Gabapentin u liječenju kronične neuropatske boli u odraslih
Abstract
Background
Gabapentin is commonly used to treat neuropathic pain (pain due to nerve damage). This review updates a review published in 2014, and previous reviews published in 2011, 2005 and 2000.
Objectives
To assess the analgesic efficacy and adverse effects of gabapentin in chronic neuropathic pain in adults.
Search methods
For this update we searched CENTRAL), MEDLINE, and Embase for randomised controlled trials from January 2014 to January 2017. We also searched the reference lists of retrieved studies and reviews, and online clinical trials registries.
Selection criteria
We included randomised, double‐blind trials of two weeks' duration or longer, comparing gabapentin (any route of administration) with placebo or another active treatment for neuropathic pain, with participant‐reported pain assessment.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with substantial pain relief (at least 50% pain relief over baseline or very much improved on Patient Global Impression of Change scale (PGIC)), or moderate pain relief (at least 30% pain relief over baseline or much or very much improved on PGIC). We performed a pooled analysis for any substantial or moderate benefit. Where pooled analysis was possible, we used dichotomous data to calculate risk ratio (RR) and number needed to treat for an additional beneficial outcome (NNT) or harmful outcome (NNH). We assessed the quality of the evidence using GRADE and created 'Summary of findings' tables.
Main results
We included four new studies (530 participants), and excluded three previously included studies (126 participants). In all, 37 studies provided information on 5914 participants. Most studies used oral gabapentin or gabapentin encarbil at doses of 1200 mg or more daily in different neuropathic pain conditions, predominantly postherpetic neuralgia and painful diabetic neuropathy. Study duration was typically four to 12 weeks. Not all studies reported important outcomes of interest. High risk of bias occurred mainly due to small size (especially in cross‐over studies), and handling of data after study withdrawal.
In postherpetic neuralgia, more participants (32%) had substantial benefit (at least 50% pain relief or PGIC very much improved) with gabapentin at 1200 mg daily or greater than with placebo (17%) (RR 1.8 (95% CI 1.5 to 2.1); NNT 6.7 (5.4 to 8.7); 8 studies, 2260 participants, moderate‐quality evidence). More participants (46%) had moderate benefit (at least 30% pain relief or PGIC much or very much improved) with gabapentin at 1200 mg daily or greater than with placebo (25%) (RR 1.8 (95% CI 1.6 to 2.0); NNT 4.8 (4.1 to 6.0); 8 studies, 2260 participants, moderate‐quality evidence).
In painful diabetic neuropathy, more participants (38%) had substantial benefit (at least 50% pain relief or PGIC very much improved) with gabapentin at 1200 mg daily or greater than with placebo (23%) (RR 1.7 (95% CI 1.4 to 2.0); NNT 6.6 (5.0 to 10); 6 studies, 1331 participants, moderate‐quality evidence). More participants (52%) had moderate benefit (at least 30% pain relief or PGIC much or very much improved) with gabapentin at 1200 mg daily or greater than with placebo (37%) (RR 1.4 (95% CI 1.3 to 1.6); NNT 6.6 (4.9 to 9.9); 7 studies, 1439 participants, moderate‐quality evidence).
For all conditions combined, adverse event withdrawals were more common with gabapentin (11%) than with placebo (8.2%) (RR 1.4 (95% CI 1.1 to 1.7); NNH 30 (20 to 65); 22 studies, 4346 participants, high‐quality evidence). Serious adverse events were no more common with gabapentin (3.2%) than with placebo (2.8%) (RR 1.2 (95% CI 0.8 to 1.7); 19 studies, 3948 participants, moderate‐quality evidence); there were eight deaths (very low‐quality evidence). Participants experiencing at least one adverse event were more common with gabapentin (63%) than with placebo (49%) (RR 1.3 (95% CI 1.2 to 1.4); NNH 7.5 (6.1 to 9.6); 18 studies, 4279 participants, moderate‐quality evidence). Individual adverse events occurred significantly more often with gabapentin. Participants taking gabapentin experienced dizziness (19%), somnolence (14%), peripheral oedema (7%), and gait disturbance (14%).
Authors' conclusions
Gabapentin at doses of 1800 mg to 3600 mg daily (1200 mg to 3600 mg gabapentin encarbil) can provide good levels of pain relief to some people with postherpetic neuralgia and peripheral diabetic neuropathy. Evidence for other types of neuropathic pain is very limited. The outcome of at least 50% pain intensity reduction is regarded as a useful outcome of treatment by patients, and the achievement of this degree of pain relief is associated with important beneficial effects on sleep interference, fatigue, and depression, as well as quality of life, function, and work. Around 3 or 4 out of 10 participants achieved this degree of pain relief with gabapentin, compared with 1 or 2 out of 10 for placebo. Over half of those treated with gabapentin will not have worthwhile pain relief but may experience adverse events. Conclusions have not changed since the previous update of this review.
PICOs
Laički sažetak
Gabapentin u liječenju kronične neuropatske boli u odraslih
Zaključak
Postoje dokazi srednje razine pouzdanosti koji pokazuju da gabapentin koji se uzima na usta u dozama od 1200 mg ili više na dan ima značajan učinak na bol kod nekih ljudi s umjerenom ili teškom neuropatskom boli uzrokovanom herpes zosterom ili dijabetesom.
Dosadašnje spoznaje
Neuropatska bol nastaje zbog oštećenja živaca. Razlikuje se od uobičajene boli koja se prenosi iz oštećenog tkiva putem zdravih živaca (primjerice uslijed pada, posjekotine ili kod artritisa koljena). Neuropatska bol često se liječi lijekovima različitima od onih koji se koriste protiv bolova koji nastaju zbog oštećenih tkiva, koje obično nazivamo analgeticima. Lijekovi koji se primjenjuju u liječenju depresije ili epilepsije mogu biti vrlo učinkoviti u nekih osoba koje pate od neuropatske boli. Jedan od takvih lijekova je gabapentin. Naša definicija dobrog rezultata liječenja je visok stupanj olakšanja boli i mogućnost kontinuiranog uzimanja lijeka bez nuspojava koje bi potaknule pacijente na prestanak uzimanja.
Značajke istraživanja
U siječnju 2017. pretražili smo medicinska ispitivanja u kojima je gabapentin primjenjivan u liječenju neuropatske boli u odraslih. Našli smo 37 ispitivanja koja su ispunjavala kriterije uključenja i nasumično rasporedila 5,914 sudionika koji su tretirani gabapentinom, placebom ili nekim drugim lijekom. Istraživanja su trajala od 4 do 12 tjedana. Većina istraživanja je pratila korisne ishode koje ljudi s neuropatskom boli smatraju važnima. Rezultati su većinom bili za bol nakon herpes zostera i uslijed oštećenja živaca u dijabetesu.
Ključni rezultati
U 3 od 10 osoba intenzitet boli nakon herpes zostera je smanjen za pola ili više uz gabapentin, naspram 2 od 10 osobe uz placebo. Bol je smanjena za trećinu ili više u 5 od 10 kod osoba koje su uzimale gabapentin i u 3 od 10 osoba koje su uzimale placebo. U 4 od 10 osoba s boli uzrokovanom dijabetesom bol se smanjila na pola ili više uz gabapentin, naspram 2 od 10 osoba uz placebo. Bol je smanjena za trećinu ili više u 5 od 10 kod osoba koje su uzimale gabapentin i u 4 od 10 osoba koje su uzimale placebo. Nije bilo pouzdanih dokaza za bilo koju drugu vrstu neuropatske boli.
Nuspojave su bile učestalije sa gabapentinom (6 od 10) nego s placebom (5 od 10). Vrtoglavica, pospanost, zadržavanje vode i otežan hod javile su se u 1 od 10 osoba koje su uzimale gabapentin. Ozbiljne nuspojave su bile rijetke i za njih nije bilo razlike između gabapentina i placeba. Nešto više osoba je prestalo uzimalti gabapentin radi nuspojava.
Gabapentin može pomoći u liječenju kronične neuropatske boli u nekih osoba. Međutim, nije moguće unaprijed znati kojim pacijentima će lijek pomoći, a kojima neće. Prema trenutnim spoznajama, preporuča se uzimanje lijeka na kraći probni period da bi se moglo procijeniti djelovanje.
Kvaliteta dokaza
Dokazi iz istraživanja većinom su bili srednje kvalitete. To znači da istraživanja daju dobar pokazatelj za vjerojatni učinak liječenja. Vjerojatnost da će stvarni učinak biti bitno različit je umjerena.
Authors' conclusions
Summary of findings
| Gabapentin compared with placebo for postherpetic neuralgia: efficacy | ||||||
| Patient or population: adults with postherpetic neuralgia Settings: community Intervention: gabapentin ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% reduction in pain or equivalent | 330 per 1000 | 190 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.9 (5.5 to 9.4) | 7 studies 2031 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any substantial pain benefit | 320 per 1000 | 170 per 1000 | RR 1.8 (1.5 to 2.1) NNT 6.7 (5.4 to 8.7) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Patient Global Impression of Change much or very much improved | 390 per 1000 | 290 per 1000 | RR1.3 (1.2 to 1.5) NNT 9.7 (6.9 to 16) | 7 studies 2013 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any at least moderate pain benefit (includes Gong 2008 at 25% pain relief) | 46 per 1000 | 25 per 1000 | RR 1.8 (1.6 to 2.0) NNT 4.8 (4.1 to 6.0) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): †Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for peripheral diabetic neuropathy: efficacy | ||||||
| Patient or population: adults with peripheral diabetic neuropathy Settings: community Intervention: ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% pain intensity reduction | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of substantial benefit (at least 50% pain intensity reduction or PGIC very much improved) | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| PGIC much or very much improved | 500 per 1000 | 300 per 1000 | RR 1.7 (1.4 to 2.0) NNT 4.9 (3.6 to 7.6) | 5 studies 695 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of moderate benefit (at least 30% pain intensity reduction or PGIC much or very much improved) | 520 per 1000 | 370 per 1000 | RR 1.4 (1.3 to 1.6) NNT 6.6 (4.9 to 9.9) | 7 studies 1439 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for neuropathic pain (all conditions pooled): adverse events and withdrawals | ||||||
| Patient or population: adults with neuropathic pain Settings: community Intervention: gabapentin 1800 mg to 3600 mg daily (gabapentin encarbil 1200 mg to 3600 mg daily) Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNH (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| Participants experiencing at least one adverse event | 630 per 1000 | 490 per 1000 | RR 1.3 (1.2 to 1.4) NNH 7.5 (6.1 to 9.6) | 18 studies 4279 participants | Moderate | Many events. Unlikely new research would change this finding |
| Adverse event withdrawals | 110 in 1000 | 82 in 1000 | RR 1.4 (1.1 to 1.7) NNH 30 (20 to 66) | 22 studies 4346 participants | High | Unlikely new research would change this finding |
| Serious adverse events | 32 in 1000 | 28 in 1000 | RR 1.2 (0.83 to 1.7) NNH not calculated | 19 studies 3948 participants | Moderate | Small number of events but no suggestion of difference |
| Death | 3 in max 3603 exposed | 5 in max 2377 exposed | Not calculated | Not calculated | Very low | Few events, relatively short duration for drug possibly taken over periods of years |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This is an update of a Cochrane Review titled 'Gabapentin for chronic neuropathic pain and fibromyalgia in adults', published in 2014 (Moore 2014a). The review has now been split and this update will consider only neuropathic pain. A separate updated review of gabapentin for fibromyalgia has been published (Cooper 2017).
Earlier versions of this review include 'Gabapentin for chronic neuropathic pain and fibromyalgia in adults' (Moore 2011a), and 'Gabapentin for acute and chronic pain' (Wiffen 2005). That was itself split out of a review previously published in the Cochrane Library on 'Anticonvulsant drugs for acute and chronic pain' (Wiffen 2000), an update of yet an older systematic review (McQuay 1995).
At a meeting in Oxford in early 2009 with Cochrane's Editor‐in‐Chief, it was decided to create separate chronic pain and acute pain reviews from the then current review on acute and chronic pain together (Wiffen 2005). The meeting was in response to controversy in the USA over the effectiveness of gabapentin as an analgesic (Landefeld 2009), together with calls for the 2005 review to be updated with the inclusion of unpublished information made available through litigation (Vedula 2009). It was agreed to update the 2005 review by splitting the earlier one into two components: one review looking at the role of gabapentin in chronic neuropathic pain (including neuropathic pain of any cause, and fibromyalgia), and a second one to determine the effects of gabapentin in acute postoperative pain. Other reviews may examine gabapentin in chronic musculoskeletal pain. The unpublished data were included in the 2011 review on chronic neuropathic pain and fibromyalgia (Moore 2011a), and in established acute postoperative pain (Straube 2010).
This latest update is based on a template for drugs to treat neuropathic pain, using current standards for Cochrane Reviews, including assessment of the reliability of the evidence with GRADE, and based on criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Moore 2013a; Appendix 1).
Description of the condition
Neuropathic pain is a consequence of a pathological maladaptive response of the nervous system to 'damage' from a wide variety of potential causes (Colloca 2017). It is characterised by pain in the absence of an noxious stimulus, or where minor or moderate nociceptive stimuli evoke exaggerated levels of pain. Neuropathic pain may be spontaneous (continuous or paroxysmal) in its temporal characteristics or be evoked by sensory stimuli (dynamic mechanical allodynia where pain is evoked by light touch of the skin).
Neuropathic pain is heterogeneous in etiology, pathophysiology, and clinical appearance. The 2011 International Association for the Study of Pain definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011), based on a definition agreed at an earlier consensus meeting (Treede 2008). Neuropathic pain is associated with a variety of sensory loss (numbness) and sensory gain (allodynia) clinical phenomena, the exact patterns of which vary between people and disease, perhaps reflecting different pain mechanisms operating in an individual person and, therefore, potentially predictive of response to treatment (Demant 2014; Helfert 2015; von Hehn 2012). A new approach of subgrouping people with peripheral neuropathic pain of different etiologies according to intrinsic sensory profiles has generated three profiles that may be related to pathophysiological mechanisms and may be useful in clinical trial design to enrich the study population for treatment responders (Baron 2017).
