Poissons larvivores pour prévenir la transmission du paludisme
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: quasi‐RCT Study location: Assab Sekir and Negado Sefer, Assab, Ethiopia Study dates: February 1987 to January 1988 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis Larval sites: domestic water containers Baseline data: February 1987 | |
| Participants | NA | |
| Interventions | Fish species: Aphanius dispar Indigenous fish species used: yes Fish source: Gibdo River, 26 km from Assab Populated sites: domestic water containers and wells; 68 stocked (32 barrels, 11 cisterns, 24 wells, 1 washbasin), 60 unstocked (33 barrels, 10 cisterns, 16 wells, 1 washbasin) Restocked: yes, as necessary during surveys that were performed either monthly or every two weeks Co‐interventions: none | |
| Outcomes | Percentage of larval sites positive for anopheline larvae Method: standard dipping procedure; 5 dips/barrel, 12 dips/cistern, 8 dips/washbasin, 3 dips buckets/well during surveys that were performed either monthly or every two weeks | |
| Source of funding | UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases; National Organisation for the Control of Malaria and Other Vectorborne Diseases, Ministry of Health, Ethiopia | |
| Notes | No environmental data collected Acceptability of fish to householders assessed by questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Quasi‐RCT: "In every other house or mosque, fish were stocked in all wells and water storage containers." |
| Site selection | Unclear risk | "A total of 54 households were selected by systematic sampling. All six mosques were also included in the study. Seven households were excluded because they had only jerrycans and buckets for water storage. They were replaced by seven other households selected by lottery system." |
| Site allocation | High risk | "In every other house or mosque, fish were stocked in all wells and water storage containers." |
| Blinding of outcome assessment (detection bias) | High risk | "During monthly or biweekly larval surveys the fish were counted and restocking was carried out as necessary to maintain the original number of fish." |
| Baseline values | Low risk | In both control and Intervention groups at prestocking (February 1987), the proportion of sites with Anopheles larvae was 0%. |
| Number of sites | Low risk | Number of sites adequate as > 20 sites per group. |
| Methods | Study design: controlled time series Study location: 2 villages, Pithai (intervention) and Anara (control), in Kheda district, Gujarat, India Study dates: December 2010 to November 2011 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. stephensi Larval sites: domestic water containers Baseline data: July 2010. More than 100 houses in each village were checked. | |
| Participants | NA | |
| Interventions | Fish species: Aphanius dispar (Rüppell) Indigenous fish species used: yes Fish source: collected from a natural habitat in a salt factory in the town of Cambay (Khambhat), Gujarat Populated sites: 295 water storage containers, such as cement tanks including underground tanks (127), kothi (big mud pots), and barrels (167), in Pithai village. 30 containers in Pithai (intervention) and 25 in Anara (control) village monitored. Only cement tanks were included in longitudinal monitoring because of declining fish populations in other containers due to frequent replenishment Restocked: no. Fish were released once during the 1‐year study period, with 10 to 25 fish/tank or per container, depending on container size. Co‐interventions: "routine intervention" | |
| Outcomes | Density of immature An. stephensi stages (larvae instars I and II; III, IV and pupae) at weekly intervals for 4 weeks, then every 2 weeks. Only total % reduction in III/IV instar and pupae shown. Method: standard larval dipper method using the mean of 3 dips. Reduction in III and IV instar larvae and pupae was calculated as per the formula: % reduction = 100 ‐ [(C1 × T2)/C2 × T1)] × 100 where: C1 = pre‐release larval density in control tanks; C2 = post‐release larval density in control tanks; T1 = pre‐release larval density in fish tanks; and T2 = post‐release larval density in fish tanks. | |
| Source of funding | Sardar Sarowar Narmadad Nigam Limited (SSNL), Gujarat | |
| Notes | Correspondence with study author: "The same person/team counting the larval density were counting the fish density in tanks. The arbitrary presence of fishes was recorded in each tank and with the help of torch in under ground tanks". The study author was unable to provide raw data on number of fish or immature Anopheles. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study |
| Site selection | Low risk | The trial authors selected 2 villages, Anara and Pithai, from 15 villages surveyed in Kheda district due to "the similar conditions in respect of type of domestic tanks, water supply and water storage practices." "Randomly, one of the Village Pithai was selected for introduction of Aphanius fish in all the tanks and water containers." |
| Site allocation | Unclear risk | The study introduced fish to 295 water storage containers, such as cement tanks including underground tanks (127), kothi (big mud pots), and barrels (167), in Pithai village. "The survival of the fish and mosquito larval was monitored in 30 containers in the experimental village and 25 in the control village." However, it is unclear how the trial selected which containers to monitor. |
| Blinding of outcome assessment (detection bias) | High risk | The assessors were not blinded to treatment. "The survival of the fish and mosquito breeding was monitored...presence of fish was monitored with the help on a bright light torch." The study author stated via email that: "The same person/team counting the larval density were counting the fish density in tanks. The arbitrary presence of fishes was recorded in each tank and with the help of torch in under ground tanks. It was observed that 2‐3 fishes were able to control the larval breeding may be because of the absence of alternate food in the domestic tanks filled with tap water." |
| Baseline values | Unclear risk | Baseline values for houses positive for mosquito larvae were comparable, but a higher number of containers were positive for mosquito in Anara (control) than in Pithai (Intervention) during baseline monitoring in July 2010 (container index 83.2 (Anara) versus 47.84 (Pithai)). It is unclear how comparable baseline values were before introduction of fish in the 2 villages in November/December 2010. We were unable to obtain further data from the corresponding study author due to "transfer from Nadiad to New Delhi HQ in 2012." |
| Number of sites | Low risk | Adequate numbers of sites in control and Intervention groups. |
| Methods | Study design: controlled interrupted time series Study location: Kisii Central District, Western Kenya Study dates: October 2003 to October 2004 Transmission intensity: endemic but highly seasonal Malaria parasite species: not specified Primary vectors: An. gambiae s. l.,An. funestus Giles Larval sites: abandoned fishponds Baseline data: October 2003 to January 2004 | |
| Participants | NA | |
