Linezolid w porównaniu z wankomycyną w leczeniu zakażeń skóry i tkanek miękkich
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open‐label, multicentred, randomised study. | |
| Participants | Location: 102 centres in the USA, Eastern and Western Europe, Latin America, South Africa, Malaysia, and Singapore. | |
| Interventions | Linezolid group (n = 537): 600 mg IV linezolid every 12 h; could be switched to oral at any time at investigator's discretion. | |
| Outcomes | Clinical outcomes: the number of cures. | |
| Notes | Aztreonam (or other antibiotic known to be inactive against Gram‐positive organisms/MRSA) and metronidazole were permitted to treat suspected Gram‐negative pathogens and anaerobic pathogens, respectively. Quote: "This study was funded by Pfizer, Inc; editorial support was provided by Elizabeth Melby Wells and Jean Turner of PAREXEL, Stamford, CT, and was funded by Pfizer, Inc; Kamal Itani has been a consultant and speaker for Pfizer; Matthew Dryden has been a member of advisory boards and has been a speaker for Pfizer, Wyeth, Bayer, and Jansen Cilag; Helen Bhattacharyya, Mark Kunkel, and Alice Baruch are employees of Pfizer; John Weigelt is a consultant to Pfizer, Ortho McNeil, and Schering‐Plough." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This prospective, randomised, open‐label, comparator‐controlled, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts: Linezolid group: 93/322 (28.9%); Vancomycin group: 108/318 (33.9%). |
| Incomplete outcome data (attrition bias) | High risk | 1052 randomised, 640 included in analysis. |
| Selective reporting (reporting bias) | Low risk | Quote: "study was conducted between October 2004 and July 2007 (Clinical Trials gov: no. NCT00087490)". |
| Other bias | Low risk | No other biases identified. |
| Methods | Randomised, multicentred, multinational, double‐blind study. | |
| Participants | Location: 58 sites in Australia, Austria, Belgium, Croatia, France, Germany, Greece, Italy, Poland, Russia, Slovenia, South Africa, Spain, and Switzerland. | |
| Interventions | Linezolid group (n = 304, 27 SSTIs): linezolid: 600 mg IV every 12 h. | |
| Outcomes | Clinical outcomes: clinical success was defined as cure (defervescence (abatement of fever) and resolution of signs and symptoms of infection), or improvement (defervescence and improvement of signs and symptoms of infection). Defervescence was defined as maximum oral temperature of ≤ 37.5 oC or axillary temperature of ≤ 36.7 oC on 3 consecutive days. Failure was defined as persistence, or progression, of clinical signs and symptoms of infection, or development of new findings. An indeterminate outcome was defined as an inability to make an assessment. | |
| Notes | SSTIs as a subgroup of Gram‐positive bacterial infection. Quote: "B.J., G.M., and J.P.‐O. received research grants from Pfizer. C.S.H., L.B.L, and K.J.T. are employed by Pfizer." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned in a 1:1 ratio". |
| Allocation concealment (selection bias) | Unclear risk | Comment: did not report whether allocation was concealed. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "To maintain blinding, a research pharmacist prepared study medications; an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". |
| Blinding (performance bias and detection bias) | Low risk | Quote: "To maintain blinding, a research pharmacist prepared study medications; an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "an unblinded co investigator monitored vancomycin or serum creatinine levels in accordance with local practice". Comment: knowledge of the intervention was unlikely to cause bias of vancomycin or serum creatinine levels, but the trial report did not state whether the assessor of signs and symptoms of infection was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: dropouts reported for all study participants, but not clear for SSTIs. dropouts: Linezolid group: 53/304 (17.4%); Vancomycin group: 64/301 (21.2%). |
| Incomplete outcome data (attrition bias) | High risk | 605 randomised, 488 included in analysis for all study participants. 47 randomised, 38 included in analysis for SSTIs. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not report whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
