氰基丙烯酸酯微生物封闭剂用于手术前皮肤准备
摘要
研究背景
手术部位感染(即切口感染)一直是医疗保健领域关注的问题。微生物密封剂是一种可在手术前立即涂抹在皮肤上的液体,它被认为可以通过密封皮肤菌群来帮助降低手术部位感染(surgical site infections, SSIs)的发生率,从而防止手术部位的污染和感染。
研究目的
评估术前使用微生物密封剂(与不使用微生物密封剂相比)对接受清洁手术的人群SSI发生率的影响。
检索策略
第二次检索更新截止至2015年5月,我们检索了下列的数据库:Cochrane创伤小组注册库(Cochrane Wounds Group Specialised Register),Cochrane临床试验注册中心(Cochrane Central Register of Controlled Trials, CENTRAL);( Cochrane图书馆 );Ovid MEDLINE;Ovid MEDLINE(包括处理中引用和其他非索引引用);Ovid EMBASE;EBSCO CINAHL。我们未对语言、研究发表日期或者研究环境设限。
纳入排除标准
如果随机对照试验(Randomised controlled trials, RCT)涉及在手术室接受清洁手术(即不涉及呼吸系统、肠道、生殖器或泌尿道或任何现有感染的身体部位的手术)的患者,并且比较了术前使用微生物密封剂和不使用微生物密封剂的情况,则符合纳入条件。
资料收集与分析
两位综述作者独立提取与研究特征、偏倚风险和结局相关的资料。
主要结果
七项试验(859名接受清洁手术的受试者)符合纳入标准。所有试验都对氰基丙烯酸酯微生物密封剂和无密封剂进行了比较。我们发现,使用微生物密封剂(23/443名受试者)的SSIs发生率低于对照对比(46/416名受试者)。合并结果时,没有证据表明使用微生物封闭剂后两组手术部位感染率存在差异(风险比(risk ratio, RR)0.53,95% CI [0.24, 1.18])。三项研究中都出现了不良事件,但这些不良事件并未被判定为是使用微生物密封剂的结果。
作者结论
在第二次更新中,仍然没有足够的证据来确定使用微生物密封剂是否可以降低手术部位感染的风险。需要进一步严格且足够有力的RCT来正确探查这一点。
PICO
简语概要
氰基丙烯酸酯微生物封闭剂用于手术前皮肤准备
研究问题
我们的目的是从接受手术的人群的随机研究中寻找证据,以证明微生物密封剂是否会对手术部位感染的数量产生影响。
研究背景
手术部位感染是手术的一个严重并发症,细菌沾染手术伤口,阻碍有效愈合,在某些情况下还会扩散到身体的其他部位。研究表明,每二十名接受手术的人中,就有一人会患上此类感染。微生物密封剂是一种在手术前立即涂抹在皮肤表面的液体,用于封住皮肤上的任何细菌。在使用密封剂之前,通常要用10%聚维酮碘消毒溶液来准备手术部位的皮肤。
研究特征
我们发现了七项试验,总共涉及859人。其中四项试验涉及心脏手术,一项涉及腹股沟疝修复,一项涉及脊柱矫正,另一项涉及腿部动脉的手术修复。
研究结果
将这些试验的结果加在一起,表明使用微生物密封剂后手术部位感染率没有差异。平均而言,每100名接受手术的患者中,使用微生物密封剂的患者手术部位感染率比未使用微生物密封剂的患者减少6例。
不良反应
没有任何研究报告使用微生物密封剂的任何危险的副作用或反应,因此我们不能确定使用微生物密封剂的风险。
局限性
我们回顾的七项研究中偏倚风险各不相同。在某些情况下,存在很高的偏倚风险,因为用于设计研究的研究方法可能让受试者和护理提供者知道患者是否属于对照组。在其他研究中,作者采取了许多措施来尽量降低这种风险。总的来说,我们不能说任何一项研究完全没有偏倚。
Authors' conclusions
Background
Description of the condition
Surgical site infections (SSIs) are a continuing concern in health care. An SSI is defined as an infection that occurs within 30 days of surgery as the result of a surgical incision, manifesting as pus or a swab with more than 106 colony forming units (cfu) per mm³ tissue, and at least one of the following signs or symptoms: pain, localised swelling, redness or heat (Mangram 1999). Surgical incisions cause 25% to 38% of hospital‐acquired infections in surgical patients (Mangram 1999; Neumayer 2007). In clean surgery, the patient's own skin flora is highly likely to be the source of bacteria that lead to SSI (Dohmen 2008a) (see Appendix 1).
