Maternal position in the second stage of labour for women with epidural anaesthesia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008070.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 noviembre 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For this update (2018), Kate Walker and Jim Thornton assessed the studies and extracted the data, Nia Jones updated the manuscript and all authors reviewed the final version.
Sources of support
Internal sources
-
University of Nottingham, UK.
Claire Kingswood and Emily Kemp worked on the 2013 version of this review as part of their BMedSci projects in 2009 and 2010
External sources
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
(2013 update)
-
National Institute of Health Research (NIHR), UK.
NIHR Cochrane Reviews of NICE Priority: Project Ref NIHR127513 (2018 update)
Declarations of interest
Kate F Walker: none known
Marion Kibuka: none known
Jim G Thornton: none known
Nia W Jones: none known
Acknowledgements
We are grateful to Emily Kemp and Claire J Kingswood for their contribution to the initial version of this review (Kemp 2013).
We would also like to thank Bita Mesgarpour for translating and assessing Amiri 2012.
This project was supported by the National Institute for Health Research, via Cochrane Reviews of NICE Priority funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
We are grateful to Anna Cuthbert (Research Associate with Cochrane Pregnancy and Childbirth) for her help in preparing the previous update. Anna assessed studies for inclusion and prepared the updated review. We thank Leanne Jones, for help in updating the 'Summary of findings' table for this update (2018).
We would like to thank Justus Hofmeyr and Joshua Vogel for providing us with a confidential pre‐publication draft of their trial so that it could be assessed for eligibility in this review (Hofmeyr 2018).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Nov 09 | Maternal position in the second stage of labour for women with epidural anaesthesia | Review | Kate F Walker, Marion Kibuka, Jim G Thornton, Nia W Jones | |
| 2017 Feb 24 | Position in the second stage of labour for women with epidural anaesthesia | Review | Marion Kibuka, Jim G Thornton | |
| 2013 Jan 31 | Position in the second stage of labour for women with epidural anaesthesia | Review | Emily Kemp, Claire J Kingswood, Marion Kibuka, Jim G Thornton | |
| 2009 Oct 07 | Position in the second stage of labour for women with epidural anaesthesia | Protocol | Marion Kibuka, Jim G Thornton, Claire J Kingswood | |
Differences between protocol and review
We have updated the methods and have incorporated the current standard methods text for Cochrane Pregnancy and Childbirth. This includes the use of GRADE and inclusion of 'Summary of findings' tables. We have restructured the Plain Language Summary using the standardised headings developed by Cochrane Pregnancy and Childbirth.
We added caesarean section and instrumental vaginal birth to our list of outcomes for GRADE assessment.
We made slight amendments to 'Types of participants'. In the original review, we had specified that we would only include singleton pregnancies at term (37 weeks + zero days). In this review we changed this to 'singleton pregnancies at 36 weeks gestation onwards'. Three of the studies (Downe 2004; Karraz 2003; Walker 2012) included women at earlier gestational time points than that prespecified in our protocol and outcomes were not available for term and preterm gestational ages separately, so we included data on women from 36 weeks onwards in the review rather than restricting to term participants only. It is unlikely that this will have significantly altered the results; the numbers of women between 36 and 37 weeks included in the review are likely to be small and the results applicable to women at term with an epidural.
We added a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesia, Epidural [*methods];
- Analgesia, Obstetrical [*methods];
- Cesarean Section [statistics & numerical data];
- Extraction, Obstetrical [methods];
- Labor Stage, Second [*physiology];
- Parturition;
- Patient Positioning [*methods];
- Posture [*physiology];
- Randomized Controlled Trials as Topic;
- Time Factors;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
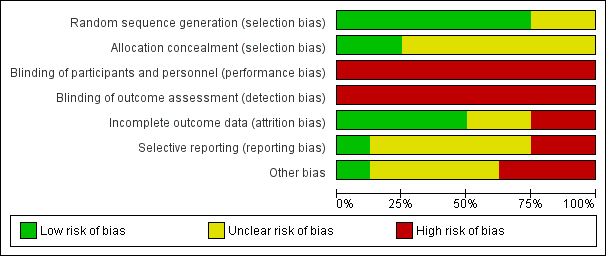
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Upright position versus recumbent position, Outcome 1 Operative birth (caesarean or instrumental vaginal).
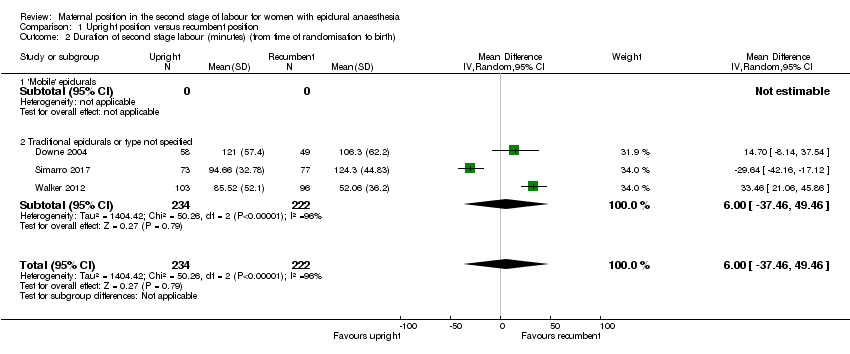
Comparison 1 Upright position versus recumbent position, Outcome 2 Duration of second stage labour (minutes) (from time of randomisation to birth).