| Methods | Open‐label, comparator‐controlled, multicentred study, 2:1 ratio randomised. | |
| Participants | Location: 84 sites in Japan. | |
| Interventions | Linezolid group (n = 100, 31 SSTIs): linezolid 600 mg IV every 12 h, could be switched to oral after a minimum of 3 days. | |
| Outcomes | Clinical outcomes: the success rate was defined as the number of cures and improvements divided by the number of cures, improvements and failures. "Cured" defined as resolution of the clinical signs and symptoms of infection when compared with baseline; "improved" defined as improvement in 2 or more, but not all, clinical signs and symptoms of infection when compared with baseline; "failed" defined as persistence or progression of baseline clinical signs and symptoms of infection; and "indeterminate" defined as unable to assess. | |
| Notes | Patients could receive aztreonam or gentamicin (or other aminoglycosides with no activity against the isolated MRSA) for Gram‐negative coverage. Quote: "This study was sponsored by Pfizer Inc. Editorial support was provided by Philip Matthews at PAREXEL and was funded by Pfizer Inc." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, comparator‐controlled, multicentre study". Comment: the trial had an open‐label design, and, therefore was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | Only reported the number of dropouts, but not the reasons. These figures were not clear for SSTIs. |
| Incomplete outcome data (attrition bias) | High risk | 151 randomised, 92 were included in analysis for all study participants. 48 randomised, 28 were included in analysis for SSTIs. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
| Methods | Randomised, double‐blind, multicentred study. | |
| Participants | Location: 7 sites in China. | |
| Interventions | Linezolid group ( n = 71, 33 SSTIs): linezolid 600 mg IV every 12 h. | |
| Outcomes | Clinical outcomes: "cured" defined as complete resolution of 4 areas identified at baseline as abnormal: (i) signs; (ii) symptoms; (iii) haematology and chemistry; and (iv) microbiology; "marked improvement" defined as resolution of 3/4 areas; "improved" defined as resolution of at least 2 areas; "failed"defined as persistence or progression of baseline. | |
| Notes | Concomitant use of aztreonam was permitted in patients with documented mixed Gram‐positive and Gram‐negative organisms. Quote: "This study was sponsored by Pfizer Inc. Editorial support was provided by Jean Turner and Elizabeth Melby Wells of PAREXEL (Stamford, CT) and was funded by Pfizer Inc." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no report of whether allocation was concealed. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study". Comment: reported to be double‐blind, but no specific details provided about who was blinded. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study" |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "This Phase 3, randomised, double‐blind, comparator controlled, multicentre study" |
| Incomplete outcome data (attrition bias) | Low risk | dropouts were adequately addressed. dropouts(for all study participants): Linezolid group: 12/71 (16.9%); Vancomycin group: 14/71 (19.7%). |
| Incomplete outcome data (attrition bias) | High risk | 142 randomised, 121 included in analysis for all study participants. 62 randomised, 59 were included in analysis for SSTIs. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and the trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
| Methods | Single‐centred, open‐label randomised study. | |
| Participants | Location: USA. | |
| Interventions | Linezolid group (n = 30): linezolid 600 mg orally every 12 h. | |
| Outcomes | Clinical outcomes: clinical cure defined as temperature normalization; presence of granulation or wound healing; resolution of pain; and decreased or resolved erythema, oedema, induration, and colour. Ulceration could persist, but lesions must appear noninfected to be defined as clinically cured. Clinical improvement defined as moderate resolution of 2 or more clinical symptoms. Clinical failure defined as persistence or progression of baseline signs and symptoms, development of new symptoms consistent with Gram‐positive infection, or inability to complete the study because of adverse events. | |