The Scottish Surveillance of Healthcare Associated Infection Programme in the UK estimated that SSI occurs in one in 20 cases of surgery (SSHAIP 2004), with an associated National Health Service (NHS) expenditure of GBP 1 billion annually. It also highlighted that the true cost of SSIs is much higher than figures would suggest, due to variations in the conduct of audits and collection of data. The reported incidence of SSI depends on a variety of factors, including: the definition of infection used, the intensity of surveillance, the nature and duration of patient follow‐up and the prevalence of risk factors in the population studied (Smyth 2000). In the UK, the National Audit Office 2004 noted that cases of infection can prolong a patient's stay in hospital by six days.
Preventative measures that can minimise SSI risk include patient skin preparation (Dumville 2015), preoperative hair removal (Tanner 2011), prophylactic antibiotics and the use of sterile disposable materials (Webster 2015).
The risk of SSI infection is influenced heavily by the nature of the surgery undertaken, and there is a widely used classification system that indicates the likelihood of SSI infection according to the risk of contamination during surgery (Appendix 1) (McLaws 2000). This review will be limited to clean surgery as SSI is least likely to occur after this type of surgery (infection rate of 3% to 5%), however, when surgery involves body cavities with infected, dead or dirty tissue ‐ for example in colorectal surgery (contaminated surgery) ‐ then SSI rates are typically higher, at between 10% and 30%.
Description of the intervention
Microbial sealants are liquids applied to the skin in the operating theatre, using an aseptic (sterile) technique, prior to surgery. The microbial sealant is applied to the surgical site immediately before surgery, after the usual preoperative skin preparation (cleansing and draping) is complete. The sealants dry to form a continuous barrier that prevents microbial migration and can be used in any type of surgery apart from that involving mucous membranes or the eyes. Instances of allergy and hypersensitivity to cyanoacrylate have been noted, and, since cyanoacrylate is an adhesive, it is suggested that accidental prolonged contact should be avoided . Cyanoacrylate sealants are supplied as a single‐use sterile pack. Integuseal is a proprietary cyanoacrylate sealant system designed to be administered via a ready‐to‐use applicator supplied in a single‐use sterile pack, with applicators offered in differing sizes depending on the surgery involved (Kimberly‐Clark 2008).
The use of cyanoacrylate microbial sealants to reduce SSI is relatively new. Cyanoacrylate forms the basis of current products that come under the term of microbial sealants. Recently the US Food and Drug Administration (FDA) gave regulatory approval for the use of topical skin adhesives, therefore, technological advances in the development of products such as cyanoacrylate may result in a number of types becoming available (Singer 2008). The focus of this review will be on cyanoacrylate‐based liquid microbial sealants.
How the intervention might work
Since the patient's own skin flora is the most common source of bacteria that cause SSIs (Nichols 1996), the aim of preoperative skin preparation is to ensure that the skin around the intended surgical site is as free as possible from endogenous bacteria, that may enter the surgical wound. Skin disinfection prior to surgery significantly reduces the number of bacteria on the skin surface, however, re‐colonisation with bacteria from deeper skin layers and hair follicles may occur during the operation (Fleischmann 1996). Cyanoacrylate‐based microbial sealant is applied before surgery in order to seal the skin flora beneath a breathable film.
Why it is important to do this review
Microbial sealants are currently being used as a method of skin preparation prior to surgery, and no systematic review exists to determine their effect on patient outcomes. Therefore, it is important to identify evidence associated with the use of microbial sealants on important outcomes such as rates of surgical site infection (SSI), the time wounds take to heal, length of stay in hospital and cost effectiveness. This review is important in determining the strength of evidence associated with the use of microbial sealants for skin preparation prior to surgery.
Objectives
To assess the effects of the preoperative application of microbial sealants (compared with no microbial sealant) on rates of SSI in people undergoing clean surgery.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished RCTs that allocate surgical participants individually either to receive microbial sealants in the immediate preoperative phase or to receive no microbial sealant. Trials were eligible for inclusion whether or not participants received usual preoperative skin preparation (e.g. chlorhexidine, povidone iodine). Quasi‐randomised trials were not included (e.g. trials that allocate treatment by sequential record number, sequential admitting number, day of the week).
Types of participants
Trials involving participants undergoing any type of clean surgery in an operating theatre.
Types of interventions
Microbial sealant applied to the surgical incision site immediately before surgery compared with no application of microbial sealant, with or without the use of traditional preoperative preparation solutions such as povidone iodine or chlorhexidine.
