cardiac death". |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Patients are analysed by intention to treat, remaining in their randomisation group even if they discontinue the drug or cross over to the alternate therapy." Quote: "No patients were lost to follow‐up, and only 8 patients in each group crossed over to the alternative therapy." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made |
| Selective reporting (reporting bias) | Low risk | According to the protocol, the published report includes all expected outcomes |
| Other bias | Unclear risk | We don't have the data regarding the outcomes divided by ICD status and pharmacological arm. The ICD status should be similar for both arms, but we don't know for sure. |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Patients with a history of severe heart failure stabilized on optimal medical therapy Country: Australia | |
| Participants | N = 34 (19 amiodarone, 15 placebo) Sex: data not available Age: mean 66‐70 years Inclusion: CHF with LVEF (threshold not stated in the report, only that they had to have a history of severe CHF; primary prevention) | |
| Interventions | Group 1: amiodarone 200 mg tid for 2 weeks and then 200 mg qd (final dose) Group 2: placebo Duration: 6 months | |
| Outcomes | All‐cause mortality, cardiac death, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[T]he double blind nature of the trial was maintained as far as possible." However, in 7 participants the 'blind' was lost. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There's no mention in the report regarding the outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | The authors have data for all of the participants at 6 months except for 3 of them (10%) |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | High risk | Due to the small number of participants, it is difficult to adequately consider other confounding factors |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with at least one documented episode of VF or VT which had been associated with a ventricular rate in excess of 130 beats per minute for at least 30 seconds with resultant syncope or the need for cardioversion Country: Australia and New Zealand | |
| Participants | N = 59 (30 amiodarone, 29 sotalol) Sex: 81% male Age: mean 59.8‐60.8 years Inclusion: resuscitated SCD (secondary prevention) | |
| Interventions | Group 1: amiodarone 1200 mg qd for 3 weeks, then 400 mg qd (final dose) Group 2: sotalol 160‐320 mg/d (640 mg/d final dose) Duration: 1 year | |
| Outcomes | Suppression of sustained ventricular tachycardia and prevention of SCD, clinically significant adverse events | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised using ". . . the next in a series of cards. . . to designate therapy with amiodarone or sotalol" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were stratified before entry. . .. This information was telephoned to the central registry." |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the sotalol arm used a different scheme than participants included in amiodarone arm. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The open design of this study was dictated by the properties of amiodarone. . . . its long half‐life made blind evaluation or a cross‐over design impractical." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All deaths and withdrawals were regarded as treatment failures and notified to the central registry." Quote: "When our results are considered by intention to treat there is no evidence that amiodarone is more effective." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | High risk | There were co‐interventions that could easily affect the study, as neither participants nor physicians were blinded. |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Patients with a recent AMI (24 hours from onset of pain) Country: Australia | |
| Participants | N = 200 (100 amiodarone, 100 placebo) Sex: 97% male Age: less than 70 years old (a exclusion criteria as being 70 years old or older) Inclusion: postacute MI (48 h after onset of chest pain; primary prevention) | |
| Interventions | Group 1: amiodarone 600 mg/d for 1 month, then 200 mg/d (final dose) Group 2: placebo Duration: 6‐42 months in the participants analysed for mortality | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Mortality data only available for 172 participants; there is no data about the final number of participants analysed in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffled envelopes |
| Allocation concealment (selection bias) | Unclear risk | It is not stated anywhere in the published report whether the envelopes were opaque or not. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Although the physicians concerned with routine care were aware of the nature of treatment, neither the subjects nor the investigators knew whether treatment was with active drug or placebo." |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated anywhere in the published report whether outcome assessors were blinded or not to the nature of the treatment |
| Incomplete outcome data (attrition bias) | High risk | 28 participants were not considered when calculating mortality rates in the report, with no explanation for it. |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | High risk | There were co‐interventions that could easily affect the study, as physicians were not blinded. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with documented myocardial infarction surviving 5 days and with EF less than 40% Country: 15 European countries | |
| Participants | N = 1486 (743 amiodarone, 743 placebo) Sex: 84‐85% male Age: mean 60 years Inclusion: LV dysfunction (LVEF < 40%) postacute MI (≥ 5 days) (primary prevention) | |