Comparison 1 Upright position versus recumbent position, Outcome 3 Caesarean section.
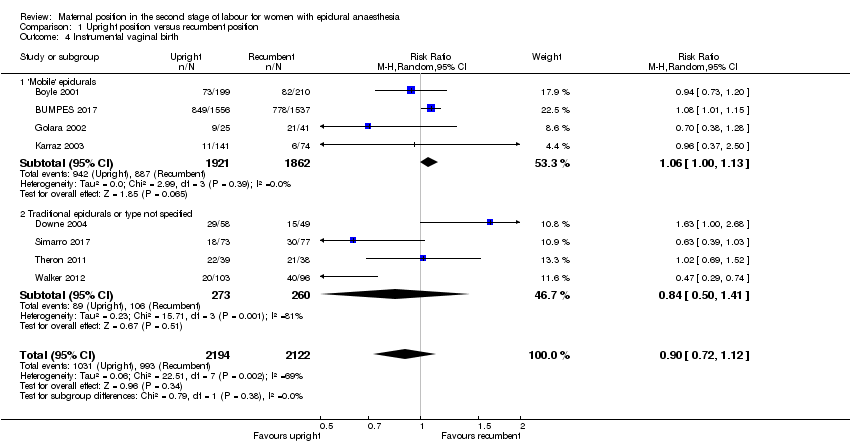
Comparison 1 Upright position versus recumbent position, Outcome 4 Instrumental vaginal birth.

Comparison 1 Upright position versus recumbent position, Outcome 5 Trauma to birth canal requiring suturing.
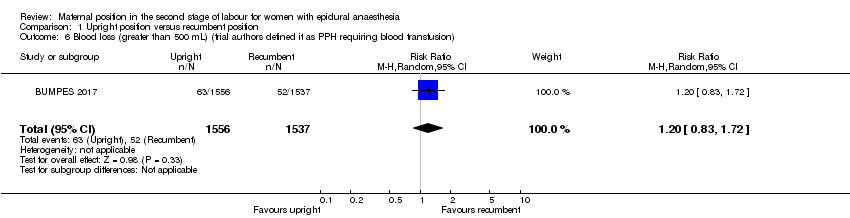
Comparison 1 Upright position versus recumbent position, Outcome 6 Blood loss (greater than 500 mL) (trial authors defined it as PPH requiring blood transfusion).

Comparison 1 Upright position versus recumbent position, Outcome 7 Prolonged second stage, defined as pushing for more than 60 minutes (trial authors report 'duration of pushing phase' in minutes.

Comparison 1 Upright position versus recumbent position, Outcome 8 Maternal experience and satisfaction of labour.