Types of outcome measures
Primary outcomes
Rates of SSI as defined by Mangram 1999, or by the study authors.
Secondary outcomes
-
All‐cause mortality.
-
Adverse reactions (e.g. contact dermatitis, anaphylaxis).
-
Other serious infection or infectious complication such as septicaemia or septic shock.
-
Length of hospital stay.
-
Rates of hospital re‐admissions.
-
Costs.
-
Postoperative antibiotic use.
Search methods for identification of studies
Electronic searches
For this update we searched the following electronic databases:
-
The Cochrane Wounds Specialised Register (searched 22 May 2015);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 4);
-
Ovid MEDLINE & Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (2013 to May 21 2015);
-
Ovid EMBASE (2013 to May 21 2015);
-
EBSCO CINAHL (2013 to May 22 2015).
The search strategies used for CENTRAL, Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3, Appendix 4 and Appendix 5 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions on the basis of date or language of publication.
Searching other resources
We searched reference lists of potentially eligible reports and review articles for further references.
We undertook the following to obtain any further data, published or unpublished for the original review but did not engage with these organisations again for the updates of this review.
-
We contacted wound care product manufacturers including Kimberly Clark, Smith & Nephew, Johnson & Johnson and 3M.
-
We contacted professional organisations including the Association of Perioperative Practitioners, the American Operating Room Nursing Organisation and the Australian College Operating Room Nurses.
Data collection and analysis
Selection of studies
All the review authors independently assessed the titles and abstracts of references identified by the search strategy, according to selection criteria, and obtained full versions of any articles that, from this initial assessment, satisfied the inclusion criteria. Independently we checked full papers to identify those that met the inclusion criteria, and resolved any disagreements by discussion. We screened reference lists of retrieved studies to identify further studies, and obtained full‐text copies for assessment. We settled any differences of opinion by consensus, or by referral to Cochrane Wounds editorial base.
For this second update one review author screened the search output, identified potential relevant studies and these were checked by a member of Cochrane Wounds editorial base.
Data extraction and management
We extracted and summarised study details using a piloted data extraction sheet. All review authors independently undertook data extraction and then discussed the findings to resolve any disagreement. If data were missing from reports, we contacted the study authors to request the missing information. Studies that had been published in duplicate we included only once, but extracted the maximum amount of data from all the study reports.
We extracted the following data from each study: study setting, number of participants, gender, mean age, predisposing risk factors, type of microbial sealant, use of prophylactic antibiotics, procedure and timing of adhesive application, period of postoperative follow‐up, all primary and secondary outcome descriptions and outcome measures reported, including infection rates and study authors' conclusions.
For this second update one review author extracted data from relevant studies and these data were checked by a member of Cochrane Wounds editorial base.
Assessment of risk of bias in included studies
In the original review and first update, all review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 6 for details of criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately. We discussed any disagreement amongst all review authors to achieve a consensus.
For this second update one review author assessed risk of bias and discussed the judgements with a member of Cochrane Wounds editorial base.
We presented assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgements in a cross‐tabulation of study by entry (Figure 1). This display of internal validity indicates the weight the reader may give the results of each study.
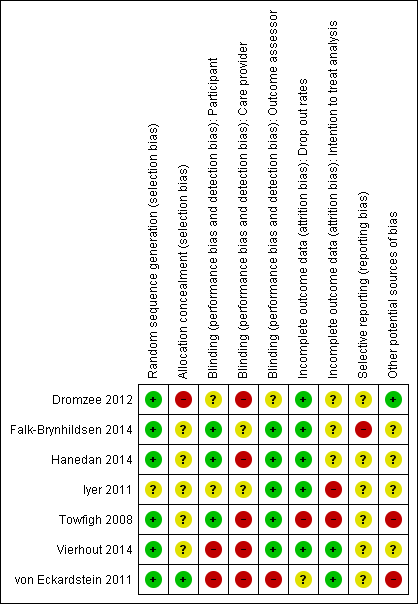
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We entered data into Cochrane Review Manager 5 software (RevMan 2014), and used this programme for the analysis. We planned to present effect measures for dichotomous outcomes (e.g. rates of infection) as risk ratio (RR) with 95% confidence intervals (CI). For continuous outcomes, we planned to use the mean difference (MD) or, if the scale of measurement differed across trials, standardised mean difference (SMD), each with 95% CI.
Assessment of heterogeneity
We planned to assess heterogeneity by first inspecting the graphical display of the estimated treatment effects. In addition we calculated the Chi2 statistic with significance set at P value less than 0.10. Any data below this threshold show evidence of heterogeneity of intervention effects. In addition, the degree of heterogeneity would have been investigated by calculating the I2 statistic, which examines the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I2 over 75% indicate a high level of heterogeneity (Higgins 2003).
