Linezolid u usporedbi s vankomicinom za liječenje infekcija kože i mekih tkiva
Appendices
Appendix 1. Glossary
| Terms | Interpretation | Abbreviations |
| Abscesses | Abscesses are localised or walled‐off accumulations of pus. They are caused by infections and can occur anywhere within the body. |
|
| β‐lactams | β‐lactam antibiotics are a broad class of antibiotics that share a similar molecular structure. |
|
| Bacteraemia | Bacteria infecting the blood. |
|
| Bioavailability | The rate at which a drug is absorbed by the body. |
|
| Carbuncles | Similar to an abscess, a carbuncle is a collection of infected hair follicles, often with multiple openings, and filled with pus and dead tissue. Carbuncles are caused by bacteria. |
|
| Cellulitis | A bacterial skin infection characterized by redness, swelling, and a feeling of heat or tenderness around the affected area. |
|
| Clinical cure | The resolution of all signs and symptoms of infections. |
|
| Complicated skin and soft tissue infection | An infection involving the deeper tissues of the body, including muscles and fat layers. Alternatively, SSTIs in patients with other illnesses such as diabetes or HIV. | cSSTI |
| Defervescence | The subsidence of fever. |
|
| Endocarditis | An inflammation of the valves and internal lining of the heart. |
|
| Erysipelas | A bacterial skin infection characterized by redness, swelling, sores and a feeling of heat or tenderness around the affected area. Erysipelas is more superficial than cellulitis. |
|
| Escherichia coli | A bacterium that belongs to the Enterobacteriaceae family. |
|
| Fascia | A layer of fibrous, connective tissue that often surrounds muscles, blood vessels and nerves. |
|
| Furuncles | Often called a boil, a furuncle is a collection of pus in the skin. Furuncles often appear in areas of friction such as underneath the belt, the fronts of the thighs, buttocks, groin, and armpits. |
|
| Gas gangrene | A bacterial infection that causes tissues to die, and gas to be produced within the tissues of the body. |
|
| Glycopeptides | A class of antibiotic. |
|
| Gram‐negative bacteria | One of two distinct types of bacteria. Gram‐negative bacteria do not turn purple when stained with a special dye. This is due to the structure of their cell walls. |
|
| Gram‐positive bacteria | One of two distinct types of bacteria. Gram‐positive bacteria turn purple when stained with a special dye. This is due to the structure of their cell walls. |
|
| Hypoderm/hypodermis | Tissue under the skin. |
|
| Iatrogenic | Illness caused by medical examination or treatment. |
|
| Impetigo | A common and highly contagious bacterial infection that causes blisters on the skin. |
|
| Meningitis | An inflammation of the membranes that surround the brain and the spinal cord. |
|
| Metastatic | The spread of a disease from one part of the body to another, non‐adjacent part. |
|
| Methicillin‐resistant Staphylococcus aureus | A strain of bacterium that has become resistant to the antibiotics commonly used to treat ordinary infections, particularly methicillin. | MRSA |
| Methicillin‐sensitive Staphylococcus aureus | A strain of bacterium that is sensitive to the commonly used antibiotic, methicillin. | MSSA |
| Microbiological cure | Eradication of bacteria in a wound; assessed by means of laboratory test or wound culture (a swab taken from the wound). |