Comparison 1 Upright position versus recumbent position, Outcome 9 Abnormal fetal heart rate patterns, requiring intervention.

Comparison 1 Upright position versus recumbent position, Outcome 10 Apgar score less than seven at five minutes.
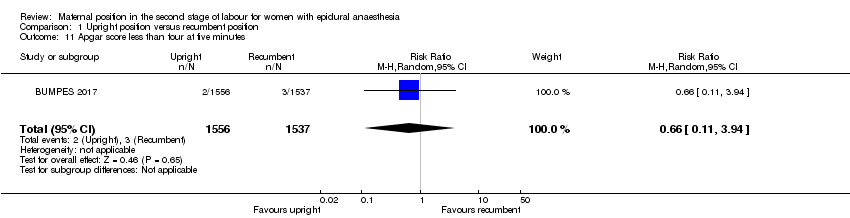
Comparison 1 Upright position versus recumbent position, Outcome 11 Apgar score less than four at five minutes.
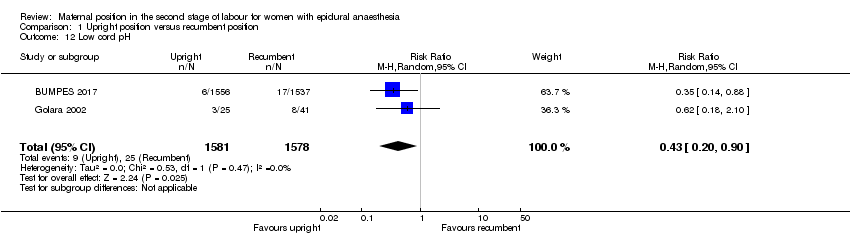
Comparison 1 Upright position versus recumbent position, Outcome 12 Low cord pH.

Comparison 1 Upright position versus recumbent position, Outcome 13 Admission to neonatal intensive care unit.

Comparison 1 Upright position versus recumbent position, Outcome 14 Need for ventilation (trial authors report 'intubation').
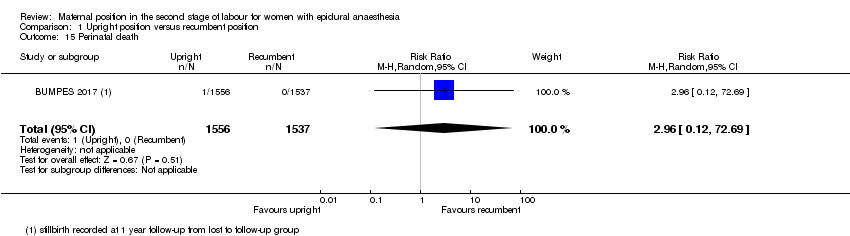
Comparison 1 Upright position versus recumbent position, Outcome 15 Perinatal death.
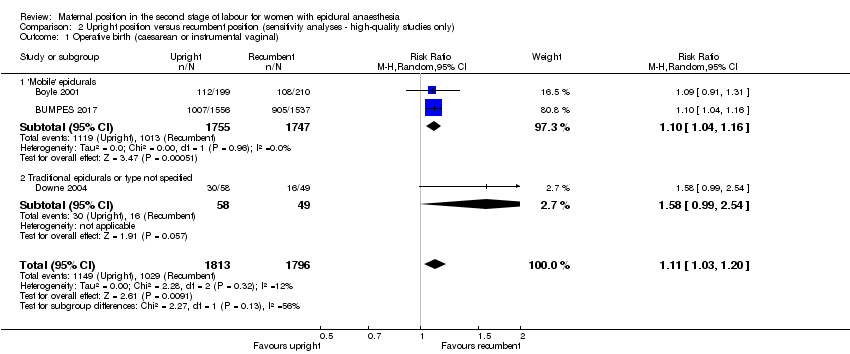
Comparison 2 Upright position versus recumbent position (sensitivity analyses ‐ high‐quality studies only), Outcome 1 Operative birth (caesarean or instrumental vaginal).
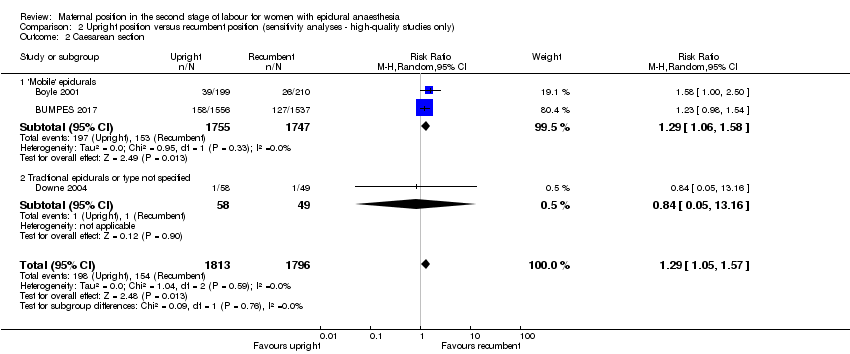
Comparison 2 Upright position versus recumbent position (sensitivity analyses ‐ high‐quality studies only), Outcome 2 Caesarean section.