Data synthesis
Results are presented with 95% confidence intervals (CI). Estimates for dichotomous outcomes (e.g. rates of infections ‐ yes or no) were reported as risk ratio (RR). The method of synthesising the studies was dependent on the quality, design and heterogeneity of the studies identified. In addition to the statistical synthesis of data we conducted a narrative review of the eligible studies.
Subgroup analysis and investigation of heterogeneity
If sufficiently similar studies had been identified, and I2 was less than 30%, a meta‐analysis would have been undertaken using a fixed‐effect model. This updated review identified four additional trials which were pooled using a random‐effects model (I2 = 42%).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The initial search identified 220 records, which included two that were identified through contact with manufacturers (Dohmen 2008b; Dohmen 2008c), and one obtained through accessing the ClinicalTrials.gov. web site (Owens 2010). We identified no additional trials through scanning reference lists. The majority of papers referred to the use of cyanoacrylate as a method of wound closure, and were not relevant to this review. Nine citations were reviewed in full, one of which was eligible for inclusion (Towfigh 2008). One citation by Wilson presented data reported in the included study and has been recorded as a secondary citation to the included study (Towfigh 2008). A study awaiting assessment in the initial review (Owens 2010) was subsequently published and included in the first update (von Eckardstein 2011). In addition we identified two further trials for the first update: one was included (Iyer 2011), and the other excluded (Dohmen 2011), as it was not randomised.
This second update identified 65 records, of which three met the inclusion criteria (Falk‐Brynhildsen 2014; Hanedan 2014; Vierhout 2014). We identified no additional trials through scanning reference lists. Four studies were awaiting assessment in the previous update; of which Dromzee 2012 was assessed and included; the three remaining studies were assessed but not included (Dohmen 2012; Doorly 2013; Waldow 2012).
Included studies
Seven studies met the inclusion criteria for the review (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011; Towfigh 2008; Vierhout 2014; von Eckardstein 2011).
Setting
All studies took place in the operating department. One was a multi‐centred US study (Towfigh 2008), one an international multi‐centred study (von Eckardstein 2011). The remainder took place in single hospitals in France (Dromzee 2012), Turkey (Hanedan 2014), Australia (Iyer 2011), or the Netherlands (Vierhout 2014). The setting of one study was not made explicit (Falk‐Brynhildsen 2014).
Participants
Dromzee 2012 included 56 participants undergoing posterior spinal correction for scoliosis. Participants had a mean age of 15 years. Falk‐Brynhildsen 2014 recruited 140 patients undergoing elective CABG with harvesting of the saphenous vein for at least two coronary arterial grafts: 110 participants were males and the mean age of all participants was 67 years. The venous harvest wound on the leg was used as the site of interest. Hanedan 2014 included 96 people undergoing cardiac surgery (specifically CABG, valve repair or repair of congenital heart disease), the mean age was 51 and 63 of the participants were male. The sternal wound site was assessed for infection. Iyer 2011 included 47 participants with a mean age of 67 years, 39 of whom were male, undergoing coronary artery bypass grafting (CABG). Randomisation was by leg, rather than by patient. The long saphenous vein site on both legs was chosen as the site of interest. Recruitment was halted at 47 participants after further ethical committee review. Towfigh 2008 included 177 participants, 170 of whom were male, undergoing an elective hernia repair in the operating theatre. The mean age was 53 years. The trial design had been informed by a sample size calculation based on the results from a porcine study, and it was estimated that 206 participants were required to demonstrate a 23% difference in wound contamination between groups, with 90% power. An interim analysis, however, was conducted after 104 participants had been recruited, and the sample size was revised to 742 participants required to detect a 10% difference in wound contamination with 80% power. The trial, however, was stopped prematurely having recruited only 177 participants, when the FDA granted regulatory approval for the product as a class II investigational device. The Vierhout 2014 study recruited 50 participants undergoing femoro‐popliteal, femoro‐crural or femoro‐femoral crossover bypass graft insertion; of these 28 participants were male and the mean age was 71. For this study an initial sample size calculation worked on the assumption of a 12% incidence of surgical site infection, meaning 180 patients would be required in both groups. However an interim analysis recorded an SSI incidence at 6% and as a result subsequent power calculations required the recruitment of 748 patients to both groups. At this point the review board giving ethical approval deemed this number of participants too large and the trial was stopped. von Eckardstein 2011 randomised 293 participants for CABG. One‐hundred and forty‐nine were male. The mean age of patients in this trial was 63 years. Both sternal and leg wounds were used as surgical sites for the study.