Pre‐clinical research hypothesises a bewildering array of possible pain mechanisms that may operate in people with neuropathic pain, which largely reflect pathophysiological responses in both the central and peripheral nervous systems, including neuronal interactions with immune cells (Baron 2012; Calvo 2012; von Hehn 2012). Overall, the treatment gains in neuropathic pain, to even the most effective of available drugs, are modest (Finnerup 2015; Moore 2013b), and a robust classification of neuropathic pain is not yet available (Finnerup 2013).
Neuropathic pain is usually classified according to the cause of nerve injury. There may be many causes, but some common causes of neuropathic pain include diabetes (painful diabetic neuropathy (PDN)), shingles (postherpetic neuralgia (PHN)), amputation (stump and phantom limb pain), neuropathic pain after surgery or trauma, stroke or spinal cord injury, trigeminal neuralgia, and HIV infection. Sometimes the cause is unknown.
Many people with neuropathic pain conditions are significantly disabled with moderate or severe pain for many years. Chronic pain conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life and employment, and increased healthcare costs (Moore 2014a). A US study found the healthcare costs were threefold higher for people with neuropathic pain than matched control subjects (Berger 2004). A UK study and a German study showed a two‐ to threefold higher level of use of healthcare services in people with neuropathic pain than those without (Berger 2012; Berger 2009). For PHN, for example, studies demonstrate large loss of quality of life and substantial costs (Scott 2006; Van Hoek 2009).
In systematic reviews, the overall prevalence of neuropathic pain in the general population is reported to be between 7% and 10% (Van Hecke 2014), and about 7% in a systematic review of studies published since 2000 (Moore 2014a). In individual countries, prevalence rates have been reported as 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008), and up to 8% in the UK (Torrance 2006). Some forms of neuropathic pain, such as PDN and post‐surgical chronic pain (which is often neuropathic in origin), are increasing (Hall 2008). The prevalence of PHN is likely to fall if vaccination against the herpes virus becomes widespread.
Estimates of incidence vary between individual studies for particular origins of neuropathic pain, often because of small numbers of cases. In primary care in the UK, between 2002 and 2005, the incidences (per 100,000 person‐years' observation) were 28 (95% confidence interval (CI), 27 to 30) for PHN, 27 (95% CI, 26 to 29) for trigeminal neuralgia, 0.8 (95% CI, 0.6 to 1.1) for phantom limb pain, and 21 (95% CI, 20 to 22) for PDN (Hall 2008). Other studies have estimated an incidence of 4 in 100,000 per year for trigeminal neuralgia (Katusic 1991; Rappaport 1994), and 12.6 per 100,000 person‐years for trigeminal neuralgia and 3.9 per 100,000 person‐years for PHN in a study of facial pain in the Netherlands (Koopman 2009). One systematic review of chronic pain demonstrated that some neuropathic pain conditions, such as PDN, can be more common than other neuropathic pain conditions, with prevalence rates up to 400 per 100,000 person‐years (McQuay 2007). It is also the case that pains not classified as neuropathic can have neuropathic features. In a community study of recent joint pain, features of neuropathic pain were common and were present in over half of those reporting pain of at least moderate severity (Soni 2013).
Neuropathic pain is difficult to treat effectively, with only a minority of people experiencing a clinically relevant benefit from any one intervention (Kalso 2013; Moore 2013b). A multidisciplinary approach is now advocated, combining pharmacological interventions with physical or cognitive (or both) interventions. The evidence for more invasive interventional therapies such as neural blockade or intrathecal medication is very weak, or non‐existent (Dworkin 2013). Conventional analgesics such as paracetamol (acetaminophen) and nonsteroidal anti‐inflammatory drugs (NSAID) are not thought to be effective, but without evidence to support or refute that view (Moore 2015a; Wiffen 2016). Some people may derive some benefit from a topical lidocaine patch or low‐concentration topical capsaicin, although evidence about benefits is uncertain (Derry 2012; Derry 2014). High‐concentration topical capsaicin may benefit some people with PHN (Derry 2017). Treatment is often by so‐called 'unconventional analgesics' (pain modulators) such as antidepressants (duloxetine and amitriptyline; Lunn 2014; Moore 2014b; Moore 2015b; Sultan 2008), or antiepileptics (gabapentin or pregabalin; Moore 2009; Moore 2014b; Wiffen 2013). Evidence for efficacy of opioids is unconvincing (Derry 2016; Gaskell 2016; Stannard 2016; Wiffen 2015).
The proportion of people who achieve worthwhile pain relief (typically at least 50% pain intensity reduction; Moore 2014c) is small, generally only 10% to 25% more than with placebo, with numbers needed to treat for an additional beneficial outcome (NNT) usually between 4 and 10 (Kalso 2013; Moore 2013b). Neuropathic pain is not particularly different from other chronic pain conditions in that only a small proportion of trial participants have a good response to treatment (Moore 2013b).
The current National Institute for Health and Care Excellence (NICE) guidance for the pharmacological management of neuropathic pain suggests offering a choice of amitriptyline (Moore 2012b), duloxetine (Lunn 2014), gabapentin, or pregabalin (Moore 2009) as initial treatment for neuropathic pain (with the exception of trigeminal neuralgia), with switching if the first, second, or third drugs tried are not effective or not tolerated (NICE 2013). This concurs with other recent guidance (Finnerup 2015).
Description of the intervention
Gabapentin is licensed for the treatment of peripheral and central neuropathic pain in adults in the UK at doses up to 3.6 grams (3600 mg) daily. It is given orally, usually as tablets or capsules, but sometimes as an oral solution (50 mg/ml). Guidance suggests that gabapentin treatment can be started at a dose of 300 mg per day for treating neuropathic pain. Based on individual patient response and tolerability, the dosage may be increased by 300 mg per day until pain relief is experienced or adverse effects make taking the drug intolerable (EMC 2017). US marketing approval for gabapentin was granted in 2002 for postherpetic neuralgia; in Europe, the label was changed to include peripheral neuropathic pain in 2006. Gabapentin has the trade name NeurontinTM, and is also available as generic products in some parts of the world.
Gabapentin has a half‐life of five to seven hours. It is absorbed through a saturable transport system, so that absorption is not linear, and the transporter is found only in the proximal small bowel. This means that the drug needs to be administered at least three times daily, and may result in plasma trough levels. Two new formulations have attempted to improve the availability of the drug. The first is an extended release, gastro‐retentive formulation, designed to provide continuous delivery at the optimal site of absorption over 8 to 10 hours (Sang 2013). The second uses an extended‐release prodrug (gabapentin encarbil) that is absorbed through a high capacity transport system found throughout the intestine, and then undergoes rapid hydrolysis to gabapentin. It is claimed to provide sustained, dose‐proportional gabapentin exposure (Backonja 2011), and can be administered twice daily.
Gabapentin can also be formulated as an aqueous solution for injection. This formulation is not available commercially or licensed for treatment of any type of neuropathic pain or fibromyalgia.
Gabapentin misuse has been reported, and the consequences documented and systematically reviewed (Evoy 2017; Quintero 2017).
How the intervention might work
Gabapentin's mechanism of action is primarily attributed to its effect on calcium channels located throughout the peripheral and central nervous systems, which modify the release of neurotransmitters and reduce excitability of nerve cells (Boyle 2014; Chang 2014). This mode of action confers antiepileptic, analgesic, and sedative effects. Research also indicates that gabapentin acts by blocking new synapse formation (Eroglu 2009).
Why it is important to do this review
Some, but not all, antiepileptics can reduce neuropathic pain (Wiffen 2010). Gabapentin is an antiepileptic widely prescribed for neuropathic pain, and it is common practice in some countries to aim for the maximum tolerated dose. There is growing controversy over whether this practice is justified by experimental evidence from double‐blind randomised trials. Guidance on prescribing typically puts gabapentin amongst the first‐line agents (Finnerup 2015; NICE 2013). Despite this guidance based on good evidence, prescribing for neuropathic pain often involves paracetamol or paracetamol combined with opioids (Hall 2013), for which there is no evidence of efficacy (Wiffen 2016).
The standards used to assess evidence in chronic pain trials have evolved substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy (Appendix 1). The most important change is the move from using mean pain scores, or mean change in pain scores, to the number of people who have a large decrease in pain (by at least 50%) and who continue in treatment, ideally in trials of 8 to 12 weeks' duration or longer. Pain intensity reduction of 50% or more correlates with improvements in co‐morbid symptoms, function, and quality of life. These standards are set out in the PaPaS Author and Referee Guidance for pain studies of Cochrane Pain, Palliative and Supportive Care (PaPaS 2012).
This Cochrane Review assesses the evidence using methods that make both statistical and clinical sense, and uses developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). Trials included and analysed meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc), and size (ideally at least 500 participants in a comparison in which the NNT is 4 or above; Moore 1998). This approach sets high standards for the demonstration of efficacy and marks a departure from how reviews were conducted previously.
Objectives
To assess the analgesic efficacy and adverse effects of gabapentin in chronic neuropathic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with double‐blind (participant and observers) assessment of participant‐reported outcomes, following two weeks of treatment or longer, although the emphasis of the review was on studies of eight weeks or longer. We required full journal publication, with the exception of online clinical trial results summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis.
We did not include short abstracts (usually meeting reports with inadequate or no reporting of data). We excluded studies of experimental pain, case reports, and clinical observations.
Types of participants
We included adult participants aged 18 years and above, with one or more chronic neuropathic pain condition including (but not limited to):
-
cancer‐related neuropathy;
-
central neuropathic pain;
-
complex regional pain syndrome (CRPS) Type II;
-
HIV neuropathy;
-
painful diabetic neuropathy;
-
phantom limb pain;
-
postherpetic neuralgia;
-
postoperative or traumatic neuropathic pain;
-
spinal cord injury;
-
trigeminal neuralgia.
Where we included studies with more than one type of neuropathic pain, we analysed results according to the primary condition if identifiable.
Types of interventions
Gabapentin in any dose, by any route, administered for the relief of neuropathic pain and compared to placebo or any other active comparator.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with most studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These were defined as:
-
at least 30% pain relief over baseline (moderate);
-
at least 50% pain relief over baseline (substantial);
-
much or very much improved on Patient Global Impression of Change scale (PGIC; moderate);
-
very much improved on PGIC (substantial).
These outcomes concentrate on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013c; O'Brien 2010).
Primary outcomes
-
Participant‐reported pain intensity reduction of 30% or greater
-
Participant‐reported pain intensity reduction of 50% or greater
-
Patient‐reported global impression of clinical change (PGIC) much or very much improved
-
Patient‐reported global impression of clinical change (PGIC) very much improved
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy, adverse events, and for any cause.
-
Participants experiencing any adverse event.
-
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
-
Specific adverse events, particularly somnolence and dizziness.
Search methods for identification of studies
Electronic searches
For this update we searched the following databases, without language restrictions:
-
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), 1 January 2014 to 16 January 2017;
-
MEDLINE via Ovid, 1 January 2014 to 16 January 2017;
-
Embase via Ovid, 1 January 2014 to 16 January 2017.
See Appendix 2 for the CENTRAL search strategy, Appendix 3 for the MEDLINE search strategy, and Appendix 4 for the Embase search strategy.
Searching other resources
We reviewed the bibliographies of any RCTs identified and review articles, and searched clinical trial databases (ClinicalTrials.gov (ClinicalTrials.gov) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTTRP) (apps.who.int/trialsearch/)) to identify additional published or unpublished data. We did not contact investigators or study sponsors.
Data collection and analysis
We performed separate efficacy analyses according to particular neuropathic pain conditions, and combined different neuropathic pain conditions in analyses for adverse events and withdrawals only.
Selection of studies
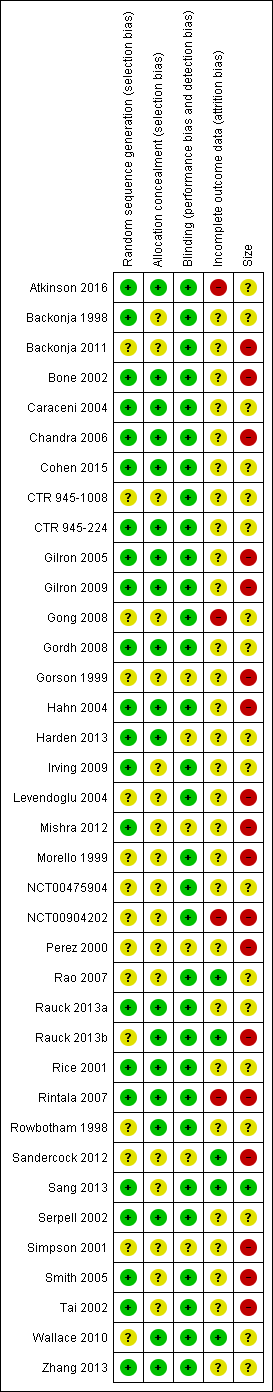
We determined eligibility by reading the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy the inclusion criteria, and we obtained full copies of the remaining studies. Two review authors made the decisions. Two review authors (RAM, SD) then read these studies independently and reached agreement by discussion. We did not anonymise the studies in any way before assessment. We have provided a PRISMA flow chart to illustrate the flow of studies (Moher 2009) (Figure 1).