| Interventions | Fish species: Oreochromis niloticus L. Indigenous fish species used: yes Fish source: local FD hatchery in Kisii town Populated sites: 3 abandoned fishponds, Pond A (104 m²), Pond C (128 m²), and Pond D (72 m²); 150 m distance from each other Restocked: no Co‐interventions: none | |
| Outcomes | Number of immature Anopheles per pond Density of immature Anopheles per pond Method: 5 larval dips (2.5 L total volume) randomly from edges of each pond, at least 1 dip/side, 5 to 7 days/week | |
| Source of funding | Government of Finland and BioVision | |
| Notes | Climatic data for study period obtained from Kenya Agricultural Research Institute. Study started with Pond B included, but as it was destroyed during the study period, the authors were unable to collect data for it for the requisite time period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Low risk | "The site has three abandoned fishponds within 150 m of each other." Author communication: "We started with a Pond B but it got destroyed during the study period so we were unable to collect data for it for the requisite time." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Low risk | Numbers of An. gambiae s. l. and An. funestus immatures comparable in Ponds A, C, and D. |
| Number of sites | High risk | Probably inadequate as < 5 sites per group; control = 1 site, intervention = 2 sites. |
| Methods | Study design: controlled time series Study location: Nyalenda, Kisumu County, Kenya Study dates: February 2008 to May 2008 Transmission intensity: not stated Malaria parasite species: not specified Primary vectors: An. gambiae Giles Larval sites: man‐made habitats (ponds or water canals) Baseline data: not recorded | |
| Participants | NA | |
| Interventions | Fish species: G. affinis Indigenous fish species used: no Fish source: colony at Kenya Medical Research Institute (KEMRI) established from a wild‐caught population provided by Kenya Marine and Fisheries Research Institute (KEMFRI). Populated sites: man‐made habitats; 30 pools (mean 1 m × 1 m × 1 m deep) or water canals (15 m × 1 m × 0.3 m deep). Pond sites and water canal sites were constructed by people for the purposes of this experiment, so can be defined as "semi‐field" studies. Restocked: no (treatment arm: ponds fish once), every 2 weeks (treatment arms: pond fish only or water canal fish only) Co‐interventions: Bacillus thuringiensis var. israelensis | |
| Outcomes | Density of early instars (L1 and L2) or late instars (L3 and L4) of anopheline mosquitoes Method: standard larval dipping procedure using 350 mL mosquito dipper (Bioquip, Gardena, CA, USA), maximum of 10 dips/habitat, estimated weekly | |
| Source of funding | The Dioraphte Foundation, The Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study. |
| Site selection | Low risk | "Thirty man‐made habitats (1 m × 1 m × 1 m) were created as mosquito larval habitats." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. In water canals: "Six treatments were randomly administered in canal habitats." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Unclear risk | Not reported. |
| Number of sites | High risk | Number of sites may be inadequate: 5 sites per group. |
| Methods | Study design: controlled interrupted time series Study location: Banwol, Suwon City, Gyeonggi Province, Korea Study dates: June to October 1989 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. sinensis Larval sites: rice fields Baseline data: none | |
| Participants | NA | |
| Interventions | Fish species: T. m. niloticus (herbivorous) with either A. latipes or Aphyocypris chinensis Indigenous fish species used: yes, except for T. m. niloticus Fish source: A. latipes: not stated; A. chinensis: holding ponds at Ansan rice fields, 2.5 km north; T. m. niloticus: fish farm at Gwagiu, Gyeonggi Populated sites: 6 rice fields (3 control sites, 3 intervention sites 500 m², 300 m², or 600 m² in size) Restocked: no Co‐interventions: none | |
| Outcomes | Mean number and percentage of reduction An. sinensis Method: larval dips using 500 mL dipper, 2 to 4 replicates per rice field | |
| Source of funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Unclear risk | "A confined field plot of ca. 20,000 m² rice field located in Banwol near Suwon City, Gyeonggi Province...three of the six paddies were taken." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Low risk | Mean number of An. sinensis larvae comparable at Intervention and control sites. |
| Number of sites | High risk | Probably inadequate number of sites. |
| Methods | Study design: controlled before‐and‐after study Study location: Kotmale oya, below Kotmale dam, Sri Lanka Study dates: May to August 2000 Transmission intensity: epidemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis (national importance), An. annularis, An. subpictus, An. tessellatus (local importance) Larval sites: pools formed in riverbed between dam and power plant Baseline data: 1 day before stocking | |
| Participants | NA | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: no Fish source: riverbed pools below the Kotmale dam and then reared in stock tanks at Regional Office Anti‐Malaria Campaign, Kandy Populated sites: 60 riverbed pools, 0.25 to 1.0 m² surface area and < 1 m depth (29 intervention, 31 control, randomly selected) Restocked: no Co‐interventions: none | |
| Outcomes | Number (percentage) of pools positive for anopheline larvae Mean number of larvae per pool Mean number of larvae per 100 dips Method: larval dipping using 100 mL dipper, 6 dips per m². Authors collected anopheline immatures but reported larval numbers only | |
| Source of funding | National Research Council, Sri Lanka (NRC Grant No. 99/09) | |
| Notes | Fish number monitored An. culicifacies not identified at any sites | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled before‐and‐after study. |
| Site selection | Unclear risk | "Sixty isolated riverbed pools...were selected and labeled." |
| Site allocation | Unclear risk | "P. reticulata was stocked in 29 randomly selected pools". Method of randomization not described. |
| Blinding of outcome assessment (detection bias) | High risk | "Visual counts of P. reticulata were made in each pool, monthly. Visual counts were possible, as the pools were small (not exceeding 1 m² surface area), shallow (< 1 m depth) and contained clean water." |
| Baseline values | Low risk | Comparable between control and intervention sites. |
| Number of sites | Low risk | Adequate numbers of sites in control (31 site) and intervention groups (29 sites). |
| Methods | Study design: controlled before‐and‐after study Study location: riverbeds below Laxapana, Kotmale 1, Kotmale 2, Nilambe, Rantembe, and Victoria dams, Sri Lanka Study dates: September 2000 to August 2002 Transmission intensity: epidemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis (national importance), An. annularis, An. subpictus, and An. tessellatus (local importance) Larval sites: pools formed in riverbed between dam and power plant Baseline data: September 2000 to August 2001 | |