Experimental intervention
Two studies used the InteguSeal IS 200 applicator to administer the sealant (Dromzee 2012; Vierhout 2014). This applicator was used following standard preoperative skin preparation, namely 5% alcoholic povidone‐iodine solution in Dromzee 2012 and 0.5% chlorhexidine in 70% isopropyl alcohol in Vierhout 2014.
In Falk‐Brynhildsen 2014, an operating room nurse used the InteguSeal IS100 applicator to apply the microbial skin sealant on the patient’s leg (at the venous harvest site) just before the surgical incision but after preoperative skin preparation (which consisted of 0.5% chlorhexidine in 70% ethanol). Iyer 2011 used the InteguSeal IS 100 applicator to apply microbial sealant to saphenous vein harvest sites on one of the legs on a random basis (the other leg acted as the participant's control). This followed application of alcoholic povidone‐iodine solution. Both substances were allowed to dry after application.
In the Towfigh 2008 trial, a microbial, film‐forming, liquid sealant was applied via a disposable sponge, to the surgical sites of participants undergoing elective hernia repair. Application was immediately prior to the surgical incision and followed the standard preoperative skin preparation procedure of an application of 10% povidone‐iodine. In Hanedan 2014 cyanoacrylate microbial (InteguSeal) was applied after standard skin preparation (10% povidone‐iodine solution).
In the von Eckardstein 2011 trial, a microbial skin sealant (InteguSeal) was applied following standard surgical preparation. The sealant was considered dry when a film formed on the skin.
Control intervention
In all studies the standard preoperative skin preparation was used in the experimental and control sites: that is, 10% povidone‐iodine (Hanedan 2014; Towfigh 2008), alcoholic povidone‐iodine solution (Iyer 2011), 0.5% chlorhexidine solution in 70% ethanol (Falk‐Brynhildsen 2014), chlorhexidine 0.5% in 70% isopropyl alcohol (Vierhout 2014) or povidone iodine or 0.7% available iodine in isopropyl alcohol 74% weight/weight (w/w) (von Eckardstein 2011). In Dromzee 2012 5% povidone‐iodine was used for standard surgical skin preparation followed by 3M Steri‐Drapes in the control group. Drapes were also used in the control group in the Hanedan 2014 trial. In the text of Hanedan 2014 the phrase "control group" is at one point used to describe the use of sealants and vice versa ‐ correspondence with the author clarifies that the control group was indeed the group receiving drapes and skin preparation but no sealants, and that cyanoacrylate microbial sealants were used only in the intervention group.
Outcome measures
In Dromzee 2012 the primary outcome measure was surgical site infection, though it is unclear from the study text how this has been defined. Secondary outcomes in Dromzee 2012 were intraoperative blood loss, intraoperative time and number of vertebral levels fused.
The primary outcome measure reported by four trials was bacterial contamination (Falk‐Brynhildsen 2014; Hanedan 2014; Towfigh 2008; von Eckardstein 2011). Falk‐Brynhildsen 2014 measured postoperative skin contamination as a primary outcome measure; secondary end‐points were time to recolonisation and surgical site infection, defined as participants being given a prescription by a doctor for antibiotics to treat wound infection. In Hanedan 2014 primary outcome measures were bacterial contamination of wound site and postoperative white blood cell (WBC) count; rates of SSI (defined as any evidence of sternal wound infection at follow‐up) were a secondary outcome measure, and adverse events and mortality were also reported. Towfigh 2008 measured secondary outcomes that included: prevalence of antibiotic‐resistant Staphylococcus aureus in the wound during surgery; any difference in postoperative SSI rates; and safety outcomes associated with microbial sealant, including adverse events. In von Eckardstein 2011 secondary outcomes were comparison of bacterial count pre‐ and post‐CABG and the proportion of SSIs in both groups according to the Centers for Disease Control Prevention National Nosocomial Infections Surveillance (NNIS) criteria.
In two studies (Iyer 2011; Vierhout 2014) the primary outcome measure was surgical wound infection graded according to the Southamption wound grading system (Bailey 1992). Secondary outcomes in Iyer 2011 were irritation and allergies. Secondary outcomes in Vierhout 2014 were not described in the methodology section but all‐cause mortality and serious complications were reported.