|
| Necrotising skin and soft‐tissue infections | A rare, but very severe, type of bacterial infection that can destroy the muscles, skin, and underlying tissue. ‘Necrotising’ refers to something that causes tissue death. |
|
| Nephrotoxicity | The poisonous effect of some substances on the kidneys. |
|
| Neutropenia | A deficiency of white blood cells in the body. |
|
| Nosocomial | Originating or taking place in a hospital, acquired in a hospital, especially in reference to an infection. |
|
| Osteomyelitis | An infection in a bone. |
|
| Ototoxicity | Damage to the ears caused by a toxin. |
|
| Oxazolidinone | A type of antibiotic. |
|
| Parenterally | A way of introducing substances such as nutrients, or medication, by a non‐oral route, for example by injection. |
|
| Pruritus | Itching. |
|
| Pseudomonas aeruginosa | A type of bacterium often found in soil or ground water. It can cause illness and infection in humans. |
|
| Red man syndrome or erythroderma | An allergic reaction characterized by reddening of the upper body and itching. |
|
| Skin and soft tissue infections | Infections involving layers of the skin and the soft tissues beneath. | SSTIs |
| Staphylococcus aureus | A type of bacterium that lives on the skin and sometimes in nasal passages. It is the most common cause of skin and soft tissue infections. |
|
| Test of cure | Evaluation of the healing of skin and soft tissue infections after treatment. | TOC |
| Thrombocytopenia | A disorder that causes a decrease of platelets in blood. Platelets help the blood to clot. |
|
| Toxicity | Toxicity refers to the ability of a substance to cause harmful effects in the body. |
|
| Vancomycin‐resistant enterococci | Bacteria that have developed resistance to many antibiotics, especially vancomycin. | VRE |
Appendix 2. Search strategies for Ovid Medline, Ovid Embase and EBSCO CINAHL
Ovid Medline
1 exp Oxazolidinones/ (3454)
2 exp Oxazolone/ (471)
3 (linezolid$ or oxazolone$).ti,ab. (3313)
4 or/1‐3 (5205)
5 exp Glycopeptides/ (24374)
6 (vancomycin$ or glycopeptide$).ti,ab. (14989)
7 or/5‐6 (32269)
8 exp Soft Tissue Infections/ (1969)
9 exp Staphylococcal Skin Infections/ (2085)
10 exp Cellulitis/ (2621)
11 exp Erysipelas/ (360)
12 exp Furunculosis/ (298)
13 exp Abscess/ (17532)
14 exp Wound Infection/ (15200)
15 exp Fasciitis, Necrotizing/ (1891)
16 exp Myositis/ (6975)
17 exp Gas Gangrene/ (356)
18 (soft tissue infection$ or skin infection$).ti,ab. (4702)
19 (cellulitis or erysipelas or furuncul$ or abscess$ or absess$ or necrotizing fasciitis or myositis or gas gangrene or (wound$ adj2 infect$)).ti,ab. (44369)
20 or/8‐19 (69951)
21 4 and 7 and 20 (216)
22 randomized controlled trial.pt. (247475)
23 controlled clinical trial.pt. (40136)
24 randomized.ab. (201843)
25 placebo.ab. (93559)
26 clinical trials as topic.sh. (80952)
27 randomly.ab. (138890)
28 trial.ti. (75242)
29 or/22‐28 (558737)
30 Animals/ (2530681)
31 Humans/ (7027945)
32 30 not 31 (1649878)
33 29 not 32 (508211)
34 21 and 33 (43)
Ovid Embase
1 exp Oxazolidinone Derivative/ (2611)
2 exp Oxazolone/ (956)
3 exp Linezolid/ (10231)
4 (linezolid$ or oxazolone$).ti,ab. (5207)
5 or/1‐4 (13224)
6 exp Vancomycin/ (43322)
7 exp Vancomycin Derivative/ (216)
8 exp Glycopeptide/ (5164)
9 (vancomycin$ or glycopeptide$).ti,ab. (22171)
10 or/6‐9 (51486)
11 exp Soft Tissue Infection/ (5340)
12 exp Skin Infection/ (77395)