| Interventions | Group 1: amiodarone (800 mg for 14 days, 400 mg for 14 weeks, and then 200 mg until the end of the study follow‐up) Group 2: placebo Duration: 6 to 24 months (median follow‐up 21 months) | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Grant from Sanofi Recherche | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation was done in balanced blocks of four patients. . ." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was assigned under masked conditions by the EMIAT Coordinating Centre, and sent by fax to the investigators. The Coordinating Centre had no access to the treatment code." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: ". . . this randomised double‐blind placebo controlled trial. . ." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The Validation Committee reviewed deaths under masked conditions" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[A]ll patients were followed up and included in the intention‐to‐treat analysis" Quote: "Data on mortality was sought for all patients at the end of the planned follow‐up" |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Patients with documented spontaneous sustained ventricular tachyarrhythmia occurring late after myocardial infarction Country: Australia | |
| Participants | N = 45 (23 amiodarone, 22 sotalol) Sex: 83‐86% male Age:mean 58‐64 years Inclusion: Sustained Ventricular Tachyarrhythmia in participants with CHD | |
| Interventions | Group 1: amiodarone, loading dose of 800 mg orally per day for 1 week followed by a maintenance dose of 400 mg per day orally Group 2: sotalol at a dose of 160 mg bid orally Duration: 3 years follow‐up | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | The primary outcome variable was the time to first episode of spontaneous sustained ventricular tachyarrhythmia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the sotalol arm used a different scheme than participants included in the amiodarone arm. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is nothing stated in the published report about the outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The results were analysed using an intention‐to‐treat analysis." There were no loss to follow‐up during the study |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome was made |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | High risk | Due to the small number of participants, it is difficult to adequately consider other confounding factors |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients at high risk for a severe ventricular arrhythmia Country: 26 countries | |
| Participants | N = 486 (53 amiodarone, 324 celivarone (50 mg/d: 109 participants, 100 mg/d: 102 participants; 300 mg/d: 113 participants), 109 to placebo) Sex: 88,7% male Age: mean 64,4 years Inclusion: resuscitated SCD with ICD, or patients that had suffered VT or VF the previous month requiring ICD intervention (secondary prevention) | |
| Interventions | Group 1: amiodarone 600 mg/d for 1 week then 200 mg/d (final dose) Group 2: celivarone 50‐300 mg/d Group 3: placebo Duration: 9 months (median follow‐up) | |
| Outcomes | All‐cause mortality, SCD, and ICD shocks | |
| Notes | Quote: "The primary objective was to assess the efficacy of celivarone . . . versus placebo, with the use of amiodarone. . . as a calibrator, for the prevention of ICD interventions or sudden death." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralized randomisation list. . . was generated." |
| Allocation concealment (selection bias) | Low risk | Quote: "A centralized randomisation list was generated with an interactive voice response system or interactive Web response system." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients were randomised to receive double‐blind, once‐daily oral therapy for at least 6 months with celivarone . . . amiodarone. . . or placebo." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An independent steering committee of academic physicians and 1 industry representative was responsible for the design and conduct of the study, data analysis, central blinded adjudication of deaths, and reporting of the study." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The main efficacy population included all randomised patients (intention‐to‐treat population)." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | High risk | Mortality reporting was inconsistent |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Survivors of cardiac arrest secondary to documented ventricular arrhythmias Country: Germany | |
| Participants | N = 189 (92 amiodarone, 97 metoprolol) Sex: 79‐82% male Age: male 56‐59 years Inclusion: resuscitated from cardiac arrest secondary to documented sustained ventricular arrhythmias (secondary prevention) | |
| Interventions | Group 1: implantable cardioverter‐defibrillator (not included in analyses) Group 2: amiodarone, loading dose of 1000 mg/d for 7 days, followed by a maintenance dose of 200 to 600 mg/d) Group 3: metoprolol (initiated at a dose of 12.5 to 25.0 mg/d and increased within 7 to 14 days to a maximum of 200 mg/d, if tolerated) Duration: 24 months | |
| Outcomes | All‐cause mortality, SCD | |
| Notes | Only the comparison amiodarone vs metoprolol is included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the metoprolol arm used a different scheme than participants included in the amiodarone arm. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is no mention in the report about how the authors handled outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[Intention‐to‐treat analysis] was used with the patients grouped as randomised." |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with a previous (10 to 60 days) AMI, left ventricular disfunction and frequent Ventricular premature depolarizations Country: Spain | |
| Participants | N = 368 (115 amiodarone, 130 metoprolol, 123 no treatment) Sex: 87‐95% male Age: mean 57‐59 years Inclusion: LV dysfunction (LVEF < 45%) postacute MI (primary prevention) | |
| Interventions | Group 1: amiodarone (200 mg/d final dose) Group 2: metoprolol (50 to 100 mg bid final dose) Group 3: no treatment Duration: 3 years | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | The participants included in the metoprolol arm used a different scheme than participants included in the amiodarone arm. Furthermore, in the control arm the participants didn't use placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is no mention in the report to the way the authors handled outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[A]ll data were analysed according to the intention‐to‐treat approach." However, the distribution per group of lost participants (7) is unclear. |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Unclear risk | The study terminated its recruitment earlier than was stated in the protocol, but continued its follow‐up for a longer period. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with congestive heart failure and frequent asymptomatic ventricular ectopy Country: UK and United States | |