| Participants | NA | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: no Fish source: not stated Populated sites: pools of 6 riverbeds below dams (2 controls, 2 fish intervention) Restocked: yes, pools that had no fish were restocked at the same rate during fortnightly larval surveys Co‐intervention: temephos treatment of all pools in 2 riverbeds | |
| Outcomes | Mean percentage of pools positive for anopheline larvae Mean number of anopheline larvae per 100 pools Mean number of anopheline larvae per 100 dips Total number of anopheline larvae Methods: larval dips, 6 dips per m² surface area of water | |
| Source of funding | National Research Council of Sri Lanka (Grant No. 99/09) | |
| Notes | Cost analysis estimation and simulations performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled before‐and‐after study. |
| Site selection | Low risk | "Six study sites, namely Laxapana, Kotmale 1, Kotmale 2, Nilambe, Rantembe and Victoria...were selected based on the occurrence of malaria outbreaks since 1985...all the pools in the riverbeds were stocked." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. "Subsequently the pools that had no fish were restocked at the same rate." |
| Baseline values | High risk | Baseline values higher in intervention group than in control group. |
| Number of sites | High risk | Probably inadequate: number of pools not specified. |
| Methods | Study design: controlled time series Study location: Gezira irrigated area, Sudan Study dates: January to December, but the years were not specified Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. arabiensis Larval sites: small temporary pools Baseline data: none | |
| Participants | NA | |
| Interventions | Fish species: G. holbrooki (note: this study refers to G. affinis holbrooki, as these fish were then considered a subspecies of G. affinis. This subspecies is now recognized as a full species) Indigenous fish species used: no Fish source: rearing ponds at Wad Medani, 20 to 25 km from trial sites Populated sites: 20 irrigation canals, 1 m in depth, 2 m in width, and 4 to 10 km in length; 5 control canals Restocked: yes Co‐intervention: none | |
| Outcomes | Mean larval density of An. arabiensis/100 dips, according to instar stage Methods: larval dipping at 2 sites per km in each canal, 10 dips per site | |
| Source of funding | Malaria Control Project, Ministry of Health, Sudan | |
| Notes | Flow of water from large branch canals was controlled by gates opened at certain times; this system deprived the Gambusia of free movement into the smaller canals, which usually are richer in mosquito larvae than the larger ones, where the fish had originally been stocked. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study. |
| Site selection | Unclear risk | "Medium size irrigation canals of about 1 m depth, 2 m width, and 4‐10 km length, officially classified as minor canals, were selected as sites for the trials. Twenty such canals were seeded with Gambusia...while five others were used as control." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Unclear risk | Not reported. Fish release in October and measurements not taken until following January. |
| Number of sites | High risk | May be inadequate, as only 5 sites in the control group. |
| Methods | Study design: controlled interrupted time series study Study location: Pondicherry Town, India Study dates: January to May 1977 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. stephensi Larval sites: wells, water tanks Baseline data: January 1977 | |
| Participants | NA | |
| Interventions | Fish species: G. affinis or A. blockii Indigenous fish species used: G. affinis: not indigenous, A. blockii: indigenous Fish source: G. affinis: mass cultured at Vector Control Research Centre (VCRC); A. blockii: collected from ponds and stored at VCRC Populated sites: 3402 to 3438 sites stocked; 317 sites unstocked Restocked: yes; if no fish were present at sites at 1, 2, or 3 months after beginning of the trial, they were restocked with G. affinis or A. blockii Co‐intervention: none | |
| Outcomes | Percentage of sites positive for anopheline larvae Methods: bucket samples taken monthly | |
| Source of funding | Not specified | |
| Notes | Number of wells where fish survived monitored Chemical analysis performed of water from wells where fish died (20) or survived (20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Low risk | "Every house with a well was marked in the experimental and comparison area." |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. |
| Blinding of outcome assessment (detection bias) | High risk | "Wells were marked according to whether the fish was present or absent...it was possible to visually observe movement of Gambusia on the surface." |
| Baseline values | High risk | Not comparable between control and intervention sites. |
| Number of sites | Low risk | Adequate numbers of sites in control and intervention groups. |
| Methods | Study design: controlled time series study Study location: Central Java Study dates: 1979 to 1984 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: not stated Larval sites: rice fields Baseline data: not recorded | |
| Participants | NA | |
| Interventions | Fish species: C. carpio and P. reticulata Indigenous fish species used: C. carpio: indigenous, P. reticulata: not indigenous Fish source: mass breeding of C. carpio in 9 ponds of 6 m² × 4 m² tended by fishery official in co‐operation with village officials. Mass breeding of P. reticulata in 2 ponds of 4 m² × 2 m² tended by local fishery official. Populated sites: number and size of control and intervention sites was not specified. Total size of area was 24.8 ha of wetland (rice fields), cultivated by 112 farmers. Restocked: fish were restocked every new rice planting season Co‐intervention: control area sprayed with fenitrothion at end of 1982 | |
| Outcomes | Mean number newly emerged adult mosquitoes/m²/day collected in traps (trap area 0.25 m²) per year | |
| Source of funding | TDR Grant UNDP/World Bank/WHO | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study. |
| Site selection | Unclear risk | Number of fields not specified. "96.4% of the total 24.8 ha were included." |
| Site allocation | Unclear risk | Numbers of control and intervention sites not specified. Size of control area not specified. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Unclear risk | Not reported. |
| Number of sites | High risk | Probably inadequate, as number of sites not specified. |
| Methods | Study design: controlled time series Study location: Vakhsh (Kirov 2 district) and Bokhtarskiy (Sadov 3 district) regions in Tajikistan Study dates: 15 July to 21 August 2007 Transmission intensity: in 2007 there were no malaria cases in Saidov, and 5 cases in Kirov; but the study authors did not provide population denominator details Malaria parasite species: not stated Primary vectors: Anopheles superpictus, Anopheles pulcherrimus,Anopheles hyrcanus Larval sites: rice fields Baseline data: no baseline data | |
| Participants | NA | |
| Interventions | Fish species: G. affinis Indigenous fish species used: no Fish source: harvested from reservoirs noted to have Gambusia Populated sites: rice field plots Restocked: implied but not explicitly stated Co‐interventions: not described | |