Study flow diagram
Data extraction and management
Three review authors (RAM, PW, SD) extracted data independently, using a standard data extraction form, and agreed data before entry into Review Manager (RevMan) 5 (RevMan 2014) or any other analysis method. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design, study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, particular adverse events, or a serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors (SD, PW) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011), and adapted from those used by Cochrane Pregnancy and Childbirth, with any disagreements resolved by discussion. We assessed the following for each study:
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process: random number table or computer random‐number generator); unclear risk of bias (when the method used to generate the sequence was not clearly stated). We excluded studies at a high risk of bias that used a non‐random process (odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively‐numbered, sealed, opaque envelopes); unclear risk of bias (when method not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (open list).
-
Blinding of participants and personnel (checking for possible performance bias), and blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study personnel and participants (all outcomes were self‐assessed) from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets, matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies at a high risk of bias that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size (Dechartres 2013; Dechartres 2014; Moore 1998; Nüesch 2010; Thorlund 2011)). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We calculated the number needed to treat for an additional beneficial outcome (NNT) as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat for an additional harmful outcome (NNH) and was calculated in the same manner. We used dichotomous data to calculate risk ratio (RR) with 95% confidence intervals (CI) using a fixed‐effect model unless significant statistical heterogeneity was found (see below). We did not use continuous data in analyses.
Unit of analysis issues
The unit of analysis was the individual participant. For cross‐over studies we planned to use first period data where possible, but otherwise to use available data and consider any potential bias that this study design presented.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned zero improvement (baseline observation carried forward (BOCF)) to missing participants wherever possible.
We paid particular attention to methods used for imputation of missing data due to withdrawals for adverse events and lack of efficacy.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually (L'Abbé 1987) and with the use of the I2 statistic (Higgins 2003). When the I² value was greater than 50%, we considered possible reasons for this.
Assessment of reporting biases
The aim of this review was to use dichotomous outcomes of known utility and of value to people with neuropathic pain (Hoffman 2010; Moore 2010a; Moore 2010b; Moore 2010c; Moore 2014c). The review did not depend on what the authors of the original studies chose to report or not, and studies that did not report dichotomous results for an outcome did not contribute to pooled analyses for that outcome. We extracted and used continuous data, which probably reflect efficacy and utility poorly, for illustrative purposes only.
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a NNT of 10 or higher in this condition; Moore 2008).
We looked for effects of possible enrichment, either complete or partial, in enrolment of participants into the studies. Enrichment typically means including participants known to respond to a therapy, and excluding those known not to respond, or to suffer unacceptable adverse effects, though for gabapentin no significant effects have been shown from partial enrichment (Straube 2008). Enriched enrolment randomised withdrawal studies, known to produce higher estimates of efficacy, would not be pooled (McQuay 2008).
Data synthesis
We used a fixed‐effect model for meta‐analysis, unless there was significant clinical heterogeneity and it was still considered appropriate to combine studies. In such cases we would use a random‐effects model.
Quality of evidence
Quality of the evidence
We used the GRADE system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 6). Two review authors (RAM, SD) independently rated the quality of the evidence for each outcome.
We paid particular attention to inconsistency, where point estimates varied widely across studies or confidence intervals (CIs) of studies showed minimal or no overlap (Guyatt 2011), and potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, where there were so few data that the results were highly susceptible to the random play of chance, or if a study used last observation carried forward (LOCF) imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we would have reported the level of evidence as very low quality (Guyatt 2013b).
In addition, we are aware that many Cochrane Reviews are based largely or wholly on small underpowered studies, and the danger of making conclusive assessments of evidence based on inadequate information (AlBalawi 2013; Brok 2009; Roberts 2015; Turner 2013).
'Summary of findings' table
We have included a 'Summary of findings' table as set out in the PaPaS author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 11, Schünemann 2011a). The table includes, where possible, outcomes equivalent to moderate or substantial benefit of at least 30% and at least 50% pain intensity reduction, PGIC (possibly at least substantial improvement and at least moderate improvement) (Dworkin 2008), withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015):
High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We planned for all analyses to be according to individual painful condition, because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009). We also planned subgroup analysis according to dose of gabapentin, and duration of study if sufficient data were available.
Sensitivity analysis
In the 2014 review we considered a sensitivity analysis for formulation of gabapentin (standard, gastroretentive, slow‐release), but there were insufficient data for meaningful analysis, and there were no additional data for these formulations. We planned no other sensitivity analyses because the evidence base was known to be too small to allow reliable analysis. Performing analyses that might inform on which patients were most likely to benefit from gabapentin treatment would require efficacy data together with detailed assessment of the exact nature and type of neuropathic pain at the individual participant level (Tölle 2013). No such data were expected to be available.
Results
Description of studies
Results of the search
In the previous version of this review we considered 36 studies in 37 reports examining oral gabapentin, involving 5483 participants with chronic neuropathic pain in various different conditions, mainly PHN, PDN, or mixed neuropathic pain.
Updated database searches from January 2014 to 17 January 2017 identified 107 potentially relevant reports in CENTRAL, 237 in MEDLINE, and 484 in Embase. No additional studies were identified in clinical trials registries or reference lists of included studies or reviews.
After de‐duplication and screening of titles and abstracts, we obtained the full text of seven reports. Of these, we included three new studies, with 468 participants (Atkinson 2016; Cohen 2015; Gong 2008). We also identified one report that was a secondary analysis of a study that was already included (Calkins 2016, see Zhang 2013), and two reports of pooled analyses of two studies that were already included (Freeman 2015 and Metha 2016, see Sang 2013; Wallace 2010).
One study that was previously ongoing has now completed. We could not identify a published article for this study, but we did find a synopsis with some results on the pharmaceutical company's website (NCT00904202). The study satisfied our inclusion criteria and was therefore included in this review (62 participants). We could not find any updated information on the remaining three ongoing studies (Fleckstein 2009; IRCT201212019014N14; NCT00674687).
We reassessed and excluded one study that had been included in the earlier review (Ho 2009). This small (18 participants) cross‐over study in small fibre sensory neuropathy used a one‐week titration period, followed by one week at the maximum dose and one week of wash‐out, then crossed over to repeat the sequence with the other treatment. We excluded it because of the very short treatment periods (only one week at a stable dose), there was some uncertainty about the dosing schedule (although the maximum dose was clearly stated), and participants could take additional gabapentin to a maximum of 1200 mg daily if they required rescue medication and paracetamol was inadequate. There was no information about the use of this additional gabapentin, or how data from participants using it were analysed. Two further studies from the previous review are in conditions not now considered neuropathic pain (Kimos 2007; Van de Vusse 2004).
Figure 1 illustrates the flow of studies for this update.
Included studies
This update therefore includes four additional studies involving 530 participants, bringing the total for the review to 37 studies involving 5914 participants, although not all of the participants took all the study medication, and not all the participants were included in results.
The majority of studies involved participants with PHN and PDN. Other neuropathic pain conditions studied were spinal cord injury, phantom limb pain, cancer, nerve injury pain, CRPS, HIV, and radicular leg pain. Four studies enrolled participants with a mixture of types of neuropathic pain.
Four studies (Irving 2009; Sandercock 2012; Sang 2013; Wallace 2010) used a gastroretentive, extended‐release formulation of gabapentin, and four others (Backonja 2011; Harden 2013; Rauck 2013a; Zhang 2013) used an extended‐release prodrug, gabapentin encarbil.
Twenty‐five studies had a parallel‐group design and 12 had a cross‐over design (Bone 2002; Gilron 2005; Gilron 2009; Gordh 2008; Gorson 1999; Harden 2013; Levendoglu 2004; Morello 1999; Rao 2007; Rintala 2007; Smith 2005; Tai 2002). We used whatever data were available from the cross‐over studies, including first period or multiple periods, though there are major issues with what constitutes the ITT denominator where there are significant withdrawals.
Parallel‐group trials were larger than cross‐over trials. The 25 parallel‐group studies involved 5298 participants (mean 204, median 162 participants, range 26 to 452), while the 12 cross‐over studies involved 621 participants (mean 48, median 40 participants, range 14 to 120). Not all studies reported the results on an ITT basis, and this was particularly the case for cross‐over studies with multiple comparisons.
Twenty‐eight studies either described enrolment processes that were not enriched, or had no exclusion criteria that would raise the possibility of enrichment (Straube 2008). Seven studies were partially enriched (Caraceni 2004; Irving 2009; Rice 2001; Sang 2013; Serpell 2002) or excluded participants with previous inadequate response to treatment with gabapentin or pregabalin as an exclusion criterion, which may have led to enrichment (Cohen 2015; Wallace 2010). Two studies enriched for tolerance to gabapentin, but not response (Backonja 2011; Harden 2013), which is probably equivalent to partial enrichment. Participants in these two studies were treated with gabapentin encarbil, a prodrug of gabapentin; these are analysed alongside the other studies, but with a view to sensitivity analysis.
Three studies reported using baseline observation carried forward (BOCF) imputation for the primary outcome (Sandercock 2012; Sang 2013; Wallace 2010), sometimes alongside last observation carried forward (LOCF) analyses, and one reported using BOCF imputation for the responder analyses (Rauck 2013b). Thirty‐one studies either made no mention of an imputation method for missing data (19) or declared use of LOCF (12). Others performed analyses on completers only (Atkinson 2016 (for responder analysis); Rintala 2007), and one presented results without imputation (Rao 2007).
Details of all eligible studies are given in the 'Characteristics of included studies' table.
Excluded studies
We excluded 25 studies from this review. The earlier review excluded 21 studies because they were open‐label studies, were studies in chronic conditions not considered for this review, investigated related acute conditions or preventive strategies, or did not have an appropriate comparator.
We excluded one new study because it did not have an appropriate comparator and did not appear to be blinded (Ding 2014). We reassessed and excluded three previously included studies, one because of its short duration, use of gabapentin as rescue medication, and unclear methods of analysis (Ho 2009), and two because definitions of chronic neuropathic pain had changed, and these two were now outside the current definitions (Kimos 2007; Van de Vusse 2004).
Risk of bias in included studies
The risk of bias assessments identified that adequate sequence generation and allocation concealment were often inadequately reported. Additional risk of bias also derived from studies being small, and rarely describing how efficacy data were handled on withdrawal (Figure 2).
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All studies were described as randomised, but only 22 adequately described the method used to generate the random sequence. Only 19 adequately described how the sequence was concealed. We judged studies with inadequate descriptions at unclear risk, although in most cases the methods were probably adequate but not reported.
Blinding
All studies were described as double‐blind (participants, who also assessed outcomes, and personnel), but six did not adequately describe the method used to achieve and maintain blinding (Gorson 1999; Harden 2013; Mishra 2012; Perez 2000; Sandercock 2012; Simpson 2001). We judged studies with inadequate descriptions at unclear risk, although in most cases the methods were probably adequate but not reported.
Incomplete outcome data
We judged four studies at high risk of bias because they reported only on participants who completed treatment phases (Atkinson 2016; Rintala 2007), did not report groups or reasons for withdrawal and used LOCF imputation where there was 7% attrition (Gong 2008), or did not report all expected outcomes in the results synopsis and used LOCF imputation (NCT00904202). We judged five studies at low risk of bias for this domain (Rao 2007; Rauck 2013b; Sandercock 2012; Sang 2013; Wallace 2010), and the remaining 28 at unclear risk, mainly because they used LOCF imputation for early withdrawals.
Other potential sources of bias
We judged one study to be at low risk of bias due to study size (more than 200 participants each treatment arm; Sang 2013), 18 at unknown risk, with between 50 and 200 participants per treatment arm, and 18 of the included studies at high risk of bias due to study size smaller than 50 participants per treatment arm.
Effects of interventions
See: Summary of findings for the main comparison Gabapentin compared with placebo for postherpetic neuralgia: efficacy; Summary of findings 2 Gabapentin compared with placebo for peripheral diabetic neuropathy: efficacy; Summary of findings 3 Gabapentin compared with placebo for neuropathic pain (all conditions pooled): adverse events and withdrawals
Appendix 7 contains details of withdrawals, efficacy, and adverse events in the individual studies.
Efficacy
We report efficacy results where data were available, or where there was sufficient information to justify analysis, defined as information from 200 participants or more, ideally from at least two studies.
Analyses 1.1 to 1.5 show results for the following outcomes: at least 50% reduction in pain (Analysis 1.1; Figure 3); Patient Global Impression of Change (PGIC) very much improved (Analysis 1.2; Figure 4); PGIC much or very much improved (Analysis 1.3; Figure 5); IMMPACT outcome of substantial improvement in pain (Analysis 1.4; Figure 6); IMMPACT outcome of at least moderate improvement in pain (Analysis 1.5; Figure 7).

