Materiales de sellado microbianos con cianocrilato para la preparación de la piel antes de la cirugía
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008062.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 mayo 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
CP, AL and PH co‐wrote the protocol. PH wrote to manufacturers and professional bodies for additional information. CP co‐ordinated the protocol development. CP and AL co‐wrote the review and all authors agreed the final submission. AL undertook the first update which was read and approved by CP, PH and ID.
The second update was undertaken by CW and approved by CP.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and protocol content. Approved the final protocol and review prior to submission.
E. Andrea Nelson, Editor: approved the review update prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the review and updated review and supervised the second update of the review.
Ruth Foxlee: designed the search strategy and edited the search methods section. For this second update Rocio Rodriguez‐Lopez ran the searches.
Sources of support
Internal sources
-
University of Glamorgan, UK.
Time and resources to prepare the systematic review
-
Department of Health Sciences, University of York, UK.
External sources
-
NIHR/Department of Health (England), (Cochrane Wounds), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Callum Wood: none known
Cheryl Phillips: none known
Acknowledgements
Thanks go to the following people who refereed the protocol, or review, for readability, relevance and methodological rigour: Cochrane Wounds editors (Susan O'Meara, Dirk Ubbink, Joan Webster and Gill Worthy) and peer referees (Duncan Chambers, Dayanithee Chetty, Iain McCallum, Jane Nadel and Kumar Samraj). Thank you also to the copy editor Denise Mitchell.
Allyson Lipp led the development of the original review and its first update but has now retired from the process. Paul Harris and Iwan Dowie were also substantially involved in the original review and the first update but were not involved in the second update. The involvement of all these review authors is acknowledged.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 May 18 | Cyanoacrylate microbial sealants for skin preparation prior to surgery | Review | Callum Wood, Cheryl Phillips | |
| 2013 Aug 21 | Cyanoacrylate microbial sealants for skin preparation prior to surgery | Review | Allyson Lipp, Cheryl Phillips, Paul Harris, Iwan Dowie | |
| 2010 Oct 06 | Cyanoacrylate microbial sealants for skin preparation prior to surgery | Review | Allyson Lipp, Cheryl Phillips, Paul Harris, Iwan Dowie | |
| 2009 Oct 07 | Cyanoacrylate microbial sealants for skin preparation prior to surgery | Protocol | Cheryl Phillips, Allyson Lipp, Paul Harris, Iwan Dowie | |
Differences between protocol and review
None.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
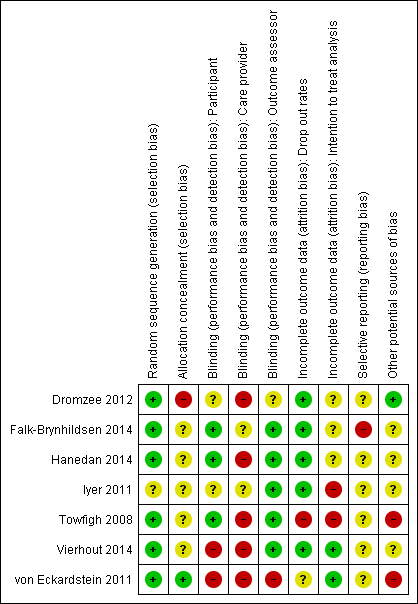
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
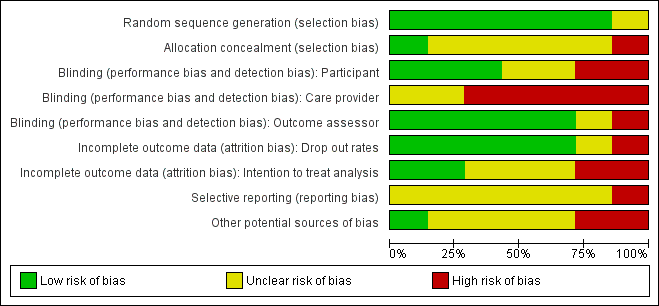
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Microbial sealant compared with no microbial sealant, Outcome 1 Surgical site infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.18] |