| Participants | N = 101 (49 amiodarone, 52 placebo) Sex: 83‐86% male Age: mean 56‐59 years Inclusion: CHF with LVEF ≤ 30% (primary prevention) | |
| Interventions | Group 1: amiodarone 400 mg/d for 4 weeks, then 200 mg/d (final dose) Group 2: placebo Duration: 1 year | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study medication was administered in a double‐blind fashion" |
| Blinding of outcome assessment (detection bias) | Unclear risk | There is nothing stated in the report regarding the outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "All data were analysed according to the intention‐to‐treat principle" 2 participants were lost (5% of sample size) |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Setting: Patients with heart failure and asymptomatic ventricular premature beats Country: United States | |
| Participants | N = 674 (336 participants were randomised to amiodarone, 338 to placebo) Sex: 99% male Age: mean 65‐66 years Inclusion: CHF with LVEF < 40% (primary prevention) | |
| Interventions | Group 1: amiodarone (800 mg/d for 2 weeks, 400 mg/d for 50 weeks, then 300 mg/d, final dose) Group 2: placebo Duration: 24 to 45 months | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was randomised, but the mechanism wasn't described in detail. |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but the allocation concealment mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Each patient was randomly assigned to receive amiodarone. . . or placebo throughout the trial." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Death and aborted cardiac arrests were reviewed in a blinded manner by a committee and classified as sudden or nonsudden deaths from cardiac causes or death from other causes." |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "All patients were followed until the completion of the study and were included in the statistical analysis according to the intention‐to‐treat principle." However, there were 78 participants lost to follow‐up, and it is not stated anywhere in the report how the authors addressed the issue |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Study characteristics | ||
| Methods | Single centre randomised controlled trial Setting: Patients with heart failure and with more than 7000 PVCs/24 h Country: Greece | |
| Participants | N = 20 (10 amiodarone + standard medical therapy, 10 standard medical therapy) Sex: 80‐100% male Age: mean 59‐62 years Inclusion: consecutive HF patients (LVEF ≤ 40%, mean LVEF: 31 ± 7%) with more than 7000 PVCs/24 h (primary prevention) | |
| Interventions | Group 1: amiodarone (200 mg/d) + standard medical therapy Group 2: standard medical therapy alone Duration: 6 months | |
| Outcomes | All‐cause mortality, cardiac mortality, SCD | |
| Notes | Presented as a poster in the 2014 Heart Failure Congress, we have unpublished data given to us by the author. At 6 months follow‐up, only 8 patients´s status was known in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from the author: "a total of 20 consecutive patients were randomised in a 1:1 treatment allocation" |
| Allocation concealment (selection bias) | Unclear risk | The trial was randomised, but allocation concealment wasn't described |
| Blinding of participants and personnel (performance bias) | High risk | Quote from the author: "Only the 24 h Holter overreading physician was blinded during the study." |
| Blinding of outcome assessment (detection bias) | High risk | From the author: The outcome assessor was not blind to the group which the patients were assigned to. |
| Incomplete outcome data (attrition bias) | High risk | There were 2 patients lost in the control group at the 6‐month follow‐up |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published report includes all expected outcomes |
| Other bias | High risk | Due to the small number of participants, it is difficult to adequately consider other confounding factors |
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: Patients with idiopathic dilated cardiomyopathy and heart failure Country: Germany | |
| Participants | N = 30 (15 amiodarone, 15 conventional antiarrhythmic or no therapy) Sex: 83% male Age: 52 years mean Inclusion: CHF with LVEF < 45% (idiopathic dilated cardiomyopathy; primary prevention) | |
| Interventions | Group 1: amiodarone 800 mg/d for 10 days then 200 mg/d (final dose) Group 2: conventional antiarrhythmic or no therapy Duration: 2 years | |
| Outcomes | Electrocardiographic alterations, all‐cause mortality, cardiac mortality, SCD | |
| Notes | Only published data, original paper in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The randomisation was incomplete: 4 participants were documented to have spontaneous continuous ventricular tachycardia or ventricular fibrillation and were therefore excluded from the randomisation. They started with amiodarone immediately. |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment mechanism wasn't described in detail. |
| Blinding of participants and personnel (performance bias) | High risk | In the control arm the participants used conventional therapy or no therapy. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated anywhere in the published report whether the outcome assessment was blinded or not. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reporting of these data is confusing. Furthermore, 2 participants withdrew from the study. It is not stated whether the authors included them in the analysis (apparently they did) |
| Incomplete outcome data (attrition bias) | Unclear risk | No description regarding any aspect of any subjective outcome |
| Selective reporting (reporting bias) | Unclear risk | There is not much clarity about this item with the information we possess |
| Other bias | High risk | Due to the small number of participants, it is difficult to adequately consider other confounding factors |