| Outcomes | Density of immature Anopheles mosquitoes by instar (data were not provided by species). Method: authors used a standard net of 20 cm diameter. The net was immersed in water and held to 0.5 m in 1 direction, then taken in the opposite direction. The net contents were rinsed and the number of fish, and mosquito larvae and pupae counted. Five such samples gave the number of fish and the immature mosquitoes/m². | |
| Source of funding | Not stated | |
| Notes | No environmental data reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series. |
| Site selection | High risk | Intervention and control areas each included 1 district with malaria cases and 1 district without malaria cases (no indication how sites for intervention and control areas were allocated). |
| Site allocation | Unclear risk | The study authors did not state how treatment was allocated to study sites. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not possible to blind (presence of fish should be obvious); unclear if those who stocked the fish also sampled for larvae. |
| Baseline values | Unclear risk | Study authors did not provide values for the interventions sites prior to introduction of fish. |
| Number of sites | High risk | There were 2 sites in the intervention group and 2 in the control group. |
| Methods | Study design: controlled interrupted time series study Study location: Grande Comore Island, Federal Islamic Republic of Comoros Study dates: November 1987 to November 1988 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. gambiae Larval sites: domestic water containers Baseline data: November 1987 | |
| Participants | NA | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: not indigenous Fish source: imported from Mayotte Island Populated sites: domestic water containers; 20 unstocked (ablution basins) for duration of trial; 59 ablution basins and 61 tanks stocked in November 1987. Stocking of basins and tanks extended, and by April 1988, all basins and tanks were treated. Total numbers of basins and tanks stocked not specified. Restocked: not clearly indicated Co‐interventions: temephos (concentration: 2 mL/m³) in tanks only, last treatment March 1988 | |
| Outcomes | Percentage of containers positive for anopheline larvae Method: surface and bottom of containers were examined for An. gambiae larvae (containers ≥ 15 cm in diameter), which were recorded monthly | |
| Source of funding | Research was undertaken with the framework of project OMS‐PNUD COM/MAL/001 | |
| Notes | No environmental data collected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Unclear risk | Unclear how sites were selected. |
| Site allocation | Unclear risk | Unclear how intervention treatment was selected. Control sites were in village of Bandamadji, 3 km from intervention site. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Low risk | Percentage of sites positive for An. gambiae larvae comparable in control and Intervention groups. |
| Number of sites | Low risk | Adequate numbers of sites in control and Intervention groups. |
| Methods | Study design: controlled interrupted time series study Study location: Great Hyderabad City, India Study dates: not stated Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. stephensi Larval sites: domestic water containers Baseline data: day 0, before release of fish | |
| Participants | NA | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: not indigenous Fish source: not stated Populated sites: 5 control and 12 intervention (50 guppies/well); 4 control and 10 intervention (100 guppies/well) Restocked: no Co‐interventions: temephos (concentration: 2 mL/m³) | |
| Outcomes | Density of immature An. stephensi stages (larvae instars I and II, III and IV, pupae) Method: 5 dips per well using a 30 cm diameter net | |
| Source of funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Unclear risk | Unclear how these particular sites were selected. |
| Site allocation | Unclear risk | Unclear how treatment was allocated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | High risk | Mean values not comparable between control and intervention groups. |
| Number of sites | High risk | Numbers of sites may be inadequate as 4 control sites were used. |
| Methods | Study design: controlled interrupted time series study Study location: Korea Study dates: June to September 1988 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. sinensis Larval sites: rice fields Baseline data: June to August 1988 | |
| Participants | NA | |
| Interventions | Fish species: A. latipes andT. m. niloticus Indigenous fish species used: A. latipes: indigenous; T. m. niloticus: not indigenous Fish source: A. latipes originated from holding ponds at Ansan rice fields (2.5 km away), T. m. niloticus sourced from fish farm in Jin‐Dong of Masan City, South Kyungsang Province Populated sites: rice fields (2 control sites, 2 intervention sites with A. latipes and T. m. niloticus, 2 intervention sites with A. latipes only, followed by Bacillus thuringiensis treatment after 3 weeks) Restocked: no Co‐interventions: see above | |
| Outcomes | Density of An. sinensis larvae determined weekly Method: larval dipping performed using a 500 mL dipper, 2 to 4 replicates per rice field usually consisting of 2 dips pooled | |
| Source of funding | WHO Medical Research Fund of the Western Pacific Region, Manila | |
| Notes | Environmental data (temperature and rainfall) recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study. |
| Site selection | Low risk | "A confined field plot of ca 1,000 m²...the rice paddy was composed of 6 similar sized (10 × 15 × 0.3 m) plots." |
| Site allocation | Unclear risk | "2 random selection of paddies was made for each group." Method of random selection not specified. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear whether assessors were blinded to treatment. |
| Baseline values | Low risk | Comparable between control and intervention sites. |
| Number of sites | High risk | Probably inadequate number of sites. |
| Methods | Study design: controlled time series Study location: Farkhor district (Kizilpakhtachi village) and Shaartuz district (Birlyash village), Tajikistan Study dates: Kizilpakhtachi village 25 June to 29 August 2008; Birlyash village: 25 June to 26 August 2008 Transmission intensity: not mentioned Malaria parasite species: not mentioned Primary vectors: An. superpictus Larval sites: rice fields Baseline data: reported values measured immediately before introduction of fish | |
| Participants | NA | |
| Interventions | Fish species: G. affinis,P. reticulata Indigenous fish species used: no. Fish source: G. affinis, not mentioned; P. reticulata bred in basic laboratory at the Republican Centers for Combating Tropical Diseases (RCCTD) Populated sites: in each paddy field, 9 rice field checks (3 m x 3 m) were used: 3 filled with P. reticulata, 3 with G. affinis, and 3 served as controls. Checks with the different species of fish were arranged in a chequer board pattern. (A rice check is a square or rectangular area of a paddy field created by low, narrow banks of earth (dykes) that serve to divide the paddy field into manageable areas and to control the flow of water.) Restocked: yes, but unclear whether for P. reticulata alone due to poor survival or both P. reticulata and G. affinis. Graphs indicated that fish were introduced twice, but text stated "Because of the problem of using guppies as larviphages, related to their much worse survival rate in the native conditions in Tajikistan than the survival rate of gambezi (which can safely be regarded as a representative of the local ichthyofauna), it was necessary to re‐release guppies into the rice checks to reduce the number of larvae." Co‐interventions: not stated | |
