Injection périanale d'agents gonflants comme traitement de l'incontinence fécale chez l'adulte
Résumé scientifique
Contexte
L'incontinence fécale est une maladie complexe et pénible aux implications médicales et sociales importantes. L'injection périanale d'agents gonflants est utilisée pour traiter les symptômes de l'incontinence fécale passive. Toutefois, différents agents ont été utilisés sans qu'il y ait une technique standardisée ; le bénéfice supposé du traitement est essentiellement de l'ordre du ouï dire et la base de recherche clinique est limitée.
Objectifs
Déterminer l'efficacité de l'injection périanale d'agents gonflants pour le traitement de l'incontinence fécale chez l'adulte.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre spécialisé d'essais cliniques du groupe Cochrane sur l’incontinence (25 mai 2012), ainsi que dans des registres d'essais cliniques et dans les références bibliographiques d'articles pertinents.
Critères de sélection
Tous les essais randomisés ou quasi‐randomisés ayant comparé l'utilisation d'agents gonflants injectables pour l'incontinence fécale à d'autres traitements ou à un placebo ont été examinés afin d'évaluer les effets thérapeutiques. Des études cas‐témoin et de cohorte ont également été examinées pour évaluer les risques et les complications associés aux traitements.
Recueil et analyse des données
Deux auteurs de la revue (YM et CN) ont évalué la qualité méthodologique des essais éligibles et ont extrait indépendamment les données des essais inclus en utilisant une série de mesures de résultat prédéfinies.
Résultats principaux
Cinq essais randomisés éligibles ont été identifiés, totalisant 382 patients. Quatre de ces essais étaient à risque incertain ou élevé de biais.
La plupart des essais avaient rendu compte d'un bénéfice à court terme des injections quel que soit le matériau utilisé, y compris l'injection de placebo salin. Une étude avait démontré que le dextranomère dans de l'acide hyaluronique stabilisé (NASHA Dx) était plus efficace que l'injection simulée, mais avec plus d'effets indésirables. Le dextranomère dans de l'acide hyaluronique stabilisé (NASHA Dx) était supérieur aux injections simulées après six mois (pour 65/136, 48% versus 48/70, 69 % des participants il n'y avait pas eu d'amélioration, c.‐à‐d. que la réduction des épisodes d'incontinence était de moins de 50%, RR 0,70 , IC à 95% 0,55 à 0,88), avec plus de jours sans incontinence (3,1 jours versus 1,7 dans le groupe à traitement simulé, DM 1,40 jours, IC à 95% 0,33 à 2,47). Une autre étude ayant comparé un matériau de silicone (PTQ™) aux injections de solution saline était de trop petite taille pour mettre en évidence un bénéfice clinique.
Un biomatériau de silicone (PTQ™) s'est avéré présenter certains avantages et était plus sûr à court terme pour le traitement de l'incontinence fécale que les billes revêtues de carbone (Durasphere®).
Il y avait de même des bénéfices à court terme de l'injection sous contrôle échographique par rapport au guidage digital.
Il n'y avait aucun élément probant sur des résultats à long terme, et les données disponibles ne permettent pas d'autres conclusions. Aucune des études n'avait rendu compte d'une évaluation des résultats par les patients et il est donc difficile d'apprécier si l'amélioration des scores d'incontinence correspond à une amélioration des symptômes pratiques qui sont importants pour les patients.
Conclusions des auteurs
Un grand essai contrôlé randomisé a montré que ce type de traitement au moyen de dextranomère dans de l'acide hyaluronique stabilisé (NASHA Dx) améliore à court terme la continence chez un peu plus de la moitié des patients. Le nombre d'essais identifiés était toutefois limité et la plupart présentaient des faiblesses méthodologiques.
Résumé simplifié
Injection d'un matériau gonflant autour de l'anus pour le traitement de l'incontinence fécale chez l'adulte
La perte de contrôle de la défécation, appelée aussi incontinence fécale, peut être un problème terrible. Elle affecte des hommes et des femmes de tous âges jusqu'à représenter 15% de la population adulte. L'incontinence fécale peut affecter radicalement la vie quotidienne, car beaucoup de personnes ne peuvent quasiment plus quitter la maison et deviennent incapables d'exécuter des tâches simples, telles que faire des courses, par crainte de fuites fécales.
L'incontinence fécale peut être due à un problème au niveau des deux muscles entourant l'anus. Ces muscles peuvent être endommagés ou s'affaiblir en raison d'une blessure à l'accouchement ou d'opérations pour le traitement d'affections anales telles que les hémorroïdes et les fistules (communications anormales entre la peau et l'anus). Les muscles de l'anus deviennent alors incapables de retenir les selles jusqu'à ce que la personne atteigne les toilettes. La bague interne ou muscle du sphincter anal interne maintient normalement l'anus fermé en permanence sauf lors de la défécation, empêchant ainsi la fuite passive de selles. Un traitement a été développé pour l'incontinence fécale, qui consiste à injecter une substance dans ou à proximité de ce muscle pour le rendre plus volumineux afin que l'anus soit mieux fermé. Il a été préconisé en tant qu'option simple et sûre.
Dans cette revue, un essai à grande échelle avait montré que l'injection de dextranomère stabilisé dans de l'acide hyaluronique améliore les symptômes de l'incontinence à court terme chez un peu plus de la moitié de ceux ayant reçu le traitement. Les quatre autres essais examinés étaient d'une utilité limitée parce qu'ils étaient généralement de qualité méthodologique médiocre et de petite taille. Aucun résultat à long terme de ce traitement n'avait été rapporté.
Authors' conclusions
Background
Description of the condition
Faecal incontinence is defined as involuntary loss of solid or liquid faeces (stools, motions). It is a distressing condition with significant social and medical implications. The prevalence of faecal incontinence may range from 1.6% to 15% depending on how it is defined, age and whether the patients are community‐dwelling or living in an institution (Whitehead 2009). The symptom has a major impact on quality of life and activities of daily living, in many cases causing extreme embarrassment and discomfort.
Faecal incontinence has many diverse causes. The maintenance of continence is a complex mechanism which consists of the interaction of an intact and functional anal sphincter complex, the consistency of the faeces, adequate cognitive ability and physical mobility, and bowel motility. Any impairment of these elements can result in incontinence.