Two studies were funded by Kimberly‐Clark Health Care, the manufacturers of the microbial sealant (Towfigh 2008; von Eckardstein 2011). In the von Eckardstein 2011 study two authors disclosed that they had a financial relationship with Kimberly‐Clark Corporation. Kimberly‐Clark Health Care provided applicators for experimental use in one study (Falk‐Brynhildsen 2014). Other studies disclosed no relationship with a manufacturer (Dromzee 2012; Hanedan 2014; Iyer 2011; Vierhout 2014).
Excluded studies
See Characteristics of excluded studies.
We excluded ten citations (not RCTs): Dohmen 2007 was a review article; Dohmen 2008b, Dohmen 2008d, and Dohmen 2009 were multiple publications of the same case‐control study; Dohmen 2008c was available in abstract form only with limited data ‐ the author was contacted but no reply was received; Pekar 2009 used unmatched cases and controls; Dohmen 2011 was a before/after trial, Dohmen 2012 was a letter, Doorly 2013 did not look at clean surgery and Waldow 2012 was not a RCT.
Risk of bias in included studies
Risk of bias in included studies
No trials were overall at low risk of bias.Though randomisation was performed well across the studies, allocation was infrequently concealed and blinding of care providers was mostly not done. See Figure 1; 'Risk of bias' summary and Figure 2 'Risk of bias' graph and Characteristics of included studies.
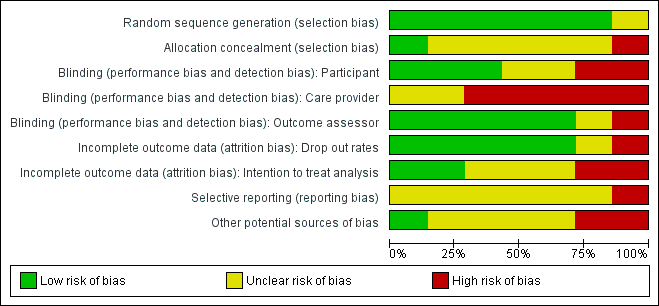
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Sequence generation
The method of generating the randomisation sequence was reported in six studies (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Towfigh 2008; Vierhout 2014; von Eckardstein 2011 ) but not reported in Iyer 2011.
Allocation concealment
The method of allocation concealment was not stated in four studies (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011). In Towfigh 2008 the sealed envelopes were not confirmed as opaque and sequentially‐numbered. In Vierhout 2014 envelopes were "blinded" but opacity and sequential numbering was not confirmed. von Eckardstein 2011 conducted allocation concealment and reported the method.
Blinding
Participant blinding was unclear in one study (Iyer 2011), and in three studies participants were blinded (Falk‐Brynhildsen 2014; Hanedan 2014; Towfigh 2008). In Vierhout 2014 patients were blinded to their allocation until 48 hours postoperatively, at which point dressings were taken down and the use of sealants inevitably revealed to the patient. No report of blinding of participants was made in Dromzee 2012. The von Eckardstein 2011 trial was described as an "open label" and therefore participants were not blinded. In five studies outcome assessors were blinded to the intervention (Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011; Towfigh 2008; Vierhout 2014). Dromzee 2012 does not describe blinding of care providers or outcome assessors. von Eckardstein 2011 was described as an open label trial therefore blinding of care providers or outcome assessors did not take place. The nature of the intervention may make blinding of care providers (viz., surgeons) quite difficult. This is acknowledged by both Dromzee 2012 and Towfigh 2008.
Incomplete outcome data
There were no drop outs in three studies (Dromzee 2012; Hanedan 2014; Iyer 2011). In Towfigh 2008 29 participants were lost to follow‐up, and data were not analysed on an intention‐to‐treat basis. Data were analysed by intention‐to‐treat analysis in von Eckardstein 2011, but 31 participants were described as ineligible for the per protocol analysis. Ten patients were lost to follow‐up (Falk‐Brynhildsen 2014), eight due to postoperative death and two due to language difficulties ‐ it is not clear from the study text that data were analysed on an intention‐to‐treat basis. There was a single postoperative death and no other drop outs in Hanedan 2014. In Vierhout 2014 three patients died postoperatively and though in the study text authors describe calculating power "on the basis of an intention‐to‐treat principle", these early postoperative deaths were excluded from subsequent analysis.
Selective outcome reporting
The presence of selective outcome reporting was unclear for all studies. Prespecified outcomes as stated in the studies were reported, but we were only able to access the study protocol for one study, Falk‐Brynhildsen 2014, which described a somewhat broader inclusion criteria and listed calculation of an ASEPSIS score as an outcome measure, though this calculation did not appear in the published report and no account was given for these differences in the study text.