| Outcomes | Outcome: density of either younger or older Anopheles larvae/m² Method of measurement: a 20 cm diameter net, or a photographic cuvette, was immersed in the water to half‐way down the rim, swept for 1 m to 1 side, trawling the superficial layers, then turned sharply and swept the other way for 1 m to trawl the bottom layers. Net contents were rinsed into a cuvette and the numbers of fish and mosquito larvae and pupae counted. 5 such samples will give the number of fish and pre‐imago mosquitoes per m². | |
| Source of funding | No information provided | |
| Notes | Article in Russian. Data were estimated from graphs In intervention checks G. affinis multiplied successfully despite the presence of predators dragonfly larvae, water bugs, water beetles, marsh frogs. P. reticulata had lower survival in the field than G. affinis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series. |
| Site selection | Unclear risk | Study authors did not state how they selected sites. |
| Site allocation | Low risk | Checks with Gambusia or Poecilia or control were arranged in a chequer board pattern. Study authors stated that allocation was random. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Presence of fish was obvious, but it was not clear whether observers of larval densities were blinded. |
| Baseline values | Unclear risk | The study authors reported baseline values taken immediately before introduction of fish. Baseline values were comparable in the Birlyash village, but not in the Kizilpakhtachi village. Authors reported mean values only. |
| Number of sites | High risk | Study authors used 2 sites, each comprised 3 checks for control, 3 for G. affinis, and 3 for P. reticulata. |
Abbreviations: NA: not applicable; RCT: randomized controlled trial; UNDP: United Nations Development Programme; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Transmission baseline data collected for < 1 year pre‐intervention. For larval population data, Anopheles and Culex populations not monitored separately. | |
| Not a fish trial. Review article. | |
| Not a fish trial. | |
| Not a fish trial. Review article. | |
| A commentary on use of larvivorous fish to control Aedes mosquitoes. | |
| Not a fish trial. Review article. | |
| Not a fish trial. Review article. | |
| Medical report, not a fish trial. | |
| Not a fish trial. Review article. | |
| No primary or secondary outcomes. | |
| No primary or secondary outcomes. | |
| Not a controlled trial. | |
| Not a fish trial. | |
| Not a fish trial. | |
| Not a fish trial. Review article. | |
| Not a fish trial. Review article. | |
| Not a fish trial. Review article. | |
| Not a fish trial. Review article. | |
| Not a controlled trial. | |
| Anopheles and Culex populations not monitored separately. No primary outcomes. | |
| Unclear study design. Unclear whether control sites were true controls or areas of no Gambusia fish in the same water body. As this study was published in 1930, we were unable to contact the study author for further details. | |
| Not a fish trial. Descriptive article. | |
| Not a fish trial. Descriptive article. | |
| No primary or secondary outcomes. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Laboratory‐based study only. | |
| Not a fish trial. | |
| Inappropriate study design. | |
| Not a fish trial. Review article. | |
| Laboratory‐based study only. | |
| Not a fish trial. | |
| Anopheles and Culex populations not monitored separately. No primary outcomes. | |
| Not a fish trial. | |
| Not a fish trial. Review article. | |
| Inappropriate study design. | |
| No primary or secondary outcomes. | |
| Laboratory‐based study only. | |
| Inappropriate study design. | |
| No primary or secondary outcomes. | |
| Not a fish trial. Review article. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Not a fish trial. | |
| Study authors reported Anopheles and Culex immature mosquito numbers combined. | |
| Inappropriate study design. | |
| Laboratory‐based study only. | |
| Not a fish trial. Review article. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Not a controlled trial. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| No primary outcomes. Secondary outcomes in Nalim 1988. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Anopheles and Culex populations not monitored separately. No primary outcomes. | |
| Inappropriate study design. | |
| Inappropriate study design. Anopheles and Culex populations not monitored separately. | |
| Not a fish trial. Review article. | |
| Inappropriate study design. | |
| Inappropriate study design. Anopheles and Culex populations not monitored separately. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| No primary outcomes. Secondary outcomes in Sabatinelli 1991. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Inappropriate study design. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| No primary outcomes. Secondary outcome follow‐up only 3 weeks in duration. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Multiple interventions, cannot determine effect of fish alone. | |
| Inappropriate study design. No control area. | |
| Not a controlled trial. | |
| Not a controlled trial. | |
| Not a fish trial. | |
| Not a fish trial. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Not a fish trial. A manual on how to evaluate fish. | |
| Inappropriate study design. | |
| Inappropriate study design. Not in malaria‐endemic area. | |
| Inappropriate study design. | |
| Not a fish trial. | |
| Inappropriate study design. | |
| Inappropriate study design. | |
| Not a fish trial. Review article. | |
| Study authors reported number of immature mosquitoes, and not specifically anopheline mosquitoes. | |
| Not a fish trial. Review article. | |
| Anopheles and Culex populations not monitored separately. Inappropriate study design. | |
| Inappropriate study design. Multiple interventions, cannot determine effect of fish alone. | |
| Inappropriate study design. | |
| Secondary outcomes in Yu 1982a. | |
| Secondary outcomes in Yu 1982a. | |
| Inappropriate study design. Culex monitored only. | |
| Inappropriate study design. Laboratory‐based experiment only. |

Larvivorous fish for preventing malaria transmission: conceptual framework.

Experimental designs that have been used to attempt to evaluate the impact of fish on the larvae of vectors in malaria‐endemic countries. In this figure, we depicted either two or six sample time points (shown by the arrows) as examples. Studies may sample at more time points, or at fewer time points in the case of time series studies.