The symptoms of incontinence have traditionally been divided into two symptom groups, urge and passive incontinence. Urge incontinence is loss of faeces following the inability to resist the urge to defaecate for long enough to reach a toilet and is primarily associated with external anal sphincter (EAS) dysfunction. Passive faecal incontinence is involuntary loss of faeces without the urge to defaecate and is predominantly associated with internal anal sphincter (IAS) dysfunction (Engel 1995 ). The multiple possible causes and contributing factors to faecal incontinence are reviewed elsewhere (Bharucha 2010; NICE 2007; Rao 2004). This review focuses specifically on treatment for passive faecal incontinence secondary to IAS weakness.
The causes of IAS dysfunction can be classified into two main groups. One is a morphologically intact but functionally weak IAS and the second is a structurally damaged IAS.
-
A structurally intact but weak IAS may be due to primary degeneration or systemic disease such as systemic sclerosis (Vaizey 1997). Radiotherapy can damage the myenteric plexus leading to diminished IAS function (Da Silva 2003).
-
Destruction of or structural damage to the IAS can be caused by anal surgery such as anal dilatation, sphincterotomy, fistula surgery and haemorrhoidectomy (Lindsey 2004). Obstetric injury such as a third or fourth degree vaginal tear may extend beyond the vagina to damage the IAS and this direct anal trauma may disrupt the structure of the IAS (Wheeler 2007).
Symptoms are likely to be exacerbated by loose faeces or incomplete rectal evacuation. The exact cause of passive soiling for an individual patient is often unclear.
Currently, the treatment for faecal incontinence associated with IAS dysfunction remains challenging. Medical management, such as modifying the consistency of the faeces by anti‐diarrhoeal agents (Cheetham 2003) or pelvic floor muscle training with or without biofeedback, is effective to a certain extent (Norton 2012) but many people do not find such conservative treatment to be an ideal long term solution. The IAS is not amenable to direct surgical repair (Felt‐Bersma 1996; Leroi 1997; Morgan 1997) and major surgery, such as implantation of an artificial bowel sphincter or dynamic graciloplasty, is associated with significant morbidity (Christiansen 1999; Wexner 1996). Such interventions may be disproportionate to symptoms. Sacral nerve stimulation is an emerging treatment option for faecal incontinence but has not yet been proven to be effective for IAS dysfunction. There is, therefore, a large gap between conservative and major surgical options for treating passive faecal incontinence.
This review focused specifically on one treatment that has been developed for the symptom of passive faecal incontinence secondary to IAS disruption or weakness. Although there has been an exploratory study injecting somatic muscle cells, using a similar technique to that to either fill in a defect or regenerate muscles for EAS injury (Frudinger 2010), it is not included within the scope of this review.
For further information on other interventions for faecal incontinence treatment, please refer to Cochrane reviews on:
-
surgery for faecal incontinence in adults (Brown 2010),
-
sacral nerve stimulation for faecal incontinence and constipation in adults (Mowatt 2007),
-
plugs for containing faecal incontinence (Deutekom 2012),
-
electrical stimulation for faecal incontinence in adults (Hosker 2007),
-
drug treatment for faecal incontinence in adults (Cheetham 2003),
-
biofeedback or sphincter exercises, or both, for the treatment of faecal incontinence in adults (Norton 2012), and
-
absorbent products for moderate‐heavy urinary or faecal incontinence, or both, in women and men (Fader 2008).
Description of the intervention
Injection of bulking agents has emerged as a new treatment for faecal incontinence following on from reported success in treating urinary incontinence. The concept is to inject a biocompatible material in order to close the anal canal to avoid faecal incontinence. This may be performed under local, regional or general anaesthetic, and can also be used as a 'minimally invasive' treatment in an outpatient clinic setting.
Ten different materials have been used (autologous fat, Teflon, bovine glutaraldehyde cross‐linked collagen, carbon‐coated zirconium beads, polydimethylsiloxane elastomer, dextranomer in non‐animal stabilised hyaluronic acid, hydrogel cross‐linked with polyacrylamide, porcine dermal collagen, synthetic calcium hydroxylapatite ceramic microspheres, and polyacrylonitrile in cylinder form). The material can be injected either via the perianal skin or via the anal mucosa. The injection may be guided by the surgeon's finger in the anal canal or by ultrasound visualisation of placement.
How the intervention might work
The aim is to inject a biocompatible material into the submucosa of the anal canal or into the space between the anal sphincters in order to bulk out the tissue around the anal canal and approximate the anal mucosa, thereby closing the anal canal or raising the pressure inside the anal canal to prevent faecal incontinence.
Why it is important to do this review
This treatment is becoming widespread following the results of observational studies as it is potentially attractive in its simplicity and minimal invasiveness. However, it is not clear if this intervention is effective, cost effective or if any effect persists in the long term. There is no consensus regarding the indications for the treatment nor the choice of optimum material. There is little information on possible adverse events (either acute such as pain and infection or chronic such as new difficulties with evacuation). We aimed to systematically review the trials published to date and combine best evidence currently available oto base recommendations for clinical practice.
Objectives
The objective of this review was to determine if the injection of bulking agents is better than currently available treatments or no treatment for faecal incontinence in adults in terms of effectiveness, complications and quality of life.
The following comparisons were made:
1. injection of bulking agents versus no intervention or a placebo or sham injection;
2. injection of bulking agent versus any single or combination conservative treatment (such as biofeedback, diet, anal plug, anti‐diarrhoeal medication, pelvic floor exercise);
3. injection of bulking agent versus another minimally invasive or surgical intervention;
4. one type of bulking agent versus another type of bulking agent;
5. one technique of administration or injection of the agent versus another technique;
6. larger volumes of agent versus smaller volumes;
7. safety outcomes related to injectable bulking agents, information from both the included trials and non‐randomised studies.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials comparing the use of injectable bulking agents for faecal incontinence due to internal anal sphincter (IAS) dysfunction with any alternative treatments or placebo. Case‐control and cohort studies were also reviewed to assess the safety (risks and complications) of the treatment.
Types of participants
All adults with faecal incontinence of any severity and any cause. Any trials in children were excluded as the causes of incontinence are more diverse and complex (for example congenital disorders).