AMI: acute myocardial infarction; BB: beta blockers; bid: bis in die, or twice a day CHD: coronary heart disease; CHF: coronary heart failure; EP: electrophysiological; HF: heart failure; ICD: implantable cardiac defibrillators; LEM: Long‐term electrocardiographs monitoring ; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PVC: premature ventricular contraction; qd: quaque die, or once a day; SCD: sudden cardiac death; tid: ter in die, or three times a day; VF: ventricular fibrillation; VT: ventricular tachycardia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study designed not for secondary prevention of SCD, but for the acute management of refractory ventricular arrhythmia | |
| Amiodarone was not randomised | |
| Not an RCT | |
| Not an RCT | |
| Not a clinical study | |
| Not a clinical study | |
| Not a RCT | |
| Treatment for less than 6 months | |
| Treatment for less than 6 months | |
| Not a clinical study | |
| Not a clinical study | |
| Lower dose of amiodarone (100 mg/day) | |
| Amiodarone used in combination with other antiarrhythmic drugs in a sequential manner | |
| Not an RCT | |
| Not a clinical study | |
| It didn't evaluate relevant outcomes | |
| Main intervention was perindopril, only sometimes combined with amiodarone | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Amiodarone used in combination with other antiarrhythmic drugs in a sequential manner | |
| Not an RCT | |
| Not an RCT | |
| Treatment for less than 6 months | |
| Not a clinical study | |
| Study carried out on dogs | |
| Not an RCT | |
| Not an RCT | |
| Not a RCT | |
| Not a clinical study | |
| Not a RCT | |
| Comparison was between Carvedilol and Metoprolol (COMET study), this was a post hoc analysis of the use of amiodarone in the study. | |
| Lower dose of amiodarone (100 mg/day) | |
| Relevant outcomes were not described, we were not able to contact the author |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Study name | Nippon ICD Plus Pharmacologic Option Necessity (NIPPON Study) |
| Methods | Multicentre, randomised controlled trial |
| Participants | 440 patients, all with spontaneous episodes of sustained VT or VF; all patients will have organic heart disease as documented either by electrocardiography, echocardiography, cardiac catherterisation, nuclear scintigraphy, computed tomography or magnetic resonance imaging; |
| Interventions | Patients will be randomly assigned to one of 2 groups; amiodarone group and non‐amiodarone group. An ICD (basically, dual chamber types) will be implanted in every patient as soon as possible after randomisation. Amiodarone loading dose of 400 mg/day, and two weeks after the loading dose period, amiodarone will be reduced to a maintenance dose of 200 mg/day |
| Outcomes | Listed as secondary end points: total death; arrhythmic death; cardiac death; impairment of patient's quality of life; occurrence of side‐effects from amiodarone |
| Starting date | Paper published in March 2006 |
| Contact information | Takashi Kurita, MD, Department of Cardiovascular Medicine, National Cardiovascular Center, 5‐7‐1 Fujishirodai, Suita 565‐8565, Japan. E‐mail: [email protected] |
| Notes | There have been no published results from this study, although the design was published in 2006. |
ICD: implantable cardiac defibrillators.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Sudden cardiac death Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.66, 0.88] |
| Analysis 1.1  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 1: Sudden cardiac death | ||||
| 1.2 Cardiac mortality Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| Analysis 1.2  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 2: Cardiac mortality | ||||
| 1.3 All‐cause mortality Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.00] |
| Analysis 1.3  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 3: All‐cause mortality | ||||
| 1.4 Sudden cardiac death subgroup post‐ AMI patients Show forest plot | 6 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.91] |
| Analysis 1.4  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 4: Sudden cardiac death subgroup post‐ AMI patients | ||||
| 1.5 Sudden cardiac death subgroup heart failure Show forest plot | 11 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.93] |
| Analysis 1.5  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 5: Sudden cardiac death subgroup heart failure | ||||
| 1.6 All‐cause mortality subgroup post‐AMI Show forest plot | 6 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| Analysis 1.6  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 6: All‐cause mortality subgroup post‐AMI | ||||
| 1.7 All‐cause mortality subgroup heart failure Show forest plot | 11 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| Analysis 1.7  Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 7: All‐cause mortality subgroup heart failure | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Sudden cardiac death Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.00] |
| Analysis 2.1  Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 1: Sudden cardiac death | ||||
| 2.2 Cardiac mortality Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.86] |
| Analysis 2.2  Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 2: Cardiac mortality | ||||
| 2.3 All‐cause mortality Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.76] |
| Analysis 2.3  Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 3: All‐cause mortality | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Sudden cardiac death Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.22] |
| Analysis 3.1  Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 1: Sudden cardiac death | ||||
| 3.2 Cardiac mortality Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.84] |
| Analysis 3.2  Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 2: Cardiac mortality | ||||
| 3.3 All‐cause mortality Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.75] |
| Analysis 3.3  Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 3: All‐cause mortality | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Sudden cardiac death Show forest plot | 2 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 4.32 [0.87, 21.49] |