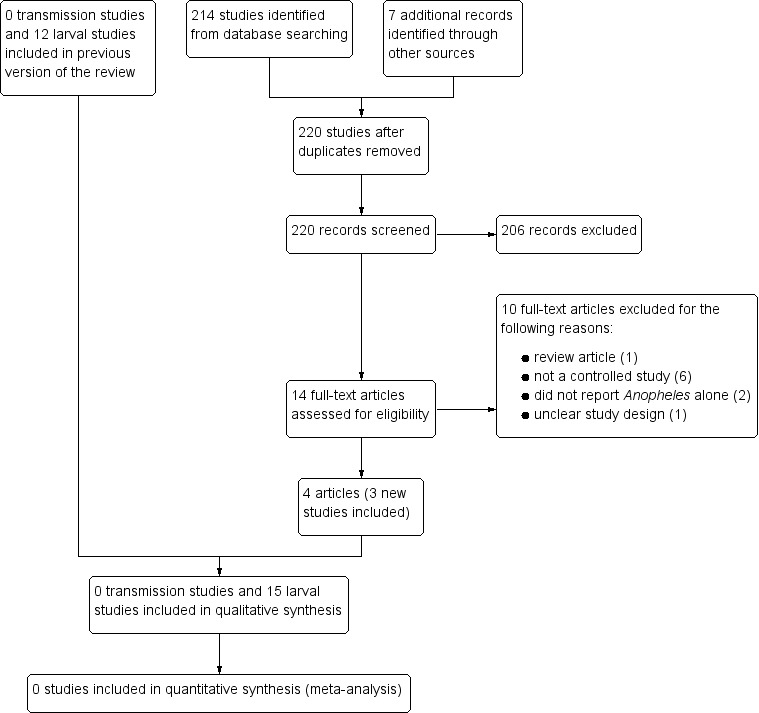
Study flow diagram
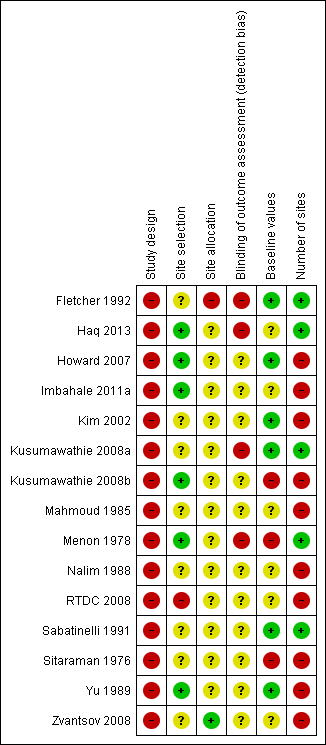
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
| Larvivorous fish for preventing malaria transmission | ||||||
| Patient or population: people living in malaria‐endemic areas Settings: malaria‐endemic areas Intervention: larvivorous fish Control: no larvivorous fish | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of studies | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Larvivorous fish | |||||
| Effects on malaria transmission | ||||||
| Clinical malaria (incidence) | — | — | — | 0 | — | No studies |
| Entomological inoculation rate | — | — | — | 0 | — | No studies |
| Density of adult malaria vectors | — | — | — | 0 | — | No studies |
| Effects on larvae at potential mosquito larval sites | ||||||
| Density of immature vector stages in water bodies Quasi‐experimental studies | — | — | Not pooled. Variable effects reported. | 12 | ⊕⊝⊝⊝ Very low1‐9 | No clear evidence whether or not larvivorous fish reduce the density of immature anopheline mosquitoes in water bodies. |
| Larval sites positive for immature vector stages Quasi‐experimental studies | — | — | Not pooled Positive effects reported | 5 | ⊕⊕⊝⊝ Low1,2,10‐12 | Larvivorous fish may reduce the number of larval sites positive for immature anopheline mosquitoes. |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Downgraded by two: the included studies were non‐randomized controlled trials. | ||||||
| Risk of bias factor | Risk of bias | ||
| High | Low | Unclear | |
| 1. Study design | Non‐RCT | RCT | Not clearly reported or not reported |
| 2. Site selection | Method of selection of sites within study area not described | Method of selection of sites within study area described | Not clearly reported or not reported |
| 3. Site allocation | Allocation of treatment not performed by random allocation | Allocation of treatment performed by random allocation | Not clearly reported or not reported |
| 4. Blinding of assessors | Not blinded | Blinded | Not clearly reported or not reported |
| 5. Baseline values comparable between sites | Not comparable | Comparable | Not clearly reported or not reported |
| 6. Number of sites | May be inadequate (5 to < 20 sites per group) Probably inadequate (< 5 sites per group or number of sites unknown) | Adequate number of sites (≥ 20 sites per group) | Not clearly reported or not reported |
| Abbreviations: RCT: randomized controlled trial. | |||
| Group | Site type | Study | Sites stocked | Unstocked | Site size | |
| Surface area | Depth | |||||
| Localized water bodies1 | Wells | 3402 to 3438 | 317 | Not stated | Not stated | |
| 10 | 4 | 1.5 m² | 1.5 to 2.5 m | |||
| Domestic water containers | 68 | 60 | Not stated | Not stated | ||
| 295 (30 monitored) | 25 monitored | Not stated | Not stated | |||
| 1205 | 20 | Not stated | Not stated | |||
| Fishponds and man‐made pools | 2 | 1 | 72 m² to 128 m² | Not stated | ||
| 25 | 5 | Mean 1 m² | 1 m | |||
| Riverbed pools below dams | 29 | 31 | 0.25 to 1 m² | < 1 m | ||
| 2 areas. Number of sites unknown | 2 areas. Number of sites unknown | Not stated | Not stated | |||
| Rice field plots | 3 | 1 | 300 m² to 600 m² | Not stated | ||
| Not specified | Not specified | 23.9 ha in total | Not stated | |||
| 2 | 2 | Not stated | Not stated | |||
| 4 | 2 | 45 m³ | 0.01 m | |||
| 2 areas, with 6 checks8 in 1 paddy field per area (3 checks treated with Gambusia affinis, 3 checks treated with Poecilia reticulata) | 2 areas. 3 checks in 1 paddy field per area | Each paddy field had 9 checks, and each check was 3 m × 3 m | Not stated | |||
| Water canals | 25 | 5 | Mean 15 m² | 0.3 m | ||
| 20 | 5 | 4 km to 10 km × 2 m wide | 1 m | |||
| 1Included wells, domestic water containers, fishponds and man‐made pools, and riverbed pools below dams. | ||||||
| Study | Fish species introduced | Stocking density | Type of site | Size of site | Size (maturity) of fish | Sex ratio male: female | Time of year fish introduced | Restocked |
| Aphanius dispar | 5 fish per barrel, 10 fish per cistern, 20 fish per well, 60 fish per washbasin; later, 10 fish per barrel and 40 fish per well | Domestic water containers | Not stated | Not stated | Not stated | February | Yes | |
| A. dispar | 10 to 25 fish per tank or container, depending on the container size | Domestic water containers | Not stated | Not stated | Not stated | November to December | No | |
| Oreochromis niloticus | 2 fish per m² pond surface area | Abandoned fishponds | 104 m² (pond A), 128 m² (pond C), 72 m² (pond D) | 1 to 2 months old | Not stated | January | No | |
| G. affinis | Total number based on feeding rate of 4 mosquito fish per 60 mosquito larvae per day | Man‐made pools or water canals | Pools (mean 1 m × 1 m × 1 m deep) or water canals (15 m × 1 m × 0.3 m deep) | 4 cm to 7 cm | Not stated | February | No (treatment arm: ponds fish once). Yes, every 2 weeks (treatment arms: pond fish only or water canal fish only). | |
| (1) A. latipes with T. m. niloticus. (2) Aphyocypris chinensis + T. m. niloticus. | (1) 1 pair T. m. niloticus/10 m² water surface + 0.8 A. latipes/m² water surface. (2) 1 A. chinensis/m² + 2 T. m. niloticus/10 m². | Rice fields. | Rice fields (1) 500 m², (2) 300 m², or 600 m². | Not stated. | Not stated. | June. | No. | |
| P. reticulata. | 5 fish/m² surface area. | Riverbed pools below dams. | 0.25 to 1 m² surface area and < 1 m depth. | Not stated. | 2:3 | May. | No. | |
| P. reticulata | 5 fish/m² surface area | Riverbed pools below dams | Not stated | Not stated | 2:3 | August | Yes | |
| G. holbrooki | Unclear. Authors stated a total of 8000 to 12,000 fish per canal depending on length and 1000 fish | Canals | 1 m depth, 2 m width, 4 to 10 km length | Not stated | Not stated | October | Yes | |