Types of interventions
Injection of bulking agents (biomaterials and autologous tissues) into the anal submucosa or intersphincteric space, either via the anal mucosa or perianally, as a treatment for faecal incontinence. This included injection of autologous fat, Teflon, bovine glutaraldehyde cross‐linked collagen, carbon‐coated zirconium beads, polydimethylsiloxane elastomer, dextranomer in non‐animal stabilised hyaluronic acid, hydrogel cross‐linked with polyacrylamide, porcine dermal collagen, microballoons, polyacrylonitrile in cylinder form and synthetic calcium hydroxylapatite ceramic microspheres, and any other materials found in the literature search.
The comparison intervention could include no treatment or placebo or sham treatment, another bulking agent, conservative treatment (for example biofeedback, anal plug, diet, anti‐diarrhoeal medication, pelvic floor exercise), minimally invasive treatment (for example sacral nerve stimulation, radio‐frequency ablation) and surgical intervention.
Different injection techniques were also compared to determine whether one technique was superior to another.
Types of outcome measures
There are a range of outcome measures which can be used to assess the effectiveness and safety of the treatment as listed below.
Primary outcomes
-
Change in incontinence scores (e.g. Wexner or Cleveland Clinic Florida Fecal Incontinence Score (CCFIS) or St Mark's incontinence score)
Secondary outcomes
Patient reported outcomes
Subjective assessment of change by the patient (improved, no change, or deterioration in incontinence); symptoms; ability to conduct activities of daily living; satisfaction or dissatisfaction with treatment; need for additional therapy (need to wear absorbent products for incontinence, use of drugs, dietary changes, repeat procedure)
Clinician observation
Physiological measures (maximum resting anal pressure, maximum squeeze anal pressure, mucosal electrosensitivity). Evidence of migration of material by imaging or examination. Further surgical intervention for faecal incontinence (including formation of colostomy)
Quality of life
Generic and condition‐specific change in quality of life scores such as Fecal Incontinence Quality of Life Scale (FIQL); measures of psychological well‐being
Adverse effects
Post‐operative complications (e.g. bleeding, infection, injection site or anal pain or discomfort, new evacuation difficulty)
Health economics
Length of hospital stay, costs
Search methods for identification of studies
This review has drawn on the search strategy developed by the Cochrane Incontinence Group. We did not impose any language or other limits on the searches.
Electronic searches
Relevant trials were identified from the Incontinence Group Specialised Register of controlled trials, which is described under the Incontinence Group module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, CINAHL and handsearching of journals and conference proceedings. The date of the most recent search of the Specialised Register for this review was 25 May 2012.
The trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
The terms used to search the Incontinence Group Specialised Register are given below:
(({DESIGN.CCT*} OR {DESIGN.RCT*}) AND {TOPIC.FAECAL*} AND {INTVENT.SURG.INJECT*})
(All searches were of the keyword field of Reference Manager 12, ISI ResearchSoft).
Additional trials were sought by one of the review authors from the UK National Research Register, Controlled Clinical Trials and the ZETOC database of conference abstracts (searching specifically the American, European, Australasian and UK Societies of Colorectal Surgeons meeting abstracts for the past 10 years). The search was conducted on 3 May 2012 using appropriate free text and MeSH terms by adapting terms drawn from the existing search strategies for the Incontinence Review Group to meet the objectives of this review. Keywords used included: fecal incontinence, durasphere, silicone, collagen, biocompatible materials, polydimethylsiloxane, polyacrylamide, dextranCopolymer, hyaluronicAcid, polyacrylonitrile, AdverseEvents.
Searching other resources
The review authors also searched all the reference lists of relevant articles.
Data collection and analysis
Two review authors (YM, CN) examined all the citations and abstracts derived from the above search strategy. Reports of relevant trials were retrieved in full. Data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data were analysed using Review Manager (RevMan Version 5.1.7).
Selection of studies
The titles and abstracts of all studies identified from the above search strategy were independently assessed for potential eligibility by two review authors (YM, CN) and the full papers were obtained for all studies considered eligible. Any disagreements were resolved by consultation with the third review author (SL). Randomised controlled trials (RCTs) and quasi‐RCTs only were included for the efficacy review.
All other study designs (for example case series of any design, any length of follow up and sample size) were reviewed for reports of adverse events and complications. This review for adverse events forms a separate section of the review and specifically alerts the reader to the different search method employed.
Data extraction and management
Data extraction from the included trials were undertaken independently by the two review authors (YM, CN). Data from RCTs and quasi‐RCTs were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) (Higgins 2011). Data were analysed using the RevMan analyses statistical programme in Review Manager. Data on adverse events and complications were extracted and entered onto an Excel spreadsheet for review and analysis by all three review authors.
Assessment of risk of bias in included studies
The methodological quality of all included RCTs and quasi‐RCTs was evaluated by two review authors (YM and CN) using the criteria recommended by the Cochrane Incontinence Review Group to assess risk of bias. In particular, trials were assessed for randomisation procedure, blinding, adequacy of allocation concealment and attrition bias.
Measures of treatment effect
If appropriate, the results of included studies were combined for each outcome in a formal meta‐analysis to produce an overall estimate of treatment effect. For dichotomous data the risk ratio (RR) and 95% confidence interval (CI) were derived using a fixed‐effect model, and for continuous data weighted mean differences (WMD), weighted by the inverse of the variance, were calculated.
Unit of analysis issues
There were no non‐standard designs.
Dealing with missing data
Trials were assessed as to whether the results were reported on attrition bias, and if not the number and reasons for withdrawals and drop‐outs were sought.
Assessment of heterogeneity
Analysis of variation across the trials was considered if the number of available trials was sufficient to conduct such an assessment.
Assessment of reporting biases
The potential bias was reported as published.
Data synthesis
It was intended to perform a meta‐analysis using a fixed‐effect model if data were available.
Subgroup analysis and investigation of heterogeneity
If data allowed, subgroup analyses would have been undertaken according to: cause of incontinence; sphincter integrity; gender; and injection technique.
Sensitivity analysis
Sensitivity analyses would have been performed if data allowed, such as according to the quality (risk of bias) of trials.
Results
Description of studies
Five randomised trials were included in this review. In total, 382 patients were randomised (Graf 2011; Maeda 2008; Siproudhis 2007; Tjandra 2004; Tjandra 2009).