13 exp Cellulitis/ (8532)
14 exp Erysipelas/ (1406)
15 exp Furunculosis/ (857)
16 exp Abscess/ (41568)
17 exp Wound Infection/ (19807)
18 exp Necrotizing Fasciitis/ (3450)
19 exp Myositis/ (15627)
20 exp Gas Gangrene/ (680)
21 (soft tissue infection$ or skin infection$).ti,ab. (7434)
22 (cellulitis or erysipelas or furuncul$ or abscess$ or absess$ or necrotizing fasciitis or myositis or gas gangrene or (wound$ adj2 infect$)).ti,ab. (65393)
23 or/11‐22 (178863)
24 5 and 10 and 23 (1789)
25 Clinical trial/ (715292)
26 Randomized controlled trials/ (29861)
27 Random Allocation/ (51197)
28 Single‐Blind Method/ (15897)
29 Double‐Blind Method/ (87219)
30 Cross‐Over Studies/ (32445)
31 Placebos/ (169756)
32 Randomi?ed controlled trial$.tw. (82914)
33 RCT.tw. (10982)
34 Random allocation.tw. (931)
35 Randomly allocated.tw. (14603)
36 Allocated randomly.tw. (1227)
37 (allocated adj2 random).tw. (266)
38 Single blind$.tw. (9897)
39 Double blind$.tw. (92147)
40 ((treble or triple) adj blind$).tw. (248)
41 Placebo$.tw. (140349)
42 Prospective Studies/ (206934)
43 or/25‐42 (1077729)
44 Case study/ (16788)
45 Case report.tw. (170882)
46 Abstract report/ or letter/ (519805)
47 or/44‐46 (703087)
48 43 not 47 (1048538)
49 animal/ (730814)
50 human/ (8821758)
51 49 not 50 (489053)
52 48 not 51 (1026150)
53 24 and 52 (546)
EBSCO CINAHL
S17 S16 and S4 and S1
S16 S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5
S15 wound* N2 infection*
S14 cellulitis or erysipelas or furuncul* or abscess* or absess* or necrotizing fasciitis or myositis or gas gangrene
S13 soft tissue infection* or skin infection*
S12 (MH "Gas Gangrene")
S11 (MH "Myositis")
S10 (MH "Fasciitis, Necrotizing")
S9 (MH "Wound Infection+")
S8 (MH "Abscess+")
S7 (MH "Furunculosis")
S6 (MH "Cellulitis")
S5 (MH "Soft Tissue Infections")
S4 (S3 or S2)
S3 glycopeptide*
S2 (MH "Vancomycin")
S1 linezolid* or oxazolone* or oxazolidinone*
Appendix 3. Assessment of risk of bias in included studies
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, because allocation based on one of the following or an equivalent method: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias.
High risk of bias
Any one of the following:
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to have introduced bias.
Unclear
Either of the following:
-
Insufficient information to permit judgement of low or high risk of bias.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers, or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
-
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
-
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
-
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
-
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
-
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified.
High risk of bias
Any one of the following:
-
Not all of the study's pre‐specified primary outcomes have been reported.
-
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
-
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
-
had a potential source of bias related to the specific study design used; or
-
had extreme baseline imbalance; or
-
has been claimed to have been fraudulent; or
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists (e.g. baseline imbalances); or
-
insufficient rationale or evidence that an identified problem will introduce bias.

Study flow diagram.