| G. affinis and A. blockii | 20 fish per negative well, 50 fish per positive well | Wells | Not stated | Not stated | Not stated | January | Yes | |
| P. reticulata and C. carpio | 9 C. carpio/10 m² and 2 P. reticulata/m² | Rice fields | 23.9 ha in total, but size of individual ponds not specified | Not stated | Not stated | Not stated | Yes | |
| G. affinis | Not clearly stated; study authors reported from 2 to 3 fish/m² (1st timepoint) up to 15 to 18 fish/m² (Vakhsh, Kirov 2) or 18 to 20 fish/m² (Bokhtarskiy, Sadov 3 districts) | Rice fields | Not stated | Not stated | Not stated | Not stated | Not clearly indicated | |
| P. reticulata | 3 to 5 fish/m3 | Domestic water containers | Size of domestic water containers (ablution basins and tanks) not clearly indicated | Not stated | Not stated | November | Not clearly indicated | |
| P. reticulata | Either 50 or 100 fish per well | Wells | 1.5 to 2.5 m depth, average square area 1.5 m² | Not stated | Not stated | Not stated | No | |
| A. latipes and T. m. niloticus | 2 A. latipes/m² and 2 T. m. niloticus/10 m² or 2 A. latipes/m² only | Rice fields | Each plot was 10 × 15 × 0.3 m, depth 10 cm | Not stated | Not stated | June | No | |
| G. affinis orP. reticulata | 5 pregnant females/m² (total of 45 females per 3 m × 3 m check) | Rice fields | Each paddy field had 9 checks, and each check was 3 m × 3 m | Only stated as adult and pregnant | Not stated | June to August | Yes, but unclear whether P. reticulata alone or both P. reticulata and G. affinis. Graphs indicated that both species of fish could have been introduced twice, but text stated, "Because of the problem of using guppies as larviphages, related to their much worse survival rate in the native conditions in Tajikistan than the survival rate of gambezi (which can safely be regarded as a representative of the local ichthyofauna), it was necessary to re‐release guppies into the rice checks." |
| Study ID | Pupae numbers reported | Distance between sites | Other larvivorous species present | Vegetation cleared |
| Recorded but not reported | < 1 km | Not reported | Not reported | |
| Only % reduction of L3 to L4 larvae and pupae combined reported | 13 km | Not reported | Not reported | |
| Only larvae and pupae combined reported | < 1 km | Not reported | Three ponds cleared of vegetation on a weekly basis | |
| Not reported | Not reported | Not reported | Not reported | |
| Not reported | < 1 km | Not reported for control site. For treatment site, no other larvivorous fish found. | Herbivorous fish Tilapia mossambicus niloticus used at intervention but not control sites | |
| Recorded but not reported | < 1 km | Not reported | Not reported | |
| Not reported | Not reported | Not reported | Not reported | |
| Not reported | Not reported | Not reported | Not reported | |
| Not reported | Not reported | Not reported | Not reported | |
| Not reported | Not reported | Not reported | Not reported | |
| Yes | Not reported | Not reported | Not reported | |
| Not reported | 3 km | Not reported | Not reported | |
| Yes | Not reported | Not reported | Not reported | |
| Not reported | < 1 km | Not reported | Herbivorous fish T. m. niloticus used in 1 study arm only | |
| Recorded but only larvae reported | Intervention and control in same paddy field in each site | Assessed, but not reported | Not clearly reported |
| Site type | Study | Intervention | Outcome | Result | |
| Localized water bodies | Wells | Intervention: Gambusia or Aplocheilus fish to 3438 wells; 50 fish per well if anopheline larvae present; 20 fish per well if no larvae present Control: 317 wells | Percentage of sites with An. stephensi larvae up to 4 months' follow‐up | Study appeared to provide evidence of a larvicidal effect of fish in wells using relatively high fish stocking levels. | |
| 100P. reticulata per well Intervention: 10 wells Control: 4 wells 50P. reticulata per well Intervention: 12 wells Control: 5 wells | A. stephensi larval and pupal densities up to 28 days (100 fish per well) or 22 days (50 fish per well) | At high fish stocking levels, larvae were eliminated in the first 4 days in wells but reappeared at lower levels from day 24 onwards. With lower fish stocking levels, there was a partial effect for 2 weeks only, with rebound. | |||
| Wells and domestic water containers | Intervention: Aphanius dispar (60 sites) Control: 51 sites | Percentage of sites with An. culicifacies adanensis larvae up to 11 months' follow‐up | Study provided evidence that fish introduction prevents an increase in the number of domestic water container sites with larvae compared with control up to 11 months' follow‐up. | ||
| Intervention: A. dispar (295 water containers, of which 30 were monitored) Control: 25 containers | Percentage reduction in An. stephensi L3‐L4 larvae and pupae up to 12 months' follow‐up | Study appeared to provide evidence that fish introduction reduces the number of L3‐L4 larvae and pupae in domestic water containers compared with control up to 12 months' follow‐up. | |||
| Intervention: P. reticulata fish (59 sites in November 1987, total number of sites not specified) Control: 20 ablution basins | Percentage of containers positive for An. gambiae larvae for 11 months' follow‐up | Study appeared to show that fish reduce the number of domestic wash basins with larvae when added to these sites for up to 11 months. | |||
| Fishponds and pools | Intervention: Oreochromis niloticus fish (2 ponds) Control: 1 pond | Number of immature An. gambiae and An. funestus mosquitoes for 5 months' follow‐up | Based on trends in the study authors' graph, data that we extracted from the graph, and the study authors' analysis, this study appeared to provide limited evidence of a possible larvicidal effect of fish in ponds. | ||