| Analysis 4.1  Comparison 4: Amiodarone versus placebo or no treatment for secondary prevention, Outcome 1: Sudden cardiac death | ||||
| 4.2 All‐cause mortality Show forest plot | 2 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.33, 7.01] |
| Analysis 4.2  Comparison 4: Amiodarone versus placebo or no treatment for secondary prevention, Outcome 2: All‐cause mortality | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Sudden cardiac death Show forest plot | 4 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.56, 3.52] |
| Analysis 5.1  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 1: Sudden cardiac death | ||||
| 5.2 Cardiac mortality Show forest plot | 2 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| Analysis 5.2  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 2: Cardiac mortality | ||||
| 5.3 All‐cause mortality Show forest plot | 5 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.42] |
| Analysis 5.3  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 3: All‐cause mortality | ||||
| 5.4 Sudden cardiac death subgroup with ICD Show forest plot | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 24.45 [2.79, 214.59] |
| Analysis 5.4  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 4: Sudden cardiac death subgroup with ICD | ||||
| 5.5 Sudden cardiac death subgroup without ICD Show forest plot | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.05] |
| Analysis 5.5  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 5: Sudden cardiac death subgroup without ICD | ||||
| 5.6 All‐cause mortality subgroup with ICD Show forest plot | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.98, 3.93] |
| Analysis 5.6  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 6: All‐cause mortality subgroup with ICD | ||||
| 5.7 All‐cause mortality subgroup without ICD Show forest plot | 3 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| Analysis 5.7  Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 7: All‐cause mortality subgroup without ICD | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Sudden cardiac death Show forest plot | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.27] |
| Analysis 6.1  Comparison 6: Amiodarone versus beta‐blockers for secondary prevention, Outcome 1: Sudden cardiac death | ||||
| 6.2 All‐cause mortality Show forest plot | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| Analysis 6.2  Comparison 6: Amiodarone versus beta‐blockers for secondary prevention, Outcome 2: All‐cause mortality | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Sudden cardiac death Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.32, 25.55] |
| Analysis 7.1  Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 1: Sudden cardiac death | ||||
| 7.2 Cardiac mortality Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.26, 7.78] |
| Analysis 7.2  Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 2: Cardiac mortality | ||||
| 7.3 All‐cause mortality Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.83] |
| Analysis 7.3  Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 3: All‐cause mortality | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Quality of life (DASI at 30 months) Show forest plot | 1 | 1160 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.56, 2.96] |
| Analysis 8.1  Comparison 8: Amiodarone and quality of life, Outcome 1: Quality of life (DASI at 30 months) | ||||
| 8.2 Quality of life (MHI‐5 at 30 months) Show forest plot | 1 | 1124 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐0.26, 4.66] |
| Analysis 8.2  Comparison 8: Amiodarone and quality of life, Outcome 2: Quality of life (MHI‐5 at 30 months) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Hyperthyroidism Show forest plot | 8 | 5972 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [1.54, 11.17] |
| Analysis 9.1  Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 1: Hyperthyroidism | ||||
| 9.2 Hypothyroidism Show forest plot | 8 | 4008 | Risk Ratio (M‐H, Random, 95% CI) | 6.13 [2.46, 15.28] |
| Analysis 9.2  Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 2: Hypothyroidism | ||||
| 9.3 Pulmonary Show forest plot | 12 | 5924 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.40] |
| Analysis 9.3  Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 3: Pulmonary | ||||
| 9.4 Discontinuation Show forest plot | 13 | 7616 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.26, 1.67] |
| Analysis 9.4  Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 4: Discontinuation | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Hyperthyroidism Show forest plot | 3 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 7.43 [1.33, 41.57] |
| Analysis 10.1  Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 1: Hyperthyroidism | ||||
| 10.2 Hypothyroidism Show forest plot | 4 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 7.77 [1.85, 32.68] |
| Analysis 10.2  Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 2: Hypothyroidism | ||||
| 10.3 Pulmonary Show forest plot | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.36, 14.67] |
| Analysis 10.3  Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 3: Pulmonary | ||||
| 10.4 Discontinuation Show forest plot | 8 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.33] |
| Analysis 10.4  Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 4: Discontinuation | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Hyperthyroidism Show forest plot | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 4.97 [0.60, 41.16] |
| Analysis 11.1  Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 1: Hyperthyroidism | ||||
| 11.2 Hypothyroidism Show forest plot | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 12.82 [0.73, 225.33] |
| Analysis 11.2  Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 2: Hypothyroidism | ||||
| 11.3 Pulmonary Show forest plot | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 14.79 [0.85, 256.43] |
| Analysis 11.3  Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 3: Pulmonary | ||||
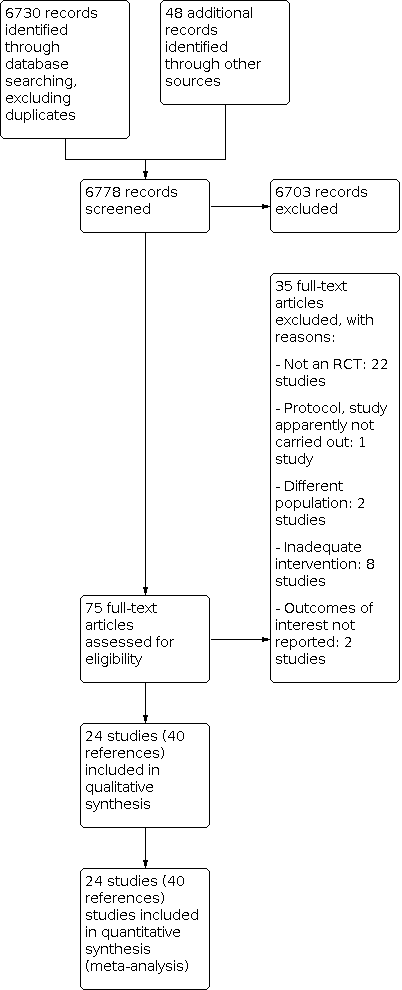
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Amiodarone versus placebo or no treatment for primary prevention, outcome: 1.1 Sudden cardiac death.