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.1 At least 50% pain reduction over baseline.
Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.2 Very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.3 Much or very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.4 IMMPACT outcome of substantial improvement.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.5 IMMPACT outcome of at least moderate improvement.
Postherpetic neuralgia (PHN)
Of the 11 studies in PHN, nine (Backonja 2011; Gong 2008; Irving 2009; NCT00475904; Rice 2001; Rowbotham 1998; Sang 2013; Wallace 2010; Zhang 2013) had a placebo control, and two (Chandra 2006; Harden 2013) an active control only. All nine placebo‐controlled studies had a parallel‐group design, with study duration of four to 12 weeks; daily gabapentin doses varied between 1800 mg and 3600 mg, while the dose of gabapentin encarbil was 1200 mg to 3600 mg daily.
In seven studies reporting the outcome, at least 50% pain intensity reduction occurred in 33% of participants given gabapentin and 19% of those given placebo by the end of the study, with considerable consistency between studies (Summary of results A; Figure 8). Available data on dosing regimens were too sparse to establish a dose‐response relationship. A number of outcomes consistent with IMMPACT recommendations for substantial and moderate benefit were reported in two or more placebo‐controlled studies, and the results showed gabapentin at doses of 1800 mg daily or more, or gabapentin encarbil at 1200 mg daily, to be more effective than placebo (Summary of results A). For a PGIC of much or very much improved, 39% of participants achieved this level of improvement with gabapentin and 29% with placebo. Other outcomes are reported in Summary of results A.