| Notes | All patients received perioperative cefazolin while awaiting culture results. Patients could receive up to 48 h of topical or systemic antibiotics before randomisation. Quote: "Supported by an unrestricted educational grant from Pfizer Inc." Comment: this study was supported by Pfizer, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty patients were randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This single‐center, open‐label study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | Comment: data concerning dropouts were not reported. The trial paper only reported the percentage cured. We calculated the number of clinical cures from this percentage. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: did not report whether ITT was undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not report whether the published reports included all expected outcomes. |
| Other bias | Unclear risk | Comment: there was baseline imbalance as the group of patients who received linezolid were significantly younger than those who received vancomycin (66 vs 76 years), this is unlikely to be clinically significant. |
| Methods | Open‐label, multicentred, randomised phase III clinical trial. | |
| Participants | Location: 104 sites in North America, Europe, Latin America and Asia. | |
| Interventions | Linezolid group (n = 240, 122 SSTI): linezolid 600 mg IV twice daily, which could be changed to oral with clinical improvement. | |
| Outcomes | Clinical outcomes: used 4 possible clinical outcomes: "cure," "treatment failure," "indeterminate," or "missing." "Cure" defined as resolution of baseline clinical signs and symptoms of infection after ≥ 5 days and ≥ 10 doses of treatment. "Treatment failure" assigned if there was persistence or progression of signs and symptoms of infection after ≥ 2 days and ≥ 4 doses of treatment, or if there was no clinical assessment at end of therapy and test‐of‐cure. "Indeterminate" assigned if there was clinical improvement, or cure, at end of therapy but no test‐of‐cure assessment, or if there was cure after receipt of < 5 days or < 10 doses of study medication. "Missing" was assigned if < 2 days or < 4 doses of treatment were received. Length of stay. | |
| Notes | SSTIs were a subset of MRSA infection. The trial also included other infection types such as bacteraemia, pneumonia, and urinary‐tract infections. Quote: "D.L.S. has received funding from Pharmacia, Pfizer Pharmaceuticals, and Wyeth‐Ayerst for investigator‐initiated research proposals". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Hospitalized patients were randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This randomised open‐label trial". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Overall, 78 (32.5%) of 240 patients in the linezolid group and 69 (31.4%) of 220 in the vancomycin group discontinued treatment. The most common reasons for discontinuation of study medication were as follows: no methicillin‐resistant pathogen detected at baseline (13.3% of patients [32/240] in the linezolid group vs 17.3% of patients [38/220] in the vancomycin group) . . ." |
| Incomplete outcome data (attrition bias) | High risk | 460 randomised, 361 included in analysis for all study participants. 230 randomised, 186 included in analysis for SSTIs. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not report whether the published reports included all expected outcomes |
| Other bias | Unclear risk | Quote: "patients who received linezolid were significantly older than those who received vancomycin (63.9 vs 59.8 yrs p = 0.0157)". Comment: baseline imbalance reported, this is unlikely to be clinically significant, therefore, judged to be at unclear risk of bias. |
| Methods | Randomised, open‐label, multicentred study. | |
| Participants | Location: Asia Pacific, South America, North America, Europe and New Zealand. | |
| Interventions | Linezolid group (n = 592): linezolid 600 mg every 12 h, IV or oral. | |