Funnel plot of comparison: 1 Amiodarone versus placebo or no treatment for primary prevention, outcome: 1.2 Cardiac mortality.

Funnel plot of comparison: 1 Amiodarone versus placebo or no treatment for primary prevention, outcome: 1.3 All‐cause mortality.

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 1: Sudden cardiac death

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 2: Cardiac mortality

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 3: All‐cause mortality

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 4: Sudden cardiac death subgroup post‐ AMI patients

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 5: Sudden cardiac death subgroup heart failure

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 6: All‐cause mortality subgroup post‐AMI

Comparison 1: Amiodarone versus placebo or no treatment for primary prevention, Outcome 7: All‐cause mortality subgroup heart failure

Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 1: Sudden cardiac death

Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 2: Cardiac mortality

Comparison 2: Amiodarone versus other antiarrhythmics for primary prevention, Outcome 3: All‐cause mortality

Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 1: Sudden cardiac death

Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 2: Cardiac mortality

Comparison 3: Amiodarone versus beta‐blockers for primary prevention, Outcome 3: All‐cause mortality

Comparison 4: Amiodarone versus placebo or no treatment for secondary prevention, Outcome 1: Sudden cardiac death

Comparison 4: Amiodarone versus placebo or no treatment for secondary prevention, Outcome 2: All‐cause mortality

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 1: Sudden cardiac death

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 2: Cardiac mortality

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 3: All‐cause mortality

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 4: Sudden cardiac death subgroup with ICD

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 5: Sudden cardiac death subgroup without ICD

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 6: All‐cause mortality subgroup with ICD