Results of the search
Twenty records were identified by the search of which four were not relevant and were excluded. Sixteen full text reports were assessed and one was excluded as it was not a randomised trial. Eight reports of five studies were eligible for inclusion in the review. Additionally, there were seven studies that still appeared to be ongoing: one (Smart 2008) was a communication about an ongoing trial and the results were not yet available in the public domain at the time of the search; one study (NCT00762047 2009) appeared to have been terminated in 2009 (reasons unknown, trialists contacted for details); and three were ongoing studies for EAS injuries using cell therapy. The flow of study selection is summarised in Figure 1.

PRISMA study flow diagram,
Included studies
The five randomised trials (Graf 2011; Maeda 2008; Siproudhis 2007; Tjandra 2004; Tjandra 2009) included a total of 382 patients. Further details are in the Characteristics of included studies table.
The participants in all five RCTs had internal anal sphincter (IAS) dysfunction or passive faecal incontinence and were reported to have failed previous conservative management with anti‐diarrhoeal drugs and pelvic floor muscle training or biofeedback (although this was not standardised and generally not reported in any detail).
The interventions tested were:
-
hydrogel cross‐linked with polyacrylamide (Bulkamid) (Maeda 2008);
-
porcine dermal collagen (Permacol) (Maeda 2008);
-
polydimethylsiloxane elastomer implants (Siproudhis 2007);
-
silicone biomaterial (PTQ) injected between the two anal sphincters (Tjandra 2009);
-
carbon‐coated beads injected into the anal submucosa (Durasphere) (Tjandra 2009);
-
two different injection techniques (endoanal ultrasound guidance or digital guidance) using a silicone biomaterial (PTQ) (Tjandra 2004);
-
dextranomer in stabilised hyaluronic acid (NASHA Dx) (Graf 2011).
The control intervention in two trials were:
-
saline (Siproudhis 2007);
-
sham injection (mimicking active treatment but without injection of any substance) (Graf 2011).
The injection sites were:
-
under the anal submucosa (Graf 2011; Tjandra 2009)
-
in between the two anal sphincters (Maeda 2008; Siproudhis 2007; Tjandra 2004; Tjandra 2009).
Excluded studies
See the Characteristics of excluded studies table. One study was not an RCT (Dehli 2007).
Risk of bias in included studies
Risk of bias was assessed in summary (Figure 2) and in each trial (Figure 3).
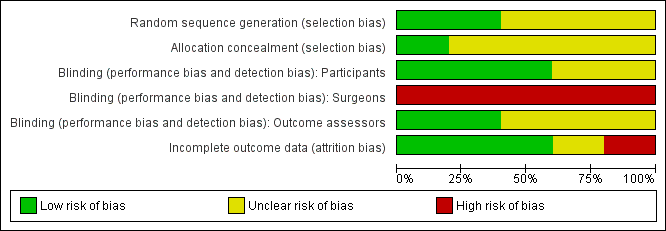
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Two trials gave sufficient details about the randomisation process to suggest freedom from bias (sequence generation in a central registry) (Graf 2011; Tjandra 2009). The quality of allocation concealment was judged to be unclear in three trials.
Blinding
In two trials (Graf 2011; Siproudhis 2007), the patients and the outcome assessors were blinded to treatment allocation. The other three trials did not specify whether the assessor was different from the person involved in the treatment.
It was not possible to blind the surgeons in any of the trials as the interventions were different.
Incomplete outcome data
Withdrawals and intention to treat
Three trials reported no drop‐outs, one presented a flow of patients with figures (Graf 2011), and one had a high drop‐out rate (Tjandra 2004). From the number of people included at the start and the results of the trial, four trials analysed data on an intention‐to‐treat basis (Graf 2011; Maeda 2008; Siproudhis 2007; Tjandra 2009) whilst one trial conducted an available case analysis (Tjandra 2004).
Follow up
Follow up varied from three to 12 months. No long term results were presented.
Selective reporting
Review authors considered there was no evidence of selective reporting.
Other potential sources of bias
There are six ongoing trials (Dehli 2007; Dehli 2009; Draganic 2008; EUCTR2010‐021463‐32‐AT 2011; Michot 2012; Norderval 2012). Five studies are ongoing with three of them aiming to regenerate muscles by cell therapy for external anal sphincter injuries, hence they are not under the scope of the current review. One study has been completed and is under preparation for publication (personal communication).
Effects of interventions
1. Injection of bulking agent versus placebo or sham injection
Two trials addressed this comparison.
Siproudhis et al (Siproudhis 2007) compared a silicone biomaterial (polydimethylsiloxane elastomer, PTQ) with a (control) normal saline injection into the intersphincteric space. The trial was too small to detect differences in any outcomes, such as failure rate (17/22, 77% versus 16/22, 72% in the control group, RR 1.06, 95% CI 0.75 to 1.50; Analysis 1.1); use of pads (Analysis 1.3) or Wexner score (Analysis 1.5). In addition, the subjective perception of treatment success was low in both groups (23% versus 27%, P = 0.73).
The study by Graf et al (Graf 2011) compared injection of dextranomer stabilised in hyaluronic acid (NASHA Dx) against sham injection (injection without actual substance). The primary endpoint was a response to treatment which was defined as a 50% or greater reduction in the number of incontinence episodes. There was a statistically significant difference between the treatment and control groups at six months (65/136, 48% versus 48/70, 69% participants not improved, defined as less than 50% reduction in incontinence episodes, RR 0.70, 95% CI 0.55 to 0.88; Analysis 1.2). Participants in the active group had statistically significantly more incontinence free days at six months (3.1 days compared with 1.7 in the sham treatment group, MD 1.40 days, 95% CI 0.33 to 2.47; Analysis 1.4). However, this difference was not shown at three months. Secondary endpoints such as number of faecal incontinence episodes, CCFIS at three and six months and FIQOL at 12 months did not show any difference except for one domain of FIQOL between the active and sham treatment groups. While there were fewer adverse events in the control group (128/136, 94% in the active group versus 29/70, 41% in the control group, RR 2.27, 95% CI 1.71 to 3.01; Analysis 1.6.2), only two in the active group were regarded as serious (rectal and prostate abscesses).
2. Injection of bulking agent versus conservative treatment
No trials were found.
3. Injection of bulking agent versus another minimally invasive or surgical intervention
No trials were found.
4. One type of bulking agent versus another type
Two trials addressed this comparison (Maeda 2008; Tjandra 2009).