Postherpetic neuralgia: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200 mg‐3600 mg daily, or placebo
Only two of these studies (Gong 2008; Rice 2001; 24% of participants) used a standard formulation of gabapentin, and removing them from the analysis did not significantly change the result. Similarly, removing the two studies using gabapentin encarbil (Backonja 2011; Zhang 2013; 21% of participants) did not affect the result. There were insufficient data for subgroup analyses based on dose or duration of studies.
We assessed the quality of evidence as moderate. Results were consistent between studies, but there were uncertainties and differences between dosing and dosing schedules, formulation, and imputation methods used.
Summary of results A. Efficacy outcomes with gabapentin in postherpetic neuralgia (PHN)
| Number of | Percent with outcome | |||||
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio | NNT |
| Substantial benefit | ||||||
| At least 50% pain intensity reduction | 7 | 2031 | 33 | 19 | 1.7 (1.4 to 2.0) | 6.9 (5.5 to 9.4) |
| PGIC very much improved | 2 | 563 | 15 | 6 | 2.7 (1.5 to 4.8) | 11 (7.0 to 22) |
| Any definition of substantial benefit (at least 50% pain intensity reduction or PGIC very much improved) | 8 | 2260 | 32 | 17 | 1.8 (1.5 to 2.1) | 6.7 (5.4 to 8.7) |
| Moderate benefit | ||||||
| At least 30% pain intensity reduction | 2 | 529 | 54 | 38 | 1.4 (1.1 to 1.7) | 6.5 (4.0 to 16) |
| PGIC much or very much improved | 7 | 2013 | 39 | 29 | 1.3 (1.2 to 1.5) | 9.7 (6.9 to 16) |
| Any definition of moderate benefit (at least 25% pain intensity reduction or PGIC much or very much improved) | 8 | 2260 | 46 | 25 | 1.8 (1.6 to 2.0) | 4.8 (4.1 to 6.0) |
In the active controlled study involving 76 participants, gabapentin at doses of up to 2700 mg daily was compared to nortriptyline at doses of up to 150 mg daily over nine weeks. At least 50% improvement in pain over baseline using a VAS pain scale was achieved by 13/38 (34%) with gabapentin and 14/38 (37%) with nortriptyline, broadly in line with event rates in placebo‐controlled studies (Chandra 2006). Harden 2013 compared two dosing regimens of gabapentin encarbil in previous low dose treatment failures and found that about 13% did respond at the 50% pain reduction level.
Painful diabetic neuropathy (PDN)
Seven of the nine studies in PDN were of parallel‐group design (Backonja 1998; CTR 945‐1008; CTR 945‐224; Perez 2000; Rauck 2013a; Sandercock 2012; Simpson 2001); two had a cross‐over design (Gorson 1999; Morello 1999). Eight had a placebo comparator, while one (Morello 1999) had an active control only. Seven placebo‐controlled parallel‐group studies had a study duration between four and 14 weeks; all but one (Sandercock 2012) of seven weeks or longer. Daily gabapentin doses varied between 600 mg and 3600 mg; doses below 1200 mg were used in two studies, 900 mg daily as the only gabapentin dose in one (Gorson 1999), and 600 mg daily in one arm of another (CTR 945‐224). Gabapentin encarbil at doses of 1200 and 3600 mg daily was compared with pregabalin 300 mg daily and placebo in one study (Rauck 2013a).
At least 50% pain intensity reduction occurred in 38% of participants given gabapentin and 23% of those given placebo by the end of the study, with considerable consistency between studies (Summary of results B; Figure 9). Available data on dosing regimens were too sparse to establish a dose‐response relationship. A number of outcomes consistent with IMMPACT recommendations for substantial and moderate benefit were reported in two or more placebo‐controlled studies, and the results showed gabapentin at doses of 1200 mg daily or more to be more effective than placebo (Summary of results B). For PGIC much or very much improved; 50% of participants achieved this level of improvement with gabapentin and 30% with placebo. We obtained very similar results when we omitted data from Simpson 2001 because of concerns one peer reviewer expressed about this study in a previous version of the review; no other efficacy outcome data were included from this study. Other outcomes are reported in Summary of results B.