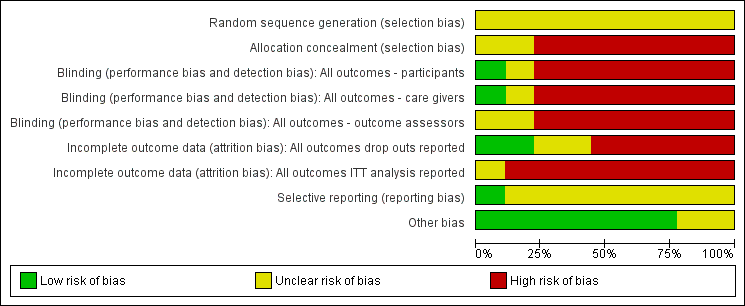
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
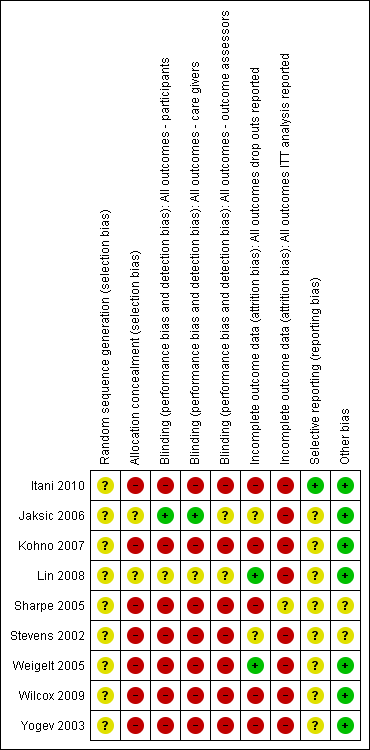
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
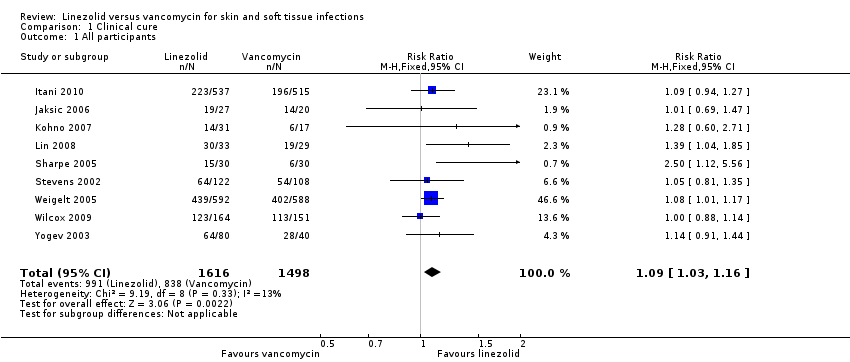
Comparison 1 Clinical cure, Outcome 1 All participants.

Comparison 1 Clinical cure, Outcome 2 Adults' subgroup (≥ 18 years).
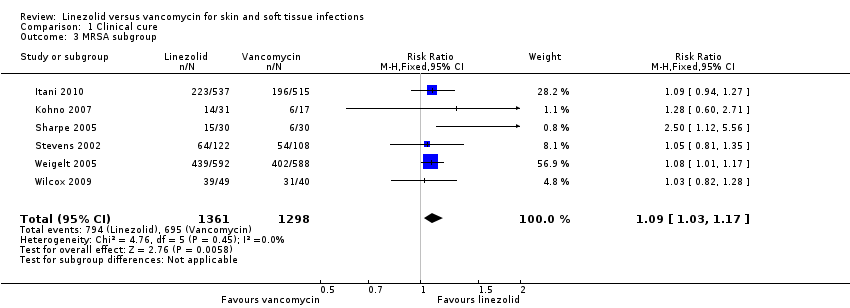
Comparison 1 Clinical cure, Outcome 3 MRSA subgroup.
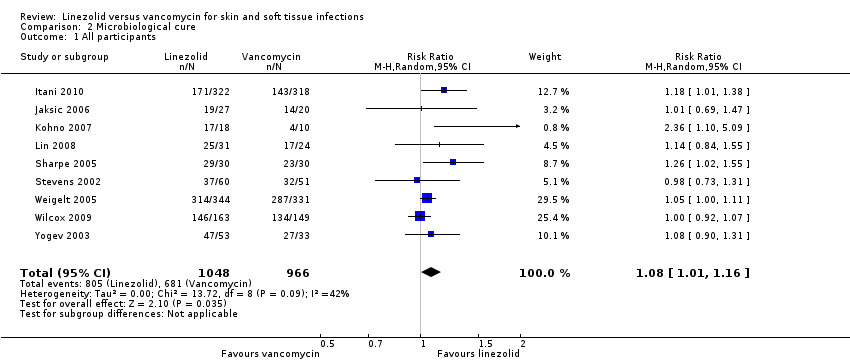
Comparison 2 Microbiological cure, Outcome 1 All participants.

Comparison 2 Microbiological cure, Outcome 2 Adults' subgroup (≥ 18 years).