| Outcomes | Clinical outcomes: patients counted as (i) "cured" if complete resolution of all pre‐therapy clinical signs and symptoms of infection (e.g. body temperature and white blood cell count) was achieved; (ii) "improved" if, at the end of treatment, 2 or more (but not all) of the pre‐therapy clinical signs and symptoms of CSSTI were resolved; (iii) "failed" if they exhibited persistence or progression of baseline clinical signs and symptoms of infection, development of new clinical findings consistent with active infection, or an inability to complete the study because of adverse events; and (iv) "indeterminate" if extenuating circumstances precluded classification to one of the above‐described categories, usually because of missed appointments. Mortality: the number of death in each group. Cause of death was judged by the investigator to be unrelated to the study drug. Duration of treatment. Length of stay. Cost. | |
| Notes | If MSSA was found, patients were switched to an appropriate antibiotic. Concomitant use of aztreonam or other antibiotics for Gram‐negative organisms was permitted. Quote: "J. Weigelt, D. Stevens, K. Itani, W. Lau, and M. Dryden have conducted research on behalf of Pfizer and have been on the Pfizer speakers' bureau. C. Knirsch is an employee of Pfizer, Inc." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a randomised, open‐label". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | dropouts were adequately addressed. |
| Incomplete outcome data (attrition bias) | High risk | 1200 randomised, 930 included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
| Methods | Open‐label, multicentred, randomised study. | |
| Participants | Location: 100 centres in Europe, USA, Latin America and Asia. | |
| Interventions | Linezolid group (n = 363, 164 SSTIs): linezolid 600 mg IV every 12 h; could be switched to oral. | |
| Outcomes | Clinical outcomes: assessed as "success" (cure with resolution of signs and symptoms or, at end of treatment only, improvement Microbiological outcomes: assessed as "success" (documented or presumed eradication based on clinical outcome) or "failure" All cause mortality: 1‐2 weeks after treatment. | |
| Notes | For methicillin‐susceptible pathogens, vancomycin could be switched to oxacillin 2 g IV, or dicloxacillin 500 mg orally, each given every 6 h. Concomitant therapy allowed on the basis of susceptibility and local practice. Quote: "M.H.W. has received honoraria for consultancy work, financial support to attend meetings, and research funding from Astra‐Zeneca, Bayer, Cerexa, Genzyme, Nabriva, Pfizer, Targanta, Vicuron" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". |
| Allocation concealment (selection bias) | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "This was a open‐label, multicenter comparative study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | dropouts (for all study participants): |
| Incomplete outcome data (attrition bias) | High risk | 726 randomised, 422 included in analysis for all study participants. 315 randomised, 296 included in analysis for SSTIs. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not stated whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
| Methods | Open label, randomised, multicentred study, randomised in a 2:1 ratio. | |
| Participants | Location: 59 sites throughout USA, Mexico and South America. | |
| Interventions | Linezolid group (n = 80): linezolid 10 mg/kg IV every 8 h; could be switched to oral after at least 3 days. | |
| Outcomes | Clinical outcomes: resolution of the signs associated with the cSSI, including lesion size, tenderness, erythema, swelling, induration, fluctuance, heat/localized warmth or discharge (purulent or nonpurulent). | |
| Notes | SSTIs as a subset of study Kaplan 2003. Quote: "From the Children's Memorial Hospital, Chicago . . . and Pharmacia Corp.". Comment: the study was funded by Pharmacia Corporation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised in a 2:1 ratio". |
| Allocation concealment (selection bias) | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Blinding (performance bias and detection bias) | High risk | Quote: "The methods for this open‐label, randomised, multicenter study". Comment: the trial had an open‐label design, and, therefore, was judged to be at high risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | Comment: data relating to dropouts were not reported. |