Painful diabetic neuropathy: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200‐3600 mg daily, or placebo
Two studies (Rauck 2013a; Sandercock 2012; 35% of participants) used the gabapentin encarbil or gastroretentive formulations. Removing these from the analysis did not change the result. There were insufficient data for subgroup analyses based on dose or duration of studies.
We assessed the quality of evidence as moderate. Results were consistent between studies, but there were uncertainties and differences between dosing and dosing schedules, formulation, and imputation methods used.
Summary of results B. Efficacy outcomes with gabapentin in painful diabetic neuropathy (PDN) (1200 mg daily or greater)
| Number of | Percent with outcome | |||||
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio | NNT |
| Substantial benefit | ||||||
| At least 50% pain intensity reduction | 6 | 1331 | 38 | 23 | 1.7 (1.4 to 2.0) | 6.6 (5.0 to 9.7) |
| PGIC very much improved | 2 | 408 | 24 | 14 | 1.9 (1.3 to 3.0) | 9.6 (5.5 to 35) |
| Any definition of substantial benefit (at least 50% pain intensity reduction or PGIC very much improved) | 6 | 1331 | 38 | 23 | 1.7 (1.4 to 2.0) | 6.6 (5.0 to 9.7) |
| Moderate benefit | ||||||
| At least 30% pain intensity reduction | 2 | 744 | 54 | 43 | 1.2 (1.1 to 1.5) | 9.4 (5.6 to 29) |
| PGIC much or very much improved | 5 | 695 | 50 | 30 | 1.7 (1.4 to 2.0) | 4.9 (3.6 to 7.6) |
| PGIC much or very much improved (excluding Simpson 2001) | 4 | 635 | 51 | 31 | 1.6 (1.3 to 2.0) | 5.1 (3.7 to 8.3) |
| Any definition of moderate benefit (at least 30% pain intensity reduction or PGIC much or very much improved) | 7 | 1439 | 52 | 37 | 1.4 (1.3 to 1.6) | 6.6 (4.9 to 9.9) |
Gabapentin 600 mg daily produced lesser effects than 1200 mg and 2400 mg daily in a study that compared them (CTR 945‐224). In one placebo‐controlled cross‐over study involving 40 randomised participants, moderate or excellent pain intensity reduction was achieved by 17/40 (43%) with gabapentin 900 mg daily over six weeks, compared with 9/40 (23%) with placebo (Gorson 1999).
In one active‐controlled study involving 25 participants, gabapentin at 1800 mg daily was compared to amitriptyline 75 mg daily over six weeks. Complete or a lot of pain relief was achieved by 6/21 (29%) with gabapentin and 5/21 (24%) with amitriptyline (Morello 1999).
Mixed neuropathic pain
One exploratory study (Rauck 2013b) examined the effects of intrathecal gabapentin in participants with chronic, intractable non cancer pain, the majority (147/170; 86%) of whom were classified as having pain of neuropathic or mixed types. Three different doses (1 mg, 6 mg, and 30 mg daily) were compared with placebo. There was no significant reduction in group mean pain scores within and between groups over the 22 day treatment period. The number of participants experiencing at least 30% reduction in pain was 4/42, 4/41, 1/41, and 2/44 for the 1 mg, 6 mg, 30 mg, and placebo groups, respectively.
Four studies examined the effects of oral gabapentin in mixed neuropathic painful conditions (Gilron 2005; Gilron 2009; NCT00904202; Serpell 2002); two included participants with PHN and PDN (Gilron 2005; Gilron 2009); in another the most common conditions were CRPS and PHN (Serpell 2002); and the fourth study enrolled participants with PHN, DN, CRPS, carpel tunnel syndrome, HIV neuropathy, idiopathic sensory neuropathy, and other peripheral neuropathy (proportions not reported, NCT00904202). One had a parallel‐group comparison with placebo over eight weeks (Serpell 2002), and one had a parallel‐group comparison with placebo, lidocaine patch, and gabapentin in combination with lidocaine patch over five weeks (NCT00904202). The others had cross‐over designs that included placebo and morphine alone and in combination with gabapentin over five weeks (Gilron 2005), and nortriptyline alone or in combination with gabapentin over six weeks (Gilron 2009).
One parallel‐group comparison with placebo used gabapentin titrated to a maximum of 2400 mg daily in 305 participants (Serpell 2002). Only for the PGIC outcome of much or very much improved was there a significant benefit of gabapentin (Summary of results C).
Summary of results C. Efficacy outcomes with gabapentin in mixed neuropathic pain (Serpell 2002)
| Number of | Percent with outcome | |||||
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio | NNT |
| At least 50% pain intensity reduction | 1 | 305 | 21 | 14 | 1.5 (0.9 to 2.4) | not calculated |
| PGIC very much improved | 1 | 305 | 12 | 6 | 2.0 (0.9 to 4.3) | not calculated |
| PGIC much or very much improved | 1 | 305 | 31 | 14 | 2.2 (1.4 to 3.4) | not calculated |
The other parallel‐group comparison used gabapentin titrated to 1800 mg daily over one week in 62 participants (NCT00904202), and did not report any of our specified efficacy outcomes. It did report the group mean change in pain intensity at the end of the study as 44% for gabapentin alone, 39% for lidocaine patch alone, 50% for the combination, and 26% for placebo (ITT analysis assumed). The number of participants who were satisfied or very satisfied with treatment were 65% for gabapentin alone, 69% for lidocaine patch alone, 69% for the combination, and 64% for placebo. There were no statistically significant differences between treatment groups.
One placebo‐controlled cross‐over study (Gilron 2005) over five weeks provided results for moderate pain relief for participants who completed a given treatment period. Gabapentin alone (target dose 3200 mg daily), morphine alone (target dose 120 mg daily), and the combination (target dose gabapentin 2400 mg plus 60 mg morphine daily) were significantly better than placebo (Summary of results D). These results were calculated from the numbers and percentages with a moderate response. The total was larger than the 57 randomised, because some participated in more than one treatment arm.
Summary of results D. Efficacy outcomes with gabapentin in mixed neuropathic pain (Gilron 2005)
| Number of | Percent with outcome | |||||
| At least moderate pain relief | Studies | Participants | Gabapentin, morphine, of their combination | Placebo | Risk ratio | NNT |
| Gabapentin alone | 1 | 96 | 61 | 25 | 2.5 (1.5 to 4.2) | not calculated |
| Morphine alone | 1 | 96 | 80 | 25 | 3.2 (1.9 to 5.2) | not calculated |
| Gabapentin plus morphine | 1 | 93 | 78 | 25 | 3.1 (1.9 to 5.1) | not calculated |
The other cross‐over study compared gabapentin alone (target dose 3600 mg daily), nortriptyline (target dose 100 mg daily) and the combination (target dose 3600 mg gabapentin plus 100 mg nortriptyline daily) over six weeks (Gilron 2009). Pain intensity was significantly lower with the combination, by less than 1 point out of 10 on a numerical rating pain scale.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Radicular leg pain
One study compared gabapentin, titrated to a maximum of 3600 mg daily, with placebo over 12 weeks in 108 participants, 46 of whom had radicular pain (Atkinson 2016). Although results were not reported separately for these participants, the investigators did report that there was no difference between those with and without radicular pain. In an exploratory analysis of completers, 36% of participants in both groups reported a 30% or more decrease in pain intensity, and 26% and 29% reported a 50% or more decrease with gabapentin (34 participants) and placebo (38 participants), respectively. There was also no difference between groups for 'patient estimation of pain improvement' at the end of the study.
Another study compared gabapentin, titrated to a target of 1800 to 3600 mg daily, with epidural steroid over three months (Cohen 2015). The study reported only group mean decreases in average and worst leg pain at the end of treatment, which ranged from 1.6 to 2.7, with large variation within groups. There were no significant differences between the groups.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Spinal cord injury
The efficacy of gabapentin in spinal cord injury pain at maximum doses of 1800 mg or 3600 mg daily was compared with placebo in three cross‐over trials (Levendoglu 2004; Rintala 2007; Tai 2002) over periods of four and eight weeks. None of the studies reported dichotomous outcomes equivalent to moderate or substantial pain relief.
One eight‐week study randomised 20 participants to a maximum of 3600 mg gabapentin daily or placebo over eight weeks (Levendoglu 2004) and reported a 62% average fall in pain with gabapentin compared with a 13% fall with placebo.
A second eight‐week study randomised 38 participants to a maximum of 3600 mg gabapentin daily, amitriptyline 150 mg daily, or placebo over eight weeks (Rintala 2007). It claimed statistical superiority for amitriptyline for the 22 participants completing all three phases, and no benefit of gabapentin over placebo.
The final study comparing gabapentin with placebo over four weeks in seven participants had no interpretable results (Tai 2002).
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Nerve injury pain
A single cross‐over study evaluated the efficacy of gabapentin at a maximum of 2400 mg daily compared with placebo over five‐week treatment periods (Gordh 2008). Among the 98 participants of the 120 randomised who completed both treatment periods, at least 50% pain intensity reduction was achieved by 13 (13%) with gabapentin and 9 (9%) with placebo, which did not reach statistical significance. At least 30% pain intensity reduction was achieved by 29 (29%) with gabapentin and 19 (19%) with placebo, which did not reach statistical significance.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Phantom limb pain
Two cross‐over studies evaluated the efficacy of gabapentin compared with placebo in phantom limb pain (Bone 2002; Smith 2005). Bone 2002 randomised 19 participants to a maximum of 2400 mg gabapentin daily, or the maximum tolerated dose, with six‐week treatment periods. Using an ITT approach, weekly VAS pain scores were lower at week six only with gabapentin, but not at any other time, nor with categorical pain measures. Smith 2005 randomised 24 participants to gabapentin titrated to a maximum daily dose of 3600 mg. A "meaningful decrease in pain" (the top of a five‐point scale) was achieved by 13 participants (54%) with gabapentin and 5 (21%) with placebo.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Cancer‐related neuropathic pain
Three studies examined gabapentin in the short term in cancer‐related neuropathic pain (Caraceni 2004; Mishra 2012; Rao 2007). A parallel‐group study (Caraceni 2004) randomised 121 participants to titration to a maximum of gabapentin 1800 mg daily or placebo, with 10 days of treatment. The average pain intensity was somewhat lower with gabapentin than with placebo, but the number of participants described as having pain under control was very similar with both treatments after six days, with 50% to 60% with pain under control over six to 10 days. A cross‐over study (Rao 2007) compared gabapentin titrated to 2700 mg daily with placebo in chemotherapy‐induced neuropathic pain over three weeks. There was no significant difference between gabapentin and placebo, but the study did recruit participants both with pain or sensory loss or paraesthesia, and baseline pain scores were only about 4/10 on a numerical rating scale. The study probably lacked sensitivity to detect any difference.
The third study compared gabapentin 1800 mg daily with pregabalin 600 mg daily and amitriptyline 100 mg daily for a total of four weeks (Mishra 2012). No dichotomous data were reported; a decrease in pain scores in all groups in all weeks was reported, together with a morphine‐sparing effect and improvement in functional capacity. Morphine‐sparing and functional capacity were significantly better with pregabalin than the other treatments.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
HIV‐associated sensory neuropathies
A single parallel‐group study compared gabapentin titrated to 2400 mg daily with placebo over four weeks in 24 participants with painful HIV‐associated neuropathies (Hahn 2004). On average, pain and sleep improved substantially with both gabapentin and placebo, though the time courses differed. After four weeks, there was no difference in median pain scores, though the placebo response had an unusual time course in 11 participants.
We assessed the quality of evidence as very low with a small number of studies, participants, and events.
Withdrawals (see Summary of results E)
We pooled data from participants with different types of neuropathic pain for analyses of withdrawals.
All‐cause withdrawals
Twenty‐two studies with 4617 participants reported on withdrawals for any cause, which occurred in 20% of participants with gabapentin at daily doses of 1200 mg or more, and in 19% with placebo (Analysis 2.1). The risk ratio was 1.0 (0.92 to 1.2). The NNH was not calculated.
Adverse event withdrawals
Twenty‐two studies with 4346 participants reported on adverse event withdrawals, which occurred in 11% of participants with gabapentin at daily doses of 1200 mg or more, and in 8.2% with placebo (Analysis 2.2). The risk ratio was 1.4 (1.1 to 1.7), and the NNH was 30 (20 to 66).
Lack of efficacy withdrawals
Fifteen studies with 3559 participants reported on lack of efficacy withdrawals, which occurred in 1.9% of participants with gabapentin at daily doses of 1200 mg or more, and in 3.3% with placebo (Analysis 2.3). The risk ratio was 0.57 (0.37 to 0.88), and the number needed to treat to prevent one withdrawal (NNTp) NNTp was 73 (41 to 360).
We assessed the quality of evidence for withdrawals as high, based on a reasonable number of events and generally good reporting.
Adverse events (see Summary of results E)
We pooled data from participants with different types of neuropathic pain for analyses of adverse events.
Participants experiencing at least one adverse event
Eighteen studies with 4279 participants reported on participants experiencing at least one adverse event, which occurred in 63% of participants with gabapentin at daily doses of 1200 mg or more, and in 49% with placebo (Analysis 3.1). The risk ratio was 1.3 (1.2 to 1.4), and the NNH was 7.5 (6.1 to 9.6). We assessed the quality of evidence as moderate, based on a reasonable number of events and consistency, but limited quality of reporting adverse events.
Serious adverse events
NIneteen studies reported on 3948 participants experiencing a serious adverse event, which occurred in 3.2% of participants with gabapentin at daily doses of 1200 mg or more, and in 2.8% with placebo (Analysis 3.2). The risk ratio was 1.2 (0.83 to 1.7). The NNH was not calculated. We assessed the quality of evidence as moderate due to the limited number of events.
Particular adverse events
Somnolence, drowsiness, or sedation was reported as an adverse event in 20 studies with 4288 participants, and it occurred in 14% of participants with gabapentin at doses of 1200 mg daily or more, and in 5.2% with placebo (Analysis 3.3). The risk ratio was 2.8 (2.3 to 3.5), and the NNH was 11 (9.4 to 14).
Dizziness was reported as an adverse event in 21 studies with 4739 participants, and it occurred in 19% of participants with gabapentin at doses of 1200 mg daily or more, and in 6.6% with placebo (Analysis 3.4). The risk ratio was 2.9 (2.4 to 3.4), and the NNH was 8.0 (7.0 to 9.4).
Peripheral oedema was reported as an adverse event in 12 studies with 3325 participants, and it occurred in 6.7% of participants with gabapentin at doses of 1200 mg daily or more, and in 1.7% with placebo (Analysis 3.5). The risk ratio was 4.1 (2.7 to 6.4), and the NNH was 20 (16 to 27).
We assessed the quality of evidence for these outcomes as moderate. While there was a reasonable number of events, definitions of adverse events and reporting was not consistent.
Ataxia or gait disturbance was reported as an adverse event in four studies with 510 participants. It occurred in 14% of participants with gabapentin at doses of 1200 mg daily or more, and in 2.6% with placebo (Analysis 3.6). The risk ratio was 5.5 (2.5 to 12), and the NNH was 8.5 (6.1 to 14).
We assessed the quality of evidence for ataxia as low. There was a small number of studies and events.
Summary of results E: Withdrawals and adverse events with gabapentin (1200 mg daily or more) compared with placebo
| Number of | Percent with outcome | |||||