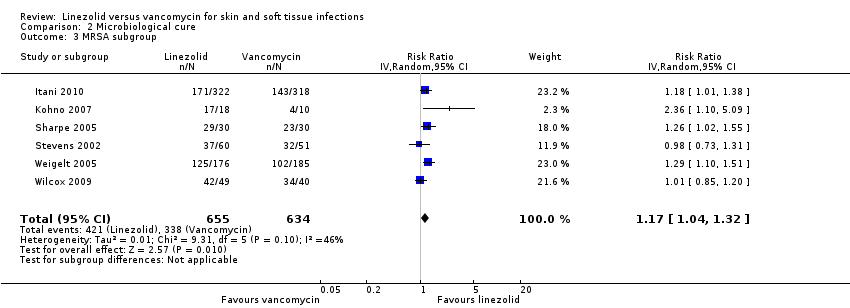
Comparison 2 Microbiological cure, Outcome 3 MRSA subgroup.
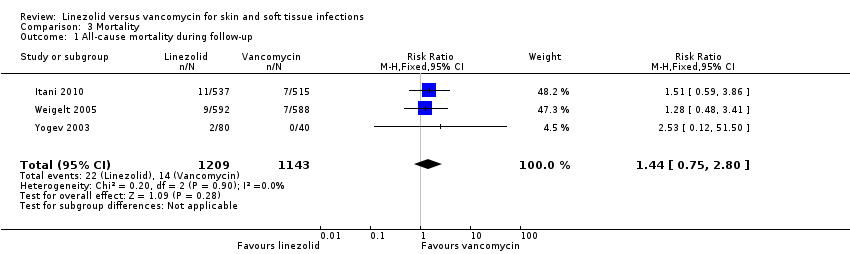
Comparison 3 Mortality, Outcome 1 All‐cause mortality during follow‐up.
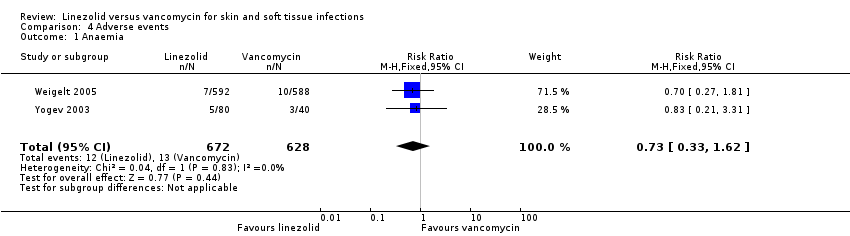
Comparison 4 Adverse events, Outcome 1 Anaemia.
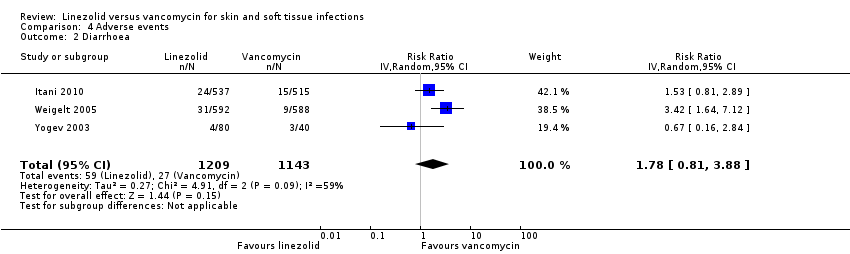
Comparison 4 Adverse events, Outcome 2 Diarrhoea.
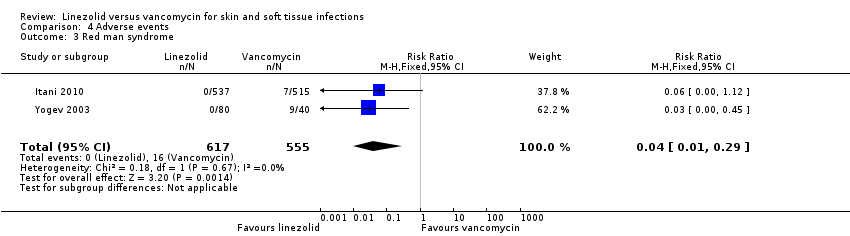
Comparison 4 Adverse events, Outcome 3 Red man syndrome.