In the trial by Tjandra and colleagues (Tjandra 2009), injection of the silicone biomaterial polydimethylsiloxane elastomer (PTQ™) and carbon‐coated beads (Durasphere®) were compared. While participants in both groups reported an improvement in Wexner's score and quality of life over the study period up to 12 months, the silicone material was better than the carbon‐coated beads in terms of: faecal incontinence at six and 12 months (for example at 12 months, RR 0.15, 95% CI 0.04 to 0.60; Analysis 2.1); Wexner's incontinence score at six and 12 months (for example at 12 months, MD ‐3.20, 95% CI ‐4.91 to ‐1.49; Analysis 2.2); and quality of life (lifestyle) index at six months (MD ‐0.56, 95% CI ‐1.01 to ‐.011; Analysis 2.3). There were more adverse effects (4/20 in the silicone group versus 8/20 in the carbon‐coated bead group; Analysis 2.4); one serious event occurred in the carbon‐coated bead group only (Analysis 2.5). It was assumed that there were no drop‐outs, though this was not explicitly stated.
Another trial with only 10 participants (Maeda 2008) compared injection of hydrogel cross‐linked with polyacrylamide (Bulkamid™) and porcine dermal collagen (Permacol™). In the hydrogel group incontinence scores improved from a median of 15 to 12 at six weeks and this was maintained at six months. In the porcine dermal collagen group, the baseline median score improved from 16 to 14 at six weeks but deteriorated to 15 at six months. The trial was too small to detect differences between the groups in terms of faecal incontinence becoming worse (Analysis 2.1), the overall St Mark's incontinence score, or the faecal incontinence quality of life score, but data were not provided in a form suitable for analysis (medians and no SDs).
5. One technique of administration of agent compared to another technique
One trial (Tjandra 2004) compared a silicone biomaterial, polydimethylsiloxane elastomer (PTQ™), injection under endoanal ultrasound guidance versus digital (finger) guidance. Using a criterion of greater than 50% decrease (improvement) in the Wexner scale, the ultrasound technique was better than the digital technique (RR 0.53, 95% CI 0.31 to 0.92; Analysis 3.1, Analysis 3.2) and improved the Quality of Life (lifestyle) index at six months (MD ‐0.60, 95% CI ‐0.92 to ‐0.28; Analysis 3.3). The only reported adverse effect, discomfort at the injection site, was rare (2/42 and 4/40 participants; Analysis 3.4). However, many of the data were reported in a form not suitable for analysis (medians and no SDs), the follow up was short (12 months), and there was a high drop‐out rate (by 12 months, 32/42 and 35/40 respectively).
6. Larger volumes of agent versus smaller volumes
No trials were found.
7. Safety data in included RCTs and other non‐randomised studies
Adverse effects were reported in four of the five included trials (Graf 2011; Siproudhis 2007; Tjandra 2004; Tjandra 2009). In the randomised trials included in this review, one trial (Siproudhis 2007) reported 18 adverse effects in nine people (Analysis 1.6). Six out of 22 people injected with silicone biomaterial (PTQ™) complained of pain at the implant site, whilst two out of 22 people in the saline group complained of pain. Anal inflammation was noted in two people in the silicone biomaterial injection group compared to none in the normal saline group. The severity of these adverse effects was categorised as 'not serious' but the duration of the events was not reported. In the trial reported by Graf et al (Graf 2011), patients treated with dextranomer stabilised in hyaluronic acid (NASHA Dx) reported 128 adverse events (number of patients not stated) whilst 29 events (number of patients not stated) were recorded in the sham treatment group (Analysis 1.6). The most common event was proctalgia (19/136 versus 2/70) followed by injection site bleeding (7/136 versus 12/70) and rectal haemorrhage (10/136 versus 1/70). Two serious events were reported in the active treatment group, one was a prostatic abscess resolved by antibiotic treatment and the other was a rectal abscess which required surgical drainage. The trial comparing silicone biomaterial (PTQ™) and carbon‐coated beads (Durasphere®) (Tjandra 2009) reported no septic or significant complications in patients injected with PTQ™, although there were four occurrences of bruising. In contrast, patients who had a Durasphere® injection complained of rectal pain (1/20), erosion of rectal mucosa (2/20) and type III hypersensitivity reaction in small joints and skin (1/20) (Analysis 2.4). One of these type III hypersensitivities was judged to be serious and resulted in hospital admission for seven days (Analysis 2.5). The trial comparing injection techniques (Tjandra 2004) reported minor discomfort in two of 42 patients who had injections with ultrasound guidance and four of 40 patients with digital guidance (Analysis 3.4); in one patient this persisted for six weeks. There were no reports of other serious adverse effects (Analysis 3.5).
The occurrence of complications was also investigated in all non‐randomised studies found to date and is summarised in Table 1. In general, the complications were poorly reported.The severity and duration of complications were rarely reported and in some studies there was no mention of whether or not there had been any complications. The complications were mostly minor (discomfort, pain, bleeding, leakage of injected material). One rectovaginal fistula was reported although it was not clear if this was related to the injection. The reason for discontinuation of one study (NCT00762047 2009) is unclear and there is no information on adverse events in this study.