Comparison 5: Amiodarone versus other antiarrhythmics for secondary prevention, Outcome 7: All‐cause mortality subgroup without ICD

Comparison 6: Amiodarone versus beta‐blockers for secondary prevention, Outcome 1: Sudden cardiac death

Comparison 6: Amiodarone versus beta‐blockers for secondary prevention, Outcome 2: All‐cause mortality

Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 1: Sudden cardiac death

Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 2: Cardiac mortality

Comparison 7: Amiodarone versus sotalol for secondary prevention, Outcome 3: All‐cause mortality

Comparison 8: Amiodarone and quality of life, Outcome 1: Quality of life (DASI at 30 months)

Comparison 8: Amiodarone and quality of life, Outcome 2: Quality of life (MHI‐5 at 30 months)

Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 1: Hyperthyroidism

Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 2: Hypothyroidism

Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 3: Pulmonary

Comparison 9: Amiodarone versus placebo (adverse effects), Outcome 4: Discontinuation

Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 1: Hyperthyroidism

Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 2: Hypothyroidism

Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 3: Pulmonary

Comparison 10: Amiodarone versus other antiarrhythmics (adverse effects), Outcome 4: Discontinuation

Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 1: Hyperthyroidism

Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 2: Hypothyroidism

Comparison 11: Amiodarone versus no treatment (adverse effects), Outcome 3: Pulmonary
| Amiodarone versus placebo or no treatment for primary prevention | |||||
| Patient or population: participants with high risk of sudden cardiac death (primary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo or no treatment | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 0.76 | 8383 | ⊕⊕⊝⊝ | |
| 91 per 1000 | 70 per 1000 | ||||
| Moderate | |||||
| 114 per 1000 | 87 per 1000 | ||||
| All‐cause mortality | Study population | RR 0.88 | 8383 | ⊕⊕⊝⊝ | |
| 203 per 1000 | 178 per 1000 | ||||
| Moderate | |||||
| 190 per 1000 | 167 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aRandomisation and allocation concealment methods not clear or not adequate in 10/16 studies, including studies with more weight. | |||||
| Amiodarone versus beta blockers | |||||
| Patient or population: beta blockers | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 0.37 | 342 | ⊕⊕⊝⊝ | |
| 56 per 1000 | 21 per 1000 | ||||
| Moderate | |||||
| 45 per 1000 | 17 per 1000 | ||||
| All‐cause mortality | Study population | RR 0.27 | 342 | ⊕⊕⊝⊝ | |
| 101 per 1000 | 27 per 1000 | ||||
| Moderate | |||||
| 76 per 1000 | 21 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aBoth studies had serious limitations, including lack of blinding for participants and unclear generation of random sequence and allocation concealment. | |||||
| Amiodarone versus other antiarrhythmics for high risk of sudden cardiac death (primary prevention) | |||||
| Patient or population: participants with high risk of sudden cardiac death (primary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Other antiarrhythmics | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 0.44 | 540 | ⊕⊕⊕⊝ | |
| 65 per 1000 | 28 per 1000 | ||||
| All‐cause mortality | Study population | RR 0.37 | 540 | ⊕⊕⊕⊝ | |
| 100 per 1000 | 37 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aAll studies had serious limitations, including lack of blinding for participants and unclear allocation concealment. | |||||
| Amiodarone compared to placebo or no treatment for high risk of sudden cardiac death (secondary prevention) | |||||
| Patient or population: participants with high risk of sudden cardiac death (secondary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo or no treatment | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 4.32 | 440 | ⊕⊝⊝⊝ | |
| 8 per 1000 | 35 per 1000 | ||||
| All‐cause mortality | Study population | RR 3.05 | 440 | ⊕⊝⊝⊝ | |
| 32 per 1000 | 99 per 1000 | ||||
| Moderate | |||||
| 35 per 1000 | 107 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aVery serious imprecision: quality of the evidence was downgraded two levels because the CI was very wide and includes both important risks and benefits, and because there was a very low number of events. | |||||
| Amiodarone versus other antiarrhythmics for high risk of sudden cardiac death (secondary prevention) | |||||
| Patient or population: participants with high risk of sudden cardiac death (secondary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Other antiarrhythmics | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 1.40 | 839 | ⊕⊝⊝⊝ | |
| 99 per 1000 | 138 per 1000 | ||||
| All‐cause mortality | Study population | RR 1.03 | 898 | ⊕⊕⊝⊝ | |
| 193 per 1000 | 198 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aAll studies had serious limitations, including 4/5 not blinded for participants. | |||||
| Amiodarone compared to beta blockers for high risk of sudden cardiac death (secondary prevention) | |||||
| Patient or population: participants with high risk of sudden cardiac death (secondary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Beta blockers | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 0.84 | 189 | ⊕⊝⊝⊝ | |
| 351 per 1000 | 294 per 1000 | ||||