Other potential sources of bias
In one study there was a baseline imbalance with a significantly longer operation time in the control group (Hanedan 2014). In two studies there was baseline imbalance between the groups in relation to obesity, with no apparent adjustment for this (Towfigh 2008; von Eckardstein 2011). In both studies there were more obese participants in the intervention group. No baseline imbalance was described in Dromzee 2012, Towfigh 2008 or Vierhout 2014.
Three trials were stopped prematurely (Iyer 2011; Towfigh 2008; Vierhout 2014). In Iyer 2011 the study was halted after further ethical review. The Towfigh 2008 trial was stopped prematurely when the FDA granted regulatory approval for InteguSeal (microbial sealant) as a class II medical device. The trial reports this as 'additional information' in the published paper. Neither Iyer 2011 nor Towfigh 2008 mentioned the effect of this early stopping on either the findings (up to the point of stopping), or on the risk of bias, neither was this event discussed in the papers. On this basis we would suggest that the studies were at high risk of bias. In Vierhout 2014 an interim analysis of SSI rates lead to an upwards revision of the sample size needed to yield adequate power, at which point the study's ethics committee halted the trial. There is no discussion of the effects of early stopping in the text, though the halting of the trial and the reasons behind this were addressed in the paper.
Effects of interventions
We identified seven trials, with 859 participants, that compared the use of microbial sealant with standard skin preparation in people undergoing clean surgery (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011; Towfigh 2008; Vierhout 2014; von Eckardstein 2011).
Primary outcome: Surgical site infection (SSI)
All seven trials reported surgical site infection (SSI) as an outcome measure, but definitions differed between trials (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011; Towfigh 2008; Vierhout 2014; von Eckardstein 2011). Overall 23/443 people developed a SSI in the groups where microbial sealant was applied compared with 46/416 in the control groups. We pooled the trials using a random‐effects model (I2 = 42%) and found there was no evidence of a difference between the groups in the rate of SSI reported (RR 0.53 95% CI 0.24 to 1.18) Analysis 1.1.
Secondary outcomes
All‐cause mortality
Mortality was reported in one study (von Eckardstein 2011), where there were four deaths (one in the intervention group and three in the control group) of cardiac/circulatory origin; none was considered to be related to the microbial sealant. Eight deaths were reported in another study (Falk‐Brynhildsen 2014) but the causes of these deaths were not discussed in the study text. Hanedan 2014 reported one postoperative mortality, though the reasons for this are not detailed in the trial report. There were two deaths due to postoperative cardiac complications and one due to bowel ischaemia reported in Vierhout 2014.
Adverse reactions
No adverse events were reported by Falk‐Brynhildsen 2014 or Iyer 2011. Towfigh 2008 reported adverse events in both groups but none were considered to be due to the microbial sealant. von Eckardstein 2011 reported adverse events (11 in the intervention group and 16 in the control group). Hanedan 2014 reported one case of sternal wound dehiscence (with no clinical or laboratory evidence of infection noted) in one patient in the microbial sealant group, requiring surgical management. Vierhout 2014 reported noninfectious lymphatic complications requiring fluid draining in two participants in the intervention group (n = 25) and two in the control group (n = 22), the authors do not state that they consider this related to the use or absence of cyanoacrylate sealants.
Other serious infection or infectious complications
In Towfigh 2008 one participant was given antibiotics for a deep infection with MRSA and was admitted to hospital for wound debridement, mesh removal and intravenous antibiotics. Four participants in Iyer 2011 required incision and drainage in the control group. In one study where wound contamination was being assessed, positive cultures were grown from samples from 38 patients, (27/68 from the microbial sealant group and 11/28 from the control group, in all of these cases there was no clinical evidence of infection (Hanedan 2014). One patient in the sealant group of the Vierhout 2014 study required further surgery at 22 days due to an infected graft following SSI.
Length of hospital stay, rates of hospital re‐admissions, costs
The above outcomes were not reported in any of the included studies.
Postoperative antibiotic use
In Falk‐Brynhildsen 2014 all patients received pre‐, intra‐ and post‐operative prophylactic antibiotics; in this trial the use of antibiotics in the two‐month postoperative period was used to define SSI. In Hanedan 2014 all patients received pre‐ and post‐operative prophylactic antibiotics. In Dromzee 2012, postoperative antibiotics and wound debridement were used to treat the six infection (event) cases. The two SSI cases amongst the control group in the Vierhout 2014 trial were treated with antibiotics (oral amoxicillin/clavulanic acid 625 mg for three days), which lead to healing of the SSI.