| Agent | Authors and Year | No of patients | Injection route | Total volume | Complications | Number of adverse effects/Number of participants |
| Autologous fat | 14 | Submucosal | 15‐20ml | Reports that there were no complications | 0/14 | |
| 1 | Perianal | 130ml | Reports that there were no complications | 0/1 | ||
| Bioplastique™/PTQ™ | 10 | Perianal | 5‐11.5ml | 4 anal canal ulcer, 1 injection site pain, 1 leakage of injected material | 6/10 | |
| 82 | Perianal | 10.0ml | 6 minor discomfort at injection site (4: digital guidance group, 2: ultrasound guidance group) | 6/82 | ||
| 7 | Perianal | Not mentioned | Reports that there were no complications | 0/7 | ||
| 20 | Perianal | 7.5ml | Not reported | Not reported | ||
| 6 | Perianal | 7.5ml | 1 recto‐vaginal fistula | 1/6 | ||
| 24 | Perianal | 7.5ml | Not reported | Not reported | ||
| 37 | Not mentioned | Not mentioned | 4 perianal abscess required surgical drainage | 4/37 | ||
| 22 | Perianal | 7.5ml | 6 pain at the implant site, 2 anal inflammation | 8/22 | ||
| 33 | Perianal | 7.5ml | Reports that there were no complications | 0/33 | ||
| 20 | Perianal | 10.0ml | 4 bruising | 4/20 | ||
| 74 | Perianal | 10.0ml | 9 minor complications | 9/74 | ||
| 50 | Perianal | Not mentioned | 2 local giant cell foreign body reaction | 2/50 | ||
| Bulkamid™ | 5 | Perianal | median 9ml (8‐9) | Reports that there were no complications | 0/5 | |
| Coaptite® | 10 | Perianal | 4ml | 1 leakage of Coaptite® | 1/10 | |
| Contigen® | 17 | Submucosal | 2ml | Reports that there were no complications | 0/17 | |
| 73 | Submucosal | 5ml | Not reported | Not reported | ||
| Durasphere®/ACYST‐TM | 7 | Submucosal | average 6.8ml | Not reported | Not reported | |
| 18 | Submucosal | mean 1.28ml at 1‐4 sites | 2 anal discomfort, 1 exacerbation of pruritis ani, 2 passage of injected Durasphere via anus | 5/18 | ||
| 33 | Submucosal | median 8.8ml (2‐19ml) | 2 anal pain, 1 Durasphere® leakage, 2 material migration | 5/33 | ||
| 11 | Perianal | mean 9ml (8‐12ml) | Local pain (most frequent, no numbers reported), 3 passage of Durasphere® | At least 3/11 | ||
| 20 | Submucosal | 10.0ml | 4 bruising, 2 erosion of rectal mucosa, 1 rectal pain, 1 type III hypersensitivity | 8/20 | ||
| Gatekeeper™ (polyacrylonitrile) | 14 | Perianal | 1 cylinder (length 21mm, diameter 1‐2mm)x4 | Reports that there were none. | 0/14 | |
| NASHA™Dx | 4 | Submucosal | 5.6ml | 1 bleeding settled with compression, 1 anal pain, 1 tenesmus. All complications settled within 2‐7 days after procedure. | 3/4 | |
| 34 | Submucosal | 4ml | Pain (26% during 1st injection procedure, 55% for second treatment), 3 suspected inflammation of anal canal, 2 sensation of obstructed defaecation | At least 5/34 | ||
| 136 | Submucosal | 4‐8ml | 128 adverse events in total. Two serious events, one prostatic abscess and one rectal abscess. | 128/136 | ||
| 115 | Submucosal | up to 4ml | 154 adverse events, including 20 serious events such as abscess and haemorrhage | 154 adverse events by 70 patients | ||
| Permacol™ | 7 | Submucosal | Not mentioned | Reports that there were no complications | 0/7 | |
| 5 | Perianal | median 15ml (15‐17.5) | Reports that there were no complications | 0/5 | ||
| Saline | 22 | Perianal | 7.5ml | 2 pain at the implant site | 2/22 | |
| Synthetic collagen | 11 | Submucosal | Not specified | One death unrelated to the treatment | 1/11 | |
| Teflon® | 11 | Submucosal | 10ml | Not reported | Not reported | |
| Urosurge® | 6 | Submucosal | up to 5 balloons | 1 bleeding, 1 lost balloon after 2 months (potential spontaneous burst) | 2/6 |
Bioplastique™/PTQ™: Silicone biomaterial
Bulkamid™: Hydrogel cross‐linked with polyacrylamide
Coaptite®: Calcium hydroxylapatite
Contigen®: Synthetic bovine collagen
Durasphere®/ACYST‐TM: Carbon‐coated microbeads
NASHA™Dx: Stabilized nonanimal hyaluronic acid with dextranomer
Permacol™: Porcine dermal collagen
Teflon®: Polytetrafluoroethylene
Urosurge®: Expandable silicone microballoon
Most studies do not appear to have systematically and prospectively collected data on adverse events, but instead reported those which came to the investigators' attention.
Discussion
Since the publication of the last version of this review, a large adequately‐sized trial has emerged which demonstrated the short term efficacy of dextranomer stabilised in hyaluronic acid (NASHA Dx) injection for faecal incontinence. The study showed 52% of patients in the active treatment group had a 50% or greater reduction in the number of incontinence episodes compared to 31% of those who had a sham injection. Although this definition of successful treatment is rather arbitrary, there were significantly more patients who achieved this primary outcome compared to sham injection.
It is interesting to note that nearly 30% of the patients in the sham injection group also achieved this primary outcome, which is compatible with another trial that used saline injection as control. The placebo effect cannot be negated as the sham group experienced improvement up to six months. The mechanism of action is obscure.
There remains a shortage of well‐designed randomised trials to evaluate the efficacy of perianal injection of bulking agents, and the studies reviewed failed to answer other important questions surrounding this treatment. There are wide variations in the agents and techniques used (submucosal versus intersphincteric, use of transanal ultrasound). Safety and potential complications have not been thoroughly assessed and the quality of reports has generally been poor. This needs further, more rigorous evaluation with longer term follow up for potential adverse events. The selection criteria for patients and the primary outcome measure, thus the definition of successful treatment, have been variable. These important questions surrounding this treatment need to be addressed in a well‐designed RCT with adequate power to assess the outcomes.
There appears to be a gap between patients' subjective evaluation of their symptoms and the incontinence scores, particularly in observational studies. Regardless of the outcome of the scores the trials in this review all showed improvement in a faecal incontinence quality of life scale, and thus this discrepancy needs to be addressed in future research.
Summary of main results
One large randomised trial has shown that dextranomer stabilised in hyaluronic acid (NASHA Dx) is efficacious to treat faecal incontinence (Graf 2011). The injection was effective up to 12 months.
Several of the studies showed that there were short term improvements in faecal incontinence after injections of a variety of materials or with injection techniques. Some materials were better than others, at least in the short term, as silicone material was better than the carbon‐coated beads (Tjandra 2009). Similarly, ultrasound‐guided injections seemed to have short term benefits over digital guidance (Tjandra 2004).
Two trials comparing active treatment against sham injection showed an improvement of symptoms in up to 27% of the control treatment group, thus the possibility of placebo effect should be noted.
Complications are common but mostly appear to be relatively minor and of short duration, resolving spontaneously in most cases. There has been no long term follow up of RCT results published to date.
Overall completeness and applicability of evidence
The number of studies available to address the objectives of this review was limited.
There is a lack of information regarding the volume, the precise location where the agent should be placed, and the choice of guidance of the needle track.