| All‐cause mortality | Study population | RR 0.96 | 189 | ⊕⊝⊝⊝ | |
| 454 per 1000 | 435 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aThe only study has serious limitations, including lack of blinding for participants. | |||||
| Amiodarone versus sotalol for high risk of sudden cardiac death (secondary prevention) | |||||
| Patient or population: participants with high risk of sudden cardiac death (secondary prevention) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Sotalol | Amiodarone | ||||
| Sudden cardiac death | Study population | RR 2.87 | 45 | ⊕⊝⊝⊝ | |
| 45 per 1000 | 130 per 1000 | ||||
| All‐cause mortality | Study population | RR 1.08 | 104 | ⊕⊝⊝⊝ | |
| 137 per 1000 | 148 per 1000 | ||||
| Moderate | |||||
| 132 per 1000 | 143 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aThe only study has serious limitations, including lack of blinding for participants. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Sudden cardiac death Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.66, 0.88] |
| 1.2 Cardiac mortality Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 1.3 All‐cause mortality Show forest plot | 17 | 8383 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.00] |
| 1.4 Sudden cardiac death subgroup post‐ AMI patients Show forest plot | 6 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.91] |
| 1.5 Sudden cardiac death subgroup heart failure Show forest plot | 11 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.93] |
| 1.6 All‐cause mortality subgroup post‐AMI Show forest plot | 6 | 3377 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 1.7 All‐cause mortality subgroup heart failure Show forest plot | 11 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Sudden cardiac death Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.00] |
| 2.2 Cardiac mortality Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.20, 0.86] |
| 2.3 All‐cause mortality Show forest plot | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Sudden cardiac death Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.22] |
| 3.2 Cardiac mortality Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.84] |
| 3.3 All‐cause mortality Show forest plot | 2 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Sudden cardiac death Show forest plot | 2 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 4.32 [0.87, 21.49] |
| 4.2 All‐cause mortality Show forest plot | 2 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.33, 7.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Sudden cardiac death Show forest plot | 4 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.56, 3.52] |
| 5.2 Cardiac mortality Show forest plot | 2 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| 5.3 All‐cause mortality Show forest plot | 5 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.42] |
| 5.4 Sudden cardiac death subgroup with ICD Show forest plot | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 24.45 [2.79, 214.59] |
| 5.5 Sudden cardiac death subgroup without ICD Show forest plot | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.05] |
| 5.6 All‐cause mortality subgroup with ICD Show forest plot | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.98, 3.93] |
| 5.7 All‐cause mortality subgroup without ICD Show forest plot | 3 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Sudden cardiac death Show forest plot | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.27] |
| 6.2 All‐cause mortality Show forest plot | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Sudden cardiac death Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.32, 25.55] |
| 7.2 Cardiac mortality Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.26, 7.78] |
| 7.3 All‐cause mortality Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Quality of life (DASI at 30 months) Show forest plot | 1 | 1160 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.56, 2.96] |
| 8.2 Quality of life (MHI‐5 at 30 months) Show forest plot | 1 | 1124 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐0.26, 4.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Hyperthyroidism Show forest plot | 8 | 5972 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [1.54, 11.17] |
| 9.2 Hypothyroidism Show forest plot | 8 | 4008 | Risk Ratio (M‐H, Random, 95% CI) | 6.13 [2.46, 15.28] |
| 9.3 Pulmonary Show forest plot | 12 | 5924 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.40] |
| 9.4 Discontinuation Show forest plot | 13 | 7616 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.26, 1.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Hyperthyroidism Show forest plot | 3 | 514 | Risk Ratio (M‐H, Random, 95% CI) | 7.43 [1.33, 41.57] |
| 10.2 Hypothyroidism Show forest plot | 4 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 7.77 [1.85, 32.68] |
| 10.3 Pulmonary Show forest plot | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.36, 14.67] |
| 10.4 Discontinuation Show forest plot | 8 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Hyperthyroidism Show forest plot | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 4.97 [0.60, 41.16] |
| 11.2 Hypothyroidism Show forest plot | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 12.82 [0.73, 225.33] |
| 11.3 Pulmonary Show forest plot | 2 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 14.79 [0.85, 256.43] |