Discussion
Microbial sealant is thought to seal skin flora beneath a continuous film prior to skin incision, thus reducing the risk of an SSI. We decided to undertake a systematic review to establish its effectiveness, as it is a relatively new product that lacks a robust evidence base.
We identified seven trials that met the inclusion criteria for the review and investigated infection rates when microbial sealant was used compared with use of a standard preoperative method of skin preparation (Dromzee 2012; Falk‐Brynhildsen 2014; Hanedan 2014; Iyer 2011; Towfigh 2008; Vierhout 2014; von Eckardstein 2011). In five of these seven trials, participants in the intervention groups developed fewer SSIs, though there was no evidence of a difference between the two groups when the trials were pooled. All trials were at high or unclear risk of bias.
The review process was carried out with no departures from the review protocol. Although a relatively small number of studies were found in the initial review and first update, the identification of four more studies suggests that research is still ongoing in this area.
Across the trials there was unclear to high risk of bias overall. In Dromzee 2012 it was unclear who was following‐up patients and whether or not they were blinded . Amongst three of the four studies identified in this most recent (2015) update (Falk‐Brynhildsen 2014; Hanedan 2014; Vierhout 2014), randomisation was done well but allocation concealment was unclear and whilst outcome assessors were usually blinded, care providers were not. This probably represents a limitation of the study design. Randomisation and allocation concealment were unclear in one study (Iyer 2011), while in another the extent of allocation concealment was unclear and 16% of those randomised were lost to follow‐up (Towfigh 2008). In von Eckardstein 2011, there was no blinding of participants or outcome assessors, and reasons for drop outs were unclear. There were no participants lost to follow up reported in Dromzee 2012. In Falk‐Brynhildsen 2014 there were eight deaths but no explanation given. There was a single death reported in the Hanedan 2014 trial. In Vierhout 2014 there were three deaths and the reasons for these were made clear in the trial report. Three trials were stopped prematurely, one because the FDA granted regulatory approval for the product as a class II investigational device (Towfigh 2008), and two others as the result of a further review by the ethical committee (Iyer 2011; Vierhout 2014). None of these trials discussed the potential implications of this lack of power due to stopping prematurely, though in the study text Vierhout 2014 reported clearly the reasons for halting the trial. The Towfigh 2008 study was supported by the Kimberly‐Clark Corporation. The Kimberly‐Clark Corporation initiated and funded the von Eckardstein 2011 study, and two of the authors were employed by them. The same corporation provided applicators for use in the Falk‐Brynhildsen 2014 trial, but not direct financial support.
In this second update there is no robust evidence that microbial sealants may reduce the number of SSIs in clean surgery. Of the seven studies assessed, none were truly double‐blinded and overall the trials were at unclear or high risk of bias. Futhermore, these studies only involved a small number of participants and although this update included four additional studies the overall conclusion remains unchanged. We cannot draw firm conclusions about the effects of this intervention without further high‐quality evidence generated from well‐powered, double‐blinded, randomised controlled trials.
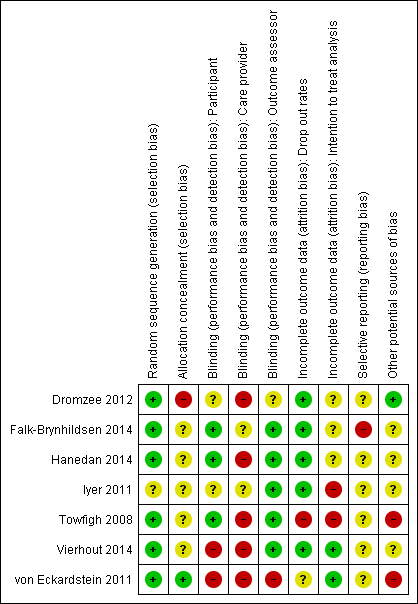
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
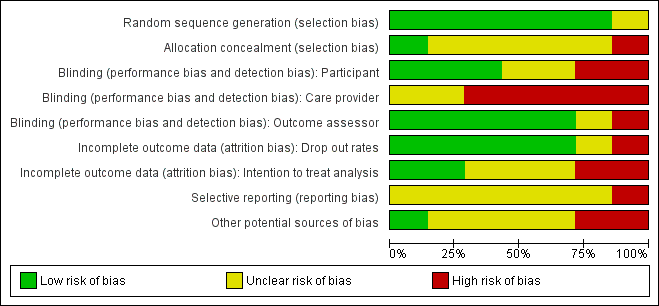
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Microbial sealant compared with no microbial sealant, Outcome 1 Surgical site infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.24, 1.18] |