Comparison 2 Upright position versus recumbent position (sensitivity analyses ‐ high‐quality studies only), Outcome 3 Instrumental vaginal birth.
| Upright position compared to recumbent position for the second stage of labour for women with epidural anaesthesia | ||||||
| Patient or population: women in the second stage of labour with epidural anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with recumbent position | Risk with upright position | |||||
| Maternal outcomes | ||||||
| Operative birth (caesarean or instrumental vaginal) | Study population | RR 0.86 | 4316 | ⊕⊕⊝⊝ | ‐ | |
| 554 per 1000 | 476 per 1000 | |||||
| Duration of second stage labour (minutes) (from time of randomisation to birth) | The mean duration of second stage labour across control groups ranged from 52.06 minutes to 124.3 minutes | MD 6.00 minutes higher (37.46 lower to 49.46 higher) | ‐ | 456 | ⊕⊝⊝⊝ | ‐ |
| Caesarean section | Study population | RR 0.94 | 4316 | ⊕⊝⊝⊝ | ‐ | |
| 86 per 1000 | 81 per 1000 | |||||
| Instrumental vaginal birth | Study population | RR 0.90 | 4316 | ⊕⊝⊝⊝ | ‐ | |
| 468 per 1000 | 421 per 1000 | |||||
| Trauma to birth canal requiring suturing | Study population | RR 1.00 | 3266 | ⊕⊕⊝⊝ | ‐ | |
| 840 per 1000 | 832 per 1000 | |||||
| Blood loss (greater than 500 mL) (trial authors defined it as PPH requiring blood transfusion) | Study population | RR 1.20 | 3093 | ⊕⊕⊕⊝ | ‐ | |
| 34 per 1000 | 41 per 1000 | |||||
| Infant outcomes | ||||||
| Abnormal fetal heart rate patterns, requiring intervention | Study population | RR 1.69 | 107 | ⊕⊝⊝⊝ | ‐ | |
| 41 per 1000 | 69 per 1000 | |||||
| Low cord pH | Study population | RR 0.43 | 3159 | ⊕⊕⊕⊝ | ‐ | |
| 16 per 1000 | 7 per 1000 | |||||
| Admission to neonatal intensive care unit | Study population | RR 0.54 | 66 | ⊕⊝⊝⊝ | ‐ | |
| 24 per 1000 | 13 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLimitations in study (no blinding possible in any of the studies, with some studies at high risk for incomplete data, selective reporting and other bias) (‐1). | ||||||
| Upright position compared to recumbent position (sensitivity analyses ‐ studies at low risk of bias only) for the second stage of labour for women with epidural anaesthesia | ||||||
| Patient or population: women in the second stage of labour with epidural anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with recumbent position (sensitivity analyses ‐ studies at low risk of bias only) | Risk with upright position | |||||
| Operative birth (caesarean or instrumental vaginal) | Study population | RR 1.11 | 3609 | ⊕⊕⊕⊕ | ‐ | |
| 573 per 1000 | 636 per 1000 | |||||
| Caesarean section | Study population | RR 1.29 | 3609 | ⊕⊕⊕⊕ | ‐ | |
| 86 per 1000 | 111 per 1000 | |||||
| Instrumental vaginal birth | Study population | RR 1.08 | 3609 | ⊕⊕⊝⊝ | ‐ | |
| 487 per 1000 | 526 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLimitations in study design (no blinding possible in any of the studies, unclear allocation concealment) – but most of the pooled effect comes from one study with low risk of bias for all domains apart from blinding – impossible to blind and so not downgraded for lack blinding as this is an objective measure. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Operative birth (caesarean or instrumental vaginal) Show forest plot | 8 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.07] |
| 1.1 'Mobile' epidurals | 4 | 3783 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.20] |
| 1.2 Traditional epidurals or type not specified | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.25] |
| 2 Duration of second stage labour (minutes) (from time of randomisation to birth) Show forest plot | 3 | 456 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐37.46, 49.46] |
| 2.1 'Mobile' epidurals | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Traditional epidurals or type not specified | 3 | 456 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐37.46, 49.46] |
| 3 Caesarean section Show forest plot | 8 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.61, 1.46] |
| 3.1 'Mobile' epidurals | 4 | 3783 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.78, 1.67] |