| Incomplete outcome data (attrition bias) | High risk | 120 randomised, 108 included in analysis for all study participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available and trial authors did not state whether the published reports included all expected outcomes. |
| Other bias | Low risk | No other biases identified. |
Abbreviations
≥ = equal to or greater than
> = greater than
≤ = equal to or less than
< = less than
cSSTI = complicated skin and soft tissue infection
h = hour(s)
IV = intravenously
ITT = intention‐to‐treat analysis
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT. | |
| Not compare linezolid vs. vancomycin. | |
| Cost‐effectiveness analysis, not a RCT. | |
| Cost‐effectiveness analysis, not a RCT. | |
| Cost‐effectiveness analysis, not a RCT. | |
| Not a RCT. | |
| A comment, not a RCT. | |
| A review; pooled data from three prospective clinical trials. | |
| Health economics analysis, not a RCT. | |
| Cost‐effectiveness analysis, not a RCT. | |
| Post‐hoc pooled data analysis, not a RCT | |
| Post‐hot pooled data analysis, not a RCT | |
| Cost‐effectiveness analysis, not an RCT. |
Abbreviation
RCT = randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 3114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.16] |
| Analysis 1.1  Comparison 1 Clinical cure, Outcome 1 All participants. | ||||
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| Analysis 1.2  Comparison 1 Clinical cure, Outcome 2 Adults' subgroup (≥ 18 years). | ||||
| 3 MRSA subgroup Show forest plot | 6 | 2659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.17] |
| Analysis 1.3  Comparison 1 Clinical cure, Outcome 3 MRSA subgroup. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.16] |
| Analysis 2.1  Comparison 2 Microbiological cure, Outcome 1 All participants. | ||||
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| Analysis 2.2  Comparison 2 Microbiological cure, Outcome 2 Adults' subgroup (≥ 18 years). | ||||
| 3 MRSA subgroup Show forest plot | 6 | 1289 | Risk Ratio (IV, Random, 95% CI) | 1.17 [1.04, 1.32] |
| Analysis 2.3  Comparison 2 Microbiological cure, Outcome 3 MRSA subgroup. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality during follow‐up Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.80] |
| Analysis 3.1  Comparison 3 Mortality, Outcome 1 All‐cause mortality during follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anaemia Show forest plot | 2 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| Analysis 4.1  Comparison 4 Adverse events, Outcome 1 Anaemia. | ||||
| 2 Diarrhoea Show forest plot | 3 | 2352 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.81, 3.88] |
| Analysis 4.2  Comparison 4 Adverse events, Outcome 2 Diarrhoea. | ||||
| 3 Red man syndrome Show forest plot | 2 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.29] |
| Analysis 4.3  Comparison 4 Adverse events, Outcome 3 Red man syndrome. | ||||
| 4 Pruritus Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| Analysis 4.4  Comparison 4 Adverse events, Outcome 4 Pruritus. | ||||
| 5 Rash Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.58] |
| Analysis 4.5  Comparison 4 Adverse events, Outcome 5 Rash. | ||||
| 6 Thrombocytopenia Show forest plot | 2 | 1300 | Risk Ratio (IV, Fixed, 95% CI) | 13.06 [1.72, 99.22] |
| Analysis 4.6  Comparison 4 Adverse events, Outcome 6 Thrombocytopenia. | ||||
| 7 Headache Show forest plot | 2 | 2232 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.59, 2.61] |
| Analysis 4.7  Comparison 4 Adverse events, Outcome 7 Headache. | ||||
| 8 Nausea Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.52, 3.94] |
| Analysis 4.8  Comparison 4 Adverse events, Outcome 8 Nausea. | ||||
| 9 Vomiting Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.96, 5.04] |
| Analysis 4.9  Comparison 4 Adverse events, Outcome 9 Vomiting. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of treatment (day) Show forest plot | 1 | 1180 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.32, 1.48] |
| Analysis 5.1  Comparison 5 Duration of treatment, Outcome 1 Duration of treatment (day). | ||||

Study flow diagram.