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio | NNH |
| Withdrawal ‐ all‐cause | 22 | 4617 | 20 | 19 | 1.0 (0.91 to 1.2) | Not calculated |
| Withdrawal due to adverse events | 22 | 4346 | 11 | 8.2 | 1.4 (1.1 to 1.7) | 30 (20 to 66) |
| At least one adverse event | 18 | 4279 | 63 | 49 | 1.3 (1.2 to 1.4) | 7.5 (6.1 to 9.6) |
| Serious adverse event | 19 | 3948 | 3.2 | 2.8 | 1.2 (0.83 to 1.7) | Not calculated |
| Somnolence/drowsiness | 20 | 4288 | 14 | 5.2 | 2.8 (2.3 to 3.5) | 11 (9.4 to 14) |
| Dizziness | 21 | 4739 | 19 | 6.6 | 2.9 (2.4 to 3.4) | 8.0 (7.0 to 9.4) |
| Peripheral oedema | 12 | 3325 | 6.7 | 1.7 | 4.1 (2.7 to 6.4) | 20 (16 to 27) |
| Ataxia/gait disturbance | 4 | 510 | 14 | 2.6 | 5.5 (2.5 to 12) | 8.5 (6.1 to 14) |
| Outcome | Studies | Participants | Gabapentin | Placebo | Risk ratio | NNTp |
| Withdrawal ‐ lack of efficacy | 15 | 3559 | 1.9 | 3.3 | 0.57 (0.37 to 0.88) | 73 (41 to 360) |
Death
Deaths were rare in these studies. Five deaths occurred in PHN studies; three with placebo: one in 231 participants (Sang 2013), one in 116 (Rowbotham 1998) and one in 133 (Wallace 2010); two with gabapentin: one in 223 participants (Rice 2001), and one in 107 (Irving 2009). An unpublished study (CTR 945‐1008) reported two deaths: one of 200 participants treated with gabapentin, and one of 189 treated with placebo. A further study reported two deaths in 152 participants taking placebo (Serpell 2002). Overall, three deaths occurred with gabapentin and five with placebo. We assessed the quality of evidence as very low due to the very small number of events.
Discussion
Summary of main results
Gabapentin is a reasonably effective treatment for a variety of neuropathic pain conditions. It has been demonstrated to be better than placebo across all studies for IMMPACT outcomes of substantial and at least moderate improvement, producing almost identical results for all trials and those in parallel‐group studies lasting six weeks or longer. Numbers needed to treat for an additional beneficial outcome (NNTs) were between 5 and 7 for substantial and at least moderate improvement in PHN and PDN (moderate‐quality evidence). Results were consistent across the major neuropathic pain conditions tested, though gabapentin was tested only in small numbers in uncommon neuropathic pain conditions. The review concentrated on doses of gabapentin of 1200 mg daily or greater, though a wide range of fixed doses and dose titration regimens were used.
Gabapentin was tested in nine different chronic pain conditions generally considered to be neuropathic in origin. For only three neuropathic pain conditions was there sufficient information to be confident that it worked satisfactorily, namely PHN, PDN, and mixed neuropathic pain, itself principally, though not exclusively, PHN and PDN.
Benefit was balanced by more withdrawals due to adverse events (high‐quality evidence), and participants taking gabapentin experienced more adverse events (high‐quality evidence), including somnolence, dizziness, peripheral oedema, and gait disturbance than did those taking placebo (moderate‐quality evidence). Serious adverse events were no more common with gabapentin than placebo (moderate‐quality evidence), and death was an uncommon finding in these studies (very low‐quality evidence).
Overall completeness and applicability of evidence
Efficacy and adverse event outcomes were not consistently reported across the studies, and this limited the analyses to some extent. However, for the most important efficacy and adverse event outcomes, analyses across all conditions were mostly based on between 1000 and about 4700 participants. All the larger studies (typically those with more than 100 participants) reported some efficacy outcome equivalent to one or both of the IMMPACT outcomes of at least moderate or substantial benefit. Clearly, analysis at the level of the individual participant would facilitate a more robust estimate (Moore 2013a). Such analysis can also demonstrate a link between benefit in terms of pain and benefit in other outcomes, including quality of life (Hoffman 2010).
Possible sources of bias that could have affected the results of the review included the following.
-
Duration ‐ NNT estimates of efficacy in chronic pain studies tend to increase (get worse) with increasing duration (Moore 2010e). However, limiting studies to those of six weeks or longer did not change the main efficacy outcomes, mainly because most participants were in longer‐duration studies.
-
Outcomes may affect estimates of efficacy, but the efficacy outcomes chosen were of participants achieving the equivalent of IMMPACT‐defined moderate or substantial improvement, and it is likely that lesser benefits, such as 'any benefit' or 'any improvement', are potentially related to lesser outcomes, though this remains to be clarified.
-
The dose of gabapentin used differed between studies, in terms of maximum allowable dose, and whether the dose was fixed, titrated to effect, or titrated up to the maximum irrespective of beneficial or adverse effects. We chose to pool data irrespective of dose, within broad limits, because it was the only practical way to deal with dose in a pooled analysis, and because of a lack of good evidence of any clear dose‐response effect for gabapentin in neuropathic pain.
-
In some circumstances cross‐over trials have been shown to exaggerate treatment effects in comparison with parallel‐group designs (Khan 1996), but the extent is unclear, and it is unlikely to be the source of major bias (Elbourne 2002). Withdrawals from cross‐over studies meant that any results were likely to be per protocol for completers rather than a true ITT analysis. Parallel‐group studies were larger than cross‐over studies, and dominated the analyses in terms of number of participants. The 25 parallel‐group studies involved 5298 participants (median 204), while the 12 cross‐over studies involved 621 participants (median 40 participants). Additionally, few cross‐over studies reported outcomes that could be used in the analyses.
-
The absence of publication bias (unpublished trials showing no benefit of gabapentin over placebo) can never be proven. However, we can calculate the number of participants in studies of zero benefit (risk ratio of 1) required for the absolute benefit to reduce beneficial effects to a negligible amount (Moore 2008). If an NNT of 10 were considered a level that would make gabapentin clinically irrelevant, then the number of participants with zero benefit would be 2448 for a moderate response and 1113 for a substantial response in PHN, and 741 for a moderate response and 887 for a substantial response in PDN. With median study size for parallel‐group studies of about 200 participants, this would require a minimum of seven unavailable studies in PHN and four in PDN. While not impossible, this seems unlikely given the paucity of new data in the last three years.
There is one important unknown for most studies, namely whether the definition of response in the trials included only participants who had both an analgesic response and were able to take gabapentin. If response included an LOCF assessment of efficacy from those who discontinued, this could have affected the results (Moore 2012a). LOCF tends to overestimate treatment effects when adverse event withdrawals with drug are higher than that with placebo. For gabapentin, the excess adverse withdrawal over placebo was about 3%. This is not likely to result in significant overestimation in treatment effect (Moore 2012a). In a similar situation, duloxetine produced little different NNTs using LOCF and BOCF in four different chronic pain conditions (Moore 2014b).
Another issue is how to deal with relatively short term, small, multiple cross‐over studies that intensively study participants on a daily basis (Gilron 2005; Gilron 2009), and do not report outcomes of clinical relevance (participants with adequate pain relief), but rather average pain scores, whose relevance has been questioned because of underlying skewed distributions (McQuay 1996; Moore 2010a; Moore 2013a). This study design can provide useful and clinically relevant information, such as the relatively rapid onset of effect of therapies in neuropathic pain, or how individual patients respond to several different drugs. However, they are difficult to include in pooled analyses, and their small size and brevity come with significant potential biases. Small size has become a particular issue, with increasing association of small study size with positive bias (Dechartres 2013; Dechartres 2014; Fanelli 2017; Nguyen 2017). Cochrane Reviews have received criticism for being overly confident with inadequate data (AlBalawi 2013; Brok 2009; Roberts 2015; Turner 2013).
There were almost no data for direct comparisons with other active treatments. It is questionable how important direct comparisons may be; they compare average efficacy rates between different active therapies, but individual people may respond to one drug, but not another (Moore 2013b).
Finally, there was no way to incorporate into the review important observations on the timing and consistency of analgesia with gabapentin in neuropathic pain. In PHN, individual participant‐level pooled analyses of several large trials have demonstrated that, judged by the proportion of participants with a 1 out of 10 point pain intensity reduction, around 20 to 40 days is needed for effects to be seen (Rauck 2013c). Early response, defined as a 30% pain intensity reduction or greater, was predictive of response after 10 weeks, while pain intensity reduction of less than 10% at week 5 was the best early predictor of lack of response at week 10 (Jensen 2012).
While there was considerable information about withdrawals and adverse events, rare but serious adverse events could not be addressed in these studies. We are aware that erectile dysfunction has been a cause for concern for younger men treated with antiepileptic drugs for epilepsy (Smalldone 2004), and anorgasmia has been reported with gabapentin (Perloff 2011). Adverse event reporting of erectile dysfunction or anorgasmia in these trials was sparse or not present, and the effects of gabapentin on sexual function may not be well represented. Moreover, the included studies did not address gabapentin misuse (Evoy 2017; Quintero 2017).
Quality of the evidence
The studies included in this review covered a large number of different painful conditions. The main quality issues involve reporting of outcomes of interest, particularly dichotomous outcomes equivalent to IMMPACT, appropriate analysis of data for participants who withdrew, and better reporting of adverse events. The earliest study was published in 1998, and the past decade or so has seen major changes in clinical trial reporting. The studies themselves appear to be well‐conducted, and individual participant analysis could overcome some of the shortcomings of reporting.
Potential biases in the review process
We know of no potential biases in the review process.
Agreements and disagreements with other studies or reviews
The results for neuropathic pain in this review have not changed noticeably since the last updates in 2014 and 2011.
Summary of results F. Comparison of NNTs (95% CI) from previous and present reviews
| 2005 review | 2011 update | 2014 Update | 2017 update | ||||
| Outcomes | Any improvement | IMMPACT moderate benefit | IMMPACT substantial benefit | IMMPACT moderate benefit | IMMPACT substantial benefit | IMMPACT moderate benefit | IMMPACT substantial benefit |
| All studies | 4.3 (3.5 to 5.7) | 5.8 (4.8 to 7.2) | 6.8 (5.6 to 8.7) | Not calculated in this review | Not calculated in this review | ||
| PHN | 3.9 (3.0 to 5.7) | 5.5 (4.3 to 7.7) | 7.5 (5.2 to 14) | 5.7 (4.6 to 7.5) | 6.8 (5.4 to 9.3) | 5 (5 to 6) | 7 (6 to 9) |
| PDN | 2.9 (2.2 to 4.3) | 8.1 (4.7 to 28) | 5.8 (4.3 to 9.0) | 6.6 (4.9 to 9.9) | 5.9 (4.6 to 8.3) | 7 (5 to 10) | 7 (5 to 10) |
Other systematic reviews
A number of guidelines based on systematic reviews have concluded that gabapentin is helpful in neuropathic pain (Finnerup 2015; Moulin 2014; NICE 2013; SIGN 2013). In PHN, a systematic review found that higher gabapentin doses may not provide greater benefit, but may increase the risk of adverse events (Wang 2017).
One other review has provided NNTs for gabapentin in different neuropathic pain conditions based on 50% pain relief, quoting NNTs of 4.7 and 4.3 for neuropathic pain and peripheral pain, and 4.6 for PHN and 3.9 for PDN (Finnerup 2005). A systematic review of therapies for PHN considered gabapentin effective, with an NNT of 4.6 (Hempenstall 2005). These efficacy estimates are more optimistic than NNTs for the IMMPACT substantial benefit calculated for this review, and more optimistic than NNTs calculated for the same outcome of at least 50% pain relief for PHN of 5.7 and PDN of 5.8. The use of more stringent criteria for efficacy, and availability of more information from longer duration studies has led to more conservative efficacy results. Both pregabalin and duloxetine have NNTs in the region of 5 to 6 for at least 50% pain relief over eight to 12 weeks compared with placebo in PHN and PDN (Lunn 2009; Moore 2009; Sultan 2008).
A number of other systematic reviews have examined the efficacy of gabapentin in neuropathic pain. Systematic reviews of gabapentin for neuropathic pain in spinal cord injury (Tzellos 2008) and fibromyalgia (Hauser 2009; Tzellos 2010) found no more studies than those reported here. An examination of the effects of enriched enrolment found no more studies, and gave similar results for withdrawals and adverse events based on a more limited data set (Straube 2008). A review comparing gabapentin and duloxetine in PDN was limited to two gabapentin studies, was statistical in nature, and restricted to average changes in some efficacy parameters (Quilici 2009). The most directly relevant review was a comparison between gabapentin and tricyclic antidepressants (Chou 2009), in which a meta‐analysis of six placebo‐controlled gabapentin studies in PHN, PDN, and mixed neuropathic pain was performed. Using a mixture of outcomes the relative benefit compared with placebo was 2.2, similar to the benefits found for the 'all studies' analysis and for analyses for PHN, PDN, and mixed neuropathic pain in this review. Phillips 2010 examined the same single study of gabapentin (Hahn 2004) as part of a wider review of pharmacological interventions for HIV neuropathy and came to similar conclusions. The UK NICE guidance on pharmacological management of neuropathic pain has gabapentin as one of four drugs to try initially, with early switching if pain relief is not forthcoming (NICE 2013).
One further review in the public domain (Perry 2008) was performed as part of a legal case in the USA ending in 2009. Perry 2008 considered similar outcomes to this review; NRS or VAS pain score was given hierarchical priority between 50% or greater reduction in pain score (higher priority) and PGIC (lower priority) mainly because it was the pre‐defined primary end point in almost all studies, and for some studies it was difficult to determine how the secondary endpoints were manipulated during post hoc changes in statistical analysis plans. The Perry conclusions are very similar to those of the present review. The likely real differences would lie in the fact that Perry excluded Perez 2000 and Simpson 2001, and did not have access to Sandercock 2012, Irving 2009, and Wallace 2010.
Perry's conclusion on effectiveness was a clinical judgement based on balancing NNH against NNT, using the Cochrane Glossary definition of effectiveness, and presuming that inherent biases in the studies (enrichment, exclusion of many typical real world patients) implied that on balance the benefit of gabapentin use on average does not exceed the harm, which is a somewhat different issue than addressed by this Cochrane Review.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.1 At least 50% pain reduction over baseline.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.2 Very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.3 Much or very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.4 IMMPACT outcome of substantial improvement.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.5 IMMPACT outcome of at least moderate improvement.