Comparison 4 Adverse events, Outcome 4 Pruritus.

Comparison 4 Adverse events, Outcome 5 Rash.

Comparison 4 Adverse events, Outcome 6 Thrombocytopenia.
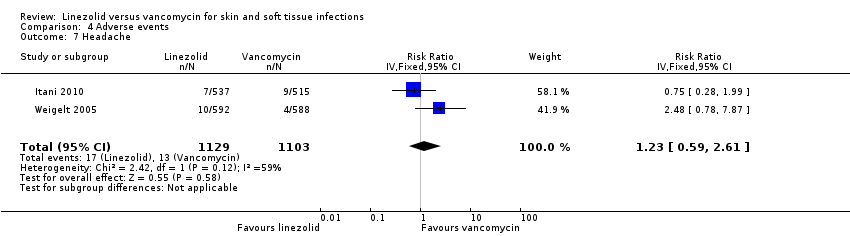
Comparison 4 Adverse events, Outcome 7 Headache.

Comparison 4 Adverse events, Outcome 8 Nausea.
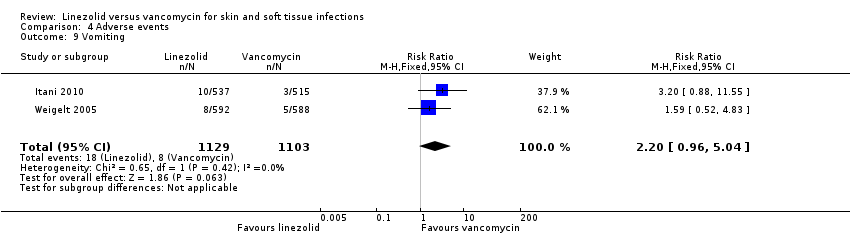
Comparison 4 Adverse events, Outcome 9 Vomiting.
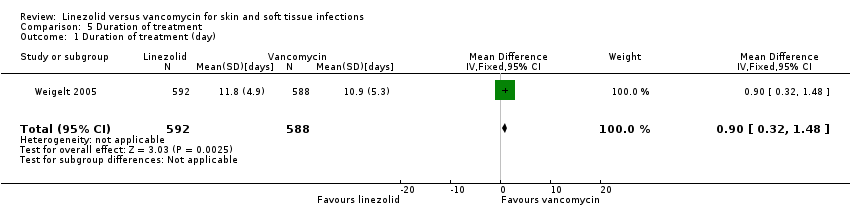
Comparison 5 Duration of treatment, Outcome 1 Duration of treatment (day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 3114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.16] |
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 2402 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| 3 MRSA subgroup Show forest plot | 6 | 2659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All participants Show forest plot | 9 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Adults' subgroup (≥ 18 years) Show forest plot | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.34] |
| 3 MRSA subgroup Show forest plot | 6 | 1289 | Risk Ratio (IV, Random, 95% CI) | 1.17 [1.04, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality during follow‐up Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.75, 2.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Anaemia Show forest plot | 2 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| 2 Diarrhoea Show forest plot | 3 | 2352 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.81, 3.88] |
| 3 Red man syndrome Show forest plot | 2 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.29] |
| 4 Pruritus Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.75] |
| 5 Rash Show forest plot | 3 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.58] |
| 6 Thrombocytopenia Show forest plot | 2 | 1300 | Risk Ratio (IV, Fixed, 95% CI) | 13.06 [1.72, 99.22] |
| 7 Headache Show forest plot | 2 | 2232 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.59, 2.61] |
| 8 Nausea Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.52, 3.94] |
| 9 Vomiting Show forest plot | 2 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.96, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of treatment (day) Show forest plot | 1 | 1180 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.32, 1.48] |