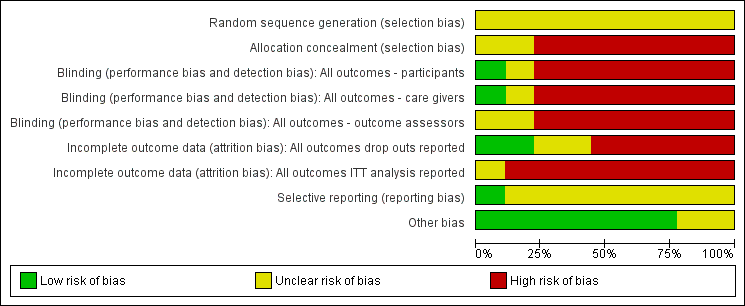
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
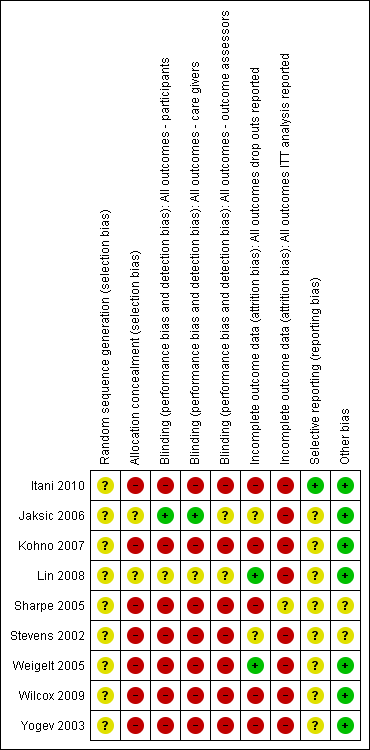
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
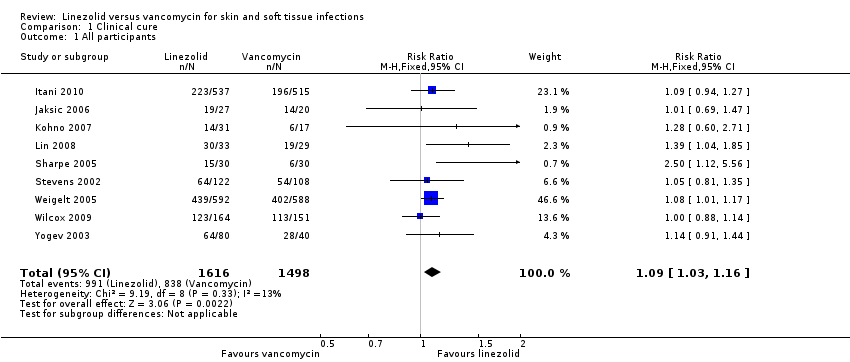
Comparison 1 Clinical cure, Outcome 1 All participants.

Comparison 1 Clinical cure, Outcome 2 Adults' subgroup (≥ 18 years).
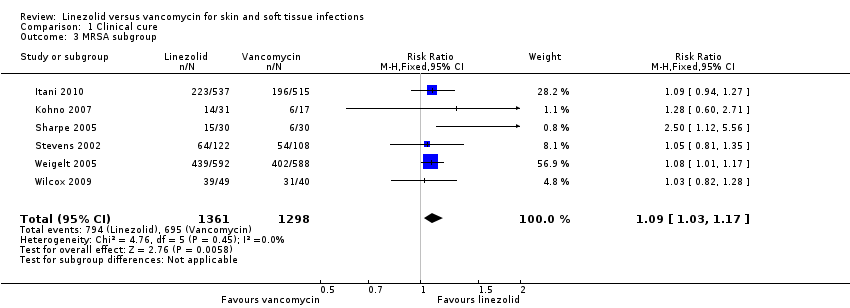
Comparison 1 Clinical cure, Outcome 3 MRSA subgroup.
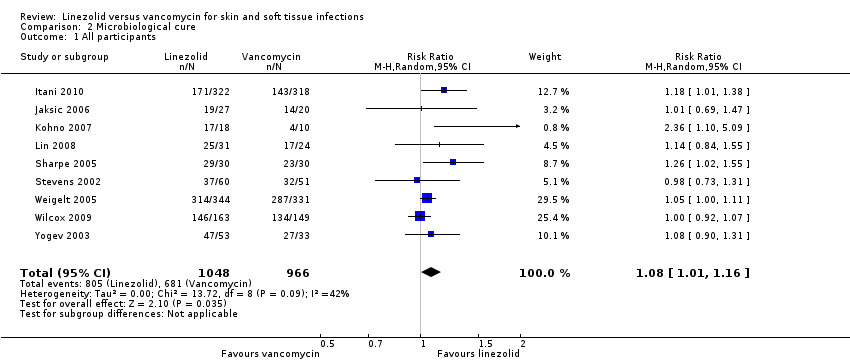
Comparison 2 Microbiological cure, Outcome 1 All participants.