Quality of the evidence
Only two of the studies provided enough data to reliably assess the chance of bias, and most of the studies were small with short duration of follow up. Some studies did not supply data in a form suitable for analysis.
Potential biases in the review process
The number of references identified was small. The limitations of this review are the variable outcome measures used in eligible trials and that meta‐analysis was not possible.
Agreements and disagreements with other studies or reviews
Similar reviews which included case‐series (Hussain 2011; Luo 2010) reached the same conclusion that evidence on the efficacy of this treatment is weak and most studies conducted for this treatment have been of poor quality.

PRISMA study flow diagram,
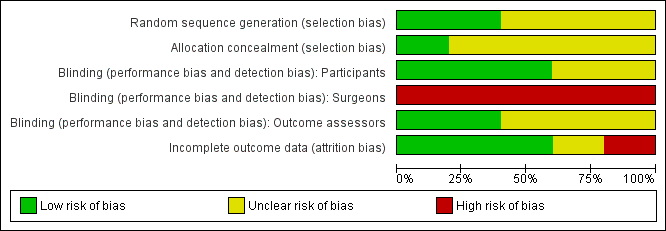
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
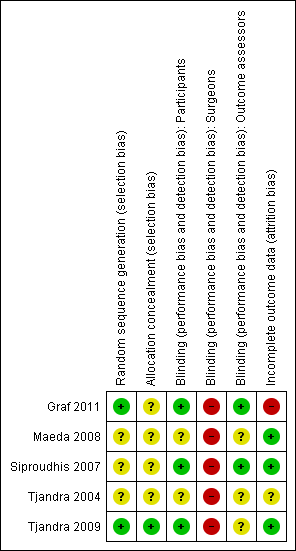
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Injectable versus placebo injection, Outcome 1 Failure (number of participants with Wexner's >8).

Comparison 1 Injectable versus placebo injection, Outcome 2 No improvement (less than 50% reduction in incontinence episodes.
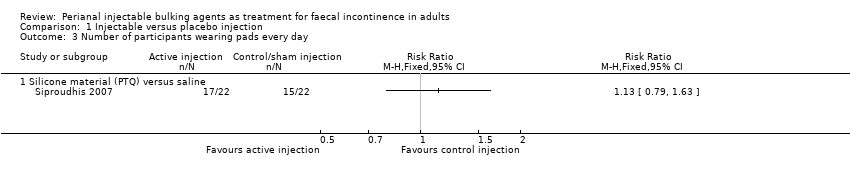
Comparison 1 Injectable versus placebo injection, Outcome 3 Number of participants wearing pads every day.

Comparison 1 Injectable versus placebo injection, Outcome 4 Number of incontinence free days at 6 months.
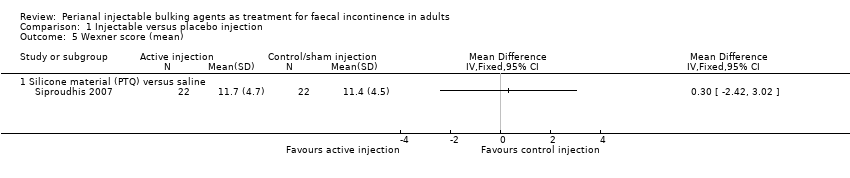
Comparison 1 Injectable versus placebo injection, Outcome 5 Wexner score (mean).

Comparison 1 Injectable versus placebo injection, Outcome 6 Number of adverse effects.

Comparison 1 Injectable versus placebo injection, Outcome 7 Serious adverse effects.

Comparison 1 Injectable versus placebo injection, Outcome 8 Need for re‐treatment at 6 months.

Comparison 2 One injectable material versus another, Outcome 1 Failure (number with worse faecal incontinence).

Comparison 2 One injectable material versus another, Outcome 2 Wexner's incontinence score.
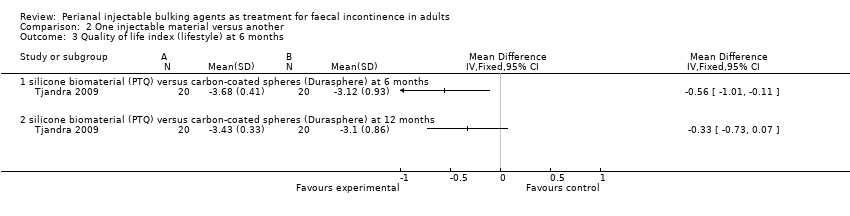
Comparison 2 One injectable material versus another, Outcome 3 Quality of life index (lifestyle) at 6 months.
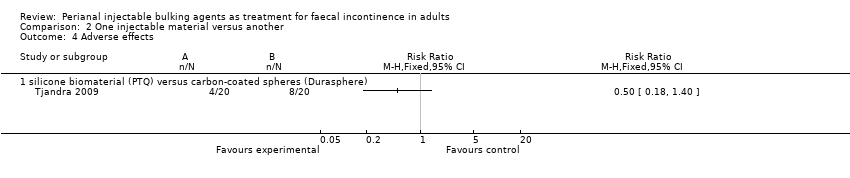
Comparison 2 One injectable material versus another, Outcome 4 Adverse effects.

Comparison 2 One injectable material versus another, Outcome 5 Serious adverse effects.
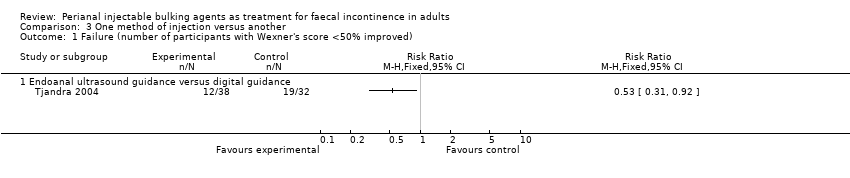
Comparison 3 One method of injection versus another, Outcome 1 Failure (number of participants with Wexner's score <50% improved).

Comparison 3 One method of injection versus another, Outcome 2 Failure (number of participants global Quality of Life score <50% improved).