Postherpetic neuralgia: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200 mg‐3600 mg daily, or placebo

Painful diabetic neuropathy: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200‐3600 mg daily, or placebo

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 1 At least 50% pain reduction over baseline.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 2 Very much improved.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 3 Much or very much improved.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 4 IMMPACT outcome of substantial improvement.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 5 IMMPACT outcome of at least moderate improvement.

Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 1 All‐cause withdrawal.

Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 2 Adverse event withdrawal.

Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 3 Lack of efficacy withdrawal.

Comparison 3 Adverse events, Outcome 1 At least one adverse event.

Comparison 3 Adverse events, Outcome 2 Serious adverse events.

Comparison 3 Adverse events, Outcome 3 Somnolence.

Comparison 3 Adverse events, Outcome 4 Dizziness.

Comparison 3 Adverse events, Outcome 5 Peripheral oedema.

Comparison 3 Adverse events, Outcome 6 Ataxia or gait disturbance.
| Gabapentin compared with placebo for postherpetic neuralgia: efficacy | ||||||
| Patient or population: adults with postherpetic neuralgia Settings: community Intervention: gabapentin ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% reduction in pain or equivalent | 330 per 1000 | 190 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.9 (5.5 to 9.4) | 7 studies 2031 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any substantial pain benefit | 320 per 1000 | 170 per 1000 | RR 1.8 (1.5 to 2.1) NNT 6.7 (5.4 to 8.7) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Patient Global Impression of Change much or very much improved | 390 per 1000 | 290 per 1000 | RR1.3 (1.2 to 1.5) NNT 9.7 (6.9 to 16) | 7 studies 2013 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any at least moderate pain benefit (includes Gong 2008 at 25% pain relief) | 46 per 1000 | 25 per 1000 | RR 1.8 (1.6 to 2.0) NNT 4.8 (4.1 to 6.0) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): †Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for peripheral diabetic neuropathy: efficacy | ||||||
| Patient or population: adults with peripheral diabetic neuropathy Settings: community Intervention: ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% pain intensity reduction | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of substantial benefit (at least 50% pain intensity reduction or PGIC very much improved) | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| PGIC much or very much improved | 500 per 1000 | 300 per 1000 | RR 1.7 (1.4 to 2.0) NNT 4.9 (3.6 to 7.6) | 5 studies 695 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of moderate benefit (at least 30% pain intensity reduction or PGIC much or very much improved) | 520 per 1000 | 370 per 1000 | RR 1.4 (1.3 to 1.6) NNT 6.6 (4.9 to 9.9) | 7 studies 1439 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for neuropathic pain (all conditions pooled): adverse events and withdrawals | ||||||
| Patient or population: adults with neuropathic pain Settings: community Intervention: gabapentin 1800 mg to 3600 mg daily (gabapentin encarbil 1200 mg to 3600 mg daily) Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNH (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| Participants experiencing at least one adverse event | 630 per 1000 | 490 per 1000 | RR 1.3 (1.2 to 1.4) NNH 7.5 (6.1 to 9.6) | 18 studies 4279 participants | Moderate | Many events. Unlikely new research would change this finding |
| Adverse event withdrawals | 110 in 1000 | 82 in 1000 | RR 1.4 (1.1 to 1.7) NNH 30 (20 to 66) | 22 studies 4346 participants | High | Unlikely new research would change this finding |
| Serious adverse events | 32 in 1000 | 28 in 1000 | RR 1.2 (0.83 to 1.7) NNH not calculated | 19 studies 3948 participants | Moderate | Small number of events but no suggestion of difference |
| Death | 3 in max 3603 exposed | 5 in max 2377 exposed | Not calculated | Not calculated | Very low | Few events, relatively short duration for drug possibly taken over periods of years |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain reduction over baseline Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Postherpetic neuralgia | 7 | 2031 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 2.00] |
| 1.2 Painful diabetic neuropathy | 6 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.02] |
| 1.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 1.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 2 Very much improved Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Postherpetic neuralgia | 2 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.51, 4.82] |
| 2.2 Painful diabetic neuropathy | 2 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 2.99] |
| 2.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.92, 4.28] |
| 2.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [1.39, 9.31] |
| 3 Much or very much improved Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Postherpetic neuralgia | 7 | 2013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.16, 1.50] |
| 3.2 Painful diabetic neuropathy | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.36, 2.03] |
| 3.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.38, 3.41] |
| 3.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.26, 3.90] |
| 4 IMMPACT outcome of substantial improvement Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postherpetic neuralgia | 8 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.49, 2.07] |
| 4.2 Painful diabetic neuropathy | 6 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.02] |
| 4.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 4.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 4.5 Phantom pain | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [1.10, 6.16] |
| 5 IMMPACT outcome of at least moderate improvement Show forest plot | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postherpetic neuralgia | 8 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.56, 2.00] |
| 5.2 Painful diabetic neuropathy | 7 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.24, 1.59] |
| 5.3 Mixed neuropathic pain | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.49, 2.95] |
| 5.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.92, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause withdrawal Show forest plot | 22 | 4617 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.16] |
| 2 Adverse event withdrawal Show forest plot | 22 | 4346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.14, 1.67] |
| 3 Lack of efficacy withdrawal Show forest plot | 15 | 3559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 18 | 4279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.22, 1.36] |
| 2 Serious adverse events Show forest plot | 19 | 3948 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.71] |
| 3 Somnolence Show forest plot | 20 | 4288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.27, 3.50] |
| 4 Dizziness Show forest plot | 21 | 4739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [2.40, 3.44] |
| 5 Peripheral oedema Show forest plot | 12 | 3325 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [2.66, 6.39] |
| 6 Ataxia or gait disturbance Show forest plot | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.53 [2.49, 12.28] |