Comparison 2 Microbiological cure, Outcome 2 Adults' subgroup (≥ 18 years).
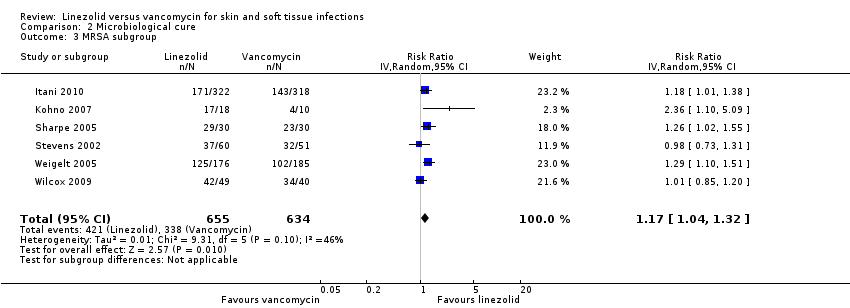
Comparison 2 Microbiological cure, Outcome 3 MRSA subgroup.
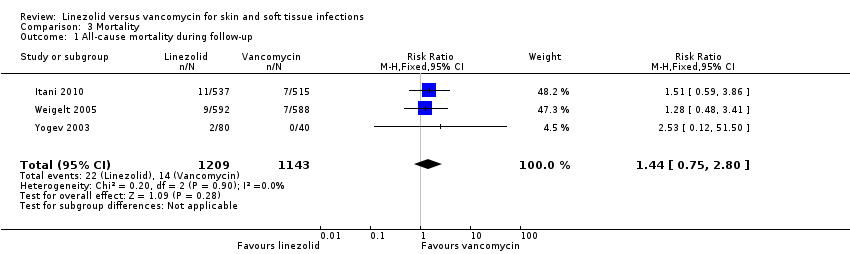
Comparison 3 Mortality, Outcome 1 All‐cause mortality during follow‐up.
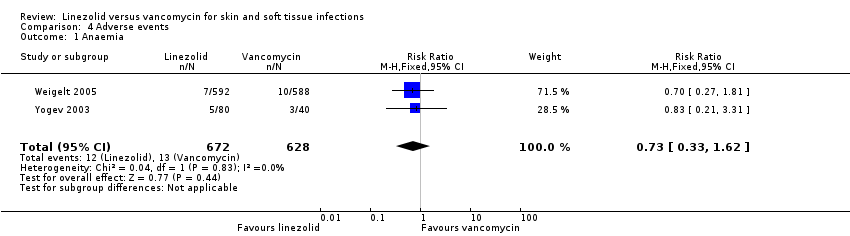
Comparison 4 Adverse events, Outcome 1 Anaemia.
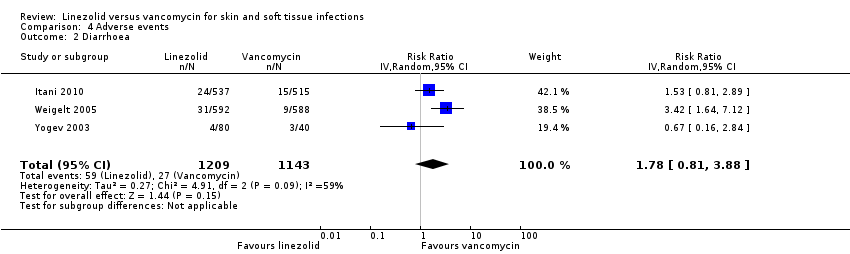
Comparison 4 Adverse events, Outcome 2 Diarrhoea.