| See the water canals section below. | |||||
| Riverbed pools below dams | Intervention: P. reticulata (29 riverbed pools) Control: 31 pools | Percentage of pools with Anopheles larvae, mean number of Anopheles larvae per pool, and mean number of Anopheles larvae per 100 dips up to 120 days' follow‐up | At follow‐up, the intervention group had greater reductions than the control group for the outcomes of percentage of pools with Anopheles larvae, mean number of larvae per pool, and mean number of larvae per 100 dips. | ||
| Intervention: P. reticulata to all riverbed pools in Laxapana and Kotmale (1 study site) Control: all riverbed pools in Kotmale 2 and Nilambe | Percentage of pools with Anopheles larvae, mean number of Anopheles larvae per pool, and mean number of Anopheles larvae per 100 dips up to 1 year follow‐up | At follow‐up, riverbed pools stocked with fish had larger reductions in terms of presence and density of larvae. | |||
| Rice field plots | Intervention: Tilapia mossambicus and A. latipes (treatment A, 1 rice field plot) or A. chinensis and Tilapia mossambicus (treatment B and treatment C, 1 rice field plot each) Control: 3 rice field plots of similar size | Number of An. sinensis larvae up to 13 weeks' (treatment A) or 7 weeks' (treatment B and C) follow‐up | In the control group and with treatments B and C, the number of An. sinensis larvae was higher at 2 weeks' pre‐intervention than at 6 weeks' pre‐intervention. At 2 weeks' follow‐up, the An. sinensis larval population in the control group was the same as at 2 weeks' pre‐intervention, but decreased at 6 weeks' follow‐up. Larvae were clearly reduced at the 2 sites where fish were introduced. For treatment A, the number of An. sinensis larvae increased between one week' and five weeks' follow‐up at both control and intervention sites. However, the number of larvae decreased by 13 weeks' follow‐up at both control and intervention sites. This shows a mean difference in larvae density between control and intervention over the entire period of observation. However, these data were weaker, as no baseline density was noted in the intervention arm, and any difference from the control could be due to chance. | ||
| Intervention: 23.9 ha of rice fields with P. reticulata and C. carpio fish Control: did not specify the size of the control area used Total numbers of control and Intervention field plots not specified | Number of An. aconitus, An. barbirostris, and An. annularis newly emerged adult mosquitoes collected/m²/day (trap area = 0.25 m²) up to 6 years' follow‐up | Effects were mixed, with some indication of an effect of fish on An. aconitus and An. annularis, but not on An. barbirostris. | |||
| Intervention: 2 rice field plots treated with G. affinis fish Control: 2 rice field plots | Number of Anopheles larvae and pupae up to 40 or 41 days' follow‐up | Study appeared to provide evidence of a larvicidal effect of fish in rice field plots up to 40/41 days' follow‐up. | |||
| Intervention: 2 plots treated with 2 species of fish (A. latipes and Tilapia mossambicus), 2 plots treated with 1 species alone (A. latipes) Control: 2 plots | Number of An. sinensis larvae up to 4 weeks' (1 fish) or 7 weeks' (2 fish) follow‐up | At 4 weeks, larvae had increased against baseline in both control and intervention plots, but the size of the increase was lower in the 2 plots treated with 1 species. Follow‐up at 4 weeks and 7 weeks showed considerably lower values in the 2 plots treated with 2 species than in the control. | |||
| 2 areas, 1 rice field per area Intervention: per rice field, 3 checks treated with G. affinis, and 3 treated with P. reticulata Control: 3 untreated checks per rice field (a rice check is a square or rectangular area of a paddy field created by low, narrow banks of earth (dykes) that serve to divide the paddy field into manageable areas and to control the flow of water) | Density of "younger" or "older" Anopheles larvae per m² up to 62 days' (Birlyash village) or 65 days' (Kizilpakhtachi village) follow‐up | Based on data that we extracted from the study authors' graphs, this study appears to provide limited evidence of a possible larvicidal effect of G. affinis fish in the rice field plots of both areas studied. P. reticulata reduced the larval density to similar levels as G. affinis in 1 district, but the effect was less sustained compared to G. affinis in the Shaartuz district, Birlyash village. | |||
| Water canals | Ponds Intervention: single (6 ponds) and multiple stocking of G. affinis (6 ponds) Control: 6 ponds Canals Intervention: G. affinis (6 canals) Control: 6 canals | Estimated marginal mean values of younger (L1 and L2) and older (L3 and L4) An. gambiae s.l. larvae up to 13 weeks' follow‐up | No difference between control and intervention groups at follow‐up, apart from the numbers of older larvae were lower in the canal intervention group. | ||
| Intervention: 20 canals treated with G. holbrooki Control: 5 canals | Density of a late larval stage of An. arabiensis (L4) up to 13 months' follow‐up | An. arabiensis density was lower in intervention canals for 2 months (5 months' and 6 months' post‐intervention) just before and at the beginning of the dry season. Larval densities dropped in both intervention and control groups in the dry season (7 months' post‐intervention) and at the end of the rainy season (13 months' post‐intervention). Fish numbers did not increase after the rainy season and during the last 6 months of the study. According to the authors, control of the flow of water from large to branch canals by gates deprived the fish of free movement. In addition, during the rainy season, rainwater pools act as suitable larval habitats for An. arabiensis. | |||