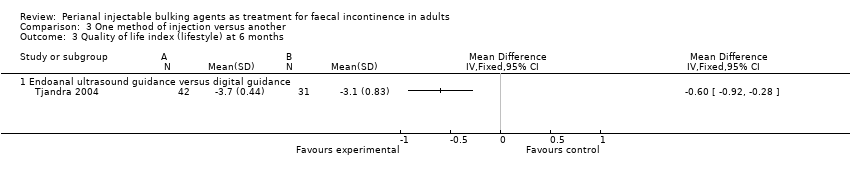
Comparison 3 One method of injection versus another, Outcome 3 Quality of life index (lifestyle) at 6 months.

Comparison 3 One method of injection versus another, Outcome 4 Discomfort at injection site.
Comparison 3 One method of injection versus another, Outcome 5 Adverse effects.
| Agent | Authors and Year | No of patients | Injection route | Total volume | Complications | Number of adverse effects/Number of participants |
| Autologous fat | 14 | Submucosal | 15‐20ml | Reports that there were no complications | 0/14 | |
| 1 | Perianal | 130ml | Reports that there were no complications | 0/1 | ||
| Bioplastique™/PTQ™ | 10 | Perianal | 5‐11.5ml | 4 anal canal ulcer, 1 injection site pain, 1 leakage of injected material | 6/10 | |
| 82 | Perianal | 10.0ml | 6 minor discomfort at injection site (4: digital guidance group, 2: ultrasound guidance group) | 6/82 | ||
| 7 | Perianal | Not mentioned | Reports that there were no complications | 0/7 | ||
| 20 | Perianal | 7.5ml | Not reported | Not reported | ||
| 6 | Perianal | 7.5ml | 1 recto‐vaginal fistula | 1/6 | ||
| 24 | Perianal | 7.5ml | Not reported | Not reported | ||
| 37 | Not mentioned | Not mentioned | 4 perianal abscess required surgical drainage | 4/37 | ||
| 22 | Perianal | 7.5ml | 6 pain at the implant site, 2 anal inflammation | 8/22 | ||
| 33 | Perianal | 7.5ml | Reports that there were no complications | 0/33 | ||
| 20 | Perianal | 10.0ml | 4 bruising | 4/20 | ||
| 74 | Perianal | 10.0ml | 9 minor complications | 9/74 | ||
| 50 | Perianal | Not mentioned | 2 local giant cell foreign body reaction | 2/50 | ||
| Bulkamid™ | 5 | Perianal | median 9ml (8‐9) | Reports that there were no complications | 0/5 | |
| Coaptite® | 10 | Perianal | 4ml | 1 leakage of Coaptite® | 1/10 | |
| Contigen® | 17 | Submucosal | 2ml | Reports that there were no complications | 0/17 | |
| 73 | Submucosal | 5ml | Not reported | Not reported | ||
| Durasphere®/ACYST‐TM | 7 | Submucosal | average 6.8ml | Not reported | Not reported | |
| 18 | Submucosal | mean 1.28ml at 1‐4 sites | 2 anal discomfort, 1 exacerbation of pruritis ani, 2 passage of injected Durasphere via anus | 5/18 | ||
| 33 | Submucosal | median 8.8ml (2‐19ml) | 2 anal pain, 1 Durasphere® leakage, 2 material migration | 5/33 | ||
| 11 | Perianal | mean 9ml (8‐12ml) | Local pain (most frequent, no numbers reported), 3 passage of Durasphere® | At least 3/11 | ||
| 20 | Submucosal | 10.0ml | 4 bruising, 2 erosion of rectal mucosa, 1 rectal pain, 1 type III hypersensitivity | 8/20 | ||
| Gatekeeper™ (polyacrylonitrile) | 14 | Perianal | 1 cylinder (length 21mm, diameter 1‐2mm)x4 | Reports that there were none. | 0/14 | |
| NASHA™Dx | 4 | Submucosal | 5.6ml | 1 bleeding settled with compression, 1 anal pain, 1 tenesmus. All complications settled within 2‐7 days after procedure. | 3/4 | |
| 34 | Submucosal | 4ml | Pain (26% during 1st injection procedure, 55% for second treatment), 3 suspected inflammation of anal canal, 2 sensation of obstructed defaecation | At least 5/34 | ||
| 136 | Submucosal | 4‐8ml | 128 adverse events in total. Two serious events, one prostatic abscess and one rectal abscess. | 128/136 | ||
| 115 | Submucosal | up to 4ml | 154 adverse events, including 20 serious events such as abscess and haemorrhage | 154 adverse events by 70 patients | ||
| Permacol™ | 7 | Submucosal | Not mentioned | Reports that there were no complications | 0/7 | |
| 5 | Perianal | median 15ml (15‐17.5) | Reports that there were no complications | 0/5 | ||
| Saline | 22 | Perianal | 7.5ml | 2 pain at the implant site | 2/22 | |
| Synthetic collagen | 11 | Submucosal | Not specified | One death unrelated to the treatment | 1/11 | |
| Teflon® | 11 | Submucosal | 10ml | Not reported | Not reported | |
| Urosurge® | 6 | Submucosal | up to 5 balloons | 1 bleeding, 1 lost balloon after 2 months (potential spontaneous burst) | 2/6 | |
| Bioplastique™/PTQ™: Silicone biomaterial Bulkamid™: Hydrogel cross‐linked with polyacrylamide Coaptite®: Calcium hydroxylapatite Contigen®: Synthetic bovine collagen Durasphere®/ACYST‐TM: Carbon‐coated microbeads NASHA™Dx: Stabilized nonanimal hyaluronic acid with dextranomer Permacol™: Porcine dermal collagen Teflon®: Polytetrafluoroethylene Urosurge®: Expandable silicone microballoon | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's >8) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 No improvement (less than 50% reduction in incontinence episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants wearing pads every day Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Silicone material (PTQ) versus saline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence free days at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Wexner score (mean) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Silicone material (PTQ) versus saline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Silicone biomaterial (PTQ) versus saline injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Need for re‐treatment at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Dextranomer stabilised in hyaluronic acid (NASHA Dx) versus sham injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number with worse faecal incontinence) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 hydrogel crosslinked with polyacrylamide versus porcine dermal collagen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Wexner's incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 silicone biomaterial (PTQ) versus carbon‐coated spheres (Durasphere) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure (number of participants with Wexner's score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Failure (number of participants global Quality of Life score <50% improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life index (lifestyle) at 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Endoanal ultrasound guidance versus digital guidance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Discomfort at injection site Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Endoanal ultrasound guidance versus digital guidance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