| 3.2 Tradtional epidurals or type not specified | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.15, 1.16] |
| 4 Instrumental vaginal birth Show forest plot | 8 | 4316 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.72, 1.12] |
| 4.1 'Mobile' epidurals | 4 | 3783 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.13] |
| 4.2 Traditional epidurals or type not specified | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.50, 1.41] |
| 5 Trauma to birth canal requiring suturing Show forest plot | 3 | 3266 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.13] |
| 6 Blood loss (greater than 500 mL) (trial authors defined it as PPH requiring blood transfusion) Show forest plot | 1 | 3093 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.83, 1.72] |
| 7 Prolonged second stage, defined as pushing for more than 60 minutes (trial authors report 'duration of pushing phase' in minutes Show forest plot | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐16.37 [‐24.55, ‐8.19] |
| 8 Maternal experience and satisfaction of labour Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Satisfaction with overall childbirth experience (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.92, 0.99] |
| 8.2 Involved in making decisions (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.00] |
| 8.3 Treated with respect by all staff (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.98, 1.01] |
| 8.4 Expectations for labour & birth were met (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 8.5 Felt safe at all times (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| 8.6 Good communication from staff (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.02] |
| 8.7 Felt in control (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.06] |
| 8.8 Able to move as much as wanted (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 8.9 Satisfied with position before pushing (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.05] |
| 8.10 Satisfied with position while pushing (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.98, 1.04] |
| 8.11 Satisfied with labour pain relief (strongly agree & agree) | 1 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| 9 Abnormal fetal heart rate patterns, requiring intervention Show forest plot | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.32, 8.84] |
| 10 Apgar score less than seven at five minutes Show forest plot | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Apgar score less than four at five minutes Show forest plot | 1 | 3093 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.11, 3.94] |
| 12 Low cord pH Show forest plot | 2 | 3159 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.20, 0.90] |
| 13 Admission to neonatal intensive care unit Show forest plot | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.02, 12.73] |
| 14 Need for ventilation (trial authors report 'intubation') Show forest plot | 1 | 3093 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.13] |
| 15 Perinatal death Show forest plot | 1 | 3093 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [0.12, 72.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Operative birth (caesarean or instrumental vaginal) Show forest plot | 3 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.20] |
| 1.1 'Mobile' epidurals | 2 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.04, 1.16] |
| 1.2 Traditional epidurals or type not specified | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.99, 2.54] |
| 2 Caesarean section Show forest plot | 3 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.05, 1.57] |
| 2.1 'Mobile' epidurals | 2 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.06, 1.58] |
| 2.2 Tradtional epidurals or type not specified | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.05, 13.16] |
| 3 Instrumental vaginal birth Show forest plot | 3 | 3609 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.30] |
| 3.1 'Mobile' epidurals | 2 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 3.2 Traditional epidurals or type not specified | 1 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.00, 2.68] |