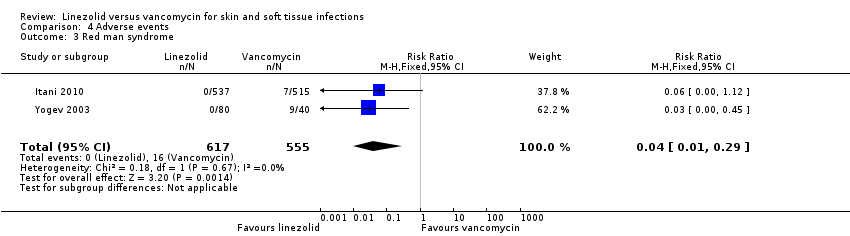
Comparison 4 Adverse events, Outcome 3 Red man syndrome.

Comparison 4 Adverse events, Outcome 4 Pruritus.

Comparison 4 Adverse events, Outcome 5 Rash.

Comparison 4 Adverse events, Outcome 6 Thrombocytopenia.
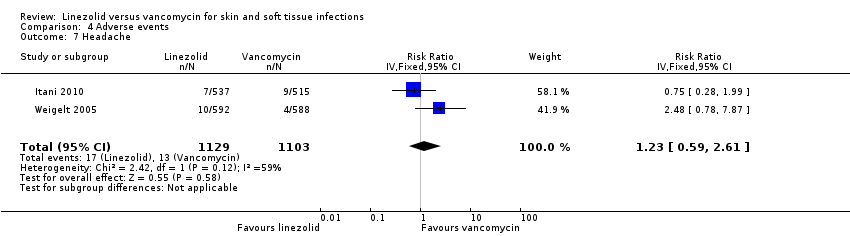
Comparison 4 Adverse events, Outcome 7 Headache.

Comparison 4 Adverse events, Outcome 8 Nausea.
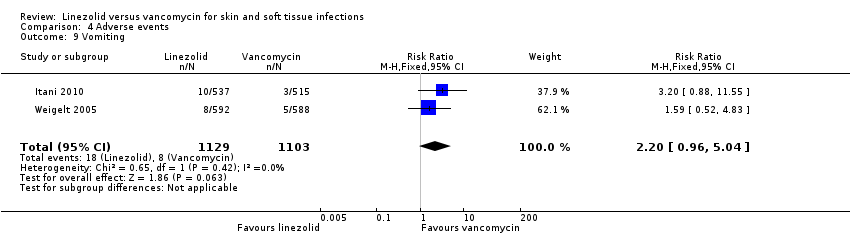
Comparison 4 Adverse events, Outcome 9 Vomiting.
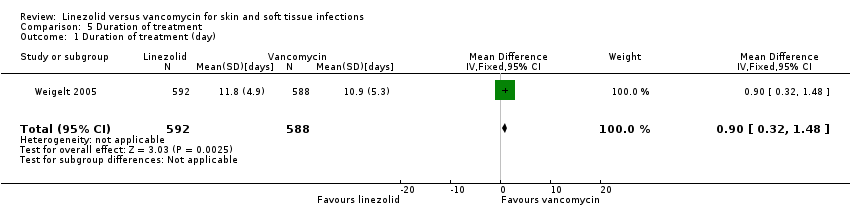
Comparison 5 Duration of treatment, Outcome 1 Duration of treatment (day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 3114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.16] |
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| 3 MRSA subgroup Show forest plot | 6 | 2659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| 3 MRSA subgroup Show forest plot | 6 | 1289 | Risk Ratio (IV, Random, 95% CI) | 1.17 [1.04, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality during follow‐up Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anaemia Show forest plot | 2 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| 2 Diarrhoea Show forest plot | 3 | 2352 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.81, 3.88] |
| 3 Red man syndrome Show forest plot | 2 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.29] |
| 4 Pruritus Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 5 Rash Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.58] |
| 6 Thrombocytopenia Show forest plot | 2 | 1300 | Risk Ratio (IV, Fixed, 95% CI) | 13.06 [1.72, 99.22] |
| 7 Headache Show forest plot | 2 | 2232 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.59, 2.61] |
| 8 Nausea Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.52, 3.94] |
| 9 Vomiting Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.96, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of treatment (day) Show forest plot | 1 | 1180 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.32, 1.48] |

